Abstract
Alzheimer's disease (AD) is characterized by an early synaptic loss, which strongly correlates with the severity of dementia. The pathogenesis and causes of characteristic AD symptoms are not fully understood. Defects in various cellular cascades were suggested, including the imbalance in production of reactive oxygen and nitrogen species. Alterations in S-nitrosylation of several proteins were previously demonstrated in various AD animal models and patients. In this work, using combined biotin-switch affinity/nano-LC-MS/MS and bioinformatic approaches we profiled endogenous S-nitrosylation of brain synaptosomal proteins from wild type and transgenic mice overexpressing mutated human Amyloid Precursor Protein (hAPP). Our data suggest involvement of S-nitrosylation in the regulation of 138 synaptic proteins, including MAGUK, CamkII, or synaptotagmins. Thirty-eight proteins were differentially S-nitrosylated in hAPP mice only. Ninety-five S-nitrosylated peptides were identified for the first time (40% of total, including 33 peptides exclusively in hAPP synaptosomes). We verified differential S-nitrosylation of 10 (26% of all identified) synaptosomal proteins from hAPP mice, by Western blotting with specific antibodies. Functional enrichment analysis linked S-nitrosylated proteins to various cellular pathways, including: glycolysis, gluconeogenesis, calcium homeostasis, ion, and vesicle transport, suggesting a basic role of this post-translational modification in the regulation of synapses. The linkage of SNO-proteins to axonal guidance and other processes related to APP metabolism exclusively in the hAPP brain, implicates S-nitrosylation in the pathogenesis of Alzheimer's disease.
The role of nitric oxide (NO)1 as a signaling molecule in the central nervous system was discovered in 1988 (1). The brain and cerebellum in particular, contain one of the highest activities of NO-forming enzyme (NO synthase, NOS) in all tissues examined (2, 3). Nitric oxide is a freely diffusible, very reactive radical molecule. It readily reacts with various endogenous substrates forming that is, iron and copper adducts in prosthetic groups of proteins (4), peroxynitrite in the reaction with reactive oxygen species, ROS (5), and S-nitrosothiols with endogenous low-molecular weight thiols like cysteine and glutathione (6). One of the aspects of NO physiology is formation of S-nitrosylated proteins. Cysteine residues, post-translationally modified by S-nitrosylation, exert control over the activity of proteins and pathways in which they are involved, analogous to the addition of a phosphate group during phosphorylation (7, 8). S-nitrosylation is a key mechanism in the transmission of NO-based cellular signals in vital cellular processes, including: transcription regulation, DNA repair, autophagy, and apoptosis (8).
The role of protein S-nitrosylation underlying pathology of various diseases, including cancer (9, 10), heart condition (11–13), and neurodegenerative disorders has been extensively reviewed (8, 14). In the brain, aging processes and environmental factors cause protein S-nitrosylation which in turn may enhance misfolding of proteins, induce apoptosis or autophagy, mitochondrial fragmentation and affect normal synaptic functions (8). S-nitrosylation of proteins plays an important role in neurons. For example, N-methyl-d-aspartate receptor (NMDAR) and caspase enzyme activity can be decreased by S-nitrosylation, thereby facilitating neuroprotection (15). This finding led to development of nitro-memantine, a nitric oxide donor and selective NMDAR interacting drug. It selectively S-nitrosylates the NMDA receptor and prevents its' hyperactivation, also observed in Alzheimer's disease (16). On the contrary, S-nitrosylation of protein-disulfide isomerase (17), dynamin-related protein 1 (18), glyceraldehyde dehydrogenase (19), cyclo-oxygenase-2 (20), N-ethylmaleimide sensitive protein (21), Parkin (22–24), Gospel (25), cyclin dependent kinase- 5 (26), mitochondrial complex I (27), stargazin (28), and serine racemase (29), has been related to severe neuropathological alterations in the brain caused by induction of: protein misfolding or aggregation, mitochondrial dysfunction, bioenergetic compromise, synaptic injury, and subsequent neuronal loss.
Alzheimer's disease is the most prevalent form of human dementia, with a frequency that progressively increases in aging societies (30). The temporal progression of AD exhibits a highly variable pattern among patients and is not fully understood (31). Environmental, age-related, and genetic factors have been proposed to contribute to pathogenesis of the disease. Defects in various signaling pathways regulated by post-translational modifications of proteins (PTM) that is, phosphorylation, were suggested to be the determinant parameter for disease progression (32–35). A pivotal role in development and progression of late-onset AD and various other age-dependent dementias has been attributed to inflammatory and oxidative stress cascades in the brain (36, 37). Reactive oxygen (ROS) and reactive nitrogen species (RNS) play a crucial role in these processes (38). The consequences of oxidative and nitrosative stress, such as lipid peroxidation, DNA oxidation, and nitrosative/oxidative PTM of brain proteins were detected in global and targeted proteomic analyses in AD patients (14, 39–43). On the other hand, a multitude of evidence suggests that physiological levels of ROS and RNS are implicated in various cell-signaling cascades (reviewed in (44–47)).
Various transgenic mouse models, based on the overexpression of mutant form(s) of human APP and recapitulating the AD phenotype are currently used to investigate mechanisms underlying disease pathology (48, 49). The present study utilized one such model, a transgenic mice expressing human APP with London mutation and its wild-type, aged-matched counterparts, for targeted, differential proteomic analysis of S-nitrosylation of proteins located at the synaptic terminals.
The biotin switch technique (BST) and its modification, SNOSID (SNO Site Identification) (50); in combination with mass spectrometry have been used in targeted proteomics studies of cellular “S-nitrosomes.” BST relies on selective ascorbate reduction of S-nitrosothiols to generate free thiol groups in the presence of other thiol derivatives (51), whereas SNOSID (SNO Site Identification) involves biotinylation of protein SNO-Cys residues, trypsin digestion, affinity purification of biotinylated-peptides, and sequencing by tandem MS (50). The majority of such studies were undertaken to identify targets of S-nitrosylation induced by nitric oxide donor treatment (52–54).
It is however, difficult to extrapolate results of donor induced studies to an in vivo situation, where cysteine S-nitrosylation is dependent on the overall redox state of a cell and its unknown levels of nitrosylating compounds (55). In this work, we optimized the BST for MS-based global analysis of endogenous protein S-nitrosylation. Our study was designed to test the hypothesis that redox imbalance and changes in activity of nitric oxide synthase(s) influence the level and pattern of endogenous S-nitrosylation of synaptic proteins. Among 138 identified synaptosomal SNO-proteins, 38 were differentially S-nitrosylated in the hAPP mouse brain. Our data suggest a possibility of sequential S-nitrosylation, similarly as for other post-translational modifications, that is, phosphorylation. Focused systematic proteomics approaches addressing the role of cysteine post-translational modifications in the brain can lead to new mechanism-based therapies for various neurodegenerative disorders including AD.
EXPERIMENTAL PROCEDURES
Reagents
Bradford reagent, sucrose, Ficoll, neocuproine, and sodium ascorbate were from Sigma. S-Methyl methanethiosulfonate (MMTS) was from Fluka. Neutravidin-agarose and biotin-HPDP were purchased from Thermo Scientific. Sequencing grade modified trypsin was obtained from Promega (Madison, WI). Complete protease inhibitor mixture was from Roche diagnostics. ECL chemiluminescence reagents were purchased from Amersham Biosciences. Antibodies directed against gamma enolase (Eno2) and glial fibrillary acidic protein (Gfap) were obtained from DAKO(Carpenteria, CA). Anti-adaptor protein complex AP-2 (Ap2a1), anti-peroxiredoxin 3 (Prdx3), anti-peroxiredoxin 6 (Prdx6), and streptavidin HRP-conjugated secondary antibodies were purchased from Abcam (Cambridge, CA). Primary antibodies, directed against neural cell adhesion molecule 1 (Ncam1), synaptotagmins-1/2 (Syt1/Syt2), neurocalcin delta (Ncald), glyceraldehyde-3-phosphate dehydrogenase (Gapdh), ras-related C3 botulinum toxin substrate 1 (Rac1), inducible nitric oxide synthase 2 (Nos2; iNos), and neuronal nitric oxide synthase 1 (Nos1; nNos), as well as secondary antibodies: rabbit anti-mouse IgG, rabbit anti-goat IgG, and goat anti-rabbit IgG antibody were purchased from Santa Cruz Biotechnology. PVDF (0.22 μm) membrane was from Millipore.
Transgenic Mice
Transgenic AD mice used in this study were generated in Prof. Fred van Leuven's laboratory (K.U. Leuven) as described by Moechars et al. (56), and kept at the Animal House of Polish Academy of Sciences Medical Research Center. The transgenic mice express human Amyloid Precursor Protein with London mutation [Tg(APPV717I)], under the control of the mouse Thy1-gene promoter (called later as hAPP mice). The study included 14–15 month female heterozygous mice. Control aged-matched mice (FVB/N, called later as FVB mice) were of the same genetic background. All experiments were performed in accordance with Polish guidelines for care and use of laboratory animals.
Synaptosome Isolation
Synaptosomes were prepared from brains of 14–15 months old, female FVB, and hAPP mice, as described (57, 58). Four mice of the same age were decapitated and their brains immediately removed and homogenized using Dounce homogenizer, in 6 ml of buffer A containing 5 mm Hepes pH 7.4, 0.32 m sucrose, 0.2 mm EDTA, 20 mm MMTS (a free thiol blocking reagent), and protease inhibitor mixture. The homogenate was centrifuged (2500 × g for 5 min) to yield pellet and supernatant fractions. Supernatant was subsequently centrifuged at 12,000 × g for 5 min. The obtained pellet was resuspended in buffer A, placed onto a discontinuous Ficoll gradient and centrifuged at 70,000 × g for 45 min. Synaptosomal fraction was collected, resuspended in buffer A and centrifuged at 20,000 × g for 20 min. Purified fraction of synaptosomes was used in all proteomic experiments.
Biotin Switch Technique
Substitution of S-nitrosylated Cys (SNO-Cys) sites with S-biotinylated Cys in synaptosomal protein lysates was based on a previously described BST procedure (51). In our study, we optimized concentration of used reagents and the time reactions to increase the specificity and sensitivity of the method. Mouse synaptosomal fractions were dissolved in HEN buffer containing 250 mm Hepes pH 7.7, 1 mm EDTA and 0.1 mm neocuproine. To avoid rearrangements of thiol modifying groups, the protein mixture was treated with two volumes of a thiol blocking solution containing 250 mm Hepes pH 7.7, 1 mm EDTA, 0.1 mm neocuproine (copper ion chelator), 5% SDS, and 20 mm MMTS at 50 °C for 20 min with agitation (in the dark). To remove excess of reagents, proteins were precipitated with acetone and resuspended in the same volume of HEN buffer containing 2.5% SDS. The obtained protein solutions were divided into two equal parts. One part was treated with a mixture of 400 μm Biotin-HPDP and 5 mm sodium ascorbate. The second half was used as a negative control for experiments and treated with 400 μm biotin HPDP without sodium ascorbate. All samples were incubated in the dark for 1.5 h at room temperature (RT). The proteins were again acetone-precipitated, resuspended in the same volume of HEN buffer containing 2.5% SDS and diluted with two volumes of neutralization buffer (20 mm Hepes, pH 7.7, 100 mm NaCl, and 1 mm EDTA). 100 μl of neutravidin-agarose beads was added to the solution and incubated for 1 h at room temperature with agitation. Afterward, the beads were washed five times with 20 mm Hepes, pH 7.7, 600 mm NaCl, and 1 mm EDTA and incubated with elution buffer containing 50 mm Tris, pH 8.0, 1 mm EDTA, and 50 mm DTT, for 20 min at RT with gentle agitation. The supernatants containing enriched SNO-proteins were used in subsequent Western blot analyses for validation of detected SNO-sites.
Western Blot Detection of nNos and iNos in FVB and hAPP Mouse Brains
Brain homogenates were analyzed for nNos and iNos protein expression using Western blot method. 20 μg of protein extracts from whole brains of FVB and hAPP mice, respectively, were separated by reducing 10% SDS-PAGE. Separated proteins were transferred onto PVDF membrane. The membranes were first blocked with caseine-based buffer (Sigma) and incubated with primary antibodies to nNos and iNos. Membranes were then probed with secondary antibodies raised against the appropriate species. Equal protein loads were assessed with antibodies against Gapdh and by Ponceau S staining on the blots. Western blots were developed using ECL chemiluminescence. The scanned blots were quantified densitometrically using GelQuant software and relative protein abundance of nNos and iNos normalized to the expression of Gapdh. Heteroschedastic two-tailed t test was used to assess the changes in expression.
Western Blot Analysis of Protein S-nitrosylation Pattern in FVB and hAPP Synaptosomes
Total synaptosomal protein fractions after BST procedure but without neutravidin-based affinity purification were resolved using reducing 10% SDS-PAGE. Selectively biotinylated proteins were captured using streptavidin-HRP conjugated antibodies and visualized by ECL chemiluminescence.
Western Blot Detection of Differential S-nitrosylation in the Brain
All fractions (including total synaptosomal fractions and those from different steps of BST procedure) were separated using 12% SDS-PAGE and transferred to PVDF membrane (0.22 μm). The membranes were first blocked with caseine-based buffer (Sigma) and incubated with primary antibodies followed by secondary HRP-conjugated antibodies. Protein bands were detected using the ECL chemiluminescence system (Amersham Biosciences). The fractions containing enriched S-nitrosylated proteins, from FVB and hAPP synaptosomes were quantified densitometrically with GelQuant software. Heteroschedastic two-tailed t test was used to statistically assess the changes in endogenous protein S-nitrosylation.
SNOSID
The synaptosomal protein fractions containing biotin labeled proteins (biotin labeling step described in the BST section) were digested using sequencing grade modified trypsin for 16 h at 37 °C. Digestion was terminated using protease inhibitors mixture. Tryptic peptides were incubated with 100 μl of neutravidin beads for 1 h at RT. Beads were then washed five times with 1 ml of wash buffer. Peptides bound to neutravidin resin were eluted with 150 μl of elution buffer containing 30 mm dithiotreitol. Eluted, cysteine-containing peptides were alkylated using 200 mm iodoacetamide. In the next step, peptides containing fractions were concentrated using SpeedVac and diluted with 1% trifluoroacetic acid/water (v/v) to a final concentration of 0.1% TFA.
LC-MS and LC-MS/MS Analysis
Each enriched SNO-peptides containing sample was measured twice: once with LC-MS/MS (tandem mass spectrometry), to identify the enriched SNO tryptic peptides, and once in LC-MS mode (resulting in profile spectrum), to attain label-free quantitative data based solely on peak intensities. All MS runs were separated by blank ones to reduce carry-over of peptides from previous samples. The measurements were carried in Nano Aquity Liquid Chromatography system (Waters, Milford, MA) coupled to LTQ-FTICR mass spectrometer (Thermo Scientific). SNO-peptides in 0.1% TFA were loaded from a cooled (10 °C) autosampler tray to a pre-column (Symmetry C18, 180 μm × 20 mm, and 5 μm Waters) and resolved on BEH130 column (C18, 75 μm x 250 mm, 1.7 μm, Waters), in a gradient of 5–30% acetonitrile/0.1% formic acid for 70 min at a 0.3 μl/min flow rate. The UPLC system was directly connected to the ion source of the mass spectrometer. The resolution of mass spectrometer was set to 50,000 for MS acquisitions with m/z measurement range of 300–2000 Th. The quantitative LC-MS runs were converted into 2D heat-maps (with retention time and m/z as vertical and horizontal axes, respectively) using in-house designed finnigan2Pipe data conversion tool (59, 60). The format of resulting data met the requirements of NMRPipe software (http://spin.niddk.nih.gov/NMRPipe).
For LC-MS/MS runs, we utilized the data dependent acquisition (DDA) mode, selecting the five most intense signals in each MS spectrum for fragmentation. Dynamic exclusion was activated, with m/z tolerance of 0.05–1.55 and duration of 15. Up to five fragmentation events were allowed for each parent ion. The peak-picking was performed using MascotDistiller software (version 2.3, MatrixScience, Boston, MA). Mascot search engine was used to survey data against UniProtKB/Swiss-Prot database version 2013_10 (45889 sequences). Mascot search parameters were set as follows: taxonomy Mus musculus, fixed modification - cysteine carbamidomethylation, variable modification - methionine oxidation, parent ion mass tolerance - 40 ppm, fragment ion mass tolerance - 0.8 Da, number of missed cleavages - 1, enzyme specificity - semi-trypsin. The false-positive rate (FDR) values for Mascot identifications were calculated using the concatenated target/decoy database search strategy (merged target/decoy databases generated with in-house developed software (http://proteom.ibb.waw.pl/decoy/index.html). This analysis demonstrated that for peptides with Mascot score > 30, the FDR did not exceed 0.29%.
Using in-house developed software Mscan (http://proteom.ibb.waw.pl/mscan), the peptides identified in all LC-MS/MS runs (both from FVB and hAPP samples) were merged into one selected peptide list (SPL). Each peptide in the SPL was characterized by its amino acid sequence, LC retention time, m/z value and charge state values of corresponding ions. The SPL was then used to localize the peptide ions on 2D heat-maps generated from LC-MS runs (supplemental Fig. S1), and to obtain the quantitative values (LC peak intensities). Peptide ion localization was performed with in-house developed TagProfile software (60). Further manual data inspection (mainly to account for retention time variation in different LC runs and deal with faulty assignment cases) and quantitative analysis was achieved using in-house developed software Msparky (http://proteom.ibb.waw.pl/msparky), a modified version of Sparky NMR software (http://www.cgl.ucsf.edu/home/sparky) (59–63). Acceptance criteria for manual data inspection included: m/z value deviation, 20 ppm; retention time deviation, 10 min, and envelope root mean squared error (a deviation between the expected isotopic envelope of the peak heights and their experimental values) - 0.7. Charge state value was also inspected.
The SPL with obtained quantitative values was then reduced so that each cysteine was represented by single peptide entry with one quantitative value. In the final step, two lists of SNO-peptides/proteins from 14 month old mice were generated, one for hAPP and one for FVB. By comparing these lists, three sets of SNO-Cysteine peptides were determined: (1) identified only in FVB (corresponding proteins named as S-nitroso-wt), (2) identified in both FVB and hAPP (corresponding proteins named as S-nitroso-all), and (3) observed exclusively in hAPP synaptosomes (corresponding proteins named as S-nitroso-diff). These sets are presented in supplemental Table S1. The annotated spectra displaying sequence information of all identified SNO-peptides are presented in supplemental Fig. S2. All raw data from LC-MS and LC-MS/MS measurements are available at the public repository ProteomicsDB (project entitled: “synaptosomes;” https://www.proteomicsdb.org/proteomicsdb/#projects/4161?accessCode=f5247f4f64cc04f627a141ffa16b4d7d836dfabaf4c33729a990b1794723ea1b).
To compare the number of identified SNO-proteins and SNO-sites in all three sets we utilized Venny (http://bioinfogp.cnb.csic.es/tools/venny/) (64). Positions of SNO-Cys sites, the number of cysteines in protein sequence, and previously identified entries with literature references are listed in the same supplemental table. Comparison of mouse and human differential SNO-peptide sequences was achieved with Uniprot/blast program (http://www.uniprot.org/blast/).
Functional Analysis of Proteomic Data
In order to connect the human orthologs of mouse differentially S-nitrosylated gene products with Amyloid Precursor Protein (APP) we utilized GeneMania (www.GeneMania.org; which indexes 1464 association networks containing 292,680,904 interactions mapped to 149,747 genes from seven organisms, last update 06/2013) (65, 66). Human orthologs of mouse genes were assigned with NCBI homologene (http://www.ncbi.nlm.nih.gov/sites/homologene/). NCBI Gene unique identifiers of human orthologs of mouse genes representing differentially mouse SNO-proteins identified in FVB or hAPP mice were used as an input for functional network analyses. The networks were calculated and drawn using GeneMania reference real- and binary-valued interaction networks consisting of physical, genetic, predicted, and pathway interaction data sets. For weighting of the networks we used Gene Ontology biological process (GO BP) algorithm from GeneMania, which assigns weights in order to maximize connectivity between all input genes in a given ontology class. The number of measured SNO-sites was included as one of the parameters in network depiction. In cases when the number of SNO-sites in a peptide after neutravidin affinity could not be unambiguously assigned, for simplicity, we chose the highest possible number of SNO-sites, according to a number of available Cys in a given sequence.
SNO-data sets were functionally analyzed using ClueGOv1.4, a Cytoscape plug-in (http://www.ici.upmc.fr/cluego/) (67), which applies GO/KEGG hierarchical characteristics for clustering of term distributions. As a reference set for term enrichment calculations we utilized genes (NCBI unique Gene identifiers), corresponding to human orthologs of MS- measured mouse synaptosomal proteins (Malinowska et al., submitted), enriched with non-redundant genes from two most comprehensive, expert- curated synaptic databases (SynsysNet; http://bioinformatics.charite.de/synsys/ (68) and SynaptomeDB; http://psychiatry.igm.jhmi.edu/SynaptomeDB/ (69)). Thus constructed “synaptic reference set” comprises of more than 5600 NCBI unique human genes. The enrichment of GO biological process functional categories in this reference set is presented in supplemental Fig. S3. A Venn diagram demonstrating the distribution of genes among three database sources in “synaptic reference set” is presented in supplemental Fig. S4. p values for term enrichment were calculated using right-sided hypergeometric test. The nodes in functionally grouped networks were linked based on their kappa score level (≥0.3) in ClueGO.
RESULTS
The Protein Expression of Nitric Oxide Synthases is Elevated in hAPP Mouse Brain
Expression levels of nNOS and iNOS in total brain cortex lysates from 14 months old FVB and hAPP mice were compared by Western blot analysis. A significant increase in the protein expression levels of iNOS (fold change = 4.94, n = 4, p = 4.74E-06), and nNOS (fold change = 2.05, n = 2) was observed in hAPP mouse brain in comparison to FVB mouse brain, consistent with previous observations (70, 71). Bands of 130 and 160 kDa corresponded to iNOS and nNOS, respectively (Fig. 1).
Fig. 1.
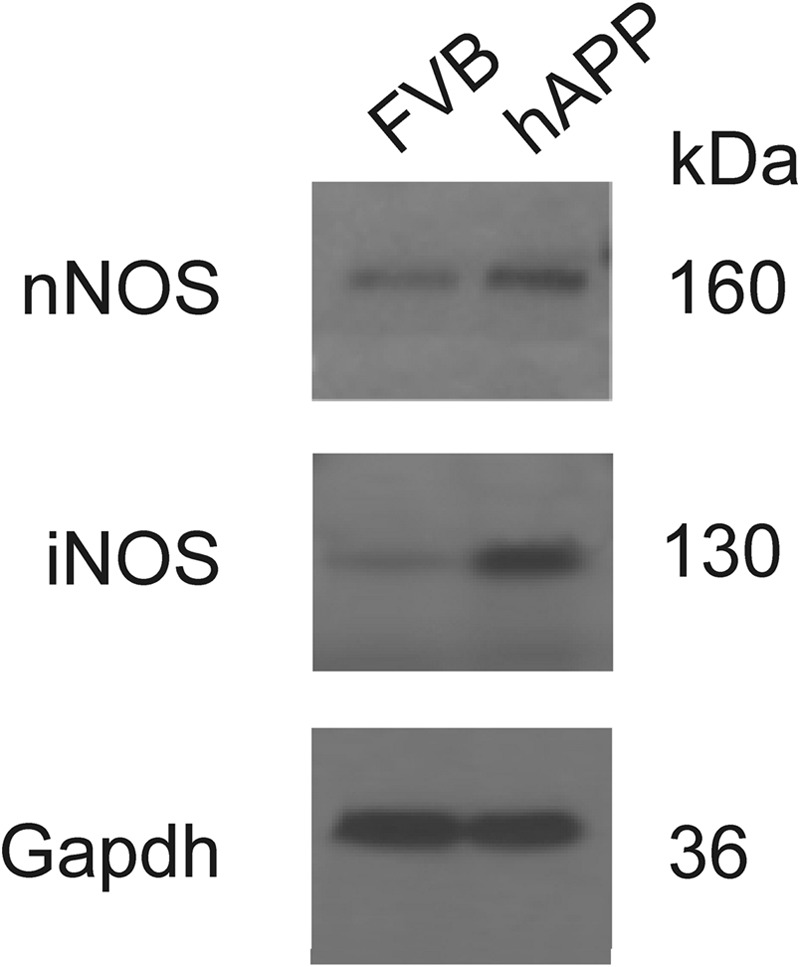
Western blot analysis of iNOS and nNOS expression in the brain. The expression of both enzymes was analyzed in wild-type (FVB, lane 1) and transgenic APP (hAPP, lane 2) total mouse brain cortex lysates. Gapdh protein expression was used for normalization. kDa- molecular weight in kilo-Daltons.
Endogenous Protein S-nitrosylation is Increased in hAPP Synaptosomes
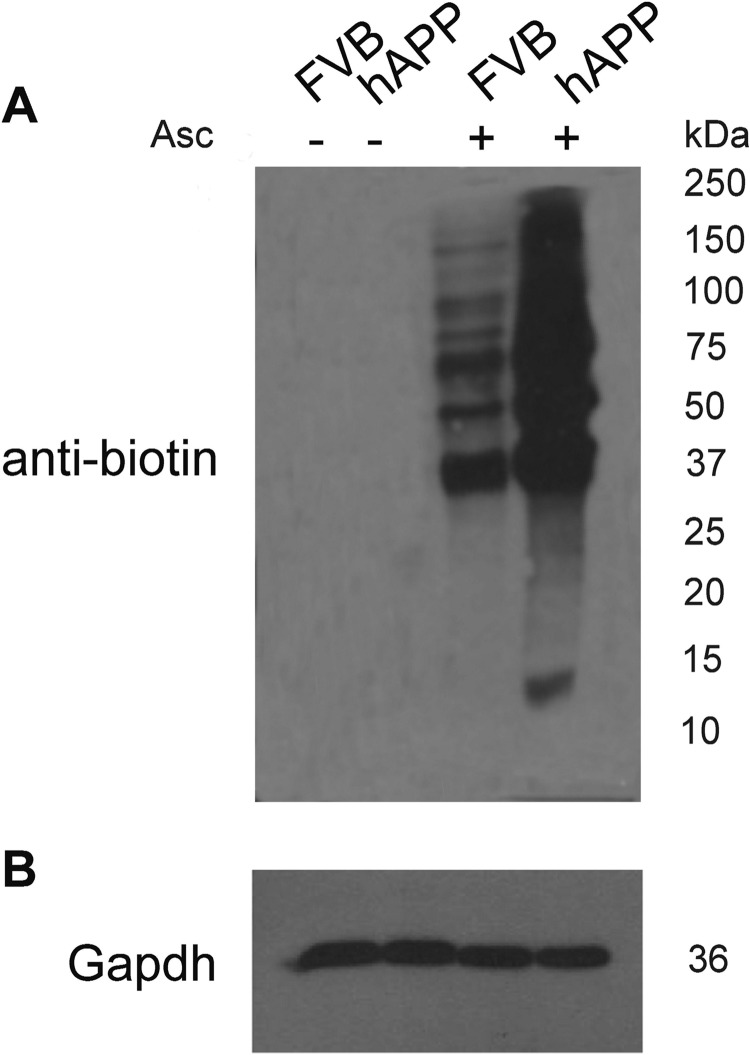
To assess whether the increased expression of nitric oxide synthases visible in the hAPP mouse brain results in pattern changes of endogenous protein S-nitrosylation, we used a BST assay in which SNO-proteins are selectively labeled by biotin, followed by Western blot detection with anti-biotin antibodies. Numerous protein bands across a broad mass range were revealed, both in FVB and hAPP derived synaptosomal fractions indicating the presence of endogenously S-nitrosylated proteins (Fig. 2, lane 3 and 4). The number and intensities of specific bands were significantly increased in hAPP synaptosomes. No bands were detected in negative control experiments confirming selectivity of the ascorbate reduction of SNO-bonds.
Fig. 2.
Western blot analysis of S-nitrosylation pattern in the FVB and hAPP brains. S-nitrosylation sites were selectively labeled with S-S-biotin (BST). Visualization of biotinylated proteins was achieved with anti-biotin antibodies. Controls were prepared without selective ascorbate (Asc) reduction of SNO bonds (lane 1 and 2). Pattern of S-nitrosylation of synaptosomal proteins from FVB and hAPP mouse brain is presented in lanes 3 and 4, respectively.
Endogenously S-nitrosylated Proteins in FVB and hAPP Synaptosomes were Identified using SNOSID-LC-MS/MS Assay
BST and anti-biotin Western blot analysis suggested that synaptosomal proteins of a wide molecular mass range are affected by S-nitrosylation.
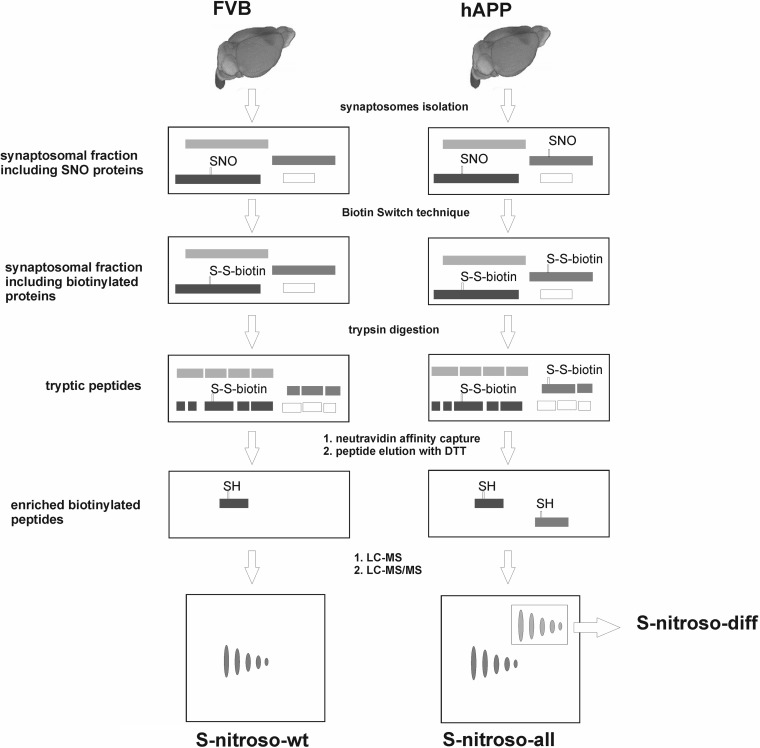
In our work, we utilized SNOSID technique developed by Hao et al. (50) for non- targeted proteomic identification of specific cysteine residues in proteins which are modified by posttranslational S-nitrosylation. The method is based on exclusive affinity capture of formerly nitrosylated, cysteine containing tryptic peptides, which are labeled with biotin similarly as in the initial steps of BST assay. The overall scheme of S-nitrosylation enrichment methodology used in this work is presented in the Fig. 3.
Fig. 3.
Scheme of S-nitrosylation enrichment methodology. Mouse brain synaptosomes were isolated and S-nitrosylated proteins enriched using Biotin Switch Technique. After trypsin digestion and enrichment on Neutravidin agarose, the SNO-peptides were analyzed by LC-MS/MS using in house developed MSparky software.
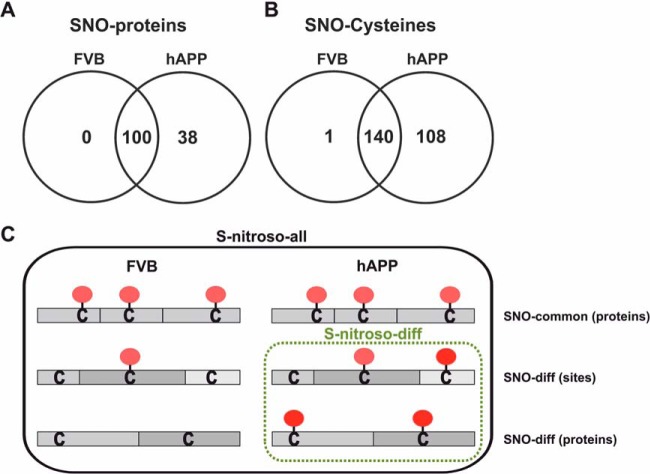
We explored SNOSID peptide enrichment technique combined with LC-MS/MS peptide identification to precisely ascertain the targets of S-nitrosylation among synaptosomal proteins. Among 138 identified SNO-proteins, 38 were present only in hAPP mice synaptosomes (Fig. 4A, Table I and supplemental Table S2). A total of 249 SNO-sites were identified, including 108 sites found exclusively in hAPP brain (Fig. 4B). Sequence alignment of mouse differentially S-nitrosylated peptide sequences with their human counterparts demonstrated that almost all SNO- Cys are conserved between both species (supplemental Table S3). 95 SNO- peptides were identified for the first time (40% of total, 33 of which were solely present in hAPP synaptosomes). 49 of the hAPP differential SNO-sites were detected in proteins S-nitrosylated in FVB mice but at a different Cys residue, that is, in calcium/calmodulin-dependent protein kinase II (CamkII), succinate-CoA ligase (Suclg1), or neurofascin (Nfasc). Interestingly, for aconitate hydratase (Aco2) we detected an exchange of a single SNO-site from Cys385 to Cys592. The number of differential SNO-peptides, that is, SNO-sites in FVB and hAPP mice differs from that of differential proteins, which suggests that some proteins are possibly sequentially S-nitrosylated.
Fig. 4.
Venn diagram comparisons of the numbers of S-nitrosylated proteins A, and S-nitrosylation sites B, identified in synaptosomes isolated from FVB and hAPP mouse brains. C, Scheme of S-nitrosylation pattern in S-nitroso-wt and S-nitroso-all sets showing differential and sequential S-nitrosylation, respectively. The details on number of SNO-Cys and SNO-sites identified in this study with respective UniProtKB/Swiss-Prot IDs and NCBI Gene Symbols are given in supplemental Fig. S5.
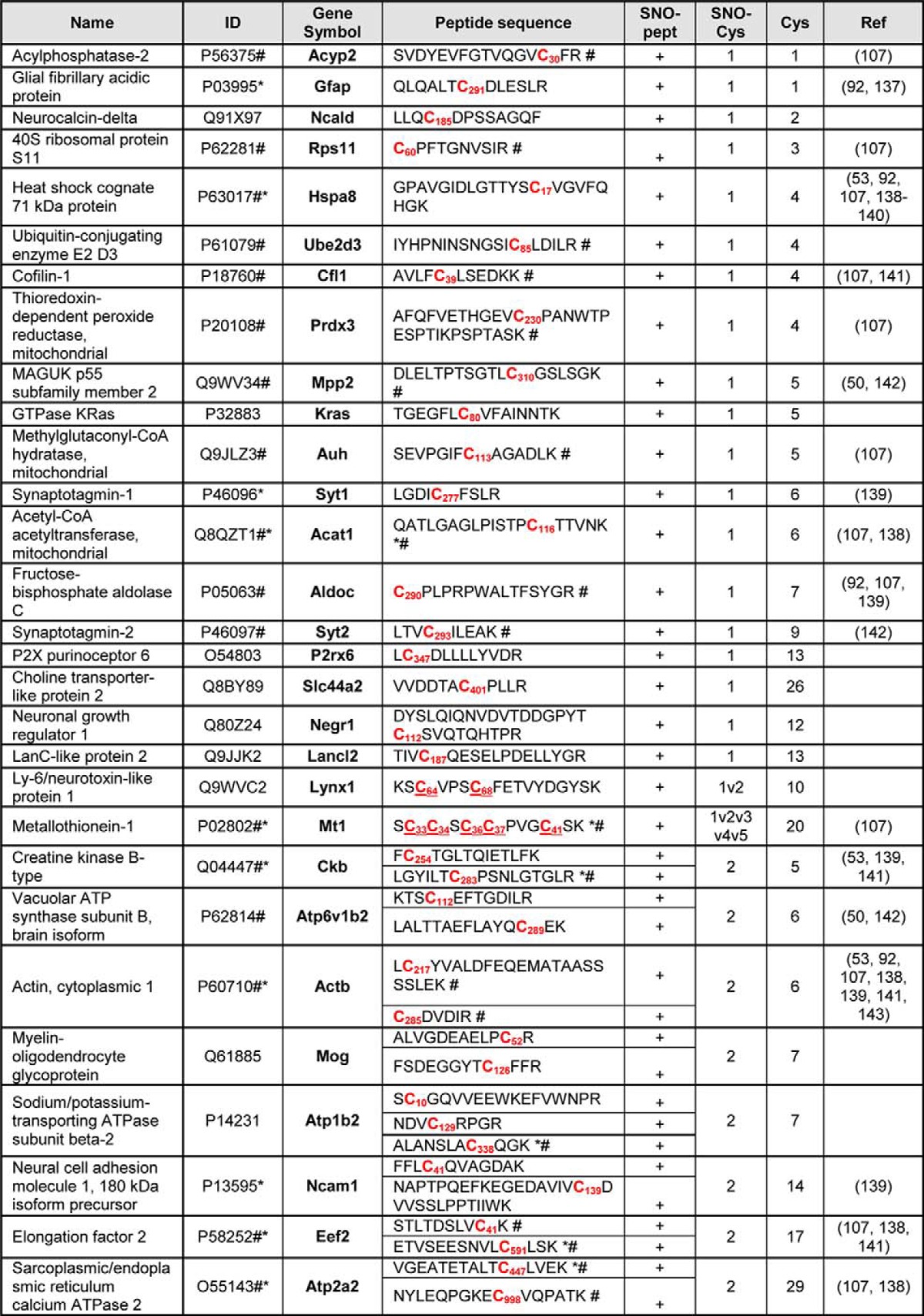
Table I. List of identified, differentially S-nitrosylated proteins and corresponding peptides in the synaptosomes from hAPP transgenic mice. Legend: Name, UniProtKB/Swiss-Prot name, ID, UniProtKB/Swiss-Prot database unique identifier; Gene Symbol, NCBI official Gene Symbol, Mus Musculus; Peptide sequence, sequence of the Cys containing peptide identified in our MS/MS experiments; SNO-peptide, SNO-peptides detected in MS/MS measurements; Cys, number of Cysteines in a given protein sequence; #, literature described endogenous S-nitrosylation of Cys from a given peptide sequence; *, literature described exogenous S-nitrosylation of Cys from a given peptide sequence. Ref, Literature citation (see references). All Cys in the sequence are bold marked. Differentially SNO-Cysteines are marked in red. The aminoacid position of Cys-SNO is given in subscript. In cases where SNO modification could not be unambiguously assigned (i.e. 2v3), the potentially SNO-Cys (in bold red) are underlined. Ref- cited literature.
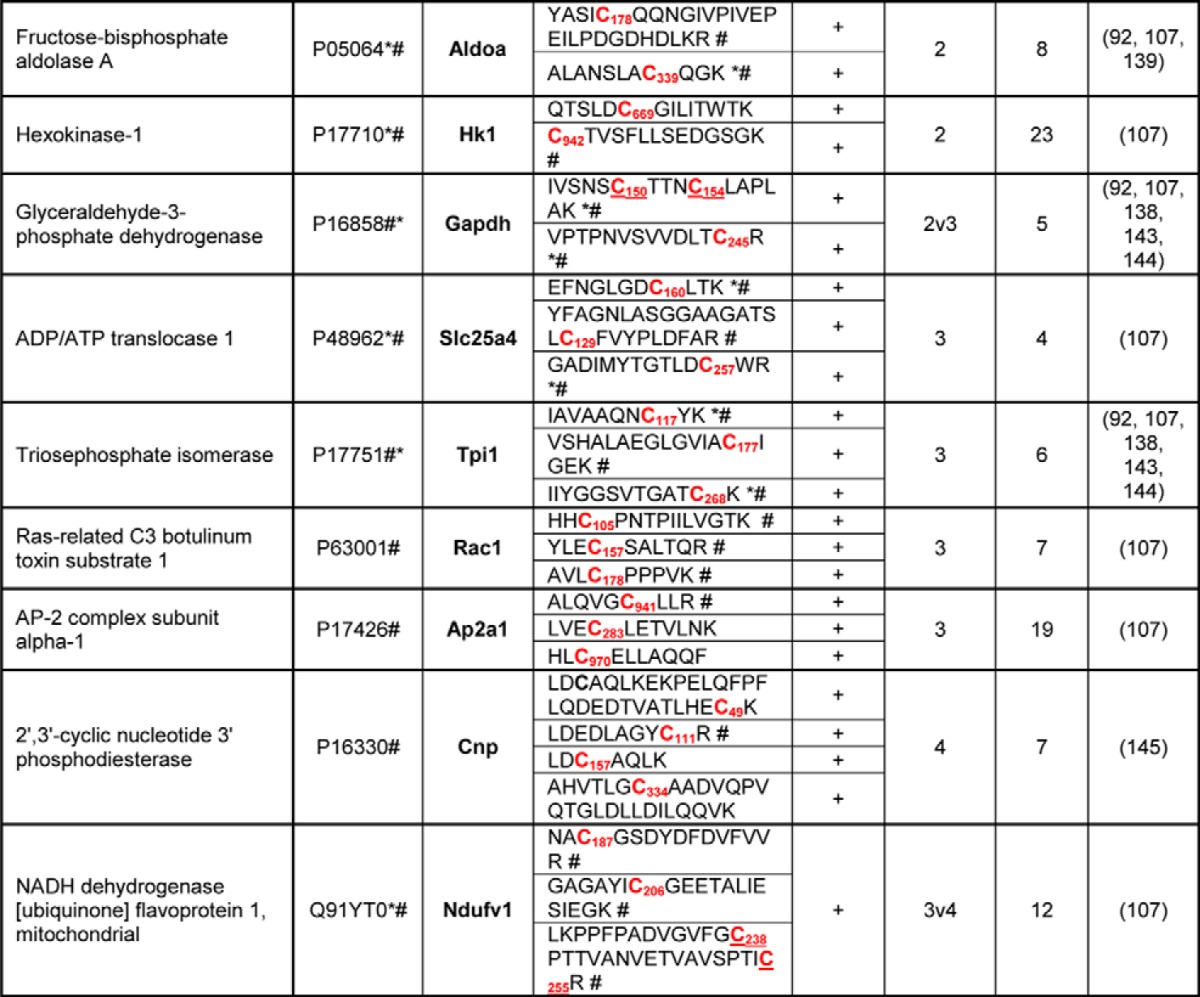
The overall pattern of S-nitrosylations in FVB and hAPP synaptosomes is depicted in supplemental Fig. S5. Fig. 4C schematically portrays the possibilities for SNO-based regulation of synaptosomal proteins as a result of the hAPP overexpression in mice.
Synaptosomal S-nitrosylated Proteins in FVB Mouse Brain Represent a Variety of Protein Classes
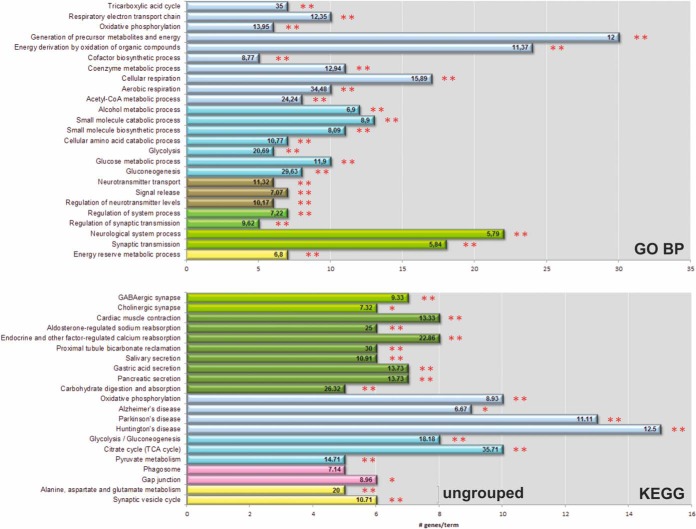
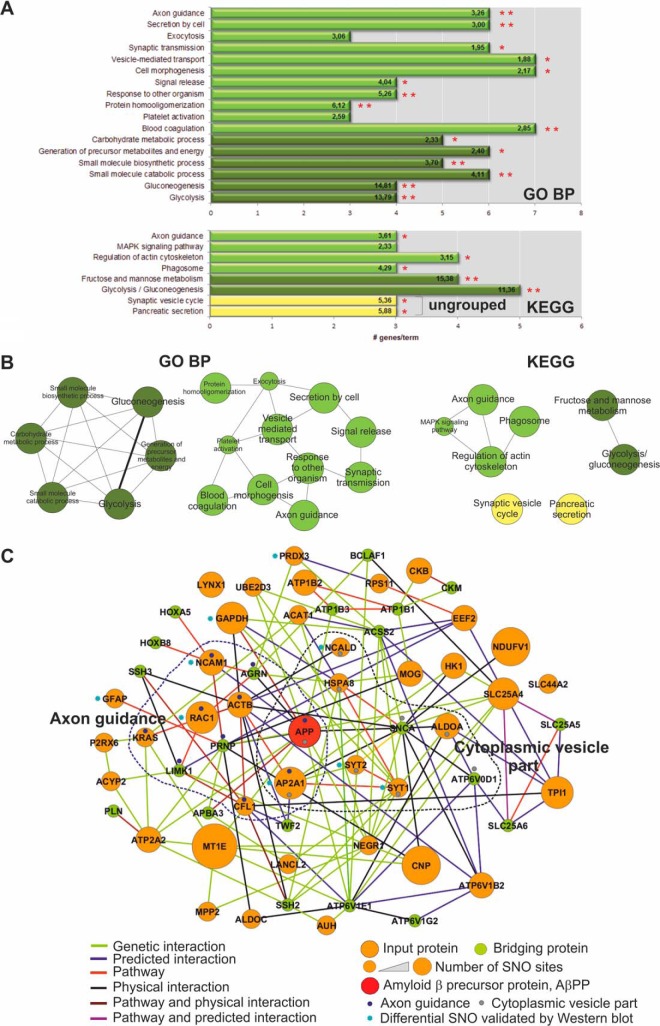
In order to decipher molecular mechanisms in the synapse, in which S-nitrosylation of proteins might play a distinct role, we performed functional enrichment analyses of terms from Gene Ontology Biological Process (GOBP) and KEGG. For this purpose, we utilized a comprehensive “synaptic reference set,” comprising over 5600 genes (see description in Material and Methods) and ClueGO algorithm. Two sets of identified human orthologs of mouse S-nitrosylated synaptosomal proteins (S-nitroso-wt and S-nitroso-diff sets) were analyzed. The analyses of S-nitroso-wt set (Fig. 5 and supplemental Tables S4) revealed multiple enriched functional categories. Terms related to: generation of precursor metabolites (p = 3.11E-15), gluconeogenesis (p = 7.41E-08), synaptic transmission (p = 1.22E-4), and neurotransmitter transport (p = 1.07E-03) were significantly statistically enriched in GO BP analyses of S-nitroso-wt set (Fig. 5 and supplemental Table S4A). In-depth analysis of KEGG pathways pinned down additional enriched terms related to other metabolic processes, that is, TCA cycle, oxidative phosphorylation and synaptic organization, to name but only a few of those most associated with the normal physiology of neurons.
Fig. 5.
ClueGO analysis of S-nitrosylated proteins from FVB mouse brain synaptosomes. GO biological process (GO BP, upper panel) and KEGG pathway (KEGG, lower panel) terms specific for S-nitrosylated proteins from the FVB brain synaptosomes (**-p ≤ 0.01, *-p ≤ 0.05). The number of corresponding genes associated with a specific term is indicated. The percentage of genes associated with a specific term is listed on the bars. Ungrouped terms are shown in yellow.
The multiplicity of enriched terms suggests that S-nitrosylation is a global post-translational modification, with a role in modulating the function of different classes of proteins within the synapse microenvironment. Interestingly, 16 SNO-proteins from FVB mice (∼12% of all identified mouse brain synaptosomal SNO-proteins; supplemental Table S4B) formed a cluster of functionally grouped terms (Fig. 5) previously implicated in Alzheimer's, Parkinson's, and Huntington's diseases.
Differentially S-nitrosylated Proteins in hAPP Mouse Brain are Linked to Axon Guidance and Vesicle Trafficking and Form a Highly Connected Network
Analysis of S-nitroso-diff proteins with ClueGO and GO BP revealed two major functional clusters. Similarly, as in the wild-type mouse brain, a six node subnetwork related to gluconeogenesis/glycolysis and generation of precursor metabolites and energy was revealed (comprising nine out of 38 analyzed proteins, supplemental Table S5A). The second subnetwork contained 11 nodes including axon guidance (GO:0007411), which presented the most enriched group term (p = 4.76E-03; Fig. 6A, 6B). Other nodes which belong to this functional cluster included that is, vesicle mediated transport and exocytosis, both of which are linked to APP neuronal trafficking (Fig. 6B and supplemental Table S5B) (72, 73). Parallel functional analyses using KEGG pathways supported the finding that proteins in the S-nitroso-diff set are linked to axon guidance and regulation of actin cytoskeleton (p = 3.71E-02, Fig. 6B and supplemental Table S5B).
Fig. 6.
A, ClueGO analysis of S-nitrosylated proteins from hAPP mouse brain synaptosomes. GO biological process/KEGG pathway terms specific for S-nitrosylated proteins from the FVB brain (*-p ≤ 0.05; **-p ≤ 0.01). The number of corresponding genes associated with a specific term is indicated as percentage (numbers on bars) and on x axis. Ungrouped terms are indicated in yellow. B, The networks of functionally grouped terms with nodes linked based on their kappa score (≥0.3). Terms not grouped are shown in yellow. C, Interaction network linking human orthologs of differentially SNO-proteins detected in hAPP synaptosomes and Amyloid β precursor protein (APP). The network was sorted according to GO BP criteria. The size of nodes, representing SNO-proteins (in orange) is proportional to the number of SNO-sites, measured in LC-MS/MS experiments. The nodes sharing two most enriched functional terms, axon guidance and cytoplasmic vesicle part are presented as constituents of network modules (represented by dotted spheres).
In this study, human APP protein expression in mouse brain was the distinguishing parameter used to model some aspects of Alzheimer disease. Therefore, we aimed to assess the connectivity of the identified differentially S-nitrosylated synaptosomal proteins to APP. We searched for human orthologs of mouse SNO-proteins (supplemental Table S1) and connected them using GeneMania. We connected 36 (out of 38) differentially S-nitrosylated orthologs with APP using multiple interaction data (Fig. 6C and supplemental Tables S6A–S6D). Fifty-nine nodes in the calculated network (supplemental Table S6A) were connected by genetic (57 links) and physical interactions (34 links), as well as pathway sharing (27 links). Thirty-four interactions in the network were derived from predicted, mostly interologous interactions (supplemental Table S6B). Five SNO-proteins were directly connected to APP either via physical interactions (Gapdh/GAPDH), genetic interactions (Ube2d3/UBE2D3, Negr1/NEGR1), and pathway sharing (Gapdh/GAPDH and Negr1/NEGR1) or predicted to interact from homologous interactions (Ncam1/NCAM1). Actb/ACTB (β-actin) has been detected as part of the same molecular complex with APP (74). The most enriched functional category of the network, participation in cytoplasmic vesicle (p = 2.74E-04) was shared by nine proteins, including APP and 6 SNO-proteins, namely AP2A1, ALDOA, HSPA8, NCALD, SYT1, and SYT2. Subsequent analysis of the axon guidance subnetwork (p = 1.73E-03) revealed that it contained 10 proteins, including APP, PRNP, LIMK1, and AGRN and six human orthologs of differentially nitrosylated proteins (from S-nitroso-diff set): ACTB, AP2A1, CFL1, KRAS, NCAM1, and RAC1 (Fig. 6C and supplemental Table S6C).
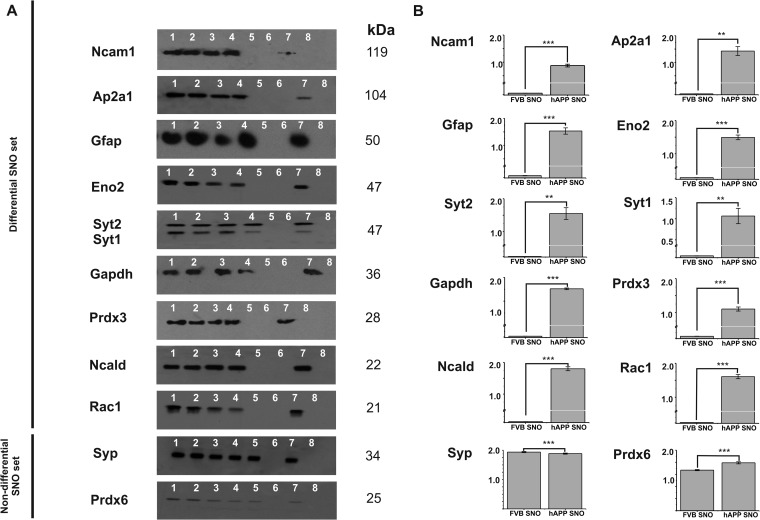
Validation of Differential Protein S-nitrosylation Detected using Biotin Switch-LC-MS/MS by Western Blotting
Western blot analysis was used to validate the results of MS based identifications of selected, differential SNO-proteins implicated in AD (75–79). Immunoreactivity was traced in different fractions during BST enrichment of S-nitrosylated synaptosomal proteins from FVB and hAPP mice. Ten differentially S-nitrosylated proteins from different functional classes were chosen for MS data validation (including a novel one described in this study, neurocalcin delta, Ncald). Fig. 7 demonstrates the results of immunoblotting with specific antibodies recognizing mouse Ncam1, Ap2a1, Gfap, Eno2, Syt1/Syt2, Gapdh, Ncald, Prdx3, and Rac1, respectively. The total expression of studied proteins was unchanged in the hAPP and FVB brains (lanes 1 and 2), which is consistent with the results of differential proteomics expression analysis in these mice (Malinowska et al., submitted). Following BST enrichment, positive signals were observed only in fractions derived from hAPP brain synaptosomes (lane 7), but not in the FVB brain (lane 5), confirming the MS-based identification of differentially SNO-proteins. An internal control in which S-nitrosylation does not change between FVB and hAPP (includes two synaptosomal proteins, synaptophysin, Syp, and peroxiredoxin, Prdx6; compare lanes 5 and 7, Fig. 7A, 7B) was included.
Fig. 7.
Western blot analysis of S-nitrosylated proteins from hAPP brain synaptosomes. A, Synaptosomal SNO-proteins enriched using BST were detected with specific antibodies. Differential SNO set: Ncam1 - Neural cell adhesion molecule, Ap2a1 - AP-2 complex subunit alpha-1, Gfap - Glial fibrillary acidic protein, Eno2 - Gamma enolase, Syt1, Syt2 - Synaptotagmin-1 and 2, Gapdh - glyceraldehyde-3-phosphate dehydrogenase, Ncald - neurocalcin-delta, Prxd3 - peroxiredoxin 3, Rac1- Ras-related C3 botulinum toxin substrate 1 precursor. Non-differential SNO set: Syp- synaptophysin, Prxd6 - peroxiredoxin 6. Lane 1 - total FVB mouse brain lysate, lane 2 - total hAPP brain lysate, lane 3 - soluble fraction (FVB), lane 4 - soluble fraction (hAPP); lane 5 - proteins enriched on neutravidin resin using BST (FVB), lane 6 - neutravidin resin after elution (FVB), lane 7 - proteins enriched on neutravidin resin using BST (hAPP), and lane 8 - neutravidin resin after elution (hAPP). kDa- molecular weight in kilo-Daltons. B, Densitometric quantitation of lanes 5 (Neutr_FVB) and 7 (Neutr_hAPP); n = 3 experiments, p values from t test. **-p ≤ 0.01; ***-p ≤ 0.001.
Interestingly, a specific monoclonal antibody against enolase 2, Eno2 was used to positively identify S-nitrosylation of this protein in the hAPP brain. In MS based analyses we could not distinguish whether this protein is differentially S-nitrosylated in hAPP mice or an additional site is modified by S-nitrosylation upon APP overexpression (supplemental Table S2A). The identified SNO-peptide in the FVB synaptosomes was also shared between three enolases, Eno1, Eno2, and Eno3, precluding a proper assignment. The results of Western blot analysis indicate that there is no S-nitrosylation of Eno2 in the wild-type mouse brain. Overall, we have validated the differential SNO of 10 proteins in hAPP synaptosomes.
DISCUSSION
Signaling by RNS is mainly carried out by targeted modifications of critical cysteine residues in proteins, including S-nitrosylation, S-oxidation, and lipid nitration (80). Despite thousands of SNO-proteins currently identified (∼3000), the observed specificity of S-nitrosylation in terms of target proteins and specific Cys residues is not entirely understood (81, 82). S-nitrosylated proteins are implicated in the pathogenesis of various neurodegenerative diseases, including Alzheimer's, Parkinson's and Huntington's diseases, amyotrophic lateral sclerosis, Friedreich ataxia, and many others, where they influence the onset or development of neurodegeneration (8, 23, 83–85).
One of the key pathological features of patients with neurodegenerative disorders including AD is impaired signaling at the synapse. Our proteomics study was designed to search for and identify endogenous regulation of synaptic proteins by S-nitrosylation. We also probed for a direct association of protein S-nitrosylation with an important aspect of AD development, namely overexpression of Aβ.
Synaptosomes are a well-recognized model for studies of synaptic complexity in the brain (86, 87). They contain complete presynaptic terminals, with postsynaptic membranes and densities, as well as other components necessary to store, release, and retain neurotransmitters. Viable mitochondria for production of ATP and active energy metabolism, are also present in synaptosomes (88). Moreover, it has recently been proposed that S-nitrosylation is effectively dependent on the subcellular localization of proteins, with short-range linkage to synaptic transmission in neurons (89). Therefore, in order to reduce the complexity of the analyzed system we decided to narrow our studies to proteins involved in synaptic functions, employing fresh synaptosomal preparations routinely used for proteomic screening of affected pathways in the brain (90, 91).
A combination of different approaches based on BST and immunoprecipitation with S-nitrosothiol antibodies, followed by 2D-electrophoresis and LC-MS/MS was recently introduced by Zahid et al. (92), to study differentially S-nitrosylated proteins in the human AD brain. However, in their study, total brain lysates from cortex, hippocampus, and substantia nigra (post-mortem frozen brain tissue) were utilized, and only 45 SNO-proteins identified (without recognizing sites of modification), impeding detailed analysis of the affected pathways in the synapse.
Human brain tissue samples are difficult targets for differential proteomics. Samples are obtained post mortem with usually longer interval times in comparison to the life-span of most SNO-proteins. Special autopsy programs for AD patients, aimed at analysis of labile PTM within 4-h post mortem interval are still rare (i.e. Rapid Autopsy Program of the University of Kentucky Alzheimer's Diseases Research Center, UKADRC (93)). Moreover, freezing and thawing of tissues leads to artifacts in tyrosine nitration and cysteine nitrosylation if homogenization is not performed in the presence of thiol blocking agents (94). As such, instead of analyzing highly variable human tissue samples (vide i.e. SNO Drp1 level analysis in AD patients (95)) we opted for a well-defined tg AD mouse model for differential analysis of synaptic SNO-proteins. Moreover, the inclusion criteria used for selection of differential protein sets proposed in our work were very stringent. A protein has been defined as differentially S-nitrosylated only if it was repeatedly observed in hAPP brain synaptosomes, and not detected in the age-matched wild-type controls. In the authors opinion, this led to highly selective identification of differentially SNO synaptic proteins in the hAPP brain.
Despite its utility in identifying SNO-Cys modification in proteins, the BST and SNOSID methods are constrained by several limitations. Each step of these techniques is a potential source of methodological errors. One of such limitations is linearity (with respect to protein input and biotinylation). The blocking step of the BST shall also be taken into consideration, as some protein thiols can be resistant to complete blocking, resulting in high levels of SNO-independent biotinylation. Another question which was raised by some authors is the specificity of the method. The other constraint is a usage of ascorbate, which was suggested to have a potential to reduce the disulfide bonds. This has been later challenged by observations that thiol-dependent reduction of dehydroascorbate to ascorbate, a scenario supported by extensive in vitro and in vivo experimentation is thermodynamically favored. Another limitation of BST method is related to the presence of metal ions, which can compromise the BST specificity, including production of ascorbate and hydroxyl radicals. Following a number of publications which discussed limitations of BST/SNOSID enrichment methods (96–99), in this work we developed special sample procedures to detect and identify S-nitrosylated proteins in the synapse. We utilized SNOSID enrichment of previously S-nitrosylated tryptic peptides to pinpoint not only the S-nitrosylated proteins but also precise sites of SNO modification. Furthermore, the aim of our study was not to identify the largest number of proteins susceptible to S-nitrosylation in the synapse milieu but rather to prove that the NO-based regulation is not random that is, related only to higher amount of produced RNS. We also aspired to demonstrate that this process is highly confined to specific key molecules and/or pathways, and is modified upon AD symptoms progression in mice.
By combining an optimized SNOSID method coupled with LC-MS/MS, we identified 138 S-nitrosylated proteins with 249 SNO-sites (Fig. 4) and corresponding changes in pattern of their S-nitrosylation. With this approach 95 SNO-peptides were identified for the first time, whereas 76% of all identified SNO-proteins were previously described to be endo/exogenously S-nitrosylated in the literature (supplemental Table S2, see literature references). We also observed that almost all SNO-Cys sites in the SNO-diff set (supplemental Table S3) were conserved between human and mouse, suggestive for importance of these sites in the PTM regulation of synaptic functions in the AD brain. An overlap of 25 S-nitrosylated-proteins (Table I and supplemental Table S2) was observed between this study and the one by Zahid et al. (92).
To establish the functional connection between identified S-nitrosylated synaptosomal proteins, we applied stringent bioinformatic filtering employing Gene Ontology and pathway data (Figs. 5 and 6). From 100 SNO-proteins identified in wild-type synaptosomes, the majority was linked to various metabolic pathways including: oxidative phosphorylation and energy derivation by oxidation of organic compounds (6 and 24 proteins respectively) and synaptic functions, involving synaptic transmission (18 proteins; Fig. 5 and supplemental Table S4A). The participation of synaptic SNO-proteins in numerous protein classes has been previously described (8, 82). Interestingly, we found for the first time that five proteins linked to synaptic transmission in wild-type synaptosomes (GO:0007268; p = 1.22E-4, supplemental Table S4A) can also be S-nitrosylated (Camk2b, Cplxn1, Kclna2, Nptn, and Synj1). S-nitrosylation of both protein kinases and phosphatases influences a wide range of signal transduction pathways mediated by phosphorylation or dephosphorylation (reviewed in (81)). In this view, regulation of Camk2b, Calcium/calmodulin-dependent protein kinase type II beta chain might be important for regulation of synaptic transmission.
To link the changes in S-nitrosylation with advancement of Alzheimer's disease we used a 14 month old transgenic hAPPV717I (hAPP) mouse strain with high neuronal expression of human transgene which recapitulates important pathological and clinical hallmarks of AD, correlated with high burden and accelerated accumulation of Aβ40/42. In order to identify a possibly full spectrum of S-nitrosylation changes we narrowed our investigations to the advanced stage at which mice start to develop Aβ pathology with neuritic plaques (56, 100).
AD patients' brains display elevated level of nitric oxide synthases (70, 71). Moreover, it was found that the iNOS reactivity, expression and calcium-independent enzymatic activity was increased in APP transgenic (Tg2576 APP) mice and related to cortical neurons and microglial cells (101). Deletion of iNOS in these mice worsened spatial memory, learning, and tau pathology, suggestive for neuroprotective effect of NO (102). Increased levels of iNOS were also detected in cortical neurons stimulated with Aβ peptide (103), and confirmed in functional experiments demonstrating that Aβ stimulated induction of long-term potentiation was inhibited in iNOS knock-out mice (104). Furthermore, treatment with resveratrol protected rats from Aβ-induced neurotoxicity by suppressing iNOS production (105). Interestingly, in another model of AD (double transgenic APP-PS1 mice) deletion of iNOS gene alleviated AD-related pathology including increased Aβ levels, plaque formation, gliosis, and premature mortality (103). We confirmed that level of iNOS expression was higher in hAPP synaptosomes as compared with aged-matched controls. Changes in the expression of iNOS and nNOS (Fig. 1) indicate aberrations in the nitric oxide production which may result in subsequent changes of protein S-nitrosylation, observed in hAPP synaptosomes (Fig. 2). Gow et al. (106) demonstrated that increased expression of various nitric oxide synthases leads to changes in the level of protein S-nitrosylation in multiple cell types and tissues.
The key factors determining S-nitrosylation sites in proteins are: 1) spatial proximity (i.e. complexing with nNOS regulates the S-nitrosylation of NMDARs and PSD-95), 2) presence of signature SNO motifs adjacent to target Cys residue, and 3) local hydrophobicity (i.e. closeness to the membrane) (8, 107). Therefore, in parallel to functional clustering analyses we linked the differentially S-nitrosylated proteins from hAPP synaptosomes using network approaches. Interestingly, the majority of SNO-proteins (95%; 36/38 of all) could be linked to APP via one bridging partner resulting in a small network with 59 nodes, when stringent GO BP filtering criteria were applied to multiple interaction data (Fig. 6). Both functional clustering and network approaches revealed that axon guidance term was one of the most enriched functional features shared by differentially S-nitrosylated proteins in hAPP synaptosomes. Axon guidance term was shared by six SNO-proteins of the interaction network (Fig. 6C), including five proteins previously described in literature as possible targets of endo/exogenous S-nitrosylation and one novel, GTPase KRas precursor (Table I). The second enriched functional term, “participation in cytoplasmic vesicle” (GO:004443) was allotted to six SNO-proteins, one of which is a novel S-nitrosylation target, Neurocalcin-delta (Ncald/NCALD). S-nitrosylation of this target has been exclusively confirmed in the hAPP brain, by Western blotting experiments with specific antibodies (Fig. 7). Involvement of APP in axonal guidance and vesicle trafficking was previously described (Fig. 6C; (73, 108–112)). Recent in silico analysis of differentially expressed genes in sporadic early onset AD revealed an alteration in biological pathways related to intracellular signaling including axon guidance among the others (113). Moreover, defects in axon guidance were linked to early stage progression of another neurodegenerative disorder, Parkinson's disease (114).
The synaptic cytoskeleton is particularly important for synaptic plasticity and plays a role in rapid activity-dependent changes of synapse volume or shape. Disruptions in the synaptic cytoskeleton affect the stability and maturation of synapses and subsequently disturb neuronal communication (115–117). Actin cytoskeletal pathology may be an early cause of transport defects in AD (118). One of the identified, differentially S-nitrosylated proteins participating in axonal guidance is β-actin (Actb). Actin microfilaments supported by actin-associated proteins, are the dominant cytoskeletal elements structuring synapses. Local β-actin synthesis in developing axons plays an important role in growth cone steering (119). S-nitrosylation of β-actin, which increases formation of short actin filaments lead to alterations in the cytoskeletal network and inhibited dopamine release (120).
Another identified SNO-protein, actin severing protein; cofilin (Cfln) affects APP transport, synaptic stability, and activity (121). siRNA knockdown of cofilin abolished both Aβ and RanBP9-induced apoptosis (122). In hippocampal neurons, fibrillar Aβ was able to alter the PAK1/LIMK1/cofilin axis and thereby actin organization (123).
Rac1, a small Rho GTPase functions as a positive regulator of neurite outgrowth downstream of growth-promoting axon guidance cues. It regulates dendritic spines and excitatory synapses, but little is known about its regulation in synapses (124). Rac1 is related to increased alterations in the actin cytoskeleton induced by fibrillar Aβ (125). It has been hypothesized that Rac1 activation exacerbates AD by shifting actin into a polymerized conformation, a phenomenon observed in various neurodegenerative disorders (125). Previous studies have also shown that the reduction of ROS generation leads to an inhibition of the Rac1 activation (126).
An important group of proteins engaged in the axon guidance are those connected to the endo/exocytosis processes. AP-2 is the major adaptor protein important for sorting of synaptic vesicle proteins during recycling (127). In neurons, AP-2-dependent trafficking of NMDA and AMPA receptors is an essential determinant of synaptic strength and plasticity (128, 129). S-nitrosylation of AMPA receptors resulted in increased endocytosis by binding AP-2 protein (130).
Levels of Neurocalcin, a calcium binding protein identified to be S- nitrosylated for the first time are reduced in AD brain, suggestive for biochemical deficits related to the synaptic degeneration (131). Taken together, the identified changes of S-nitrosylation of proteins linked to axon guidance and related to endo/exocytosis processes underlie an important role of this modification in the regulation of synapses in the AD brain.
Another significantly enriched category of the differentially S-nitrosylated proteins identified in hAPP synaptosomes constituted of those involved in energy metabolism and oxidative phosphorylation (Fig. 6A, 6B and supplemental Table S5). Modulation of protein function through PTM is probably an important feature of energy production at the synapses. Although the brain represents only 2% of the body weight, it uses ∼20% of the total body basal oxygen consumption (132). Previous proteomic analyzes reported significant decrease in level of glycolytic enzymes in the AD brain and increase in the oxidation of proteins involved in the glycolysis and TCA cycle (37, 41).
In the current study, we showed changes in the S-nitrosylation of seven proteins involved in energy metabolism (supplemental Table S5A). We, for example, identified aberrant S-nitrosylation of GAPDH a key enzyme in glycolysis process which catalyzes NAD-mediated oxidative phosphorylation of glyceraldehyde phosphate to 1,3-diphosphoglycerate. However, apart from its' classical role in glycolysis, GAPDH takes part in highly diverse, non-glycolytic functions. For example, S-nitrosylation of GAPDH enhances binding to SIAH1 protein, an E3 ubiquitin ligase, and the complex is translocated to nucleus where it activates apoptosis. In the nucleus, SNO-GAPDH not only mediates apoptosis but also serves as a trans-nitrosylase of other nuclear proteins, including SIRT1, HDAC2, or DNAPK (19). SNO-GAPDH dependent molecular pathway leading to neuronal apoptosis may contribute to Alzheimer's disease pathogenesis (76).
A recent study demonstrated that both GAPDH and gamma enolase were oxidatively modified in post mortem AD patients brains (76, 133). Oxidative modifications of these enzymes led to inhibition of glycolytic pathways. This is consistent with the altered glucose tolerance and metabolic changes confirmed in PET analyses of AD patients (134). The impact of S-nitrosylation on the function of those proteins is unclear. Aberrant S-nitrosylation of a large number of glycolytic enzymes suggests that synapses may be sensitive to glycolytic perturbation, which in turn exacerbates Aβ toxicity.
We also found a large group of mitochondrial proteins with altered S-nitrosylation. There is mounting evidence that mitochondrial dysfunction accompanies AD progression and development (135). Mitochondrial dysfunctions are consequences of aberrant redox reactions triggered by high level of NO. Disturbances in the activity of Complexes I and IV caused by S-nitrosylation have been reported in the AD brain (reviewed in (136)). Additionally, a decrease in the activity of F1 ATPAse caused by S-nitrosylation was reported in cardiomyocytes (54). We found that S-nitrosylated Ndufv1/NDUFV1 and Uqcrc1/UQCRC1, mitochondrial complex I and III proteins were either uniquely present (Ndufv1) or more nitrosylated (Uqcrc1/UQCRC1) in APP brains (supplemental Table S2).
Summarizing, these results suggest that altered S-nitrosylation of proteins involved in energy metabolism might be one of the main events associated with AD, leading to reduced activity of metabolic pathways and therefore decreased ATP production, confirming the previous observations.
Cellular “S-nitrosome” homeostasis is regulated by enzymatic and non-enzymatic nitrosylation, denitrosylation, and the overall redox milieu. The current work was based on systematic profiling of S-nitrosylations in brain synaptosomes from wild-type and AD mice. We have identified several endogenous SNO-sites exclusively in the hAPP brain, which further contributes to our understanding of synaptic complexity and its alterations in AD. It also implicates S-nitrosylation in the interplay of various PTM controlling the signaling cascades in the brain, in normal and neurodegenerative conditions, and identifies novel putative drug targets for therapeutic interventions. Further research is necessary to decipher the precise role of S-nitrosylation in the function of identified synaptic proteins.
Supplementary Material
Acknowledgments
We thank Prof. Marc Baumann, MSc. Janusz Dębski and MSc. Enzo Scifo for critical comments on the manuscript. We would also like to thank: Prof. Fred van Leuven for transgenic mouse strain hAPPV717I; PhD Anna Kowalczyk for help with mouse breeding; MSc. Anna Fogtman for heat-map analysis; MSc Michał Kistowski for help in bioinformatics, MSc. Sławomir Januszewski for help with tissue preparation, MSc. Jacek Olędzki for technical help with mass spectrometry and MSc. Liliya Zhukova for laboratory technical support.
Footnotes
Author contributions: M.Z., M.D., and A.W. designed research; M.Z. performed research; M.Z. contributed new reagents or analytic tools; M.Z., A.S., and M.L. analyzed data; M.Z., A.W., and M.L. wrote the paper; M.D., A.W., and M.L. conceived and supervised the project.
* This study was supported by a grant from Foundation for Polish Science TEAM program (TEAM/2011-7/1), CEPT (POIG.02.02.00-14-024/08-00) and Ministry of Science and Education (2543/B/P01/2007/33).
 This article contains supplemental Figs. S1 to S5 and Tables S1 to S6.
This article contains supplemental Figs. S1 to S5 and Tables S1 to S6.
1 The abbreviations used are:
- Aco2
- aconitate hydratase
- AD
- Alzheimer's disease
- APP
- Amyloid β Precursor Protein
- ACTB
- actin- beta
- AP-2
- alpha 2-adaptin
- Aβ
- Amyloid β peptide
- BST
- biotin switch technique
- CamkII
- calcium/calmodulin-dependent protein kinase II
- CFL1
- cofilin 1
- Cox-2
- cyclo-oxygenase −2
- DRP1
- dynamin-related protein 1
- eFAD
- early-onset autosomal dominant familial Alzheimer's disease
- eNOS
- endothelial nitric oxide synthase
- Eno2/ENO2
- enolase 2, gamma neuronal
- Gapdh/GAPDH
- glyceraldehyde dehydrogenase
- Gfap/GFAP
- glial fibrillary acidic protein
- iNOS
- inducible nitric oxide synthase
- Kyoto Encyclopedia of Genes and Genomes (KEGG)
- late-onset Alzheimer's disease (LOAD)
- MMTS
- S-Methyl methanethiosulfonate
- Ncald/NCALD
- neurocalcin delta
- NCAM1
- neural cell adhesion molecule 1
- Nfasc
- neurofascin
- NMDA
- N-methyl-D-aspartate
- NMDAR
- N-methyl-D-aspartate receptor
- nNOS
- neuronal nitric oxide synthase
- NO
- nitric oxide
- NSE
- neuronal specific enolase
- Prdx3/PRDX3
- peroxiredoxin-3
- Prdx6/PRDX6
- peroxiredoxin-6
- PS1
- presenilin 1
- PS2
- presenilin 2
- PTM
- post-translational modifications
- Rac1/RAC1
- ras-related C3 botulinum toxin substrate 1
- RNS
- reactive nitrogen species
- ROS
- reactive oxygen species
- SNO
- S-nitrosothiols
- SNO-Cys
- S-nitrosylated cysteines
- SNOSID
- SNO Site Identification
- SPL
- Selected peptides list
- Suclg1
- succinate-CoA ligase
- Syt1/Syt2
- Synaptotagmin-1/2
- TFA
- 2,2,2-Trifluoroacetic acid.
REFERENCES
- 1. Garthwaite J., Charles S. L., Chess-Williams R. (1988) Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336, 385–388 [DOI] [PubMed] [Google Scholar]
- 2. Bredt D. S., Glatt C. E., Hwang P. M., Fotuhi M., Dawson T. M., Snyder S. H. (1991) Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron 7, 615–624 [DOI] [PubMed] [Google Scholar]
- 3. Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. (1991) Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc. Natl. Acad. Sci. U. S. A. 88, 7797–7801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drapier J. C., Bouton C. (1996) Modulation by nitric oxide of metalloprotein regulatory activities. Bioessays 18, 549–556 [DOI] [PubMed] [Google Scholar]
- 5. Pryor W. A., Squadrito G. L. (1995) The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 268, L699-L722 [DOI] [PubMed] [Google Scholar]
- 6. Chiueh C. C., Rauhala P. (1999) The redox pathway of S-nitrosoglutathione, glutathione and nitric oxide in cell to neuron communications. Free Radic. Res. 31, 641–650 [DOI] [PubMed] [Google Scholar]
- 7. Lane P., Hao G., Gross S. S. (2001) S-nitrosylation is emerging as a specific and fundamental posttranslational protein modification: head-to-head comparison with O-phosphorylation. Sci. STKE 2001, re1. [DOI] [PubMed] [Google Scholar]
- 8. Nakamura T., Tu S., Akhtar M. W., Sunico C. R., Okamoto S., Lipton S. A. (2013) Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron 78, 596–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aranda E., Lopez-Pedrera C., De La Haba-Rodriguez J. R., Rodriguez-Ariza A. (2012) Nitric oxide and cancer: the emerging role of S-nitrosylation. Curr. Mol. Med. 12, 50–67 [DOI] [PubMed] [Google Scholar]
- 10. Wang Z. (2012) Protein S-nitrosylation and cancer. Cancer Lett. 320, 123–129 [DOI] [PubMed] [Google Scholar]
- 11. Martinez-Ruiz A., Lamas S. (2004) S-nitrosylation: a potential new paradigm in signal transduction. Cardiovasc. Res. 62, 43–52 [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez D. R., Treuer A., Sun Q. A., Stamler J. S., Hare J. M. (2009) S-Nitrosylation of cardiac ion channels. J. Cardiovasc. Pharmacol. 54, 188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murphy E., Kohr M., Sun J., Nguyen T., Steenbergen C. (2012) S-nitrosylation: a radical way to protect the heart. J. Mol. Cell Cardiol. 52, 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakamura T., Lipton S. A. (2011) Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases. Cell Death Differ. 18, 1478–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi Y. B., Tenneti L., Le D. A., Ortiz J., Bai G., Chen H. S., Lipton S. A. (2000) Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat. Neurosci. 3, 15–21 [DOI] [PubMed] [Google Scholar]
- 16. Nakamura T., Lipton S. A. (2010) Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: possible pharmacological strategies. Cell Calcium 47, 190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uehara T., Nakamura T., Yao D., Shi Z. Q., Gu Z., Ma Y., Masliah E., Nomura Y., Lipton S. A. (2006) S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441, 513–517 [DOI] [PubMed] [Google Scholar]
- 18. Nakamura T., Cieplak P., Cho D. H., Godzik A., Lipton S. A. (2010) S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion 10, 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kornberg M. D., Sen N., Hara M. R., Juluri K. R., Nguyen J. V., Snowman A. M., Law L., Hester L. D., Snyder S. H. (2010) GAPDH mediates nitrosylation of nuclear proteins. Nat. Cell Biol. 12, 1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tian J., Kim S. F., Hester L., Snyder S. H. (2008) S-nitrosylation/activation of COX-2 mediates NMDA neurotoxicity. Proc. Natl. Acad. Sci. U. S. A. 105, 10537–10540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang Y., Man H. Y., Sekine-Aizawa Y., Han Y., Juluri K., Luo H., Cheah J., Lowenstein C., Huganir R. L., Snyder S. H. (2005) S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron 46, 533–540 [DOI] [PubMed] [Google Scholar]
- 22. Sunico C. R., Nakamura T., Rockenstein E., Mante M., Adame A., Chan S. F., Newmeyer T. F., Masliah E., Nakanishi N., Lipton S. A. (2013) S-Nitrosylation of parkin as a novel regulator of p53-mediated neuronal cell death in sporadic Parkinson's disease. Mol. Neurodegener. 8, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung K. K., Thomas B., Li X., Pletnikova O., Troncoso J. C., Marsh L., Dawson V. L., Dawson T. M. (2004) S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304, 1328–1331 [DOI] [PubMed] [Google Scholar]
- 24. Ozawa K., Komatsubara A. T., Nishimura Y., Sawada T., Kawafune H., Tsumoto H., Tsuji Y., Zhao J., Kyotani Y., Tanaka T., Takahashi R., Yoshizumi M. (2013) S-nitrosylation regulates mitochondrial quality control via activation of parkin. Sci. Rep. 3, 2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sen N., Hara M. R., Ahmad A. S., Cascio M. B., Kamiya A., Ehmsen J. T., Agrawal N., Aggrawal N., Hester L., Dore S., Snyder S. H., Sawa A. (2009) GOSPEL: a neuroprotective protein that binds to GAPDH upon S-nitrosylation. Neuron 63, 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qu J., Nakamura T., Cao G., Holland E. A., McKercher S. R., Lipton S. A. (2011) S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc. Natl. Acad. Sci. U. S. A. 108, 14330–14335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clementi E., Brown G. C., Feelisch M., Moncada S. (1998) Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. U. S. A. 95, 7631–7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Selvakumar B., Huganir R. L., Snyder S. H. (2009) S-nitrosylation of stargazin regulates surface expression of AMPA-glutamate neurotransmitter receptors. Proc. Natl. Acad. Sci. U. S. A. 106, 16440–16445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mustafa A. K., Kumar M., Selvakumar B., Ho G. P., Ehmsen J. T., Barrow R. K., Amzel L. M., Snyder S. H. (2007) Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc. Natl. Acad. Sci. U. S. A. 104, 2950–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abbott A. (2012) Cognition: The brain's decline. Nature 492, S4–S5 [DOI] [PubMed] [Google Scholar]
- 31. Komarova N. L., Thalhauser C. J. (2011) High degree of heterogeneity in Alzheimer's disease progression patterns. PLoS Comput. Biol. 7, e1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. da Cruz e Silva O. A., Henriques A. G., Domingues S. C., da Cruz e Silva E. F. (2010) Wnt signalling is a relevant pathway contributing to amyloid beta- peptide-mediated neuropathology in Alzheimer's disease. CNS Neurol. Disord. Drug Targets 9, 720–726 [DOI] [PubMed] [Google Scholar]
- 33. Liu Y., Liu F., Grundke-Iqbal I., Iqbal K., Gong C. X. (2011) Deficient brain insulin signalling pathway in Alzheimer's disease and diabetes. J. Pathol. 225, 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munoz L., Ammit A. J. (2010) Targeting p38 MAPK pathway for the treatment of Alzheimer's disease. Neuropharmacology 58, 561–568 [DOI] [PubMed] [Google Scholar]
- 35. Silvestrelli G., Lanari A., Parnetti L., Tomassoni D., Amenta F. (2006) Treatment of Alzheimer's disease: from pharmacology to a better understanding of disease pathophysiology. Mech. Ageing Dev. 127, 148–157 [DOI] [PubMed] [Google Scholar]
- 36. Agostinho P., Cunha R. A., Oliveira C. (2010) Neuroinflammation, oxidative stress, and the pathogenesis of Alzheimer's disease. Curr. Pharm. Des. 16, 2766–2778 [DOI] [PubMed] [Google Scholar]
- 37. Sultana R., Butterfield D. A. (2010) Role of oxidative stress in the progression of Alzheimer's disease. J. Alzheimers Dis. 19, 341–353 [DOI] [PubMed] [Google Scholar]
- 38. Malinski T. (2007) Nitric oxide and nitroxidative stress in Alzheimer's disease. J. Alzheimers Dis. 11, 207–218 [DOI] [PubMed] [Google Scholar]
- 39. Aluise C. D., Robinson R. A., Cai J., Pierce W. M., Markesbery W. R., Butterfield D. A. (2011) Redox proteomics analysis of brains from subjects with amnestic mild cognitive impairment compared to brains from subjects with preclinical Alzheimer's disease: insights into memory loss in MCI. J. Alzheimers Dis. 23, 257–269 [DOI] [PubMed] [Google Scholar]
- 40. Barone E., Di Domenico F., Cenini G., Sultana R., Coccia R., Preziosi P., Perluigi M., Mancuso C., Butterfield D. A. (2011) Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer's disease and amnestic mild cognitive impairment. J. Alzheimers Dis. 25, 623–633 [DOI] [PubMed] [Google Scholar]
- 41. Butterfield D. A., Sultana R. (2007) Redox proteomics identification of oxidatively modified brain proteins in Alzheimer's disease and mild cognitive impairment: insights into the progression of this dementing disorder. J. Alzheimers Dis. 12, 61–72 [DOI] [PubMed] [Google Scholar]
- 42. Robinson R. A., Lange M. B., Sultana R., Galvan V., Fombonne J., Gorostiza O., Zhang J., Warrier G., Cai J., Pierce W. M., Bredesen D. E., Butterfield D. A. (2011) Differential expression and redox proteomics analyses of an Alzheimer disease transgenic mouse model: effects of the amyloid-beta peptide of amyloid precursor protein. Neuroscience 177, 207–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sultana R., Robinson R. A., Di Domenico F., Abdul H. M., St Clair D. K., Markesbery W. R., Cai J., Pierce W. M., Butterfield D. A. (2011) Proteomic identification of specifically carbonylated brain proteins in APP(NLh)/APP(NLh) x PS-1(P264L)/PS-1(P264L) human double mutant knock-in mice model of Alzheimer disease as a function of age. J. Proteomics 74, 2430–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Finkel T. (2011) Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fransen M., Nordgren M., Wang B., Apanasets O. (2012) Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim. Biophys. Acta 1822, 1363–1373 [DOI] [PubMed] [Google Scholar]
- 46. Valko M., Leibfritz D., Moncol J., Cronin M. T., Mazur M., Telser J. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84 [DOI] [PubMed] [Google Scholar]
- 47. Westerblad H., Allen D. G. (2011) Emerging roles of ROS/RNS in muscle function and fatigue. Antioxid. Redox Signal 15, 2487–2499 [DOI] [PubMed] [Google Scholar]
- 48. Games D., Buttini M., Kobayashi D., Schenk D., Seubert P. (2006) Mice as models: transgenic approaches and Alzheimer's disease. J. Alzheimers Dis. 9, 133–149 [DOI] [PubMed] [Google Scholar]
- 49. Marchetti C., Marie H. (2011) Hippocampal synaptic plasticity in Alzheimer's disease: what have we learned so far from transgenic models? Rev. Neurosci. 22, 373–402 [DOI] [PubMed] [Google Scholar]
- 50. Hao G., Derakhshan B., Shi L., Campagne F., Gross S. S. (2006) SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc. Natl. Acad. Sci. U. S. A. 103, 1012–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jaffrey S. R., Snyder S. H. (2001) The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 2001, pl1. [DOI] [PubMed] [Google Scholar]
- 52. Martinez-Ruiz A., Lamas S. (2004) Detection and proteomic identification of S-nitrosylated proteins in endothelial cells. Arch. Biochem. Biophys. 423, 192–199 [DOI] [PubMed] [Google Scholar]
- 53. Ohtake K., Shimada N., Uchida H., Kobayashi J. (2009) Proteomic approach for identification of protein S-nitrosation in mouse gastric mucosa treated with S-nitrosoglutathione. J. Proteomics 72, 750–760 [DOI] [PubMed] [Google Scholar]
- 54. Sun J., Morgan M., Shen R. F., Steenbergen C., Murphy E. (2007) Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ. Res. 101, 1155–1163 [DOI] [PubMed] [Google Scholar]
- 55. Tannenbaum S. R., White F. M. (2006) Regulation and specificity of S-nitrosylation and denitrosylation. ACS Chem. Biol. 1, 615–618 [DOI] [PubMed] [Google Scholar]
- 56. Moechars D., Dewachter I., Lorent K., Reverse D., Baekelandt V., Naidu A., Tesseur I., Spittaels K., Haute C. V., Checler F., Godaux E., Cordell B., Van Leuven F. (1999) Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 274, 6483–6492 [DOI] [PubMed] [Google Scholar]
- 57. Linetska M. V., Storchak L. G., Tarasenko A. S., Himmelreich N. H. (2004) Involvement of membrane GABA transporter in alpha-latrotoxin-stimulated [3H]GABA release. Neurochem. Int. 44, 303–312 [DOI] [PubMed] [Google Scholar]
- 58. Tarasenko A. S., Kostrzhevska O. G., Storchak L. G., Linetska M. V., Borisova T. A., Himmelreich N. H. (2005) Phenylarsine oxide is able to dissipate synaptic vesicle acidic pool. Neurochem. Int. 46, 541–550 [DOI] [PubMed] [Google Scholar]
- 59. Bakun M., Karczmarski J., Poznanski J., Rubel T., Rozga M., Malinowska A., Sands D., Hennig E., Oledzki J., Ostrowski J., Dadlez M. (2009) An integrated LC-ESI-MS platform for quantitation of serum peptide ladders. Application for colon carcinoma study. Proteomics Clin. Appl 3, 932–946 [DOI] [PubMed] [Google Scholar]
- 60. Sikora J., Towpik J., Graczyk D., Kistowski M., Rubel T., Poznanski J., Langridge J., Hughes C., Dadlez M., Boguta M. (2009) Yeast prion [PSI+] lowers the levels of mitochondrial prohibitins. Biochim. Biophys. Acta 1793, 1703–1709 [DOI] [PubMed] [Google Scholar]
- 61. Bakun M., Niemczyk M., Domanski D., Jazwiec R., Perzanowska A., Niemczyk S., Kistowski M., Fabijanska A., Borowiec A., Paczek L., Dadlez M. (2012) Urine proteome of autosomal dominant polycystic kidney disease patients. Clin. Proteomics 9, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mikula M., Gaj P., Dzwonek K., Rubel T., Karczmarski J., Paziewska A., Dzwonek A., Bragoszewski P., Dadlez M., Ostrowski J. (2010) Comprehensive analysis of the palindromic motif TCTCGCGAGA: a regulatory element of the HNRNPK promoter. DNA Res. 17, 245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Orlowska K. P., Klosowska K., Szczesny R. J., Cysewski D., Krawczyk P. S., Dziembowski A. (2013) A new strategy for gene targeting and functional proteomics using the DT40 cell line. Nucleic Acids Res. 41, e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Oliveros J. C. (2007) VENNY. An interactive tool for comparing lists with Venn Diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html [Google Scholar]
- 65. Warde-Farley D., Donaldson S. L., Comes O., Zuberi K., Badrawi R., Chao P., Franz M., Grouios C., Kazi F., Lopes C. T., Maitland A., Mostafavi S., Montojo J., Shao Q., Wright G., Bader G. D., Morris Q. (2010) The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 38, W214–W220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zuberi K., Franz M., Rodriguez H., Montojo J., Lopes C. T., Bader G. D., Morris Q. (2013) GeneMANIA prediction server 2013 update. Nucleic Acids Res. 41, W115–W122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bindea G., Mlecnik B., Hackl H., Charoentong P., Tosolini M., Kirilovsky A., Fridman W. H., Pages F., Trajanoski Z., Galon J. (2009) ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. von Eichborn J., Dunkel M., Gohlke B. O., Preissner S. C., Hoffmann M. F., Bauer J. M., Armstrong J. D., Schaefer M. H., Andrade-Navarro M. A., Le Novere N., Croning M. D., Grant S. G., van Nierop P., Smit A. B., Preissner R. (2013) SynSysNet: integration of experimental data on synaptic protein-protein interactions with drug-target relations. Nucleic Acids Res. 41, D834–D840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pirooznia M., Wang T., Avramopoulos D., Valle D., Thomas G., Huganir R. L., Goes F. S., Potash J. B., Zandi P. P. (2012) SynaptomeDB: an ontology-based knowledgebase for synaptic genes. Bioinformatics 28, 897–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Luth H. J., Holzer M., Gartner U., Staufenbiel M., Arendt T. (2001) Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer's disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res. 913, 57–67 [DOI] [PubMed] [Google Scholar]
- 71. Fernandez-Vizarra P., Fernandez A. P., Castro-Blanco S., Encinas J. M., Serrano J., Bentura M. L., Munoz P., Martinez-Murillo R., Rodrigo J. (2004) Expression of nitric oxide system in clinically evaluated cases of Alzheimer's disease. Neurobiol. Dis. 15, 287–305 [DOI] [PubMed] [Google Scholar]
- 72. Vella L. J., Sharples R. A., Nisbet R. M., Cappai R., Hill A. F. (2008) The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur. Biophys. J 37, 323–332 [DOI] [PubMed] [Google Scholar]
- 73. Groemer T. W., Thiel C. S., Holt M., Riedel D., Hua Y., Huve J., Wilhelm B. G., Klingauf J. (2011) Amyloid precursor protein is trafficked and secreted via synaptic vesicles. PLoS One 6, e18754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cottrell B. A., Galvan V., Banwait S., Gorostiza O., Lombardo C. R., Williams T., Schilling B., Peel A., Gibson B., Koo E. H., Link C. D., Bredesen D. E. (2005) A pilot proteomic study of amyloid precursor interactors in Alzheimer's disease. Ann. Neurol. 58, 277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aisa B., Gil-Bea F. J., Solas M., Garcia-Alloza M., Chen C. P., Lai M. K., Francis P. T., Ramirez M. J. (2010) Altered NCAM expression associated with the cholinergic system in Alzheimer's disease. J. Alzheimers Dis. 20, 659–668 [DOI] [PubMed] [Google Scholar]
- 76. Butterfield D. A., Hardas S. S., Lange M. L. (2010) Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to neurodegeneration. J. Alzheimers Dis. 20, 369–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim S. H., Fountoulakis M., Cairns N., Lubec G. (2001) Protein levels of human peroxiredoxin subtypes in brains of patients with Alzheimer's disease and Down syndrome. J. Neural Transm. Suppl, 223–235 [DOI] [PubMed] [Google Scholar]
- 78. Krapfenbauer K., Engidawork E., Cairns N., Fountoulakis M., Lubec G. (2003) Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 967, 152–160 [DOI] [PubMed] [Google Scholar]
- 79. Rudinskiy N., Grishchuk Y., Vaslin A., Puyal J., Delacourte A., Hirling H., Clarke P. G., Luthi-Carter R. (2009) Calpain hydrolysis of alpha- and beta2-adaptins decreases clathrin-dependent endocytosis and may promote neurodegeneration. J. Biol. Chem. 284, 12447–12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Calabrese V., Cornelius C., Rizzarelli E., Owen J. B., Dinkova-Kostova A. T., Butterfield D. A. (2009) Nitric oxide in cell survival: a janus molecule. Antioxid. Redox Signal 11, 2717–2739 [DOI] [PubMed] [Google Scholar]
- 81. Hess D. T., Stamler J. S. (2012) Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem. 287, 4411–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Seth D., Stamler J. S. (2011) The SNO-proteome: causation and classifications. Curr. Opin. Chem. Biol. 15, 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jeon G. S., Nakamura T., Lee J. S., Choi W. J., Ahn S. W., Lee K. W., Sung J. J., Lipton S. A. (2014) Potential effect of S-Nitrosylated protein disulfide isomerase on Mutant SOD1 aggregation and neuronal cell death in amyotrophic lateral sclerosis. Mol. Neurobiol. 49, 796–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Walker A. K., Farg M. A., Bye C. R., McLean C. A., Horne M. K., Atkin J. D. (2010) Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain 133, 105–116 [DOI] [PubMed] [Google Scholar]
- 85. Haun F., Nakamura T., Shiu A. D., Cho D. H., Tsunemi T., Holland E. A., La Spada A. R., Lipton S. A. (2013) S-Nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in huntington's disease. Antioxid. Redox Signal 19, 1173–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bai F., Witzmann F. A. (2007) Synaptosome proteomics. Subcell Biochem. 43, 77–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Morciano M., Beckhaus T., Karas M., Zimmermann H., Volknandt W. (2009) The proteome of the presynaptic active zone: from docked synaptic vesicles to adhesion molecules and maxi-channels. J. Neurochem. 108, 662–675 [DOI] [PubMed] [Google Scholar]
- 88. Raiteri L., Raiteri M. (2000) Synaptosomes still viable after 25 years of superfusion. Neurochem. Res. 25, 1265–1274 [DOI] [PubMed] [Google Scholar]
- 89. Martinez-Ruiz A., Araujo I. M., Izquierdo-Alvarez A., Hernansanz-Agustin P., Lamas S., Serrador J. M. (2013) Specificity in S-Nitrosylation: A Short-Range Mechanism for NO Signaling? Antioxid. Redox Signal 19, 1220–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Banerjee S., Liao L., Russo R., Nakamura T., McKercher S. R., Okamoto S., Haun F., Nikzad R., Zaidi R., Holland E., Eroshkin A., Yates J. R., 3rd, Lipton S. A. (2012) Isobaric tagging-based quantification by mass spectrometry of differentially regulated proteins in synaptosomes of HIV/gp120 transgenic mice: implications for HIV-associated neurodegeneration. Exp. Neurol. 236, 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Koch S., Scifo E., Rokka A., Trippner P., Lindfors M., Korhonen R., Corthals G. L., Virtanen I., Lalowski M., Tyynela J. (2013) Cathepsin D deficiency induces cytoskeletal changes and affects cell migration pathways in the brain. Neurobiol. Dis. 50, 107–119 [DOI] [PubMed] [Google Scholar]
- 92. Zahid S., Khan R., Oellerich M., Ahmed N., Asif A. R. (2014) Differential S-nitrosylation of proteins in Alzheimer's disease. Neuroscience 256, 126–136 [DOI] [PubMed] [Google Scholar]
- 93. Schmitt F. A., Nelson P. T., Abner E., Scheff S., Jicha G. A., Smith C., Cooper G., Mendiondo M., Danner D. D., Van Eldik L. J., Caban-Holt A., Lovell M. A., Kryscio R. J. (2012) University of Kentucky Sanders-Brown healthy brain aging volunteers: donor characteristics, procedures, and neuropathology. Curr. Alzheimer Res. 9, 724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Daiber A., Daub S., Bachschmid M., Schildknecht S., Oelze M., Steven S., Schmidt P., Megner A., Wada M., Tanabe T., Munzel T., Bottari S., Ullrich V. (2013) Protein tyrosine nitration and thiol oxidation by peroxynitrite-strategies to prevent these oxidative modifications. Int. J. Mol. Sci. 14, 7542–7570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cho D. H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., Lipton S. A. (2009) S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324, 102–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Forrester M. T., Foster M. W., Benhar M., Stamler J. S. (2009) Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic. Biol. Med. 46, 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Forrester M. T., Foster M. W., Stamler J. S. (2007) Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J. Biol. Chem. 282, 13977–13983 [DOI] [PubMed] [Google Scholar]
- 98. Raju K., Doulias P. T., Tenopoulou M., Greene J. L., Ischiropoulos H. (2012) Strategies and tools to explore protein S-nitrosylation. Biochim. Biophys. Acta 1820, 684–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Torta F., Usuelli V., Malgaroli A., Bachi A. (2008) Proteomic analysis of protein S-nitrosylation. Proteomics 8, 4484–4494 [DOI] [PubMed] [Google Scholar]
- 100. Dewachter I., van Dorpe J., Spittaels K., Tesseur I., Van Den Haute C., Moechars D., Van Leuven F. (2000) Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice. Exp. Gerontol. 35, 831–841 [DOI] [PubMed] [Google Scholar]
- 101. Rodrigo J., Fernandez-Vizarra P., Castro-Blanco S., Bentura M. L., Nieto M., Gomez-Isla T., Martinez-Murillo R., MartInez A., Serrano J., Fernandez A. P. (2004) Nitric oxide in the cerebral cortex of amyloid-precursor protein (SW) Tg2576 transgenic mice. Neuroscience 128, 73–89 [DOI] [PubMed] [Google Scholar]
- 102. Wilcock D. M., Lewis M. R., Van Nostrand W. E., Davis J., Previti M. L., Gharkholonarehe N., Vitek M. P., Colton C. A. (2008) Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J. Neurosci. 28, 1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nathan C., Calingasan N., Nezezon J., Ding A., Lucia M. S., La Perle K., Fuortes M., Lin M., Ehrt S., Kwon N. S., Chen J., Vodovotz Y., Kipiani K., Beal M. F. (2005) Protection from Alzheimer's-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J. Exp. Med. 202, 1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang Q., Rowan M. J., Anwyl R. (2004) Beta-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J. Neurosci. 24, 6049–6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Huang T. C., Lu K. T., Wo Y. Y., Wu Y. J., Yang Y. L. (2011) Resveratrol protects rats from Abeta-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS One 6, e29102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gow A. J., Chen Q., Hess D. T., Day B. J., Ischiropoulos H., Stamler J. S. (2002) Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J. Biol. Chem. 277, 9637–9640 [DOI] [PubMed] [Google Scholar]
- 107. Kohr M. J., Aponte A. M., Sun J., Wang G., Murphy E., Gucek M., Steenbergen C. (2011) Characterization of potential S-nitrosylation sites in the myocardium. Am. J. Physiol. Heart Circ. Physiol. 300, H1327–H1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chivet M., Javalet C., Hemming F., Pernet-Gallay K., Laulagnier K., Fraboulet S., Sadoul R. (2013) Exosomes as a novel way of interneuronal communication. Biochem. Soc. Trans. 41, 241–244 [DOI] [PubMed] [Google Scholar]
- 109. Kins S., Lauther N., Szodorai A., Beyreuther K. (2006) Subcellular trafficking of the amyloid precursor protein gene family and its pathogenic role in Alzheimer's disease. Neurodegener. Dis. 3, 218–226 [DOI] [PubMed] [Google Scholar]
- 110. Magdesian M. H., Gralle M., Guerreiro L. H., Beltrao P. J., Carvalho M. M., Santos L. E., de Mello F. G., Reis R. A., Ferreira S. T. (2011) Secreted human amyloid precursor protein binds semaphorin 3a and prevents semaphorin-induced growth cone collapse. PLoS One 6, e22857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mizumaru C., Saito Y., Ishikawa T., Yoshida T., Yamamoto T., Nakaya T., Suzuki T. (2009) Suppression of APP-containing vesicle trafficking and production of beta-amyloid by AID/DHHC-12 protein. J. Neurochem. 111, 1213–1224 [DOI] [PubMed] [Google Scholar]
- 112. Sosa L. J., Bergman J., Estrada-Bernal A., Glorioso T. J., Kittelson J. M., Pfenninger K. H. (2013) Amyloid precursor protein is an autonomous growth cone adhesion molecule engaged in contact guidance. PLoS One 8, e64521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Antonell A., Llado A., Altirriba J., Botta-Orfila T., Balasa M., Fernandez M., Ferrer I., Sanchez-Valle R., Molinuevo J. L. (2013) A preliminary study of the whole-genome expression profile of sporadic and monogenic early-onset Alzheimer's disease. Neurobiol. Aging 34, 1772–1778 [DOI] [PubMed] [Google Scholar]
- 114. Lin L., Lesnick T. G., Maraganore D. M., Isacson O. (2009) Axon guidance and synaptic maintenance: preclinical markers for neurodegenerative disease and therapeutics. Trends Neurosci. 32, 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. De Vos K. J., Grierson A. J., Ackerley S., Miller C. C. (2008) Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 31, 151–173 [DOI] [PubMed] [Google Scholar]
- 116. Lazarov O., Morfini G. A., Pigino G., Gadadhar A., Chen X., Robinson J., Ho H., Brady S. T., Sisodia S. S. (2007) Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer's disease-linked mutant presenilin 1. J. Neurosci. 27, 7011–7020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stokin G. B., Lillo C., Falzone T. L., Brusch R. G., Rockenstein E., Mount S. L., Raman R., Davies P., Masliah E., Williams D. S., Goldstein L. S. (2005) Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science 307, 1282–1288 [DOI] [PubMed] [Google Scholar]
- 118. Penzes P., Vanleeuwen J. E. (2011) Impaired regulation of synaptic actin cytoskeleton in Alzheimer's disease. Brain Res. Rev. 67, 184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Welshhans K., Bassell G. J. (2011) Netrin-1-induced local beta-actin synthesis and growth cone guidance requires zipcode binding protein 1. J. Neurosci. 31, 9800–9813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lu J., Katano T., Okuda-Ashitaka E., Oishi Y., Urade Y., Ito S. (2009) Involvement of S-nitrosylation of actin in inhibition of neurotransmitter release by nitric oxide. Mol. Pain 5, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Maloney M. T., Bamburg J. R. (2007) Cofilin-mediated neurodegeneration in Alzheimer's disease and other amyloidopathies. Mol. Neurobiol. 35, 21–44 [DOI] [PubMed] [Google Scholar]
- 122. Woo J. A., Jung A. R., Lakshmana M. K., Bedrossian A., Lim Y., Bu J. H., Park S. A., Koo E. H., Mook-Jung I., Kang D. E. (2012) Pivotal role of the RanBP9-cofilin pathway in Abeta-induced apoptosis and neurodegeneration. Cell Death Differ. 19, 1413–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mendoza-Naranjo A., Contreras-Vallejos E., Henriquez D. R., Otth C., Bamburg J. R., Maccioni R. B., Gonzalez-Billault C. (2012) Fibrillar amyloid-beta1–42 modifies actin organization affecting the cofilin phosphorylation state: a role for Rac1/cdc42 effector proteins and the slingshot phosphatase. J. Alzheimers Dis. 29, 63–77 [DOI] [PubMed] [Google Scholar]
- 124. Oh D., Han S., Seo J., Lee J. R., Choi J., Groffen J., Kim K., Cho Y. S., Choi H. S., Shin H., Woo J., Won H., Park S. K., Kim S. Y., Jo J., Whitcomb D. J., Cho K., Kim H., Bae Y. C., Heisterkamp N., Choi S. Y., Kim E. (2010) Regulation of synaptic Rac1 activity, long-term potentiation maintenance, and learning, and memory by BCR and ABR Rac GTPase-activating proteins. J. Neurosci. 30, 14134–14144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mendoza-Naranjo A., Gonzalez-Billault C., Maccioni R. B. (2007) Abeta1–42 stimulates actin polymerization in hippocampal neurons through Rac1 and Cdc42 Rho GTPases. J. Cell Sci. 120, 279–288 [DOI] [PubMed] [Google Scholar]
- 126. Nozoe M., Hirooka Y., Koga Y., Sagara Y., Kishi T., Engelhardt J. F., Sunagawa K. (2007) Inhibition of Rac1-derived reactive oxygen species in nucleus tractus solitarius decreases blood pressure and heart rate in stroke-prone spontaneously hypertensive rats. Hypertension 50, 62–68 [DOI] [PubMed] [Google Scholar]
- 127. Traub L. M. (2009) Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10, 583–596 [DOI] [PubMed] [Google Scholar]
- 128. Kastning K., Kukhtina V., Kittler J. T., Chen G., Pechstein A., Enders S., Lee S. H., Sheng M., Yan Z., Haucke V. (2007) Molecular determinants for the interaction between AMPA receptors and the clathrin adaptor complex AP-2. Proc. Natl. Acad. Sci. U. S. A. 104, 2991–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lavezzari G., McCallum J., Lee R., Roche K. W. (2003) Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology 45, 729–737 [DOI] [PubMed] [Google Scholar]
- 130. Selvakumar B., Jenkins M. A., Hussain N. K., Huganir R. L., Traynelis S. F., Snyder S. H. (2013) S-nitrosylation of AMPA receptor GluA1 regulates phosphorylation, single-channel conductance, and endocytosis. Proc. Natl. Acad. Sci. U. S. A. 110, 1077–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Shimohama S., Chachin M., Taniguchi T., Hidaka H., Kimura J. (1996) Changes of neurocalcin, a calcium-binding protein, in the brain of patients with Alzheimer's disease. Brain Res. 716, 233–236 [DOI] [PubMed] [Google Scholar]
- 132. Mink J. W., Blumenschine R. J., Adams D. B. (1981) Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am. J. Physiol. 241, R203–R212 [DOI] [PubMed] [Google Scholar]
- 133. Butterfield D. A., Lange M. L. (2009) Multifunctional roles of enolase in Alzheimer's disease brain: beyond altered glucose metabolism. J. Neurochem. 111, 915–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kuntzelmann A., Guenther T., Haberkorn U., Essig M., Giesel F., Henze R., Schroeter M. L., Schroder J., Schonknecht P. (2013) Impaired cerebral glucose metabolism in prodromal Alzheimer's disease differs by regional intensity normalization. Neurosci. Lett. 534, 12–17 [DOI] [PubMed] [Google Scholar]
- 135. Swerdlow R. H., Burns J. M., Khan S. M. (2013) The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochim. Biophys. Acta [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nakamura T., Lipton S. A. (2013) Emerging role of protein-protein transnitrosylation in cell signaling pathways. Antioxid. Redox Signal 18, 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Riederer I. M., Schiffrin M., Kovari E., Bouras C., Riederer B. M. (2009) Ubiquitination and cysteine nitrosylation during aging and Alzheimer's disease. Brain Res. Bull. 80, 233–241 [DOI] [PubMed] [Google Scholar]
- 138. Doulias P. T., Greene J. L., Greco T. M., Tenopoulou M., Seeholzer S. H., Dunbrack R. L., Ischiropoulos H. (2010) Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc. Natl. Acad. Sci. U. S. A. 107, 16958–16963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Huang B., Chen C. (2010) Detection of protein S-nitrosation using irreversible biotinylation procedures (IBP). Free Radic. Biol. Med. 49, 447–456 [DOI] [PubMed] [Google Scholar]
- 140. Tello D., Tarin C., Ahicart P., Breton-Romero R., Lamas S., Martinez-Ruiz A. (2009) A “fluorescence switch” technique increases the sensitivity of proteomic detection and identification of S-nitrosylated proteins. Proteomics 9, 5359–5370 [DOI] [PubMed] [Google Scholar]
- 141. Paige J. S., Xu G., Stancevic B., Jaffrey S. R. (2008) Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chem. Biol. 15, 1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Liu M., Hou J., Huang L., Huang X., Heibeck T. H., Zhao R., Pasa-Tolic L., Smith R. D., Li Y., Fu K., Zhang Z., Hinrichs S. H., Ding S. J. (2010) Site-specific proteomics approach for study protein S-nitrosylation. Anal. Chem. 82, 7160–7168 [DOI] [PubMed] [Google Scholar]
- 143. Gao C., Guo H., Wei J., Mi Z., Wai P. Y., Kuo P. C. (2005) Identification of S-nitrosylated proteins in endotoxin-stimulated RAW264.7 murine macrophages. Nitric Oxide 12, 121–126 [DOI] [PubMed] [Google Scholar]
- 144. Forrester M. T., Thompson J. W., Foster M. W., Nogueira L., Moseley M. A., Stamler J. S. (2009) Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat. Biotechnol. 27, 557–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Doulias P. T., Tenopoulou M., Greene J. L., Raju K., Ischiropoulos H. (2013) Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci. Signal. 6, rs1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.