SUMMARY
Metastatic dissemination is often initiated by the reactivation of an embryonic development program referred to as epithelial-mesenchymal transition (EMT). The transcription factor SNAIL promotes EMT and elicits associated pathological characteristics, such as invasion, metastasis and stemness. To better understand the post-translational regulation of SNAIL, we performed a luciferase-based genome-wide E3 ligase siRNA library screen and identified SCF-FBXO11 as an important E3 which targets SNAIL for ubiquitylation and degradation. Furthermore, we discovered that SNAIL degradation by FBXO11 is dependent on Serine-11 phosphorylation of SNAIL by protein kinase D1 (PKD1). FBXO11 blocks SNAIL-induced EMT, tumor initiation and metastasis in multiple breast cancer models. These findings establish the PKD1-FBXO11-SNAIL axis as a mechanism of post-translational regulation of EMT and cancer metastasis.
INTRODUCTION
The majority of cancer-related deaths can be attributed to the spread of cancer cells to distant vital organs (Wan et al., 2013). Epithelial-mesenchymal transition (EMT), a crucial process in embryonic development that allows epithelial cells to lose apical-basal polarity and cell-cell contacts while gaining mesenchymal phenotypes, is believed to be utilized by cancer cells to gain mobility and invasiveness during metastasis (Brabletz, 2012; De Craene and Berx, 2013; Nieto, 2011). A hallmark of EMT is the functional loss of E-cadherin, while additional cellular changes, such as reduced expression of epithelial markers cytokeratins and ZO-1, and the upregulation of mesenchymal markers N-cadherin, Vimentin and Fibronectin, are also frequently observed.
SNAIL protein is among the first transcription factors discovered to repress the CDH1 gene (encoding E-cadherin protein) transcription and induce EMT (Batlle et al., 2000; Cano et al., 2000). Recent studies suggest that SNAIL has a much broader impact on cancer progression. In mammary epithelial cells, overexpression of SNAIL induces EMT, coupled with increased tumor initiating properties (Mani et al., 2008). In melanoma, SNAIL promotes tumor metastasis by suppressing host immune surveillance (Kudo-Saito et al., 2009). SNAIL also cooperates with chromatin-modifying enzymes to inhibit fructose-1,6-biphosphatase (FBP1) expression, which results in increased glucose uptake, macromolecule biosynthesis, and maintenance of ATP production under hypoxic conditions (Dong et al., 2013). Given the importance of SNAIL in cancer progression, it is not surprising that many signaling pathways have been implicated in the regulation of SNAIL gene expression, including TGF-β, NOTCH and WNT pathways, reactive oxygen species (ROS), and hypoxic stress (reviewed by De Craene and Berx, 2013). A better understanding of the regulatory mechanisms for SNAIL will provide critical information on how to block EMT and related processes in cancer progression.
Many transcription factors are labile proteins with short half lives and are actively degraded through the ubiquitin-proteosome pathway. Interestingly, in many cases, E3 ligases recognize and ubiquitylate transcription factor substrates by interacting with their transcriptional activation/repressor domain. This allows the coupling of the transcriptional activity with the protein degradation process to prevent hyper-activation of important transcription factors (Muratani and Tansey, 2003). For instance, Mdm2 binds to the transactivation domain of p53, targeting it for ubiquitylation and degradation (Momand et al., 1992). Likewise, E3 ligase FBW7 interacts with KLF5 transactivation domain for its degradation (Liu et al., 2010; Zhao et al., 2010). Although prior studies have identified two E3 ubiquitin ligases, FBXL14 (Ppa in Xenopus) (Lander et al., 2011) and FBXW1 (also called β-TRCP or BTRC) (Zhou et al., 2004), responsible for SNAIL ubiquitylation and degradation, neither of them interacts with the SNAG transcriptional repression domain of SNAIL. This leads us to suspect that there may be additional critical E3 ligase(s) that targets the SNAIL protein for degradation through interacting with the SNAG domain. Identifying such E3 ligase(s) and the signaling events regulating SNAIL ubiquitination will provide possible new windows for therapeutic targeting of SNAIL.
RESULTS
A luciferase based screening system for SNAIL protein turnover regulation
To confirm that the SNAIL protein is post-translationally controlled by the ubiquitin-proteosome system (UPS) in breast cancer, we first blocked protein synthesis using cycloheximide (CHX) and pulse-chased the SNAIL protein in the SUM1315 human breast cancer cell line. Indeed, SNAIL is degraded rapidly and became undetectable within 4 hr of CHX treatment (Figure 1A and Figure S1A). Furthermore, treatment of cells with proteosomal inhibitor MG132 increased stable SNAIL protein level, confirming SNAIL is degraded through the UPS (Figure 1A and Figure S1A).
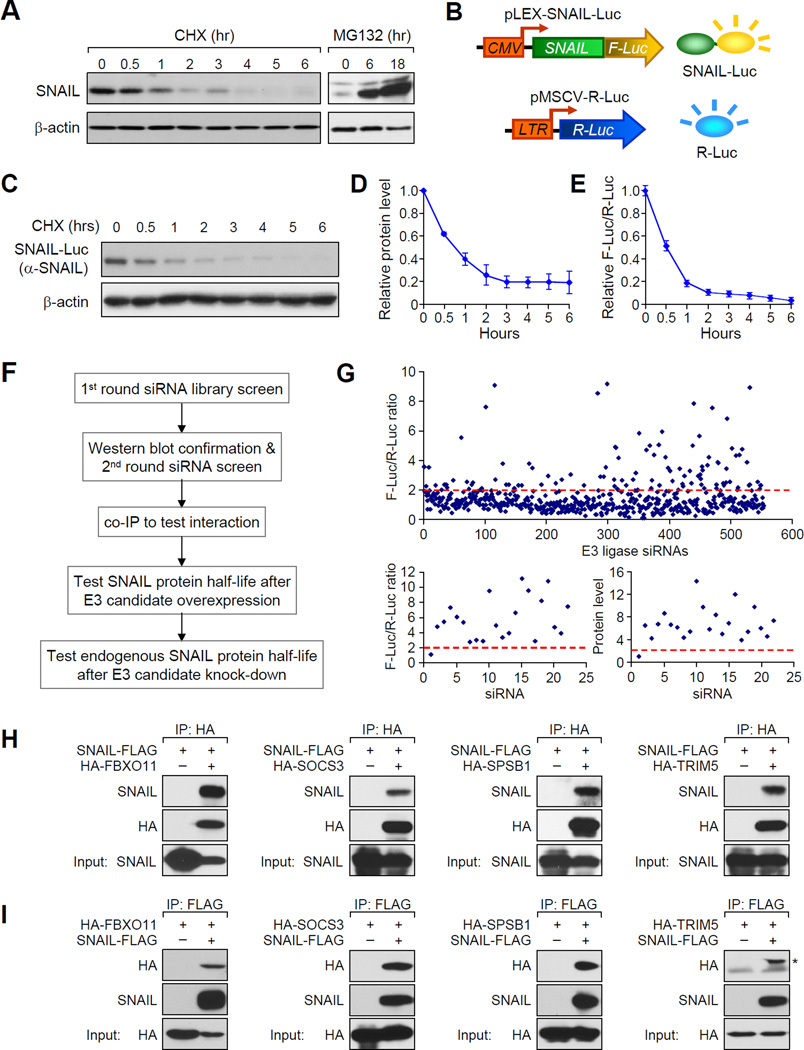
Figure 1. A genome-wide functional screen for E3 ubiquitin ligase(s) targeting SNAIL protein for degradation.
(A) In SUM1315 cells, endogenous SNAIL protein was detected by western blot after CHX (left panel) or MG132 treatment (right panel) for the indicated hours. Western blot data was quantified in Figure S1A. (B) Illustration of dual-luciferase reporter screening system for SNAIL-targeting E3 ligases. (C, D) CHX pulse-chase experiment demonstrated the degradation of SNAIL-F-Luc fusion protein in SUM-SNAIL-Luc/R-Luc cells. Western blot data was quantified in (D). Data is presented as mean ± SEM. (E) Degradation of SNAIL-F-Luc protein was monitored by normalized luciferase activity measurement. Data is presented as mean ± SEM. (F) Experimental procedure flow chart for identification of the E3 ubiquitin ligase(s) targeting SNAIL protein. See text for details. (G) Luciferase based siRNA library screen against human E3 ligases identified multiple E3 candidates, when knocked down in SUM-SNAIL-Luc/R-Luc cells, increased luciferase activity by more than 2 fold (upper panel). A second round of siRNA screening (lower left panel) and immunoblotting (lower right panel) was performed for confirmation of candidates. (H, I) 293T cells were transfected according to each panel labeling. Co-IP experiment was performed using either an HA antibody to pull down HA-tagged E3 ligase proteins (H) or an anti-FLAG antibody against SNAIL-FLAG protein. See also Figure S1 and Table S1 and S2.
The identification of E3 ligases responsible for certain protein substrate’s degradation process has traditionally relied on serendipitous discoveries or candidate gene approaches. In order to identify potential E3 ligase(s) responsible for SNAIL degradation in an unbiased manner, we designed a luciferase based siRNA screening strategy, which can be easily adapted in future discovery efforts to identify new E3 ligase-protein substrate pairs. We fused the SNAIL protein coding sequence in frame with the Firefly luciferase gene coding sequence (SNAIL-Luc for abbreviation) to produce the SNAIL-Luciferase fusion reporter protein (Figure 1B). This reporter allowed us to monitor the SNAIL protein stable level and its degradation dynamics by monitoring the luciferase activity. The SUM1315 cell line was transduced first with a lentiviral vector containing the SNAIL-Luc fusion gene, and subsequently with the renilla-Luciferase retrovirus to generate a dual luciferase reporter cell line with renilla luciferase (R-Luc) serving as an internal control. This cell line is denoted as “SUM-SNAIL-Luc/R-Luc” to facilitate descriptions below (Figure 1B). The SNAIL-Luc fusion protein is localized in the nucleus (Figure S1B and S1C) and has a degradation dynamics similar to that of the endogenous SNAIL protein (Figure 1C, 1D and Figure S1D). In contrast, the F-Luc protein alone is very stable (Figure S1E and S1F). The luciferase activity of SNAIL-Luc fusion protein is also readily detectable by the standard firefly luciferase reporter assay, and correlates with the fusion protein level (Figure 1E). Thus, the firefly luciferase activity in this cell line reliably represents the stable level of the SNAIL-Luc protein, while R-Luc activity is used as internal control for cell number and viability.
siRNA library screening identified potential E3 ligases targeting SNAIL protein for degradation
We followed the procedures outlined in Figure 1F to identify potential E3 ligase candidates for SNAIL protein. We first knocked down individual human E3 ligase by pooled siRNA (3 siRNAs/gene) in SUM-SNAIL-Luc/R-Luc cells and looked for E3 ligase genes, whose knockdown (KD) resulted in more than 2-fold increase of luciferase activity of the SNAIL-Luc fusion protein (Figure 1F). The cell lysates from these positive hits were immunoblotted for SNAIL-Luc fusion protein to confirm protein stabilization. Positive E3 ligase hits were then examined by a second round of siRNA screening to confirm the results. We then cloned all the positive E3 ligase genes and determined whether they can interact with SNAIL protein by co-immunoprecipitation assay (co-IP) and reciprocal co-IP for further confirmation. Only the proteins that interact with SNAIL were considered as E3 candidates for SNAIL protein and selected for further functional testing to determine whether they can affect exogenous and endogenous SNAIL protein degradation.
The E3 siRNA library screening revealed that siRNA-mediated inhibition of multiple E3 ligase genes increased the level of SNAIL-Luc protein. Among the 554 known and predicted human E1, E2, and E3 ligases (Table S1), siRNA knockdown of more than 100 genes increased the luciferase activity by more than 2 fold (Figure 1G, upper panel). This large initial pool of potential hits may include false positive candidates that do not directly regulate SNAIL protein stability, therefore further validation of these candidates are needed. Interestingly, in this screening, knockdown of FBXW1, a previously known E3 ligase for SNAIL (Zhou et al., 2004), increased SNAIL-Luc protein level by 2.6 fold, while no change was observed after knockdown of FBXL14, another SNAIL E3 identified in the Xenopus model (Lander et al., 2011). The cell lysates from these initial positive hits were then subjected to immunoblotting for SNAIL-Luc fusion protein (Figure S1G and S1H), for most of which an upregulation of SNAIL-Luc protein was observed. Among them, 21 E3 candidates, when individually knocked down, increased SNAIL-Luc protein level by at least 6 fold in luciferase assay or in immunoblot analyses, the stringent criteria we set for identifying potential candidates (Table S2). A second round of siRNA screening for these 21 genes was performed, confirming that 21 siRNAs were all able to consistently increase the luciferase activity and protein stable level (Figure 1G, lower panels and Figure S1I). We cloned the cDNA coding sequences of 13 candidate genes into a lentiviral expression vector with an N-terminal HA-epitope (Figure S1J). The remaining 8 genes were not cloned successfully. To test the interactions between these 13 E3 ubiquitin ligases and SNAIL, we performed co-IP experiment to test interactions between these E3 candidates with SNAIL. Only four E3 ligases were able to interact with SNAIL, including FBXO11, SOCS3, SPSB1, and TRIM5 (Figure S1J). This result was first confirmed by repeating the co-IP experiment for these four E3 ligase proteins using HA antibody (Figure 1H), and then by reciprocal IP of the SNAIL-FLAG protein with an anti-FLAG antibody (Figure 1I). We thus focused on these four E3 genes in our further functional study.
FBXO11 is a bona fide E3 ligase targeting SNAIL protein for ubiquitylation and degradation
As an initial step in functionally testing these 4 E3 ubiquitin ligase candidates, we co-transfected the SNAIL-Luc gene with individual E3 genes and examined the SNAIL-Luc protein stable level. FBXO11 expression strongly reduced stable SNAIL-Luc protein level by up to 70% (Figure S2A and S2B). In CHX pulse-chase analysis of SNAIL-Luc, FBXO11 accelerated SNAIL-Luc protein degradation, while SOCS3, SPSB1, and TRIM5 had only modest or negligible effects on SNAIL-Luc protein degradation (Figure 2A; Note that in this and other similar pulse-chase figures, western blot images with different exposure time were used so that the basal SNAIL-Luc protein band intensity at time = 0 hr is the same across the experimental group, in order to illustrate the difference in the kinetics of degradation).
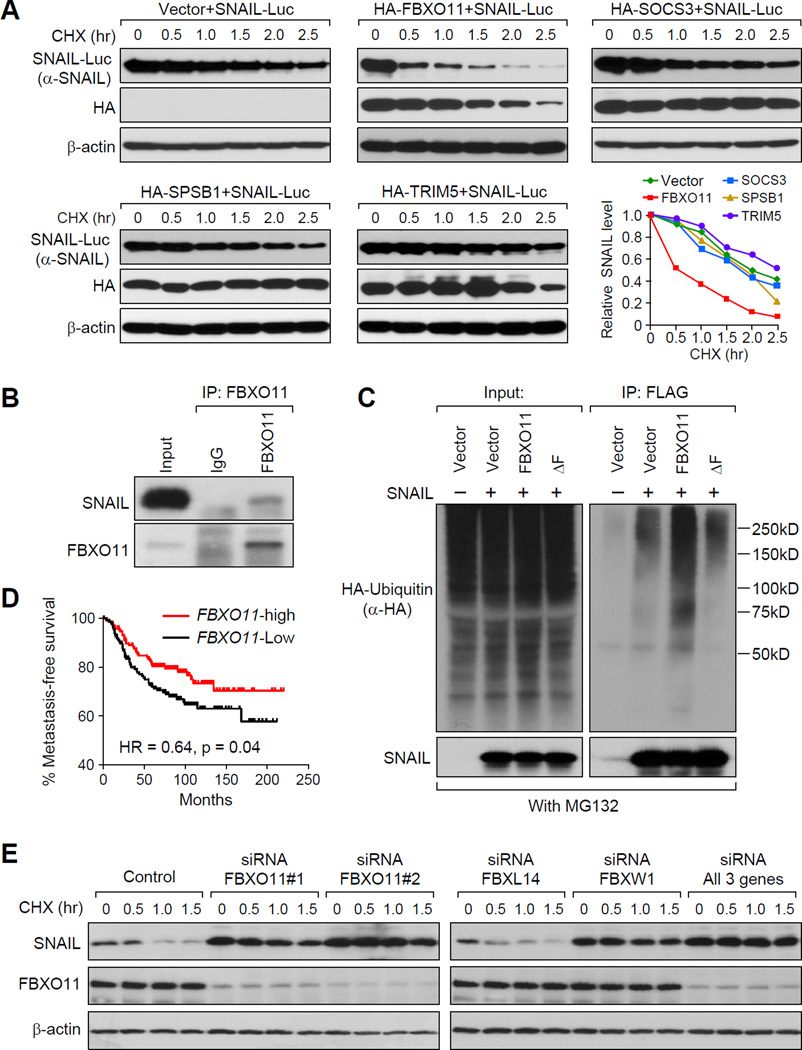
Figure 2. FBXO11 is a bona fide E3 ligase targeting SNAIL protein for ubiquitination and degradation.
(A) Four E3 ligase candidates or control pLEX-vector were co-transfected with SNAIL-Luc plasmid into 293T cells and CHX pulse-chase assay was performed. Western blot data was quantified using ImageJ software. (B) LM2 cells were treated with 10 µM MG132 for 6 hr before the cell lysate was immunoprecipitated with FBXO11 antibody or IgG control and subjected to western blot analysis. (C) 293T cells were co-transfected by HA-Ub, SNAIL-Flag, together with vector, FBXO11 or FBXO11 - ΔF. Cells were treated with MG132 for 6 hr before cell lysates were immunoprecipitated using a denature IP protocol to pull down SNAIL protein, and the polyubiquitylated SNAIL protein was detected by anti-HA antibody. (D) Kaplan-Meier plots of metastasis-free survival of patients stratified by expression of FBXO11. Data obtained from the NKI breast cancer dataset (van de Vijver et al., 2002). (E) MCF10A cells were transfected with indicated siRNAs, followed by the CHX pulse-chase assay. Quantification data is shown in Figure S2P See also Figure S2.
To directly test the effect of FBXO11 on endogenous SNAIL protein degradation, we generated FBXO11-overexpressing stable cell lines using SUM1315 and the LM2 subline of MDA-MB-231 (Minn et al., 2005). In both cell lines, we observed dramatic increase of endogenous SNAIL protein degradation (Figure S2C–F). Next, we confirmed the interaction between endogenous SNAIL and FBXO11 proteins by co-IP experiments using MG132 treated LM2 cell lysate (Figure 2B). To further investigate whether FBXO11 functions as a bona fide E3 ligase that ubiquitylates the SNAIL protein, we co-transfected 293T cells with SNAIL-FLAG, FBXO11, and HA-Ubiquitin and treated the cells with MG132 for 6 hr to prevent protein degradation before performing an ubiquitylation assay. A significant increase of polyubiquitynated SNAIL protein was observed in FBXO11 transfected cells, whereas an F-box (SCF complex formation domain) deletion mutant of FBXO11 (FBXO11-ΔF) was not able to increase the SNAIL ubiquitylation (Figure 2C).
Since SNAIL protein has been reported as poor-prognosis marker with its high expression correlated with worse patient outcome (Moody et al., 2005), we hypothesized that E3 ligase(s) targeting SNAIL should be a good-prognosis factor. Indeed, using the NKI295 breast cancer data set (van de Vijver et al., 2002), we found that higher FBXO11 gene expression is significantly correlated with longer metastasis-free survival (Figure 2D), while SPSB1, SOCS3, and TRIM5 are not correlated with metastasis-free survival (Figure S2G).
To test the effect of reducing endogenous FBXO11 expression on SNAIL protein degradation, we used siRNAs to knock down FBXO11 gene expression in MCF10A cells. The stable level of SNAIL protein was found to be upregulated by more than 2 fold after the siRNA treatment (Figure 2E, left panel, without CHX treatment). Furthermore, SNAIL protein was much more stable after FBXO11 KD in CHX pulse-chase assay (Figure 2E). Stabilization of SNAIL after FBXO11 KD was also observed in the LM2 cell line (Figure S2H, I). Taken together, our results suggested that SCF-FBXO11 is a bona fide E3 ubiquitin ligase targeting SNAIL protein for ubiquitylation and degradation.
We confirmed that, two previously reported E3s for SNAIL, FBXW1 and FBXL14 also interact with SNAIL, while other closely related F-box family members did not bind to SNAIL (Figure S2J–L). Interestingly, FBXW1 promoted SNAIL protein degradation, while FBXL14 has no effect on SNAIL stability (Figure S2M, N). Conversely, FBXW1 KD increased SNAIL stability while no effect was observed after FBXL14 KD. Importantly, simultaneous KD of FBXO11, FBXW1 led to increased SNAIL stabilization compared to either FBXO11 or FBXW1 KD alone (Figure 2E, right panel, Figure S2O, P). These results suggest that both FBXO11 and FBXW1 are capable of promoting SNAIL degradation and may function collectively to control SNAIL stability.
FBXO11 blocks SNAIL-induced EMT and tumorigenesis
SNAIL has been shown to induce EMT and increases tumor initiating capability in non-transformed or oncogene transformed HMLE human mammary epithelial cells (Mani et al., 2008). We tested whether FBXO11 can block SNAIL-induced EMT by transducing Neu-tranformed HMLEN cells with either SNAIL alone or together with FBXO11. SNAIL expression induced strong EMT phenotypes in HMLEN cells, with loss of E-CADHERIN and upregulation of VIMENTIN and FIBRONECTIN (Figure 3A, S3A and S3B). When SNAIL and FBXO11 were co-expressed in HMLEN cells, the stable level of SNAIL protein was significantly decreased compared to cells only expressing SNAIL (Figure S3A). The reduced level of SNAIL led to the reversal of the EMT phenotype in HMLEN cells, with the gain of E-CADHERIN at the cell-cell junctions and the loss of VIMENTIN and FIBRONECTIN expression (Figure 3A, S3A and S3B).
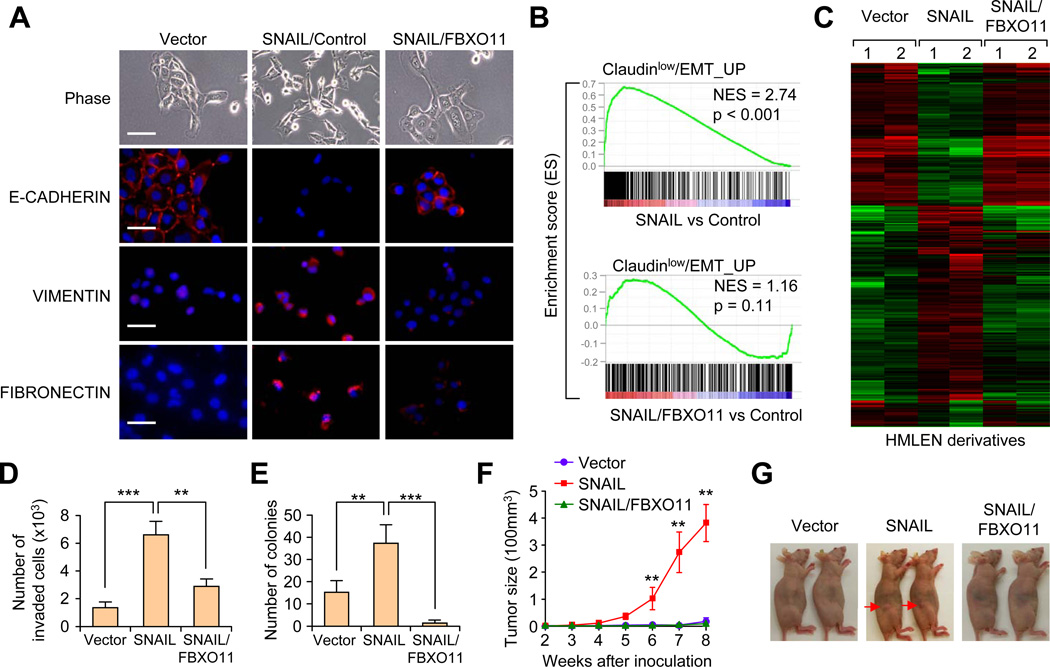
Figure 3. FBXO11 blocks SNAIL-induced EMT and reduces tumorigenesis capability in HMLEN cells.
(A) Phase contrast and immunofluorescent images of HMLEN cells with stable overexpression of SNAIL or SNAIL together with FBXO11. Scale bar, 100 µm in phase contrast images and 25 µm in IF images. (B) GSEA analysis of enriconment of EMT gene signature in HMLEN cells after overexpression of SNAIL with or without FBXO11. (C) Heatmap was generated using hierarchical clustering of the microarray data for the indicated HMLEN cell lines. The 2327 gene probes used for clustering were those showing >2 folds expression changes upon SNAIL overexpression. Experiment was performed in duplicates. (D) Boyden chamber invasion assay of HMLEN cells with SNAIL or SNAIL/FBXO11 overexpression. The data were shown as mean of collected data from 3 triplicate wells of three independent experiments. Data is presented as mean ± SEM, **p < 0.01, ***p < 0.001. (E) Quantification of colony formation assay results for the indicated HMLEN cell lines. Data is presented as mean ± SEM, **p < 0.01, ***p < 0.001. (F, G) 2×104 of the indicated HMLEN cells were orthotopically injected into nude mice (n = 8) and primary mammary tumor growth was measured weekly after injection, with representative tumor images shown in (G). Data represent mean ± s.d **p < 0.01 by two-tailed Student’s t test. See also Figure S3.
To evaluate the global transcriptomic changes associated with expression of SNAIL and FBXO11, we performed gene expression microarray profiling and gene set enrichment analysis (GSEA). Using four previously published EMT/cancer stem cell-related gene signatures (see Supplemental Information), we found that the SNAIL-overexpressing cells significantly enriched for EMT gene signatures (Figure 3B using the Hennessy et al. Claudin-low/EMT signature, and data not shown using the other three signatures), while SNAIL/FBXO11 cells lost such enrichments. We then generated a gene expression heatmap using a list of 2327 genes showing >2 fold differential expression between the SNAIL-overexpressing cells and the control cells. As shown in Figure 3C, FBXO11 expression largely reversed the gene expression changes induced by SNAIL. Taken together, these results suggest that FBXO11 can block SNAIL induced EMT program and associated global gene expression pattern.
Since cells that undergo EMT often gain migratory and invasive capability, we tested matrigel invasion ability of these cells. As expected, SNAIL expression significantly increased the invasiveness of HMLEN cells while FBXO11 expression blocked SNAIL-induced invasion (Figure 3D). HMLE cells that have undergone EMT have also been reported to acquire breast cancer stem cell characteristics (Mani et al., 2008). Indeed, HMLEN-SNAIL cells showed a significant increase of the CD44high/CD24low population, while FBXO11 decreased this population (Figure S3C, S3D). In line with the population shift, HMLEN-SNAIL showed increased colony formation efficiency, while FBXO11 suppressed SNAIL-induced colony formation (Figure 3E, S3E). To further test the effect of FBXO11 on tumor initiation, we injected 20,000 tumor cells orthotopically into the mammary fat pads of nude mice. HMLEN cells had minimal tumor forming capability; however, significant formation of primary tumors can be observed 8 weeks after inoculation of SNAIL-overexpressing HMLEN cells. Again, FBXO11 completely blocked SNAIL-induced tumor initiation (Figure 3F, G). In aggregate, our results demonstrate a prominent effect of FBXO11 in inhibiting SNAIL-induced EMT and tumorigenesis in the HMLEN model, and this effect is likely due to FBXO11 mediated ubiquitylation and degradation of SNAIL protein.
FBXO11 blocks SNAIL-induced EMT and lung metastasis in vivo
To test the functional impact of FBXO11 on SNAIL-induced EMT in breast cancer metastasis, we decided to use epithelial-like 4T1 and EpRas mouse mammary tumor cell lines, which are sensitive to Snail-induced EMT and have much more robust primary tumor growth capability than HMLEN. As expected, FBXO11 expression accelerated SNAIL degradation in 4T1 cells (Figure S4A). SNAIL overexpression in epithelial-like 4T1 and EpRas cell lines induced an EMT-like phenotype, including the reduction of E-cadherin and upregulation of N-cadherin or Vimentin expression at both mRNA and protein levels (Figure 4A, 4B, and Figure S4B–D). Strikingly, FBXO11 expression blocked SNAIL-induced EMT in both cell lines. Gene expression profiling revealed that the enrichment of EMT gene signatures in SNAIL overexpressing cells was reversed by FBXO11 overexpression (Figure 4C). Likewise, overexpression of FBXO11 suppressed the SNAIL expression signature in 4T1 cells (Figure 4D). Consistent with cellular phenotype and gene expression changes, the increased invasive capability of SNAIL-overexpressing 4T1 cells was inhibited by FBXO11 (Figure S4E). Importantly, while SNAIL overexpression did not affect primary tumor growth when 105 of tumor cells were injected orthotopically (Figure S4F), its expression significantly increased spontaneous lung metastasis in vivo (Figure 4E and 4F). The lung metastasis-promoting effect of SNAIL was reversed after FBXO11 overexpression (Figure 4E and 4F), again without any significant alteration in primary tumor growth rate (Figure S4F, G).
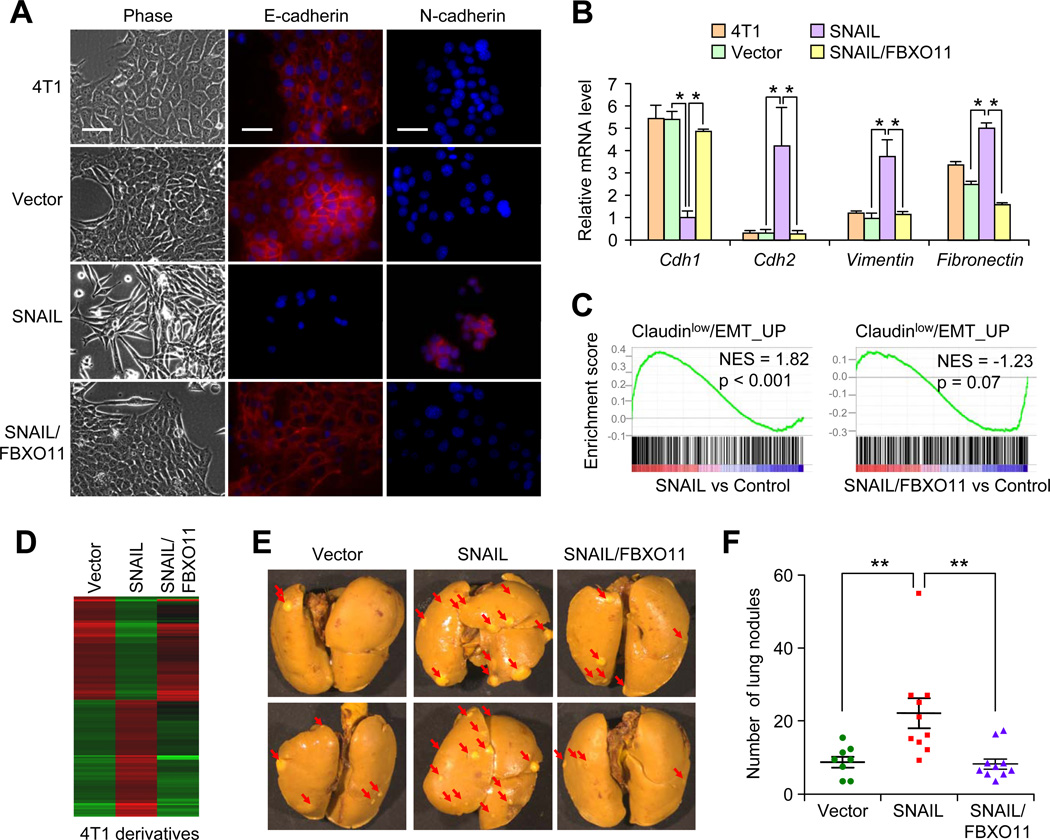
Figure 4. FBXO11 blocked SNAIL-induced EMT and metastasis in 4T1 breast cancer cell line.
(A) Phase contrast and IF images for 4T1 cells transduced with indicated lentiviruses. SNAIL-induced strong EMT program in 4T1 cells with downregulation of E-cadherin and upregulation of N-cadherin expression, while FBXO11 blocked SNAIL-induced EMT. Scale bars, 25µm. (B) qRT-PCR analysis of EMT marker mRNAs in the indicated 4T1 cell lines. Results were normalized to GAPDH mRNA level. Data represent average ± SEM, *p < 0.05 by Student's t test. (C) GSEA analysis demonstrated FBXO11 expression inhibited SNAIL induced EMT gene signature in 4T1 cells. (D) Heatmap was generated using hierarchical clustering. The 1400 gene probes used for clustering were those showing >2 folds expression changes in SNAIL overexpressing cells. (E) 105 cells was orthotopically injected into Balb/c mice (n = 10). Mice were sacrificed 3 weeks later. Representative lung metastasis nodule images were presented. (F) Numbers of lung metastasis lesions of mice injected with the indicated 4T1 cell lines. **p < 0.01 by Mann–Whitney U test. See also Figure S4.
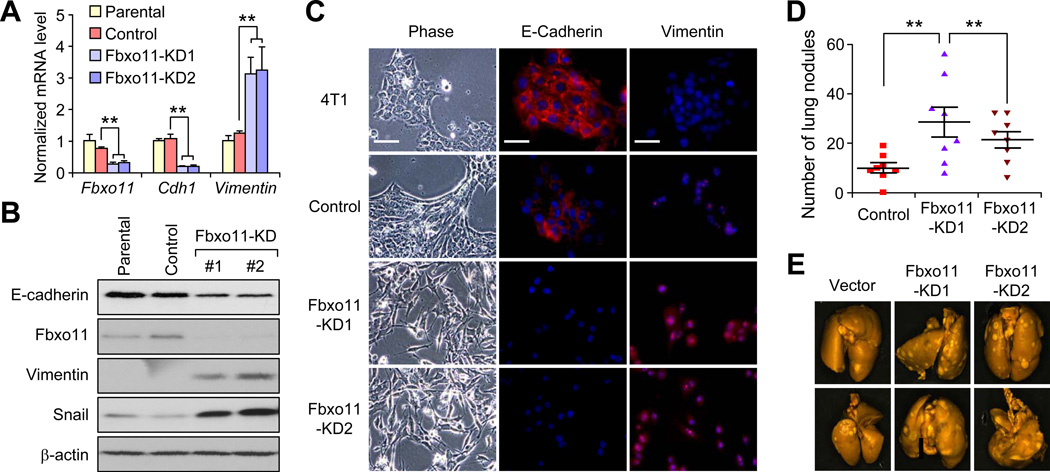
To complement these findings of using SNAIL and FBXO11 overexpressing cells, we knocked down endogenous Fbxo11 in 4T1 cells by shRNAs (Figure 5A). Western blot analysis revealed a five-fold up-regulation of Snail protein after stable knockdown of Fbxo11 (Figure 5B). Fbxo11 KD in 4T1 cells induced EMT-like cellular changes, including reduction of E-cadherin and increase of Vimentin at the mRNA (Figure 5A) and protein levels by western blot and immunofluorescence analyses (Figure 5B and 5C). Importantly, Fbxo11 KD significantly increased lung metastasis (Figure 5D and 5E), despite no difference in primary tumor growth (data not shown). Western blot analysis of primary tumors confirmed that Snail expression was up-regulated and E-cadherin expression was reduced in Fbxo11 KD tumors (Figure S5A), consistent with in vitro analysis. Fbxo11 KD and Snail upregulation were also maintained in lung metastasis nodules (Figure S5B). These results indicate that FBXO11 inhibits metastasis by promoting the ubiquitylation and degradation of the EMT-inducing SNAIL protein.
Figure 5. Knockdown of endogenous FBXO11 promotes breast cancer metastasis.
(A) Real-time PCR analysis of Fbxo11, Cdh1 and Vimentin expression in the parental and control 4T1 cell line, and two Fbxo11-KD cell lines. Data represent mean ± SD. **: p < 0.01 by two-tailed Student’s t test. (B) Western blot analysis of Snail, E-cadherin and Vimentin expression in the indicated 4T1 cell lines. (C) Phase contrast images and immunofluorescent images of EMT markers in the indicated 4T1 cell lines. Scale bars, 25µm. (D) 105 tumor cells from various 4T1 cell lines were orthotopically injected into Balb/c mice and primary tumors were removed 10 days later. Lung metastasis nodules were counted after sacrificing the mice at 38 days post injection. **p < 0.01 by Mann–Whitney U test. (E) Representative lung metastasis nodule images were presented. See also Figure S5.
Phosphorylation of Serine-11 on SNAG domain is essential for SNAIL to be recognized by FBXO11
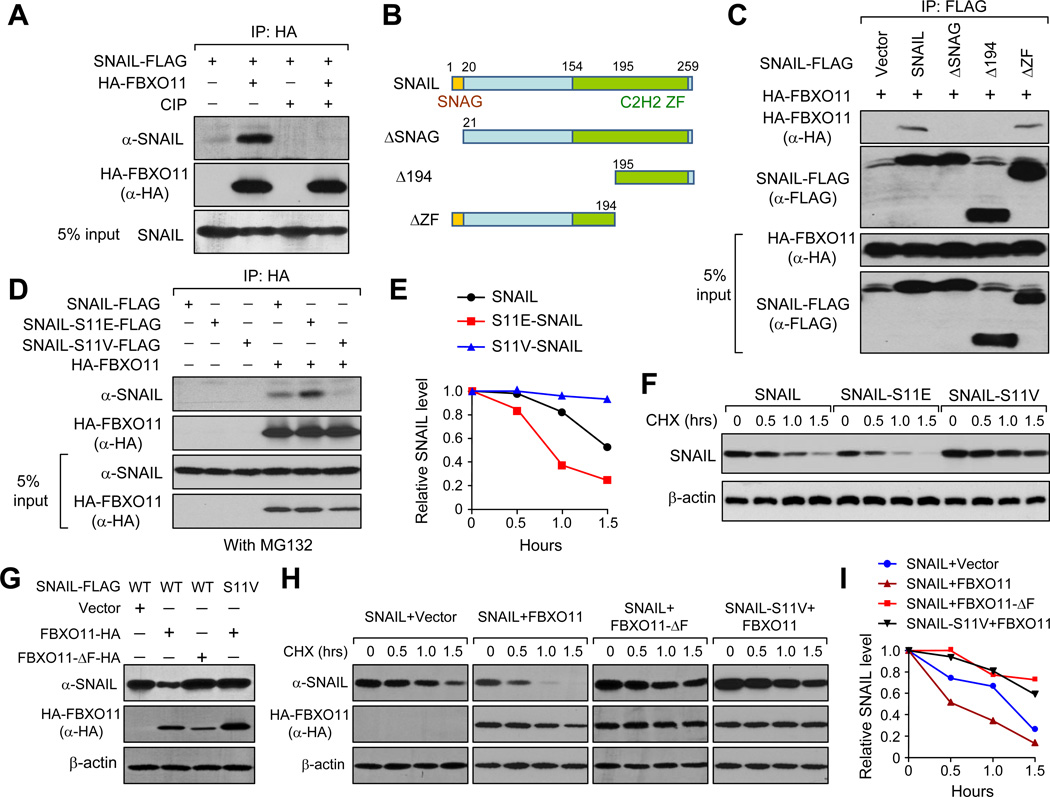
FBXO11 belongs to a large group of the F-box protein super-family. These proteins usually exert their E3 ligase function through forming a 4-subunit functional complex with Cullin, Skp and Rbx proteins (SCF complex) with F-box proteins as substrate recognition component. Interestingly, F-Box proteins generally recognize the protein substrate when they are phosphorylated (Cardozo and Pagano, 2004). It is thus plausible that SNAIL protein also needs to be phosphorylated before being recognized by FBXO11. To investigate this, we performed a modified co-IP experiment, in which cell lysate from 293T transfected with SNAIL and FBXO11 was treated without or with alkaline phosphatase (CIP) to remove protein phosphorylation before IP. SNAIL was pulled down together with FBXO11 protein, and this interaction was completely disrupted by CIP treatment (Figure 6A), suggesting that phosphorylation modification of either or both of SNAIL and FBXO11 might be critical for the SNAIL-FBXO11 protein interaction. To identify the potential region within the SNAIL protein that is phosphorylated and responsible for SNAIL-FBXO11 interaction, we generated several SNAIL truncation mutations and performed the co-IP experiment. Interestingly, only two truncation mutants — ΔSNAG and Δ194, both lacking the first 20 amino acids SNAG domain on the SNAIL protein (Figure 6B), lost the ability to interact with FBXO11 (Figure 6C). Immunofluoresence analysis indicated that ΔSNAG remained localized in the nucleus, suggesting that loss of interaction between ΔSNAG and FBXO11 was not due to localization change (Figure S7A). Thus, the SNAG domain of SNAIL protein is critical for its interaction with FBXO11.
Figure 6. Serine-11 in the SNAG domain of SNAIL is responsible for interaction with FBXO11.
(A) 293T cells were transfected with SNAIL-Flag and HA-FBXO11 for 2 days. Cell lysates were collected and treated either with or without CIP for 1 hour before subjected to co-IP experiment. (B) Schematic representation of SNAIL protein structure and the deletion mutation constructs generated to map out the interaction domain for SNAIL-FBXO11 interaction. (C) Wild-type FLAG-tagged SNAIL and SNAIL truncation mutants were co-transfected with HA-FBXO11 into 293T cells, cell lysates were collected for co-IP experiment using anti-FLAG antibody. (D) Wild-type SNAIL and SNAIL–S11E, SNAIL-S11V single amino acid mutants were co-transfected with HA-FBXO11 into 293T cells, cell lysates were collected for co-IP experiment using anti-HA antibody. (E) CHX pulse-chase assay for wild-type SNAIL, SNAIL-S11E, and SNAIL-S11V in the absence of exogenously expressed FBXO11 in 293T cells. Data was quantified using ImageJ software. (F) Western blot images of experiments in (E). (G) 293T cells were transfected with the indicated expression plasmids and cell lysates were collected for immunoblotting two days after transfection. (H, I) 293T stable cells expressing corresponding wild type SNAIL or mutant SNAIL were transfected with the indicated FBXO11 expression plasmids. Pulse-chase assay was performed 2 days later. Data was quantified in (I) using ImageJ software. See also Figure S6.
As recent literature suggests, there are at least 5 kinases that phosphorylate SNAIL protein at distinct amino acids and fine-tunes the functions of SNAIL differentially (Bastea et al., 2012; Du et al., 2010; Eiseler et al., 2012; Pon et al., 2008; Yang et al., 2005; Zhang et al., 2012a; Zhou et al., 2004). Phosphorylation of the Serine-11 site by protein kinase D1 (PKD1, also known as PKCµ) lies within the domain that we identified to be responsible for SNAIL-FBXO11 interaction. Interestingly, aberrant upregulation of PKD1 has been suggested to block SNAIL-induced EMT and anchorage independent growth (Bastea et al., 2012; Du et al., 2010; Eiseler et al., 2012). We thus tested whether PKD1 consensus phosphorylation sequence LXRXXS in the SNAG domain is essential for SNAIL-FBXO11 interaction. We generated L6A-, R8A-, and S11A-SNAIL alanine scanning mutants in the PKD1 consensus sequence and found that none of these mutants could interact with FBXO11 anymore (Figure S6A, B). Importantly, they also became stabilized compared to wild type SNAIL protein (Figure S6C). We further generated two additional mutants in Ser11, SNAIL-S11E and SNAIL-S11V, to mimic and disrupt the phosphorylation of the SNAIL protein at Serine-11, respectively. The phosphorylation-disrupting S11V mutant completely lost the interaction with FBXO11 protein, whereas the phosphorylation-mimicking mutant S11E still interacted with FBXO11 (Figure 6D). Previous literature suggests that GSK-3β dependent phosphorylation on SNAIL protein is critical for its recognition by FBXW1, another E3 ligase targeting SNAIL protein (Zhou et al., 2004). We acquired several SNAIL mutants including 6SA mutant, which can no longer be phosphorylated by GSK-3β, and performed co-IP experiment with FBXO11. Our results demonstrated that these mutants did not disrupt the interaction between SNAIL and FBXO11 protein (Figure S6D). Therefore, while both FBXO11 and FBXW1 can destabilize SNAIL through UPS, distinct protein kinases are involved in producing different phosphorylated SNAIL proteins for recognition by these E3s.
To further test the effects of these mutations on SNAIL protein degradation, we transfected these mutants either alone or together with wild-type FBXO11 or FBXO11-ΔF expression vectors into 293T cells and performed the CHX pulse-chase experiment. In the absence of exogenous FBXO11, SNAIL-S11E degraded much faster than WT SNAIL protein, while SNAIL-S11V protein was stabilized (Figure 6E and 6F). In the presence of exogenous FBXO11, the stable level of SNAIL protein was significantly lower than S11V-SNAIL mutant (Figure 6G). In CHX pulse-chase experiment, SNAIL protein turnover was accelerated by co-transfection with FBXO11, but not the FBXO11-ΔF mutant lacking the SCF complex formation domain. As expected, SNAIL-S11V was more stable compared to wild-type SNAIL protein, even at the presence of FBXO11 (Figure 6H and 6I). Taken together, our results suggest that Serine-11 is the critical amino acid for SNAIL-FBXO11 interaction.
PKD1 dependent phosphorylation of Ser-11 is critical for SNAIL degradation
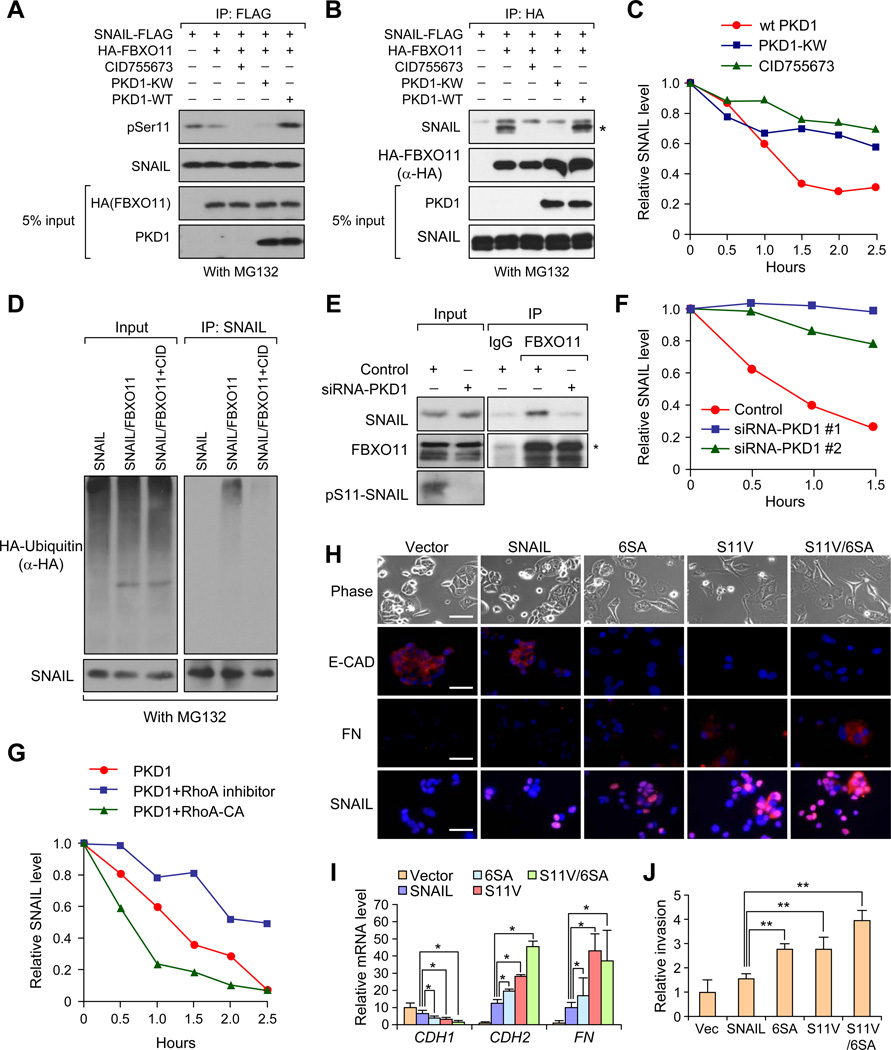
To directly test whether Serine-11 is phosphorylated by PKD1 in our system, we co-transfected 293T cells with SNAIL-FLAG, HA-FBXO11 and different wild-type or mutant PKD1 plasmids. In one set of cell lysates, we first performed IP with an anti-Flag antibody to pull down SNAIL protein and examined the Serine-11 phosphorylation status by an antibody that specifically recognizes pS11-SNAIL. SNAIL protein presented a very strong phosphorylation band at the correct size, while PKD1 inhibitor CID755673 treatment results in a lost of this phosphorylation band. PKD1 dominant negative mutant (PKD1-KW), but not the wild-type PKD1, also almost completely abolished SNAIL protein phosphorylation (Figure 7A). In the remaining set of cell lysates, we performed co-IP experiments with HA antibody to pull down FBXO11 protein. PKD1 inhibitor, as well as dominant negative PKD1-KW mutant disrupted the interaction between SNAIL and FBXO11 protein (Figure 7B) and stabilized SNAIL protein in the CHX pulse-chase experiment (Figure 7C and Figure S7A) without changing the localization of SNAIL (Figure S7B). Consistent with these results, PKD1 inhibitor reduced poly-ubiquitylated SNAIL protein bands compared to control (Figure 7D). Importantly, both pS11-SNAIL and FBXO11 are localized in the nucleus based on immunofluorescent staining (Figure S7C), suggesting that PKD1-dependent targeting of SNAIL by FBXO11 may occur in the nucleus.
Figure 7. PKD1 dependent phosphorylation of SNAIL Ser-11 is critical for SNAIL protein degradation.
(A) Detection of Ser11 phosphorylation of immunoprecipitated SNAIL protein when SNAIL was co-transfected with indicated plasmids or treated with PKD1 inhibitor CID755673 in 293T cells. (B) 293T cells were transfected or treated similarly to (A) and then lysed, and co-IP experiment was performed using anti-HA antibody. (C) In 293T cells stably transfected with SNAIL, SNAIL protein turnover rates were determined by CHX pulse-chase assays after cells were transfected with FBXO11 together with the indicated plasmids or inhibitor CID755673. Western blot images in Figure S8A were quantified using Image J software. (D) 293T cells were transfected with the indicated plasmids. Cells were treated with DMSO or PKD1 kinase inhibitor CID755673 before lysing in denature lysis buffer. Cell lyslates were subjected to denature IP with SNAIL antibody. Poly-ubiquitylated SNAIL protein was visualized by HA antibody blotting against HA-Ubiquitin. (E) MCF10A cells were treated with either 10nM control or PKD1 -targeting siRNA before subjected to co-IP and western blot analysis using the indicated antibodies. In (A), (B), (D) and (E), cells were treated with MG132 for 6 hr before co-IP experiments. (F) MCF10A cells were transfected with control or two PKD1 siRNAs. Two days later, endogenous SNAIL degradation was determined by CHX pulse-chase assay. Western blot images in Figure S7E were quantified using Image J software. (G) 293T-SNAIL stable cell line was transfected with HA-PKD1, together with either vector or GST-tagged constitutively active RhoA-CA, and CHX pulse-chase assay was performed for SNAIL. Western blot images in Figure S7H were quantified using Image J software. (H) Representative phase contrast images and immunofluorescent images of MCF7 cells stably expressing SNAIL or SNAIL mutants. Scale bars: 25µm. (I) qRT-PCR was performed for CDH1, CDH2, VIM, and FN in the indicated MCF7 stable cell lines. Data represent mean ± s.d., *p < 0.05 by two-tailed Student’s t test. (J) Boyden chamber invasion assay of MCF7 cells stably expressing SNAIL or SNAIL stabilization mutants. **p < 0.01 by Student's t test. See also Figure S7.
We further investigated the role of endogenous PKD1 in regulating SNAIL protein stability. When we knocked down PKD1 expression by siRNAs (Figure S7D), we observed a significant decrease of Serine-11 phosphorylation level, loss of interaction between SNAIL and FBXO11 (Figure 7E) and stabilization of the endogenous SNAIL protein (Figure 7F and Figure S7E). Previous reports demonstrated RhoA as a physiological activator for PKD1 kinase (Eiseler et al., 2009). We therefore used a constitutively activated RhoA (CA), and a RhoA inhibitor, Exoenzyme C3 transferase protein (C3), to test their effects on the SNAIL protein degradation. We first confirmed PKD1 activation status by probing p-PKD1-744/748 in PKD1-pull down samples (IP with HA antibody) (Figure S7F). SNAIL and FBXO11 interaction was also significantly diminished by RhoA inhibitor (Figure S7G). Consistently, SNAIL was stabilized after RhoA inhibition by C3 while SNAIL degradation was accelerated in cells co-transfected with RhoA-CA mutant (Figure 7G and Figure S7H). Taken together, our results demonstrate that SNAIL is phosphorylated by PKD1 at Serine-11, which promotes the interaction, ubiquitylation, and degradation of SNAIL protein.
Previous results demonstrated that WT SNAIL failed to induce EMT in MCF7 cells, possibly due to its rapid turnover rate and low stable protein level in this cell line (Zhou et al., 2004). On the other hand, SNAIL-6SA mutant, with disruption of the GSK-3β phosphorylation sites (binding site for FBXW1), was able to induce EMT in MCF7 cells. Since SNAIL-S11V mutant disrupted the PKD1 phosphoryaltion site and also stabilized SNAIL protein, we sought to determine whether S11V mutant is also able to induce EMT in MCF7 cells. Similar to what has been reported before (Zhou et al., 2004), while SNAIL failed to induce complete EMT in MCF7 cells, 6SA mutant induced strong EMT phenotypes in MCF7 cells (Figure 7H). S11V and the double S11V/6SA mutants also induce a significant EMT program in MCF7 cell (Figure 7H). Immunofluorescent staining revealed a decrease of E-CADHERIN at the cell membrane, and an increase of FIBRONECTIN in 6SA, S11V, and S11V/6SA transduced MCF7 cells, but not in control or wild-type SNAIL transduced MCF7 cells (Figure 7H). Furthermore, while SNAIL was able to reduce CDH1 expression and increase CDH2 and FN levels, these changes were minimal compared to that of 6SA, S11V, and S11V/6SA induced changes. 6SA and S11V seems to have similar effect on repressing CDH1 expression and inducing CDH2/FN expression, while combining these two mutants presented strongest EMT phenotype based on mRNA profiles (Figure 7I). To functionally test the effect of these mutants on tumor invasion ability we performed a matrigel invasion assay, and revealed that the S11V- and 6SA-SNAIL mutants induced a much stronger invasive capability when compared to WT SNAIL or vector control (Figure 7J). In aggregate, our results demonstrate that PKD1 is responsible for phosphorylation of Ser-11 on SNAIL protein, and this phosphorylation promoted SNAIL protein ubiquitylation and degradation.
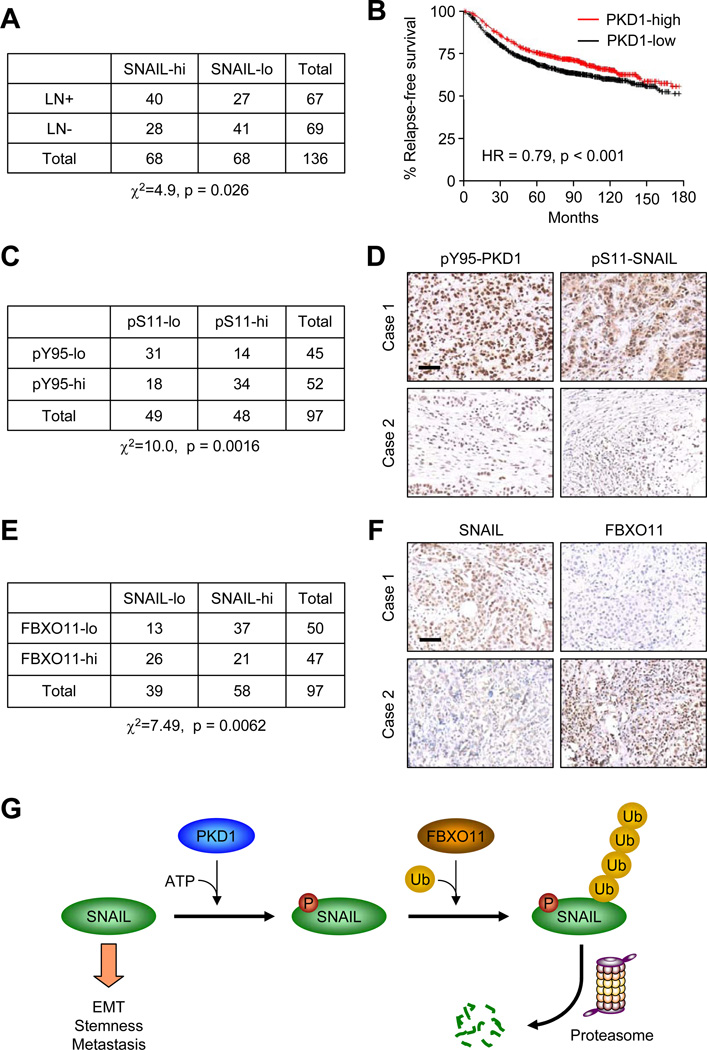
Negative correlation of PKD1 and FBXO11 with SNAIL in clinical breast cancer samples
We used immunohistochemistry staining (IHC) to examine the SNAIL protein expression level with the invasive property in 136 breast tumor samples collected at Sun Yat-Sen University Cancer Center. Nuclear SNAIL protein expression is positively correlated with lymph node invasion (Figure 8A, Pearson χ2=4.9, p = 0.026). Based on our finding that PKD1 phosphorylation promotes SNAIL degradation, we next investigated the prognosis value of PKD1. Consistent with its role in promoting SNAIL protein degradation, higher PKD1 expression level is correlated with longer relapse-free survival (Figure 8B, HR = 0.79, p < 0.001) in a large public clinical microarray database of breast tumors from 1354 patients (Gyorffy et al., 2010). Next, we tested the possible correlation between activated PKD1 (pY95-PKD1 antibody staining) and p-Ser-11 SNAIL protein levels using a tissue microarray containing 100 breast cancer patient samples. Consistent with a previous report (Bastea et al., 2012), activated PKD1 expression is positively correlated with p-Ser-11 SNAIL protein expression (Figure 8C and 8D, χ2=10.0, p = 0.0016). Finally, we tested the correlation between FBXO11 and SNAIL expression in the same breast tumor samples, and observed a strong negative correlation between FBXO11 and nuclear SNAIL expression, as over 70% of FBXO11-low samples have high SNAIL level (Figure 8E and 8F, χ2=7.49, p =0.0062).
Figure 8. SNAIL protein expression level is positively correlated with lymph node invasion and negatively correlated with FBXO11 expression level in breast cancer patients.
(A) Correlation study of SNAIL expression level with lymph node invasion in 136 breast tumor specimens. SNAIL-lo: SNAIL IHC staining lower than median; SNAIL-hi: SNAIL IHC staining higher than median; LN-: without lymph node invasion; LN+: with lymph node invasion. χ2=4.9, p = 0.026 by chi-square test. (B) Kaplan-Meier plots of distant relapse-free survival of patients, stratified by expression of PKD1. Data obtained from the Kaplan-Meier plotter database (Gyorffy et al., 2010). (C) Correlation study of activated PKD1 (pY95-PKD1) and S11-SNAIL in a breast tumor tissue microarray (US Biomax BC081120), χ2=10.0, p = 0.0016 by chi-square test. (D) Representative IHC images of pY95-PKD1 and pS11 -SNAIL in breast tumors. Scale bars, 100µm. (E) Correlation study of SNAIL and FBXO11 in the same breast tumor tissue microarray. χ2=7.49, p = 0.0062 by chi-square test. (F) Representative IHC images of SNAIL and FBXO11 in breast tumors. Scale bars: 100µm. (G) Schematic model for PKD1-dependent SNAIL protein ubiquitylation and degradation by FBXO11. SNAIL protein is first phosphorylated by PKD1 kinase at Serine-11 residue before it can be recognized and ubiquitylated by SCF-FBXO11 E3 ligase complex. Poly-ubiquitylated SNAIL protein is then degraded through 26S proteosomal degradation pathway.
In conclusion, our study reveals a functionally important posttranslational control mechanism of EMT master regulator SNAIL. Our results suggest a model that SNAIL protein is phosphorylated by PKD1 kinase at the Serine-11 residue. Phosphorylated SNAIL protein is then recognized and poly-ubiquitylated by SCF-FBXO11 E3 ligase complex for proteosomal degradation, thereby limiting its ability to induce EMT, tumor initiation and metastasis (Figure 8G).
DISCUSSION
SNAIL is a conserved transcription factor that plays an essential role in EMT during cancer metastasis. Recent discoveries also reveal that SNAIL has a much broader effect on cancer progression, linking the SNAIL gene not only to EMT, but also to cancer stem cell properties (Mani et al., 2008), immune evasion (Kudo-Saito et al., 2009), cancer metabolism (Dong et al., 2013), and breast cancer relapse (Moody et al., 2005). Given these important roles, a better understanding of the regulatory mechanisms for SNAIL protein level will provide additional avenues to inhibit SNAIL-induced tumor malignancy in breast cancer.
Previous results demonstrated that SNAIL is a labile protein with a very short half-life (Zhou et al., 2004). While E3 ligases often recognize their transcriptional factor substrate by binding to their transcriptional regulatory domains, neither one of the two previously identified E3 ligases for SNAIL, FBXW1/β-TRCP and FBXL14, interacts with the SNAG transcriptional repression domain of SNAIL (Lander et al., 2011; Zhou et al., 2004), leading us to suspect that there exists additional E3 ligase(s) targeting the SNAG domain under the control of important kinase signaling pathways. Conventionally, identifying such E3 ligase is a time-consuming and often serendipitous process. Proteomic analyses have been used to identify protein substrates for certain E3 ligases (Emanuele et al., 2011). However, this method is not suitable to search for E3 ligases that can degrade a specific protein substrate of interest. We developed a luciferase-based screening method to discover candidate E3 ligases for a specific protein substrate (SNAIL). It is worth noting that other candidate SNAIL-targeting E3s identified in our initial screen, including SOCS3, SPSB1, and TRIM5, can directly interact with SNAIL and the knockdown of these E3s increased the steady state level of SNAIL. However, overexpression of these three E3s did not accelerate SNAIL degradation. It is possible that these E3s are already expressed at relative high levels in 293T cells and further overexpression of these three E3 ligases will not further enhance their activity. The other potential explanation is the off-target effect of siRNA knockdown, which is the reason why we only consider an E3 as a true candidate when they have strong effects on SNAIL protein degradation in both knockdown and overexpression experiments. Overall, our study serves as proof-of-principle for adopting such a screening and validation strategy to effectively identify other E3 ligase-substrate pairs.
Functionally, FBXO11 blocks SNAIL-induced EMT in HMLEN cells, a commonly used breast cancer EMT model, through promoting SNAIL protein degradation. While HMLEN-SNAIL cells become more mesenchymal-like with enhanced tumor initiating properties, FBXO11 co-expression completely reverses these phenotypes. FBXO11 also prevents SNAIL-induced EMT and in vivo metastasis in 4T1 and EpRas mammary tumor cell lines by ubiquitylating and degrading SNAIL protein. Knockdown of endogenous Fbxo11 also stabilizes SNAIL protein and induces EMT in 4T1 cells and promotes lung metastasis. In line with its role as a negative regulator of pro-metastatic protein SNAIL, higher FBXO11 expression is correlated with longer metastasis-free survival in breast cancer patients. In our preliminary analysis, we didn’t find any correlation of FBXO11 expression with the breast cancer subtype using the publically available NKI295 dataset, which has limited sample size. However, future studies using larger datasets are needed to further analyze whether FBXO11 is negatively correlated with claudin-low subtype in which the gene expression signature of tumor samples resemble that of normal and cancerous stem cells of the mammary gland.
Previous literature identified five kinases that can phosphorylate SNAIL protein; in our search, we identified PKD1 dependent phosphorylation of Serine-11 on SNAG domain is the rate limiting step for FBXO11 mediated SNAIL protein ubiquitylation and degradation. Mutation of the consensus PKD1 phosphorylation site on SNAIL, particularly Serine-11, blocks its phosphorylation by PKD1 and disrupts the interaction of SNAIL with FBXO11. Stabilized SNAIL-S11V protein promotes EMT in MCF7 breast cancer cell lines, while the wild-type SNAIL fails to do so. The SNAIL-FBXO11 complex is likely formed in the nucleus, where both pSer11-SNAIL and FBXO11 are detected. Although pSer11-SNAIL is most abundant in the nucleus, we can not rule out the possibility that PKD1 dependent phosphorylation can still occur in the cytoplasm as many transcriptional factors are constantly shuttling between the nucleus and cytoplasm.
Our result is also consistent with previously established functional role of PKD1 during EMT process. PKD1 was reported to maintain epithelial phenotype and inhibit EMT by phosphorylation of SNAIL protein. PKD1 phosphorylates Ser-11 on SNAIL, causing the repression of E-cadherin expression by triggering SNAIL nuclear export (Du et al., 2010), or by forming an inactive DNA/SNAI1 complex that shows decreased interaction with its co-repressor Ajuba (Bastea et al., 2012). Our findings demonstrate that the phosphorylation at Ser-11 by PKD1 serves another previously unknown distinct functional role in regulating SNAIL-induced EMT and metastasis by promoting SNAIL protein ubiquitylation and degradation. Our further analysis reveals that FBXO11 interacts with the PKD1-phosphorylated SNAG transcription repressor domain, suggesting FBXO11 indeed is an important “classical” E3 ligase targeting SNAIL protein for degradation through coupling the transcriptional activity with the degradation process. Clinical sample analysis also confirms a positive correlation of activated PKD1 and p-Ser-11 SNAIL expression. Importantly, we also observe a strong negative correlation of SNAIL protein and FBXO11 protein expression in breast cancer patients. Interestingly, it was recently shown that PKD1 level was regulated at the epigenetic level (Borges et al., 2013). The PKD1 promoter is highly methylated, leading to reduced PKD1 level in metastatic breast cancer cells compared to non-metastatic breast cancer. This suggests a possible scenario in which PKD1 silencing may lead to increased level of SNAIL and subsequent increase of the metastatic ability of cancer cells.
During cancer progression, the ubiquitylation and deubiquitylation system have been shown in recent years to play critical roles by aberrant signaling amplification (Zhang et al., 2012b), promoting survival (Lau et al., 2012; Zhao et al., 2008), and coping with DNA repair (Wu et al., 2012). Specifically, FBXO11 has also been identified to play multifaceted roles in cancer. In human B cell lymphoma, SCF-FBXO11 targets BCL6, a commonly over-expressed gene in aggressive diffuse large B-cell lymphoma (DLBCL), for ubiquitylation and proteosomal degradation (Duan et al., 2012). Importantly, FBXO11 was found to be deleted or mutated in multiple DLBCL cell lines, and inactivation of FBXO11 correlates with increased level of BCL6. These observations suggest that FBXO11 may be an important tumor suppressor in DLBCL. More recently, SCF-FBXO11 was shown to interact with CDT2, a DCAF protein that controls cell-cycle progression, and to promote CDT2 proteosomal degradation. This regulatory mechanism also has important implications in cell cycle exit and cell migration (Abbas et al., 2013; Duan et al., 2012). Our study extended the multifaceted role FBXO11 in cancer from a tumor suppressor to a key metastasis inhibitor by suppressing SNAIL-mediated EMT, invasion and metastasis.
Experimental Procedures
Animal Studies
All procedures involving mice and experimental protocols were approved by Institutional Animal Care and Use Committee (IACUC) of Princeton University. For orthotopic primary tumor formation, female BALB/c mice or athymic nude mice at 4–6 week old were anaesthetized and a small incision was made to reveal the mammary gland. Tumor cells suspended in 10µl PBS were injected directly into the mammary fat pad. The primary tumor growth was monitored weekly by measurement of tumor size. In 4T1 FBXO11-KD lung metastasis assay primary tumors were removed after 10 days, Lung metastases nodes were examined after euthanizing the mice at experimental end point.
Clinical Data Set Analysis
NKI295 gene expression data were downloaded from the Stanford Microarray Database (http://microarray-pubs.stanford.edu/wound_NKI/explore.html). For E3 candidate survival analyses (FBXO11, SPSB1, SOCS3), distant metastasis-free survivals, stratified by expression of the gene of interest, were presented as Kaplan−Meier plots and tested for significance using log-rank tests in NKI295 dataset. TRIM5 gene was not included in NKI295 dataset, and thus it was analyzed using online KMplot database (www.KMplot.com) (Gyorffy et al., 2010). PKD1 survival analysis was also done in KMplot database using relapse-free survival.
Human breast cancer TMAs
Formalin-fixed paraffin-embedded microarrays of breast cancer tissues were from US Biomax (BC081120) and from Sun Yat-Sen University Cancer Center. Both sample sets used de-identified tumor samples and were considered exempt by the Institutional Review Boards of Princeton University and Sun Yat-Sen University Cancer Center.
Statistical Analysis
Results were reported as mean ± SD (standard deviation) or mean ± SEM (standard error of the mean), as indicated in the figure legend. Statistical comparisons were performed using unpaired two-sided Student’s t-test with unequal variance assumption. Statistical comparison for 4T1 lung metastasis assay was performed using Mann–Whitney U-test. Clinical correlation of SNAIL staining and lymph node invasion was performed using chi-square test. Clinical correlation between SNAIL and FBXO11, or between pY95-PKD1 and p-Ser-11 SNAIL was also performed using chi-square test. All the experiments with representative images (including western blot and immunofluorescence) have been repeated at least twice and representative images were shown. The histology images in Figure 8 are representative of tumor specimens of the same category (SNAIL-high or SNAIL-low).
Supplementary Material
HIGHLIGHTS.
A luciferase-based screen for identifying E3 ligase(s) of specific substrates
SCF-FBXO11 inhibits EMT by promoting SNAIL ubiquitylation and degradation
FBXO11 binding to SNAIL depends on PKD1 phosphorylation of SNAIL at Serine-11
Reduced FBXO11 or PKD1 expression correlates with poor clinical outcomes
SIGNIFICANCE.
SNAIL is one of the most well established master transcriptional regulators of EMT, however, a comprehensive understanding of the post-translational regulations of these factors is lacking. We developed a high throughput luciferase-based RNAi screening strategy to identify E3 ligase(s) targeting the SNAIL protein for ubiquitylation and degradation. We identified FBXO11 as a crucial post-translational regulator that controls the stability of the SNAIL protein. We further uncovered that this regulation is dependent on Serine-11 phosphorylation of SNAIL by PKD1. Importantly, functional studies and clinical sample analysis established a crucial role of the PKD1-FBXO11-SNAIL axis in regulating breast cancer EMT and metastasis, and indicated potential therapeutic value of targeting this pathway.
ACKNOWLEDGEMENTS
We thank Dr. R.A. Weinberg for kindly provide HMLEN cells and Dr. B.P. Zhou for providing SNAIL-2SA, SNAIL-4SA, and SNAIL-6SA plasmids. We thank Dr. M. Pagano for providing FLAG tagged FBXO11, FBXO1, FBXO5, FBXO10, FBXO31, FBXW1, and FBXW9 and helpful advice. We thank B. Ell, M.B. Esposito, B.I. Koh, L. Wan, R. Chakrabarti and other lab members for helpful discussions, M. Yuan for technical assistance and C. DeCoste for assistance with flow cytometry. We thank L. Cong at the Tissue Analytic Service Core of Cancer Institute of New Jersey for optimizing the conditions for IHC staining of clinical tumor samples. This research was supported by a CINJ Research Development Award, the Brewster Foundation, the Champalimaud Foundation, and grants from the U. S. Department of Defense (BC123187) and the National Institute of Health (R01CA134519 and R01CA141062) to Y.K., National Science Foundation of China to Y.K. and H-Y.W., and from National Institute of Health to P.S.(GM086435 and CA140182). H.Z. is a recipient of a Komen for the Cure postdoctoral fellowship (KG111164) and G. R. is a recipient of a DOD Postdoctoral Fellowship (BC123284). M.S. is supported by the 111 Project of China (B13026, Department of Education) and an international exchange program of Zhejiang University.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBERS
The raw and normalized microarray data have been deposited in the Gene Expression Ominbus (GEO) database under accession number GSE50889.
SUPPLEMENTAL INFORMATION
Supplemental Information including Supplemental Experimental Procedures, eight Supplemental Figures, and 2 Supplemental Tables can be found with this article online.
REFERENCES
- Abbas T, Mueller AC, Shibata E, Keaton M, Rossi M, Dutta A. CRL1-FBXO11 Promotes Cdt2 Ubiquitylation and Degradation and Regulates Pr-Set7/Set8-Mediated Cellular Migration. Mol Cell. 2013 doi: 10.1016/j.molcel.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastea LI, Doppler H, Balogun B, Storz P. Protein kinase D1 maintains the epithelial phenotype by inducing a DNA-bound, inactive SNAI1 transcriptional repressor complex. PLoS One. 2012;7:e30459. doi: 10.1371/journal.pone.0030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Borges S, Doppler H, Perez EA, Andorfer CA, Sun Z, Anastasiadis PZ, Thompson EA, Geiger XJ, Storz P. Pharmacologic reversion of epigenetic silencing of the PRKD1 promoter blocks breast tumor cell invasion and metastasis. Breast Cancer Res. 2013;15:R66. doi: 10.1186/bcr3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T. To differentiate or not--routes towards metastasis. Nature reviews Cancer. 2012;12:425–436. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nature reviews Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al. Loss of FBP1 by Snail-Mediated Repression Provides Metabolic Advantages in Basal-like Breast Cancer. Cancer Cell. 2013 doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Zhang C, Hassan S, Biswas MH, Balaji KC. Protein kinase D1 suppresses epithelial-to-mesenchymal transition through phosphorylation of snail. Cancer Res. 2010;70:7810–7819. doi: 10.1158/0008-5472.CAN-09-4481. [DOI] [PubMed] [Google Scholar]
- Duan S, Cermak L, Pagan JK, Rossi M, Martinengo C, di Celle PF, Chapuy B, Shipp M, Chiarle R, Pagano M. FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature. 2012;481:90–93. doi: 10.1038/nature10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseler T, Doppler H, Yan IK, Kitatani K, Mizuno K, Storz P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat Cell Biol. 2009;11:545–556. doi: 10.1038/ncb1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseler T, Kohler C, Nimmagadda SC, Jamali A, Funk N, Joodi G, Storz P, Seufferlein T. Protein kinase D1 mediates anchorage-dependent and -independent growth of tumor cells via the zinc finger transcription factor Snail1. J Biol Chem. 2012;287:32367–32380. doi: 10.1074/jbc.M112.370999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele MJ, Elia AE, Xu Q, Thoma CR, Izhar L, Leng Y, Guo A, Chen YN, Rush J, Hsu PW, et al. Global identification of modular cullin-RING ligase substrates. Cell. 2011;147:459–474. doi: 10.1016/j.cell.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast cancer research and treatment. 2010;123:725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Lander R, Nordin K, LaBonne C. The F-box protein Ppa is a common regulator of core EMT factors Twist, Snail, Slug, and Sip1. J Cell Biol. 2011;194:17–25. doi: 10.1083/jcb.201012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AW, Fukushima H, Wei W. The Fbw7 and betaTRCP E3 ubiquitin ligases and their roles in tumorigenesis. Front Biosci. 2012;17:2197–2212. doi: 10.2741/4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Li H, Li S, Shen M, Xiao N, Chen Y, Wang Y, Wang W, Wang R, Wang Q, et al. The Fbw7/human CDC4 tumor suppressor targets proproliferative factor KLF5 for ubiquitination and degradation through multiple phosphodegron motifs. J Biol Chem. 2010;285:18858–18867. doi: 10.1074/jbc.M109.099440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Muratani M, Tansey WP. How the ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol. 2003;4:192–201. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annual review of cell and developmental biology. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- Pon YL, Zhou HY, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase promotes epithelial to mesenchymal transition through snail induction in ovarian cancer cells. Cancer Res. 2008;68:6524–6532. doi: 10.1158/0008-5472.CAN-07-6302. [DOI] [PubMed] [Google Scholar]
- van de Vijver MT, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. The New England journal of medicine. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nature medicine. 2013;19:1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang X, Zhang L, Wu CY, Rezaeian AH, Chan CH, Li JM, Wang J, Gao Y, Han F, et al. Skp2 E3 ligase integrates ATM activation and homologous recombination repair by ubiquitinating NBS1. Mol Cell. 2012;46:351–361. doi: 10.1016/j.molcel.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res. 2005;65:3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rodriguez-Aznar E, Yabuta N, Owen RJ, Mingot JM, Nojima H, Nieto MA, Longmore GD. Lats2 kinase potentiates Snail1 activity by promoting nuclear retention upon phosphorylation. EMBO J. 2012a;31:29–43. doi: 10.1038/emboj.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou F, Drabsch Y, Gao R, Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu CX, ten Dijke P. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-beta type I receptor. Nat Cell Biol. 2012b;14:717–726. doi: 10.1038/ncb2522. [DOI] [PubMed] [Google Scholar]
- Zhao D, Zheng HQ, Zhou Z, Chen C. The Fbw7 tumor suppressor targets KLF5 for ubiquitin-mediated degradation and suppresses breast cell proliferation. Cancer Res. 2010;70:4728–4738. doi: 10.1158/0008-5472.CAN-10-0040. [DOI] [PubMed] [Google Scholar]
- Zhao X, Heng JI, Guardavaccaro D, Jiang R, Pagano M, Guillemot F, Iavarone A, Lasorella A. The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nat Cell Biol. 2008;10:643–653. doi: 10.1038/ncb1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.