Summary
Crystal arthropathies are among the most common causes of painful inflammatory arthritis. Gout, the most common example, has been associated with cardiovascular and renal disease. In the last years, evidence on these associations and those involving other comorbidities, such as the metabolic syndrome, have emerged and established the importance of asymptomatic hyperuricemia. This review article presents an update on evidence, both experimental and clinical, describing associations between hyperuricemia, gout, and several comorbidities. Causality on calcium pyrophosphate arthropathy and associated comorbidities is also briefly reviewed.
Keywords: crystal arthropathies, comorbidities, gout, hyperuricemia, cardiovascular disease, metabolic syndrome, renal disease, calcium pyrophosphate arthropathy
Crystal arthropathies are amongst the most common cause of arthritis worldwide. Of these, gout represents the highest known burden of crystal-induced arthritis and is likely the most common type of inflammatory arthritis in adults in the United States.1,2 Calcium pyrophosphate arthropathies, initially described as pseudogout by McCarty and colleagues, and other calcium crystal arthropathies are less commonly recognized than gout.3 Although initially observed only as a painful inflammatory arthropathy, in the past years more evidence has been building up the case for an association between gout and hyperuricemia and important cardiovascular-metabolic conditions.4–8 This article will present an updated review of the evidence on these associations, as well as comorbidities associated with calcium crystal arthropathies.
COMORBIDITIES ASSOCIATED WITH HYPERURICEMIA AND GOUT
Hyperuricemia, defined as a serum urate (SU) concentration above the point of saturation of 6.8 milligrams per deciliter (mg/dL) or more,9 is the most common biochemical abnormality associated with the development of gout, but is not a sufficient causative factor. Individuals in whom SU concentrations are elevated above saturation levels but have not developed clinical manifestations of gout are considered to have asymptomatic hyperuricemia. Data from the Unites States National Health and Nutrition Examination Survey (NHANES) 2007–2008 study estimated a gout prevalence of 3.9% (5.9% for men; 2.0% for women) but a higher hyperuricemia prevalence of 21.4% (21.2% for men; 21.6% for women).5 In the sections below, we summarize the experimental and epidemiological evidence linking gout and various comorbidities and their complex inter-relationships.
Cardiovascular disease
Urate and the endothelium – laboratory and animal studies
In vitro studies that used urate concentrations similar to in vivo levels have shown several potential vascular effects. These include suppression of nitric oxide (NO) levels,10,11 increased platelet-derived growth factor expression, local thromboxane production, cyclooxygenase-2 stimulation, as well as induction of endothelial proliferation, angiotensin II production and increased markers of oxidative stress.12–14 The key role of the renin-angiotensin system (RAS) was proven by the reversibility of these effects by adding captopril or losartan.13 Other significant in vitro observations include the increased production of endothelin-1, a powerful vasoconstrictor, on human aortic smooth muscle cells and cardiac fibroblasts under different urate concentrations.15,16 All of these effects are facilitated by the entry of urate to vascular smooth muscle cells via the urate anion transporter-1 (URAT-1), an integral membrane protein that serves as a urate transporter and was initially described in afferent renal arterioles.17
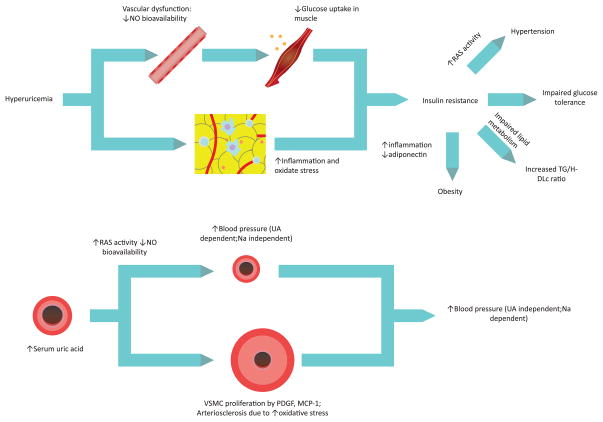
In vivo animal models have also supported data from in vitro studies. Hyperuricemia was induced in rats through the administration of oxonic acid, a uricase inhibitor, which led to renal vascular disease characterized by cortical vasoconstriction, afferent arteriolar swelling, and glomerular hypertension. 18,19 Partial attenuation of these abnormalities was obtained through the administration of the non-reversible xanthine oxidase inhibitor, febuxostat.20,21 Other animal models supported these observations and have also shown that although early hypertension can be corrected with SU reduction, after prolonged hyperuricemia urate reduction doesn’t translate into control of blood pressure and avoidance of arteriolar thickening. Prolonged hyperuricemia results in an irreversible sodium-sensitive urate-insensitive hypertension.14,22 These observations have pointed to a two stage model, with an early hypertension mediated by increased renal renin activity and reduction of circulating plasma nitrates, and a later irreversible phase secondary to an altered intrarenal vascular architecture [see Figure 1].23
Figure 1.
Proposed two-stage Urate-mediated hypertension
An initial stage of urate-dependent vasoreactive hypertension is induced. Later, when anatomical changes that include wall thickening and smooth muscle proliferation have occurred, a second and definitive sodium-dependent hypertension is established. Adapted with permission from Feig, DI, The role of urate in the pathogenesis of hypertension in the young. J Clin Hypertens (Greenwich), 2012. 14(6): p. 346–52.
RAS = renin-angiotensin system; NO = nitric oxide; UA = urate; VSMC = vascular smooth muscle cell; PDGF = platelet-derived growth factor; MCP-1 = monocyte chemotactic protein-1; Na = sodium
Hypertension
In 1999, a study of the Framingham cohort reported an association between hyperuricemia and hypertension, which has been confirmed by other epidemiological studies in different populations.24–31 Recently, the National Health and Nutrition Examination Survey (NHANES) 2007–2008, analyzed the prevalence of gout, hyperuricemia and comorbidities in non-institutionalized adults. Hypertension was present in 74% with gout, and in 47% with hyperuricemia (defined as a SU >7.0mg/dl for men and >5.7 mg/dl for women) but no history of gout, in comparison to a population-estimated prevalence of 24% among normouricemic patients. Prevalence amongst gout population with and without hyperuricemia was 77.7% and 70.8% respectively, higher in comparison to individuals with asymptomatic hyperuricemia. The prevalence of hypertension was significantly higher amongst individuals in the higher SU category (SU ≥ 10mg/dL) in comparison to those in the lowest SU category (SU <4 md/dL).5
A meta-analysis that pooled 11 studies showed a significantly increased risk ratio for incident hypertension of 1.41 (95% CI 1.23–1.58) among individuals with hyperuricemia, after adjusting for traditional risk factors, including age, BMI, alcohol and tobacco use. This risk appeared more pronounced in younger individuals and women. An increased pooled relative risk for incident hypertension of 1.13 (95% CI 1.06–1.20) per each mg/dL increase in SU was calculated from six studies32. Another meta-analysis including 8 prospective studies also reported an increased risk, pooled RR 1.55 (95% CI 1.32–1.82), for hypertension when comparing the highest quartile to the lowest one of SU. However, the analysis presented showed significant heterogeneity (p<0.05).33
Most interventional studies come from adolescent or pediatric population. A relationship between primary hypertension and high SU levels has been observed in even at concentrations below the supersaturarion value of 6.8 mg/dL, and one study has reported elevated SU levels (>5.5 mg/dL) in up to 90% in children with primary hypertension.34–36 A small randomized, controlled, crossover trial of 30 treatment-naïve adolescents (11–17 years old) with stage I hypertension and hyperuricemia, randomized individuals to receiving oral allopurinol 200 mg daily or placebo undergo a washout period and then crossover intervention arm. After treatment, 20 of 30 participants achieved normal blood pressures in comparison to one participant taking placebo.37 A randomized, double-blind, placebo-controlled study compared allopurinol with probenecid, in pre-hypertensive obese adolescents. Both treatment arms had a significant decrease in SU, and led to a 10.2 and 9.0 mm Hg reduction in systolic and diastolic blood pressure, respectively. These results suggested that reduction in blood pressure was related to the urate lowering effect and not to the decreased xanthine oxidase activity.38 A similar study with a small sample of hyperuricemic adults receiving allopurinol 300mg daily also supported the blood pressure reduction with urate lowering therapy (ULT).39 Despite these encouraging results, a recent Cochrane review on pharmacotherapy for hyperuricemia and the reduction of blood pressure concluded that evidence is still insufficient to recommend ULT.40
Atherosclerosis, coronary heart disease and peripheral arterial disease
Several mechanisms such as maintenance of a pro-inflammatory state, promoting a proliferative response in vascular smooth muscle cells, and alterations in the renin-angiotensin system and promotion of hypertensive state may explain the link between urate concentrations and cardiovascular disease. Contributing to this association, elevated levels of monosodium urate have also been observed in atherosclerotic plaques.41 Carotid intima-media thickness (IMT), regarded as a surrogate marker for atherosclerosis, has been shown to have a significant association with SU levels in cohort of healthy postmenopausal women, and also in a different cohort of hypertensive individuals with and without hyperuricemia.42,43 Another study also reported a dose response relation between SU and carotid atherosclerotic plaques in men with and without cardiovascular risk factors.44 However, the same group didn’t find any association between the levels of SU and coronary artery calcification (CAC).45 The associations between serum urate, CAC, and IMT were re-evaluated in an analysis of 5115 young adults aged 18–30 years at followed for 25 years. Using CAC and carotid IMT as markers of subclinical atherosclerosis, the investigators reported increased risks for CAC progression from years 15 to 25 with respect to baseline SU. For carotid IMT, SU at year 15 significantly predicted greater IMT at year 20, but this association only remained significant in men after adjusting for BMI. Greater increments in SU concentrations from years 0 to 15 were associated with higher risks of CAC progression and IMT.46 These findings supported a role for serum urate as a potential biomarker of subclinical atherosclerosis in young adults.
Urate- induced endothelial dysfunction secondary to reduced NO production, actually precedes plaque formation47 and may play a more direct role [see Figure 2]. A recent review and meta-analysis of the use of xanthine oxidase inhibitors for the treatment of cardiovascular disease, evaluated three outcome parameters (brachial artery flow mediated dilation, forearm blood flow responses to acetylcholine infusion and circulating markers of oxidative stress) and showed a significant improvement in each of them in patients with, or at risk of, cardiovascular disease.48 However, data on this aspect is still not conclusive.
Figure 2.
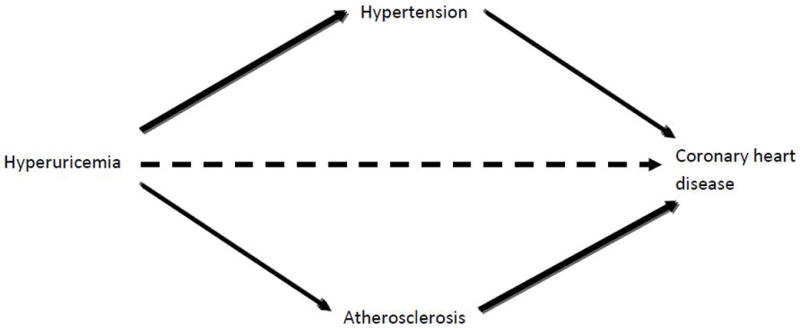
Association and causality between hyperuricemia and coronary heart disease
Hyperuricemia has an indirect effect in the development of coronary heart disease through the development of hypertension and atherosclerosis. However, evidence on a direct and independent effect (dashed arrow) on the development of coronary heart disease is still not conclusive. Adapted from Gaffo, A.L., N.L. Edwards, and K.G. Saag, Gout. Hyperuricemia and cardiovascular disease: how strong is the evidence for a causal link? Arthritis Res Ther, 2009. 11(4): p. 240.
Since the Framingham Heart Study in 1999 failed to identify a significant association between SU, cardiovascular disease, and cardiovascular death;49 several other population studies have presented contradictory evidence. An NHANES 2007–2008 analysis reported a 14% prevalence of myocardial infarction among individuals with gout, with an age- and sex-adjusted OR of 2.68 (1.45 for men; 6.86 for women) compared to non-gout individuals. Prevalence of myocardial infarction in individuals with hyperuricemia was 5.7%, with an OR of 1.21 in comparison to normouricemic individuals. Myocardial infarction prevalence was significantly higher in hyperuricemic or normouricemic individuals with gout (11.6% and 14.1% respectively), in comparison to hyperuricemic individuals with no diagnosis of gout (5.7%).5 Other studies also support an increased risk of coronary heart disease (CHD) in women with gout.50 Recently, a population-based study of a Taiwanese cohort also reported gout as an independent risk factor for myocardial infarction, and stated that this risk was greater in younger individuals without cardiovascular risk factors.51
An initial meta-analysis on the subject reported a 13% increased risk (RR 1.13 95% CI 1.07–1.20) of CHD among those in the top tertile of SU levels compared to those in the lowest tertile.52 A more recent meta-analysis of 26 studies with 402,997 adults reported a modest but significant increased risk of CHD incidence and mortality on hyperuricemic individuals, 1.09 (95% CI 1.03–1.16) and 1.16 (95% CI 1.05–1.19) respectively, even after adjusting for traditional risk factors. A non-significant increased CHD mortality RR of 1.12 for was reported for each 1mg/dL SU increase, with only the women subgroup analysis having a statistically significant but modest increase.53 So, although statistically significant, evidence so far has show a small increase in risk of both incidence and mortality in CHD with hyperuricemia.
Peripheral arterial disease (PAD) is another manifestation of atherosclerosis, and scarce evidence regarding an association with SU has been published. A cross sectional study among 3987 participants in NHANES 1999–2009, found that higher SU levels were significantly associated with PAD, independently from traditional cardiovascular risk factors.54 Another study analyzing data from the Multiple Risk Factor Intervention Trial, showed an increased, although non-significant, odds of having PAD in association with hyperuricemia with an OR of 1.23 (95% CI 0.98–1.54). However, a history of gout was associated with an OR of 1.33 (95% CI 1.07–1.66), even after adjustment of underlying hyperuricemia.55 These findings are regarded as insufficient in order to assume a possible therapeutic approach.56,57
Congestive Heart Failure
Increasing SU levels and hyperuricemia have been associated with increased incidence of CHF58,59 and increased mortality in patients with established CHF.60–64 Data from the NHANES 2007–2008 estimated increased point prevalences for CHF in hyperuricemic individuals compared with normouricemic individuals with an OR of 2.52 (95% CI 1.58–4.04), and in individuals with gout compared with non-gout with an OR of 2.68 (95% CI 1.88–3.83).5 An analysis of the Framingham offspring cohort of 4989 adults, with no clinical CHF at baseline, showed that individuals with gout had a 2–3 times higher incidence of CHF and echocardiographic measures of systolic dysfunction. Median follow-up time was 15.9 years. Mortality was increased in participants with gout with an adjusted HR of 1.58 (95% CI 1.40–1.78), compared to people without gout, and this effect was also observed in subgroup analysis comparing individuals with gout and CHF in comparison to those with heart failure but without gout.65
However, evidence suggests that increased xanthine-oxidase activity in damaged myocardial tissue results in the production of urate precursors and radical oxygen species, which are responsible for cardiac hypertrophy, myocardial fibrosis, left ventricular remodeling, and contractility impairment.66 This poses urate as a marker of increased xanthine-oxidase activity rather than actual causative factor. Support for this hypothesis was provided by an analysis on CHF outcomes in patients with and without chronic kidney disease (CKD), which concluded that hyperuricemia is associated with a poor outcome in CHF without CKD but not in patients with CHF and CKD. In the former case, hyperuricemia would be secondary to increased xanthine oxidase activity, rather than impaired excretion like in CKD.63 Although there are few therapeutic trials, and data is suggestive of improvements in myocardial function and ejection fraction being secondary to xanthine-oxidase inhibition rather the lowering of SU.67–71
Cerebrovascular disease
New evidence has emerged regarding an association between SU and cerebrovascular disease. A study using brain magnetic resonance images (MRI) evaluated the aggregate volume of white matter hyperintense signals in a sample of 177 adults. High normal SU (SU >5.75 mg/dL for men; >4.8 mg/dL for women) concentrations were associated with a significantly increase in white matter hyperintense signals compared to participants with low to moderate SU levels. This association was still significant after adjusting for traditional risk factors.72 SU has also been postulated as a predictor of poor prognosis and recurrent events in stroke survivors.73,74
Results from the NHANES 2007–2008 showed an increased incidence of stroke in individuals with gout with an OR of 2.02 (95% CI 0.98–4.19) and hyperuricemia 1.74 (95% CI 1.16–2.59), in comparison to the control population. Although the risk was increased in women, this difference was not significant.5 A systematic review and meta-analysis pooled a total of 16 studies including 238,449 adults and after adjusting for known risk factors, hyperuricemia was associated with a 47% (95% CI 1.19–1.76) increased risk of stroke and a 26% (95% CI 1.12–1.39) increased mortality. In this analysis, no significant difference by sex was observed.75 The intervention trials with ULT have had conflicting results on subclinical parameters,76–78 and no evidence supporting use of ULT in stroke patients is still available.
In summary, an association between serum urate concentrations and cardiovascular disease is becoming firmly established for hypertension and is an evolving field with still insufficient evidence for atherosclerosis, coronary artery disease, stroke, and congestive heart failure. The first attempts at a leap to causality are being made by the development of interventional clinical trials aimed at lowering serum urate and affecting cardiovascular outcomes.
Renal disease
Urate and renal disease – laboratory and animal models
Almost 90% of the filtered urate is reabsorbed at the proximal tubule by the urate-anion exchanger URAT-1, located at the apical membrane of tubular cells.17,79 Urate regulation at the tubular level is a complex process that involves several other transporters, and conditions such as Lesch-Nyhan syndrome and tumor lysis syndrome, in which SU levels rise over 10 mg/dl, cause renal damage through urate deposition in the tubuli.79–81 Deposition of crystals within the tubuli has also been mentioned as an initial phase of the translocation of urate crystals to the interstitium and medulla, a component of the crystal-related nephropathy. This mechanism, which leads to tubular atrophy and vascular degeneration, used to be considered as the explanation for renal damage in gout patients. However, with the decrease in incidence and severity of crystal nephropathy, this diagnosis is now considered only for specific subgroups which include lead intoxication or genetic defeats leading to increased urate production.79
However, animal models have shown significant evidence of renal injury and disease in absence of crystal deposition. Systemic and glomerular hypertension with increased vascular resistance and reduced renal blood flow secondary to increased oxidative stress and endothelial dysfunction, was observed in rats with oxonic acid-induced hyperuricemia. In two of these models, changes were reversed by using tempol (a superoxide scavenger) and L-arginine (a substrate for endothelial nitric oxide synthase).18,82,83 Activation of the renin-angiotensin system (RAS) also contributes to the development of vascular disease of the afferent arteriolar system and glomerular hypertrophy.22,84 The development of arteriolopathy leads to glomerular hypoxia and ineffective autoregulation mechanisms, which further increases the damage to the glomerulus.14,19 These changes result from specific mechanisms that involve stimulation of NADPH oxidases with mitochondrial dysfunction,85 production of reactive oxygen species,86 activation of the RAS,13 smooth-muscle cell proliferation,13 and induction of proinflammatory cytokines.87 Recent data have also shown a direct effect of urate in tubular cells, promoting a phenotypic transition of renal tubular cells such as epithelial-to-mesenchymal transition by decreasing expression of E-cadherin synthesis.88 Epithelial-to-mesenchymal transition is considered one of the initial phenomena in renal fibrosis.89
Hyperuricemia-induced renal damage has been shown to have a significant effect in animal models with pre-existing renal disease. This has been proven in nephrectomy injury models, were ULT was shown to improve blood pressure, renal function and decrease histologic changes.21,90 In a model of cyclosporine nephropathy, increasing urate worsened renal disease and ULT ameliorated renal damage.91,92 An animal model of diabetic mice also showed that reducing SU improved diabetic nephropathy by reducing tubulointerstitial injury with no effect on glomerular damage.93
Chronic kidney disease
An association between hyperuricemia, including gout, and CKD has been frequently described in the literature and population studies. Data from NHANES 2007–2008 described a prevalence of CKD stage 3 or more in 19.9% of individuals with gout, associated with an OR of 2.32 (95% CI 1.65–3.26) when compared to individuals without gout. Prevalence in hyperuricemic population was also significantly increased compared to normouricemic population (14.8% vs. 3.3%, respectively), with an OR of 3.96 (95% CI 2.63–5.97). Increased prevalence was observed with increasing values for SU.5 Although the causality of these observations has been difficult to confirm, due to the increasing SU with declining renal function, evidence from experimental data explained a possible role of SU in the incidence and progression of CKD.
The largest epidemiological study to date, that included 177,570 adults from the U.S. Renal Data System (USRDS) for 25 years, reported an independent association between SU and risk for end-stage renal disease with a HR of 2.14 (1.65–2.77) when comparing the highest with the lowest quartile.94 Another large cohort study evaluating 21 547 adults of the Vienna Health Screening project, reported a almost double (OR 1.74 [95% CI 1.45–2.09]) increased risk of kidney disease in individuals with SU levels between 7.0–8.9 mg/dL and a triple risk in individuals with levels above 9.0 mg/dL (OR 3.12 (95% CI [2.29–4.25]).95 A pooled study from the Atherosclerosis Risks in Communities and the Cardiovascular Health Study cohorts and two analyses from the Okinawa General Health Maintenance Association Study cohort also support an association between SU and the development of end-stage renal disease.96–98
In IgA nephropathy, elevated SU has also been reported as an independent predictor for the development of CKD.99–101 In diabetic patients, elevated SU levels have been described as independent predictor of the development of diabetic nephropathy,102 micro- and macroalbuminuria,103 and declining renal function104 in type 1 diabetes patients. Hyperuricemia has also been associated, after adjusting for possible confounders, with an increased risk of incident CKD (OR 2.10 [95% CI 1.16–3.76]) among type 2 diabetes patients with normal kidney function.105 However, data analyzing the association between progression of CKD and SU is still not conclusive. A recent study of stage 3–5 CKD middle-aged and elderly Taiwanese adults concluded that elevated urate increased the risk of renal disease only in stage 3 CKD but not in more advanced stages. 106 SU has also been reported as an independent risk factor for progression of kidney disease by other studies,107,108 while no association has also been reported.109,110 This information may point to urate as a stronger risk factor for incidence rather progression of CKD.111
Treatment with allopurinol in hyperuricemic individuals with normal renal function have shown a beneficial effect on estimated glomerular filtration rate.39,112 Interventional trials on CKD patients, although scarce and small, have also shown supporting results. A randomized study of allopurinol and placebo in 54 patients with stage 3–4 CKD showed a slowing in disease progression in the treatment arm compared to placebo.113 Another study that included 113 patients with estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2, randomized patients either to allopurinol 100 mg/day or placebo for 24 months. After 24 months, there was no significant change in eGFR the allopurinol group, while a significant decrease in eGFR was noticed in the control group. Allopurinol treatment slowed renal disease progression estimating a HR reduction of 0.53 (95% CI 0.28–0.99). A beneficial effect on cardiovascular endpoints was also observed.114 A study using a different approach randomized 50 patients with CKD 3–4 already on allopurinol for treatment of mild hypeuricemia, to either continuation or withdrawal of allopurinol. After allopurinol withdrawal, there was a significant acceleration in the decline of renal function as well as worsening hypertension.115 In a post-hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAAL) trial, where the intervention was treatment with the antihypertensive losartan, a reduction of 6% of renal events was observed for every 0.5 mg/dL reduction of SU. The impact of SU reduction over time on losartan’s renoprotective effect was estimated by adjusting for the residual SU in the analysis of renal events, and after observing a mild reduction on losartan’s effect (from 22% to 17%), the investigators concluded that 1/5 of the observed losartan’s renoprotective effect was attributed to its uricosuric effect.116 In diabetic patients with diabetic nephropathy, a small randomized placebo-controlled trial of 40 patients showed a reduction of proteinuria in patients treated with allopurinol.117
Acute kidney injury
Hyperuricemia and acute kidney injury, via crystal-dependent mechanisms, have usually been associated in the context of tumor lysis syndrome. However, based on the observations of experimental models on crystal-independent renal injury, a possible association of SU and acute kidney injury (AKI) has been described. In a small trial evaluating the incidence of postoperative AKI in patients undergoing high-risk cardiovascular surgery, SU greater than 6 mg/dL was associated with a four-fold increase for AKI (OR 3.98 [95% CI 1.10–14.33]).118 Poor survival after coronary artery bypass grafting has also been associated with increasing SU levels.119 A more recent study of 190 patients who underwent cardiovascular surgery reported increasing incidences of AKI (defined as absolute increase in SCr ≥0.3 mg/dL from baseline within 48 hours after surgery) with increasing levels of SU. In the multivariate analysis, SU levels starting from equal or above 5.5 mg/dL were associated with an increased risk, ascending up to a 35-fold (OR 35.4 95% [CI 9.7–128.7]) with SU equal or above 7 mg/dL.120 A double-blind, placebo-controlled, randomized interventional trial using pre-operative rasburicase in hyperuricemic patients undergoing high-risk cardiovascular surgery showed no benefit on postoperative serum creatinine. However, a decrease in of the urine neutrophil-associated lipocalcin (a predictive marker of AKI in cardiovascular surgery patients) was reported in the rasburicase-treated subjects.121 Information on this subject is still scarce and inconclusive.
Urolithiasis
Urate nephrolithiasis represents 7 to 10% of all nephrolithiases in the United States. 122 The vast majority of individuals suffering from urate kidney stones are neither hyperuricemic or suffer from gout, since the usual abnormality observed in these individuals is consistently acidic urine (pH <5.5). 123,124 However, a large population study did report an association between the diagnosis of gout and an increased risk of incident kidney stones (RR 2.12 95% CI [1.22–3.68]).125 Hyperuricosuria, decreased urinary pH and low urinary volume are considered the three main factors involved in the development of urate nephrolithiasis.80 The role of hyperuricemia and gout as risk factors may still not be clear, especially since studies have shown that most gout patients are urate underexcretors.124,126 A stronger association between urate nephrolithiasis with type 2 diabetes mellitus and obesity has been described and explained in the basis of predisposition to an acidic urinary environment.80,122
Evidence on urate crystal-independent mechanisms of renal injury, coupled to epidemiological data, show a role for urate in the development of CKD. However, its importance in the progression of the disease and the potential use of ULT in CKD patients is still unclear. Further clinical trials in CKD patients are needed, as well as further information on the possible role of SU as a risk factor for AKI.
Metabolic disease
Urate pathways and fructose – laboratory and animal models
The increased renal reabsorption of SU at the proximal tubules secondary to hyperinsulinemia, has been regarded as the main hypothesis for the association between hyperuricemia and the metabolic syndrome (MS).127,128 However, models incorporating fructose metabolism are uncovering the contributory role of urate in the metabolic syndrome. Fructose consumption, either in the forms of table sugar or high-fructose corn syrup (in beverage and food sweetening), has increased in the last 30 years and epidemiological data has shown similar increments in obesity and associations with certain components of the MS.129–133 Fructose is phosphorylated by the enzyme fructokinase, which by having no negative-feedback mechanism works uninterruptedly, causing intracellular phosphate and ATP depletion and increased activity by the adenosine monophosphate deaminase, leading to increased levels of urate.134,135 An overlap of the increasing consumption of fructose and a corresponding trend of SU has been recognized.136 Fructose-induced hyperuricemia has been proven to result in the development of insulin resistance (IR), hypertension and renal injury in several animal models.137–140 This process has also been reversed by the administration of xanthine oxidase inhibitors (allopurinol and febuxostat) and uricosuric drugs (benzodiarone), suggesting a dependency on SU concentrations and not xanthine oxidase activity.139,140 Recent data on a trial using oxonic acid on rats fed with physiologic concentrations of fructose, showed that although hyperuricemia did not increase body weight, blood pressure or triglyceride levels, it did cause structural renal damage and significant increase in plasma insulin levels.141 The latter, through development of IR, is regarded as the potential central promoter of the MS.142–144
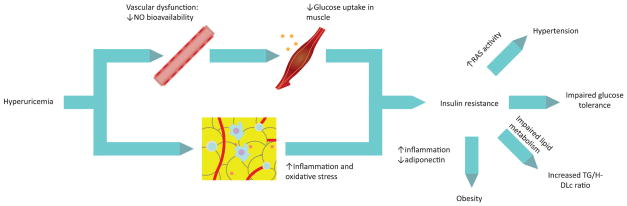
Based on findings and observations from experimental data, two different mechanisms that could explain the induction of IR, and hence hyperinsulinemia, have been proposed [see Figure 3].111 First, hyperuricemia reduces endothelial NO bioavailabilty.10,11 Since NO is necessary for glucose uptake in skeletal muscle, alterations in carbohydrate metabolism occurs secondary to this deficiency.145 Hypertension, another result of reduced NO bioavailability and damage to the endothelium, has also been pointed as a possible mediator of the MS.146 A second important mechanism results from the inflammatory and oxidative changes in adipose tissue secondary to exposure to elevated concentrations of urate. Intracellular urate in adipocytes, probably after translocation by URAT-1 transporters, increase oxidative stress by an increase in the enzymatic activity of the reduced form of the nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, giving rise to reactive oxygen species which lead to protein nitrosylation, lipid peroxidation and further NO reduction.86 This also induces macrophage infiltration, liberation of other inflammatory molecules such as MCP-1 and reduction of adiponectin.146,147 These inflammatory and oxidative changes in adipocytes have been shown to cause MS in obese mice.148 A study with obese hyperuricemic mouse hyperuricemic showed that after lowering urate levels with allopurinol the proinflammatory endocrine imbalance was improved, with reduction of macrophage infiltration in adipose tissue, increase in adiponectin levels and reduction in IR.147
Figure 3.
Hyperuricemia, insulin resistance and the metabolic syndrome
Experimental models have shown that hyperuricemia-induced vascular dysfunction, inflammation and increased oxidative stress lead to insulin resistance, which later leads to impaired glucose tolerance and predisposes to the other components of the metabolic syndrome.
NO = nitric oxide; TG = triglycerides; HDLc = high-density lipoprotein-cholesterol; RAS = renin-angiotensin system.
Insulin resistance and diabetes
As already seen in animal models, human studies have also shown an inverse correlation between high urate levels and insulin sensitivity.149,150 A large population study on 53,477 Korean adults showed that SU levels were independently correlated with IR and that this risk, as that for the metabolic syndrome, was maintained when patients were within the normal range.151 Although a causal association is still in debate, a recent 15-year follow-up study on 5,012 US non-diabetic adults has supported the contribution of SU to IR. After using regression models, it demonstrated that individuals with hyperuricemia (defined as >7.0 mg/dL) were more likely to develop IR and pre-diabetes, HR 1.36 (95% CI 1.23–1.51) and 1.25 (95% CI 1.04–1.52), respectively.152 Another study also supported a higher risk of developing hyperinsulinemia with increase baseline urate levels in non-diabetic patients.153 Although interventional studies are still missing, a clinical trial of ULT with benzbromarone in patients with CHF, lowering SU showed an improvement in IR.71
Type 2 diabetes mellitus, the final expression of IR, has also been associated with increasing serum urate concentrations in epidemiological studies. A 15-year follow-up study reported an increased risk for the development of diabetes in individuals with hyperuricemia (HR 1.87 [95% CI 1.33–2.62]), hyperuricemia.152 An increased risk has also been reported by different cohorts.29,154–158 Data from NHANES 2007–2008 showed that individuals with hyperuricemia presented an increased risk for diabetes (OR 1.63 [95% CI 1.13–2.34]), and that this risk had a dose-dependent association with SU levels. Diabetes prevalence among individuals with gout was also increased in comparison to individuals without gout, 25.7% and 7.8% respectively, reflecting an increased risk for diabetes among individuals with gout.5 However, data on this relation is still not conclusive with several studies indicating either no association between SU and diabetes,159 or even an inverse relationship between both.160–162 Although based on epidemiological data causality is still controversial, use of SU as a predictor of all-cause mortality in type 2 diabetic patients was recommended in a study carried out on a cohort of 535 diabetic adults. This association remained significant after adjusting for other co-variables.163
Metabolic syndrome
The MS represents a cluster of physiological and anthropometric abnormalities (IR, elevated blood pressure, truncal obesity, hypertriglyceridemia and low high-density lipoprotein-cholesterol (HDL) and is regarded as a risk factor for the development of diabetes and cardiovascular diseases. An association between MS and hyperuricemia has been already been well-described. An analysis of NHANES III data showed an increasing prevalence of the MS with increasing levels of SU, equal or above 10 mg/dL in comparison to the individuals with levels less than 6.0 mg/dL (70.7% and 18.9% respectively). A significant difference in prevalence between individuals with and without gout was also shown (62.8% and 25.4% respectively).164 The increasing risk of MS in individuals with higher SU levels has also been described by other studies,165,166 and even the elevated SU levels have also been observed to be significantly increased by the number of components of MS.165,167,168 An increased risk has even been described in individuals with high-normal SU levels in a Korean cohort.169
Besides IR and hypertension, associations between SU and other individual components of the MS have also been described. In a study on 11,182 subjects over 65 years old, triglyceride levels and waist circumference have shown a positive correlation with urate levels, while HDLc showed a negative correlation.170 These observations had been previously reported on patients with high cardiovascular risk and a population based study in Spain.171,172 A strong relationship between hyperuricemia, gout and obesity has been well documented, and data from a NHANES 1988–1994 and 2007–2010 analysis of has shown a progressively greater prevalence of gout in higher BMI categories.173 However, direct causality is still not clear and it may involve leptin, adiponectin and inflammation on adipocytes [see Figure 3].174 Interestingly, a study analyzing data from individuals with and without hyperuricemia in Taiwan, identified obesity and hypertriglyceridemia as possible potentiating factors on SU for the development of gout.175
Neurological disorders
Antioxidants effects of urate
In comparison to other mammals, higher levels of urate are secondary to the evolutionary loss of urate oxidase.176,177 This mutation was seen by as beneficial and part of adaptation by several authors, that postulated the presence of urate as an stimulant with positive effects in cognition, alertness and motivation,178 or an anti-aging effect through its ability to prevent oxidative damage.179 Although the theories of urate’s antioxidant capacity were ignored due to the association of high urate levels and cardiovascular risk, evidence on its neuroprotective effects and association with neurodegenerative disorders have resurfaced this aspect.
Urate can act as a powerful scavenger for peroxynitrite,180 peroxyl and hydroxyl radicals,181 and has been shown to reduce oxidative damage in DNA molecules.182 In studies using cellular models of neurodegeneration, reduced oxidative stress and cell death have been associated with urate, and even one study using a mouse model of Parkinson’s disease showed suppressed oxidative stress and death of dopaminergic cells.183,184 In 1994, the first study reporting an association of SU and Parkinson disease in post-mortem human tissue samples showed that urate was significantly reduced in the substantia nigra of Parkinson patients, and its addition decreased oxidation of dopamine in the caudate nucleus and substantia nigra.185
Parkison’s disease and other neurodegenerative conditions
The largest prospective study evaluating the relationship between hyperuricemia and the risk of developing Parkinson’s disease analyzed data from 18,018 men from the Health Professional Follow-up Study. During the observation period, 84 subjects were diagnosed with Parkinson’s disease and after adjusting for other variables, the rate ratio for the highest quartile of uricemia compared with the lowest was 0.43 (95% CI 0.18–1.02).186 Data from a more recent study, the Atherosclerosis Risk in Communities (ARIC) study, reported a similar OR, 0.4 (95% CI 0.2–10), when comparing extreme quartiles of plasma urate.187 Data regarding risk of Parkinson’s disease on individuals with gout has also been reported by two studies. An analysis of the General Practice Research Database, which includes over 3 million adults in the UK, reported that individuals with a previous history of gout had a lower risk, OR 0.69 (95% CI 0.48–0.99) of developing Parkinson’s disease. This association was only seen in men.188 Another study based on an 8-year median follow up of a Canadian cohort, reported an adjusted relative risk of 0.70 (95% CI 0.59–0.83) among those with gout.189 A recent review on this subject suggests, although still not at a clinical level, the feasibility of using SU as risk, diagnostic and prognostic marker for Parkinson’s disease.176
Data supporting this association has also been observed in other neurodegenerative diseases. In multiple sclerosis, a study showed that lower urate levels correlated with a worst prognosis, this expressed as relapses.190 Meanwhile a study on Huntington’s disease that higher SU levels correlated with a slower disease progression, and evidenced a trend of decreased worsening of motor function with increasing urate.191 Although evidence on interventional trials is lacking, a small trial with 11 multiple sclerosis patients treated with inosine (aimed at increasing SU levels) showed clinical improvement in 3 of the patients, and no disease progression on the rest.192 Evidence on this association is still lacking and further studies are needed in order to determine the actual nature of these observations.
COMORBIDITIES ASSOCIATED TO CALCIUM PYROPHOSPHATE DIHYDRATE CRYSTAL DEPOSITION DISEASE
Pseudogout, as initially described by McCarty and colleagues, is just part of the spectrum of CPPD crystal deposition disease that also includes pyrophosphate arthropathy, asymptomatic chondrocalcinosis and unusual presentations such as pseudo-rheumatoid arthritis or crowned dens syndrome.3,193,194 Population studies using radiological evidence of chondrocalcinosis have estimated prevalence ranging between 7% and 10%, usually in population over 60 years, and have proven a positive association with age.195–198 However, prevalence can vary depending on the method of identification. Studies examining synovial fluid of osteoarthritic joints at the time of joint replacement have reported a 25–43% prevalence of CPPD crystals.3 Data on CPPD, as well as on the associations with other comorbidities is still scarce. Most relevant evidence on these associations is presented in the following sections.
Osteoarthritis
An association between CPPD deposition disease and osteoarthritis (OA) has been discussed and suggested for years; however the precise nature of this relationship is still unclear, and the existence of common risk factors (e.g. aging and joint injury), makes studying the relationship quite complex and challenging.199,200 CPPD and other calcium crystals, such as basic calcium phosphate crystals, have been shown to generate calcium oscillations in articular chondrocytes201 and a prolonged inflammatory response which can contribute or amplify articular degeneration and joint damage.202,203 Increased transcription of progressive ankylosis homolog gene (ANKH), whose mutations have been described in familial forms of CPPD disease,204 has been reported in OA meniscal cells.205
The European League Against Rheumatism (EULAR) recommendations on CPPD deposition disease, analyzed the association with OA taking into account four cross-sectional and five case-control studies. It estimated that people with OA were almost three times more likely to have CPPD, OR 2.66 (95% CI 2.00–3.54).199 A study included two cohorts of people with knee OA and radiographic Kellgren scores of greater than or equal to 2 used MRI to explore the relation between radiographic chondrocalcinosis and OA progression. After 30 months of imaging and follow up, cartilage loss, used as a proxy for progressive OA, showed no correlation with the presence of chondrocalcinosis.206 Evidence on this subject is still not conclusive, and although calcium crystals may be involved in the process of OA, more studies are needed.
Metabolic and endocrine disorders
A relationship between hemochromatosis and CPPD disease has been described. A study of 178 patients diagnosed with hereditary hemochromatosis and not yet treated with phlebotomy, showed a 30% prevalence of chondrocalcinosis and a positive correlation between the number of joints involved with age, ferritin level and PTH 44–68.207 An older case series of 54 patients with hemochromatosis, reported a significant association, OR 6.81 (95% CI 2.02–22.95), with chondrocalcinosis.208 Although still in debate, the actual significance of this association may be relatively small. This was shown by a study that carried out systematic genetic testing in 128 patients with chondrocalcinosis and pseudogout, showing a low prevalence of C282Y homozygotes and C282Y/H63D compound heterozygotes (1.6% and 3.1%, respectively).209
The 2011 EULAR report on CPPD disease pooled data from five studies described an association between CPPD deposition disease and hyperparathyroidism, showing that patients with hyperparathyroidism were three times more likely to have CPPD than controls (OR 3.03 95% CI [1.15–8.02]).199 Triggering of pseudogout attacks by parathyroidectomy has also been reported been reported by some studies.210,211 However data on this association is still scarce.
A cross sectional study of 72 patients with intestinal failure and in parenteral nutrition, showed a significant association between hypomagnesemia and chondrocalcinosis. Compared to healthy controls, these patients with chronic hypomagnesemia presented a higher prevalence of chondrocalcinosis (16.6% vs 2.7% in controls), and prevalence of chondrocalcinosis was significantly higher, OR 13.5 (95% CI [2.76–127.3]), in patient with lower serum magnesium levels.212 Reports on Gitelman’s syndrome and chondrocalcinosis support this association.213,214 CPPD arthropathy or pseudogout can be the onset of presentation of Gitelman’s syndrome, and this disease should be considered in the differential of younger patients presenting with CPPD deposition disease.215
Hypophosphatasia, gout, ochronosis, familial hypocalciuric hypercalcaemia, X-linked hypophostatemic rickets, Wilson’s disease, and acromegaly are additional diseases that have been linked to CPPD disease. However, data on these associations are only based on case reports.216
CONCLUSIONS
The clinical significance of asymptomatic hyperuricemia and gout as risk factors for various comorbidities is being recently supported by the emergence of new data from experimental, epidemiological and clinical intervention trials (See Table 1). Evidence available is supportive of a causal role of hyperuricemia on hypertension, leaving SU as a possible therapeutic target, especially on early stages. Although interventional data from small trials is available and does suggest a benefit, larger trials are needed to collect enough evidence to support indication of ULT. Evidence on CHD and stroke although suggesting, is still not clear and required further studies. The association between CHF and urate is probably indirect and related to an increased activity by xanthine oxidase, and benefit of xanthine-oxidase inhibition is still not conclusive. Cardiovascular comorbidities are nowadays important to be considered in the management of gout patients.
Table 1.
Comorbidities and their association with hyperuricemia and gout
| Comorbidity | Evidence on association and causality | Evidence on impact of urate therapies |
|---|---|---|
| Hypertension | Several population studies have proven an independent association between SU and development of hypertension.32,33 SU plays a key role in the initial stages of the development of hypertension.217 |
Small-sampled controlled studies have shown BP reduction with ULT in young individuals37,38 and adults.39 However, evidence to recommend use of ULT is still not conclusive. 40 |
| Coronary heart disease | Although still not conclusive, evidence shows a small but significant increased risk of CHD in individuals with hyperuricemia,52,53 and patients with gout. 51 | Trials on the use of allopurinol and angina exist, although evidence on CHD incidence and prognosis is still lacking. |
| Congestive heart failure | Although a constant association with increased incidence58,59,65 and mortality,60,61 causality is still in debate since hyperuricemia may just reflect increased xanthine oxidase activity.66,63 | Small trials showing improvement in myocardial function and ejection fraction with allopurinol suggest benefits secondary to xanthine-oxidase inhibition rather than SU lowering.69,70,71 |
| Stroke | Associations with cerebral ischemia, 72 increased incidence of stroke75 and poorer prognosis in stroke patients have been reported.73,74 However causality is still not clear. | Trials using ULT have shown conflicting results in subclinical parameters.76–78 |
| Chronic kidney disease | Experimental evidence on crystal-independent renal injury models82–84 have supported epidemiological evidence for a causative role of hyperuricemia on CKD.95–97 However, data on effects of disease progression are still not conclusive.106,110 | Interventional studies with allopurinol have shown improvement in eGFR in normal individuals39,112 and a decrease in renal function deterioration in CKD patients.113–115 Data is still scarce to generalize recommendations for use of ULT. |
| Insulin resistance and metabolic syndrome | Experimental data has shown a causative role for UA in the development on IR and an association with the development of IR151,152 and the MS has been established.165,166,169 Associations with dyslipidemia170 and obesity have been reported. 173 | A role for ULT in animal models with MS has been shown,140,147 and improvement in IR was seen in one trial with CHF patients.71 Further trials are needed. |
| Type 2 diabetes mellitus | Increasing incidence in individuals with hyperuricemia has been shown.152,154,155 Studies studying an association between gout and diabetes are still not conclusive, with some studies even showing an inverse relation between both.159,160,162 | Data on this subject is still lacking to consider further recommendations. |
| Neuro-degenerative disorders | An association between hyperuricemia and a decreased incidence with Parkinson’s Disease186–188 as a slower development of multiples sclerosis190 and Huntington’s disease has been reported. Data on the subject is still scarce.191 | A small trial in multiple sclerosis patients showed clinical improvements in 3 of the patients while increasing SU with inosine.192 Further trials are needed. |
SU = urate; UA = urate; ULT = urate-lowering therapy; CHD = coronary heart disease; eGFR = estimated glomerular filtration rate; CKD = chronic kidney disease; IR = insulin resistance; MS = metabolic syndrome; CHF = congestive heart failure.
Although SU was initially not considered to be a causal factor for the development of the metabolic syndrome, fructose-fed animal models have shown evidence to involve urate in the pathological process of IR. This association requires further studies but poses an interest relation between the overlapping growths of their respective prevalences. An association between hyperuricemia, gout and diabetes however, is still not clear. The relationship between hyperuricemia and CKD incidence is also becoming clearer in the light of new experimental and epidemiological evidence, however the relation of effects on CKD progression are still not conclusive. Recent data on AKI deserves further attention. The associations between SU and neurodegenerative diseases are still not clear, and further evidence is needed to show if this just represents an observation or a potential therapeutic strategy.
Calcium deposition diseases, such as CPPD, still represent a low proportion of crystal arthropathies and studies on the subject are still scarce. Although interest on the role of calcium crystals in the development of OA is arising, data is still lacking in order for conclusion to be drawn. The association between CPPD deposition disease and several metabolic and endocrine disorders has been established based on several small studies; however the association is still small to consider an active search of these in patients with CPPD disease.
Key points.
Recent evidence has shown that asymptomatic hyperuricemia, as well as hyperuricemia in gout patients, plays a significant role in the development of cardiovascular comorbidities.
In addition to an already proven association between hypertension and hyperuricemia, interventional trials are now showing a positive effect of urate lowering therapy in early stages of hypertension in young individuals.
An association between hyperuricemia and other cardiovascular diseases such as coronary heart disease, congestive heart failure, and stroke is still not clear.
A link between hyperuricemia, insulin resistance and the metabolic syndrome has been shown by fructose-fed animal models, and may explain the association between two overlapping and increasing diseases.
Hyperuricemia is associated with an increased risk of chronic kidney disease, but the use of urate lowering therapy in these patients is still not clear.
Evidence regarding calcium pyrophosphate arthropathy and associated comorbidities is still scarce and not conclusive.
Acknowledgments
Grant support
J.A.S. is supported by grants from the Agency for Health Quality and Research Center for Education and Research on Therapeutics (CERTs), National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS), National Institute of Aging (NIA) and National Cancer Institute (NCI) and the resources and use of facilities at the Birmingham VA Medical Center, Alabama, USA.
Footnotes
Competing interests
There are no financial conflicts related directly to this study. JAS has received research grants from Takeda and Savient; and consultant fees from Savient, Takeda, Allergan and Regeneron. DGL has received royalties/speaker fees from Zimmer, has been a paid consultant to Zimmer and has received institutional research funds from DePuy, Stryker and Zimmer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Contributor Information
Sebastian E Sattui, Email: sattui@uab.edu, University of Alabama, School of Medicine, Division of Clinical Immunology and Rheumatology, Department of Medicine, Faculty Office Tower 813, 510 20th Street S, Birmingham, AL 35294, USA, +1 205 975 4177.
Jasvinder A Singh, Email: jasvinder.md@gmail.com, Medicine Service and Center for Surgical Medical Acute Care Research and Transitions (C-SMART), Birmingham VA Medical Center, Birmingham, AL, USA. University of Alabama, School of Medicine, Division of Clinical Immunology and Rheumatology, Department of Medicine, Faculty Office Tower 805B, 510 20th Street S, Birmingham, AL 35294, USA, +1 205 934 8158; +1 205 996 9685. Mayo Clinic College of Medicine, Department of Orthopedic Surgery, Rochester, MN, USA.
Angelo L Gaffo, Email: agaffo@uab.edu, Rheumatology Section at the Veterans Affairs Medical Center. Birmingham, AL, US. University of Alabama, School of Medicine, Division of Clinical Immunology and Rheumatology, Department of Medicine, Shelby Building 201, 1825 University Blvd, Birmingham, AL 35294, USA, +1 205 975 6410; +1 205 996 6734.
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis and rheumatism. 2011;63:3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 3.Richette P, Bardin T, Doherty M. An update on the epidemiology of calcium pyrophosphate dihydrate crystal deposition disease. Rheumatology (Oxford, England) 2009;48:711–5. doi: 10.1093/rheumatology/kep081. [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Annals of the rheumatic diseases. 2008;67:1310–6. doi: 10.1136/ard.2007.081604. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. The American journal of medicine. 2012;125:679–87. e1. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Annemans L, Spaepen E, Gaskin M, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Annals of the rheumatic diseases. 2008;67:960–6. doi: 10.1136/ard.2007.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson PC, Merriman TR, Herbison P, et al. Hospital admissions associated with gout and their comorbidities in New Zealand and England 1999–2009. Rheumatology (Oxford, England) 2013;52:118–26. doi: 10.1093/rheumatology/kes253. [DOI] [PubMed] [Google Scholar]
- 8.Sari I, Akar S, Pakoz B, et al. Hyperuricemia and its related factors in an urban population, Izmir, Turkey. Rheumatology international. 2009;29:869–74. doi: 10.1007/s00296-008-0806-2. [DOI] [PubMed] [Google Scholar]
- 9.Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis and rheumatism. 1972;15:189–92. doi: 10.1002/art.1780150209. [DOI] [PubMed] [Google Scholar]
- 10.Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. American journal of physiology Cell physiology. 2008;295:C1183–90. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney international. 2005;67:1739–42. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 12.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. The Journal of biological chemistry. 1991;266:8604–8. [PubMed] [Google Scholar]
- 13.Corry DB, Eslami P, Yamamoto K, et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. Journal of hypertension. 2008;26:269–75. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 14.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. American journal of physiology Renal physiology. 2002;282:F991–7. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 15.Chao HH, Liu JC, Lin JW, et al. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta pharmacologica Sinica. 2008;29:1301–12. doi: 10.1111/j.1745-7254.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 16.Cheng TH, Lin JW, Chao HH, et al. Uric acid activates extracellular signal-regulated kinases and thereafter endothelin-1 expression in rat cardiac fibroblasts. International journal of cardiology. 2010;139:42–9. doi: 10.1016/j.ijcard.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–52. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Lozada LG, Tapia E, Santamaria J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney international. 2005;67:237–47. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Lozada LG, Tapia E, Avila-Casado C, et al. Mild hyperuricemia induces glomerular hypertension in normal rats. American journal of physiology Renal physiology. 2002;283:F1105–10. doi: 10.1152/ajprenal.00170.2002. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Lozada LG, Tapia E, Soto V, et al. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:1179–85. doi: 10.1093/ndt/gfm783. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Lozada LG, Tapia E, Soto V, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiology. 2008;108:p69–78. doi: 10.1159/000127837. [DOI] [PubMed] [Google Scholar]
- 22.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–6. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 23.Feig DI. The role of uric acid in the pathogenesis of hypertension in the young. Journal of clinical hypertension (Greenwich, Conn) 2012;14:346–52. doi: 10.1111/j.1751-7176.2012.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundstrom J, Sullivan L, D’Agostino RB, et al. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan E, Kwoh CK, Schumacher HR, et al. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49:298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 26.Masuo K, Kawaguchi H, Mikami H, et al. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–80. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 27.Mellen PB, Bleyer AJ, Erlinger TP, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension. 2006;48:1037–42. doi: 10.1161/01.HYP.0000249768.26560.66. [DOI] [PubMed] [Google Scholar]
- 28.Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertension research: official journal of the Japanese Society of Hypertension. 2004;27:835–41. doi: 10.1291/hypres.27.835. [DOI] [PubMed] [Google Scholar]
- 29.Nakanishi N, Okamoto M, Yoshida H, et al. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. European journal of epidemiology. 2003;18:523–30. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 30.Gaffo AL, Jacobs DR, Jr, Sijtsma F, et al. Serum urate association with hypertension in young adults: analysis from the Coronary Artery Risk Development in Young Adults cohort. Annals of the rheumatic diseases. 2012 doi: 10.1136/annrheumdis-2012-201916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48:1031–6. doi: 10.1161/01.HYP.0000248752.08807.4c. [DOI] [PubMed] [Google Scholar]
- 32*.Grayson PC, Kim SY, LaValley M, et al. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis care & research. 2011;63:102–10. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Sun K, Yang Y, et al. Plasma uric acid and hypertension in a Chinese community: prospective study and metaanalysis. Clinical chemistry. 2009;55:2026–34. doi: 10.1373/clinchem.2009.124891. [DOI] [PubMed] [Google Scholar]
- 34.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–52. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viazzi F, Antolini L, Giussani M, et al. Serum uric Acid and blood pressure in children at cardiovascular risk. Pediatrics. 2013;132:e93–9. doi: 10.1542/peds.2013-0047. [DOI] [PubMed] [Google Scholar]
- 36.Hongo M, Hidaka H, Sakaguchi S, et al. Association between serum uric acid levels and cardiometabolic risk factors among Japanese junior high school students. Circulation journal: official journal of the Japanese Circulation Society. 2010;74:1570–7. doi: 10.1253/circj.cj-09-0837. [DOI] [PubMed] [Google Scholar]
- 37*.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA: the journal of the American Medical Association. 2008;300:924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60:1148–56. doi: 10.1161/HYPERTENSIONAHA.112.196980. [DOI] [PubMed] [Google Scholar]
- 39.Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clinical journal of the American Society of Nephrology: CJASN. 2011;6:1887–94. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gois PH, Souza ER. Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane database of systematic reviews (Online) 2013;1:CD008652. doi: 10.1002/14651858.CD008652.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Patetsios P, Rodino W, Wisselink W, et al. Identification of uric acid in aortic aneurysms and atherosclerotic artery. Annals of the New York Academy of Sciences. 1996;800:243–5. doi: 10.1111/j.1749-6632.1996.tb33318.x. [DOI] [PubMed] [Google Scholar]
- 42.Montalcini T, Gorgone G, Gazzaruso C, et al. Relation between serum uric acid and carotid intima-media thickness in healthy postmenopausal women. Internal and emergency medicine. 2007;2:19–23. doi: 10.1007/s11739-007-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavil Y, Kaya MG, Oktar SO, et al. Uric acid level and its association with carotid intima-media thickness in patients with hypertension. Atherosclerosis. 2008;197:159–63. doi: 10.1016/j.atherosclerosis.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Neogi T, Ellison RC, Hunt S, et al. Serum uric acid is associated with carotid plaques: the National Heart, Lung, and Blood Institute Family Heart Study. The Journal of rheumatology. 2009;36:378–84. doi: 10.3899/jrheum.080646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neogi T, Terkeltaub R, Ellison RC, et al. Serum urate is not associated with coronary artery calcification: the NHLBI Family Heart Study. The Journal of rheumatology. 2011;38:111–7. doi: 10.3899/jrheum.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Wang H, Jacobs DR, Jr, Gaffo AL, et al. Longitudinal association between serum urate and subclinical atherosclerosis: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Journal of internal medicine. 2013 doi: 10.1111/joim.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabelink TJ, Luscher TF. Endothelial nitric oxide synthase: host defense enzyme of the endothelium? Arteriosclerosis, thrombosis, and vascular biology. 2006;26:267–71. doi: 10.1161/01.ATV.0000196554.85799.77. [DOI] [PubMed] [Google Scholar]
- 48.Higgins P, Dawson J, Lees KR, et al. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovascular therapeutics. 2012;30:217–26. doi: 10.1111/j.1755-5922.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 49.Culleton BF, Larson MG, Kannel WB, et al. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Annals of internal medicine. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 50.De Vera MA, Rahman MM, Bhole V, et al. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Annals of the rheumatic diseases. 2010;69:1162–4. doi: 10.1136/ard.2009.122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo CF, Yu KH, See LC, et al. Risk of myocardial infarction among patients with gout: a nationwide population-based study. Rheumatology (Oxford, England) 2013;52:111–7. doi: 10.1093/rheumatology/kes169. [DOI] [PubMed] [Google Scholar]
- 52.Wheeler JG, Juzwishin KD, Eiriksdottir G, et al. Serum uric acid and coronary heart disease in 9,458 incident cases and 155,084 controls: prospective study and meta-analysis. PLoS medicine. 2005;2:e76. doi: 10.1371/journal.pmed.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis care & research. 2010;62:170–80. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shankar A, Klein BE, Nieto FJ, et al. Association between serum uric acid level and peripheral arterial disease. Atherosclerosis. 2008;196:749–55. doi: 10.1016/j.atherosclerosis.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 55.Baker JF, Schumacher HR, Krishnan E. Serum uric acid level and risk for peripheral arterial disease: analysis of data from the multiple risk factor intervention trial. Angiology. 2007;58:450–7. doi: 10.1177/0003319707303444. [DOI] [PubMed] [Google Scholar]
- 56.Langlois M, De Bacquer D, Duprez D, et al. Serum uric acid in hypertensive patients with and without peripheral arterial disease. Atherosclerosis. 2003;168:163–8. doi: 10.1016/s0021-9150(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 57.Tseng CH. Independent association of uric acid levels with peripheral arterial disease in Taiwanese patients with Type 2 diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2004;21:724–9. doi: 10.1111/j.1464-5491.2004.01239.x. [DOI] [PubMed] [Google Scholar]
- 58.Ekundayo OJ, Dell’Italia LJ, Sanders PW, et al. Association between hyperuricemia and incident heart failure among older adults: a propensity-matched study. International journal of cardiology. 2010;142:279–87. doi: 10.1016/j.ijcard.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnan E. Hyperuricemia and incident heart failure. Circulation Heart failure. 2009;2:556–62. doi: 10.1161/CIRCHEARTFAILURE.108.797662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strasak AM, Kelleher CC, Brant LJ, et al. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. International journal of cardiology. 2008;125:232–9. doi: 10.1016/j.ijcard.2007.11.094. [DOI] [PubMed] [Google Scholar]
- 61.Strasak A, Ruttmann E, Brant L, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83,683 Austrian men. Clinical chemistry. 2008;54:273–84. doi: 10.1373/clinchem.2007.094425. [DOI] [PubMed] [Google Scholar]
- 62.Chen JH, Chuang SY, Chen HJ, et al. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis and rheumatism. 2009;61:225–32. doi: 10.1002/art.24164. [DOI] [PubMed] [Google Scholar]
- 63.Filippatos GS, Ahmed MI, Gladden JD, et al. Hyperuricaemia, chronic kidney disease, and outcomes in heart failure: potential mechanistic insights from epidemiological data. European heart journal. 2011;32:712–20. doi: 10.1093/eurheartj/ehq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamariz L, Harzand A, Palacio A, et al. Uric acid as a predictor of all-cause mortality in heart failure: a meta-analysis. Congestive heart failure (Greenwich, Conn) 2011;17:25–30. doi: 10.1111/j.1751-7133.2011.00200.x. [DOI] [PubMed] [Google Scholar]
- 65.Krishnan E. Gout and the risk for incident heart failure and systolic dysfunction. BMJ open. 2012;2:e000282. doi: 10.1136/bmjopen-2011-000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bergamini C, Cicoira M, Rossi A, et al. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. European journal of heart failure. 2009;11:444–52. doi: 10.1093/eurjhf/hfp042. [DOI] [PubMed] [Google Scholar]
- 67*.Hare JM, Mangal B, Brown J, et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. Journal of the American College of Cardiology. 2008;51:2301–9. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 68.Cingolani HE, Plastino JA, Escudero EM, et al. The effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata Study. Journal of cardiac failure. 2006;12:491–8. doi: 10.1016/j.cardfail.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 69.George J, Carr E, Davies J, et al. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–16. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 70.Noman A, Ang DS, Ogston S, et al. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375:2161–7. doi: 10.1016/S0140-6736(10)60391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogino K, Kato M, Furuse Y, et al. Uric acid-lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled crossover preliminary study. Circulation Heart failure. 2010;3:73–81. doi: 10.1161/CIRCHEARTFAILURE.109.868604. [DOI] [PubMed] [Google Scholar]
- 72.Schretlen DJ, Inscore AB, Vannorsdall TD, et al. Serum uric acid and brain ischemia in normal elderly adults. Neurology. 2007;69:1418–23. doi: 10.1212/01.wnl.0000277468.10236.f1. [DOI] [PubMed] [Google Scholar]
- 73.Wong KY, MacWalter RS, Fraser HW, et al. Urate predicts subsequent cardiac death in stroke survivors. European heart journal. 2002;23:788–93. doi: 10.1053/euhj.2001.2970. [DOI] [PubMed] [Google Scholar]
- 74.Weir CJ, Muir SW, Walters MR, et al. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke; a journal of cerebral circulation. 2003;34:1951–6. doi: 10.1161/01.STR.0000081983.34771.D2. [DOI] [PubMed] [Google Scholar]
- 75*.Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis and rheumatism. 2009;61:885–92. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muir SW, Harrow C, Dawson J, et al. Allopurinol use yields potentially beneficial effects on inflammatory indices in those with recent ischemic stroke: a randomized, double-blind, placebo-controlled trial. Stroke; a journal of cerebral circulation. 2008;39:3303–7. doi: 10.1161/STROKEAHA.108.519793. [DOI] [PubMed] [Google Scholar]
- 77.Khan F, George J, Wong K, et al. Allopurinol treatment reduces arterial wave reflection in stroke survivors. Cardiovascular therapeutics. 2008;26:247–52. doi: 10.1111/j.1755-5922.2008.00057.x. [DOI] [PubMed] [Google Scholar]
- 78.Dawson J, Quinn TJ, Harrow C, et al. The effect of allopurinol on the cerebral vasculature of patients with subcortical stroke; a randomized trial. British journal of clinical pharmacology. 2009;68:662–8. doi: 10.1111/j.1365-2125.2009.03497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cameron JS. Uric Acid and renal disease. Nucleosides, nucleotides & nucleic acids. 2006;25:1055–64. doi: 10.1080/15257770600890954. [DOI] [PubMed] [Google Scholar]
- 80.Cameron MA, Sakhaee K. Uric acid nephrolithiasis. The Urologic clinics of North America. 2007;34:335–46. doi: 10.1016/j.ucl.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Cameron JS, Moro F, Simmonds HA. Gout, uric acid and purine metabolism in paediatric nephrology. Pediatric nephrology (Berlin, Germany) 1993;7:105–18. doi: 10.1007/BF00861588. [DOI] [PubMed] [Google Scholar]
- 82.Sanchez-Lozada LG, Soto V, Tapia E, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. American journal of physiology Renal physiology. 2008;295:F1134–41. doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanchez-Lozada LG, Tapia E, Lopez-Molina R, et al. Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. American journal of physiology Renal physiology. 2007;292:F1238–44. doi: 10.1152/ajprenal.00164.2006. [DOI] [PubMed] [Google Scholar]
- 84.Nakagawa T, Mazzali M, Kang DH, et al. Hyperuricemia causes glomerular hypertrophy in the rat. American journal of nephrology. 2003;23:2–7. doi: 10.1159/000066303. [DOI] [PubMed] [Google Scholar]
- 85.Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, et al. Uric Acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Experimental nephrology. 2012;121:e71–8. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sautin YY, Nakagawa T, Zharikov S, et al. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. American journal of physiology Cell physiology. 2007;293:C584–96. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 87.Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–93. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 88.Ryu ES, Kim MJ, Shin HS, et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. American journal of physiology Renal physiology. 2013;304:F471–80. doi: 10.1152/ajprenal.00560.2012. [DOI] [PubMed] [Google Scholar]
- 89.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. Journal of molecular medicine (Berlin, Germany) 2004;82:175–81. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- 90.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. Journal of the American Society of Nephrology: JASN. 2002;13:2888–97. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 91.Mazali FC, Johnson RJ, Mazzali M. Use of uric acid-lowering agents limits experimental cyclosporine nephropathy. Nephron Experimental nephrology. 2012;120:e12–9. doi: 10.1159/000330274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mazzali M, Kim YG, Suga S, et al. Hyperuricemia exacerbates chronic cyclosporine nephropathy. Transplantation. 2001;71:900–5. doi: 10.1097/00007890-200104150-00014. [DOI] [PubMed] [Google Scholar]
- 93.Kosugi T, Nakayama T, Heinig M, et al. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. American journal of physiology Renal physiology. 2009;297:F481–8. doi: 10.1152/ajprenal.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsu CY, Iribarren C, McCulloch CE, et al. Risk factors for end-stage renal disease: 25-year follow-up. Archives of internal medicine. 2009;169:342–50. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Obermayr RP, Temml C, Gutjahr G, et al. Elevated uric acid increases the risk for kidney disease. Journal of the American Society of Nephrology: JASN. 2008;19:2407–13. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weiner DE, Tighiouart H, Elsayed EF, et al. Uric acid and incident kidney disease in the community. Journal of the American Society of Nephrology: JASN. 2008;19:1204–11. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iseki K, Ikemiya Y, Inoue T, et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2004;44:642–50. [PubMed] [Google Scholar]
- 98.Iseki K, Oshiro S, Tozawa M, et al. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertension research: official journal of the Japanese Society of Hypertension. 2001;24:691–7. doi: 10.1291/hypres.24.691. [DOI] [PubMed] [Google Scholar]
- 99.Ohno I, Hosoya T, Gomi H, et al. Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron. 2001;87:333–9. doi: 10.1159/000045939. [DOI] [PubMed] [Google Scholar]
- 100.Syrjanen J, Mustonen J, Pasternack A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2000;15:34–42. doi: 10.1093/ndt/15.1.34. [DOI] [PubMed] [Google Scholar]
- 101.Shi Y, Chen W, Jalal D, et al. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney & blood pressure research. 2012;35:153–60. doi: 10.1159/000331453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hovind P, Rossing P, Tarnow L, et al. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58:1668–71. doi: 10.2337/db09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:1865–9. doi: 10.1093/ndt/gfp740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes care. 2010;33:1337–43. doi: 10.2337/dc10-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zoppini G, Targher G, Chonchol M, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes care. 2012;35:99–104. doi: 10.2337/dc11-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang HY, Tung CW, Lee PH, et al. Hyperuricemia as an independent risk factor of chronic kidney disease in middle-aged and elderly population. The American journal of the medical sciences. 2010;339:509–15. doi: 10.1097/MAJ.0b013e3181db6e16. [DOI] [PubMed] [Google Scholar]
- 107.Chonchol M, Shlipak MG, Katz R, et al. Relationship of uric acid with progression of kidney disease. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2007;50:239–47. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 108.Madero M, Sarnak MJ, Wang X, et al. Uric acid and long-term outcomes in CKD. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney international. 1997;51:1908–19. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 110.Sturm G, Kollerits B, Neyer U, et al. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Experimental gerontology. 2008;43:347–52. doi: 10.1016/j.exger.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 111.Gustafsson D, Unwin R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC nephrology. 2013;14:164. doi: 10.1186/1471-2369-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. International urology and nephrology. 2007;39:1227–33. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- 113*.Siu YP, Leung KT, Tong MK, et al. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;47:51–9. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 114.Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clinical journal of the American Society of Nephrology: CJASN. 2010;5:1388–93. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. American journal of nephrology. 2007;27:435–40. doi: 10.1159/000105142. [DOI] [PubMed] [Google Scholar]
- 116.Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [DOI] [PubMed] [Google Scholar]
- 117.Momeni A, Shahidi S, Seirafian S, et al. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iranian journal of kidney diseases. 2010;4:128–32. [PubMed] [Google Scholar]
- 118.Ejaz AA, Beaver TM, Shimada M, et al. Uric acid: a novel risk factor for acute kidney injury in high-risk cardiac surgery patients? American journal of nephrology. 2009;30:425–9. doi: 10.1159/000238824. [DOI] [PubMed] [Google Scholar]
- 119.Hillis GS, Cuthbertson BH, Gibson PH, et al. Uric acid levels and outcome from coronary artery bypass grafting. The Journal of thoracic and cardiovascular surgery. 2009;138:200–5. doi: 10.1016/j.jtcvs.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 120.Lapsia V, Johnson RJ, Dass B, et al. Elevated uric acid increases the risk for acute kidney injury. The American journal of medicine. 2012;125:302e9–17. doi: 10.1016/j.amjmed.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 121.Ejaz AA, Dass B, Lingegowda V, et al. Effect of uric acid lowering therapy on the prevention of acute kidney injury in cardiovascular surgery. International urology and nephrology. 2013;45:449–58. doi: 10.1007/s11255-012-0192-2. [DOI] [PubMed] [Google Scholar]
- 122.Mehta TH, Goldfarb DS. Uric acid stones and hyperuricosuria. Advances in chronic kidney disease. 2012;19:413–8. doi: 10.1053/j.ackd.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 123.Kamel KS, Cheema-Dhadli S, Halperin ML. Studies on the pathophysiology of the low urine pH in patients with uric acid stones. Kidney international. 2002;61:988–94. doi: 10.1046/j.1523-1755.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- 124.Sakhaee K, Adams-Huet B, Moe OW, et al. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney international. 2002;62:971–9. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 125.Kramer HJ, Choi HK, Atkinson K, et al. The association between gout and nephrolithiasis in men: The Health Professionals’ Follow-Up Study. Kidney international. 2003;64:1022–6. doi: 10.1046/j.1523-1755.2003.t01-2-00171.x. [DOI] [PubMed] [Google Scholar]
- 126.Alvarez-Nemegyei J, Medina-Escobedo M, Villanueva-Jorge S, et al. Prevalence and risk factors for urolithiasis in primary gout: is a reappraisal needed? The Journal of rheumatology. 2005;32:2189–91. [PubMed] [Google Scholar]
- 127.Facchini F, Chen YD, Hollenbeck CB, et al. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA: the journal of the American Medical Association. 1991;266:3008–11. [PubMed] [Google Scholar]
- 128.Reaven GM. The kidney: an unwilling accomplice in syndrome X. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1997;30:928–31. doi: 10.1016/s0272-6386(97)90106-2. [DOI] [PubMed] [Google Scholar]
- 129.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. The American journal of clinical nutrition. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 130.Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–8. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 131.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 132.Nguyen S, Choi HK, Lustig RH, et al. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. The Journal of pediatrics. 2009;154:807–13. doi: 10.1016/j.jpeds.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes care. 2010;33:2477–83. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fox IH, Kelley WN. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism: clinical and experimental. 1972;21:713–21. doi: 10.1016/0026-0495(72)90120-5. [DOI] [PubMed] [Google Scholar]
- 135.van den Berghe G, Bronfman M, Vanneste R, et al. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. The Biochemical journal. 1977;162:601–9. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rho YH, Zhu Y, Choi HK. The epidemiology of uric acid and fructose. Seminars in nephrology. 2011;31:410–9. doi: 10.1016/j.semnephrol.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nakayama T, Kosugi T, Gersch M, et al. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. American journal of physiology Renal physiology. 2010;298:F712–20. doi: 10.1152/ajprenal.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sanchez-Lozada LG, Tapia E, Jimenez A, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. American journal of physiology Renal physiology. 2007;292:F423–9. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 139.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. American journal of physiology Renal physiology. 2006;290:F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 140.Sanchez-Lozada LG, Tapia E, Bautista-Garcia P, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. American journal of physiology Renal physiology. 2008;294:F710–8. doi: 10.1152/ajprenal.00454.2007. [DOI] [PubMed] [Google Scholar]
- 141.Lanaspa MA, Tapia E, Soto V, et al. Uric acid and fructose: potential biological mechanisms. Seminars in nephrology. 2011;31:426–32. doi: 10.1016/j.semnephrol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 142.Ferrannini E. Is insulin resistance the cause of the metabolic syndrome? Annals of medicine. 2006;38:42–51. doi: 10.1080/07853890500415358. [DOI] [PubMed] [Google Scholar]
- 143.Gallagher EJ, Leroith D, Karnieli E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. The Mount Sinai journal of medicine, New York. 2010;77:511–23. doi: 10.1002/msj.20212. [DOI] [PubMed] [Google Scholar]
- 144.Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. The Medical clinics of North America. 2007;91:1063–77. viii. doi: 10.1016/j.mcna.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 145.Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. The American journal of physiology. 1998;274:E692–9. doi: 10.1152/ajpendo.1998.274.4.E692. [DOI] [PubMed] [Google Scholar]
- 146.Simao AN, Lozovoy MA, Dichi I. The uric acid metabolism pathway as a therapeutic target in hyperuricemia related to metabolic syndrome. Expert opinion on therapeutic targets. 2012;16:1175–87. doi: 10.1517/14728222.2012.723694. [DOI] [PubMed] [Google Scholar]
- 147.Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–69. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vuorinen-Markkola H, Yki-Jarvinen H. Hyperuricemia and insulin resistance. The Journal of clinical endocrinology and metabolism. 1994;78:25–9. doi: 10.1210/jcem.78.1.8288709. [DOI] [PubMed] [Google Scholar]
- 150.Meshkani R, Zargari M, Larijani B. The relationship between uric acid and metabolic syndrome in normal glucose tolerance and normal fasting glucose subjects. Acta diabetologica. 2011;48:79–88. doi: 10.1007/s00592-010-0231-3. [DOI] [PubMed] [Google Scholar]
- 151.Yoo TW, Sung KC, Shin HS, et al. Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circulation journal: official journal of the Japanese Circulation Society. 2005;69:928–33. doi: 10.1253/circj.69.928. [DOI] [PubMed] [Google Scholar]
- 152*.Krishnan E, Pandya BJ, Chung L, et al. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15-year follow-up study. American journal of epidemiology. 2012;176:108–16. doi: 10.1093/aje/kws002. [DOI] [PubMed] [Google Scholar]
- 153.Carnethon MR, Fortmann SP, Palaniappan L, et al. Risk factors for progression to incident hyperinsulinemia: the Atherosclerosis Risk in Communities Study, 1987–1998. American journal of epidemiology. 2003;158:1058–67. doi: 10.1093/aje/kwg260. [DOI] [PubMed] [Google Scholar]
- 154.Bhole V, Choi JW, Kim SW, et al. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. The American journal of medicine. 2010;123:957–61. doi: 10.1016/j.amjmed.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kramer CK, von Muhlen D, Jassal SK, et al. Serum uric acid levels improve prediction of incident type 2 diabetes in individuals with impaired fasting glucose: the Rancho Bernardo Study. Diabetes care. 2009;32:1272–3. doi: 10.2337/dc09-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Niskanen L, Laaksonen DE, Lindstrom J, et al. Serum uric acid as a harbinger of metabolic outcome in subjects with impaired glucose tolerance: the Finnish Diabetes Prevention Study. Diabetes care. 2006;29:709–11. doi: 10.2337/diacare.29.03.06.dc05-1465. [DOI] [PubMed] [Google Scholar]
- 157.Chien KL, Chen MF, Hsu HC, et al. Plasma uric acid and the risk of type 2 diabetes in a Chinese community. Clinical chemistry. 2008;54:310–6. doi: 10.1373/clinchem.2007.095190. [DOI] [PubMed] [Google Scholar]
- 158.Choi HK, De Vera MA, Krishnan E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology (Oxford, England) 2008;47:1567–70. doi: 10.1093/rheumatology/ken305. [DOI] [PubMed] [Google Scholar]
- 159.Taniguchi Y, Hayashi T, Tsumura K, et al. Serum uric acid and the risk for hypertension and Type 2 diabetes in Japanese men: The Osaka Health Survey. Journal of hypertension. 2001;19:1209–15. doi: 10.1097/00004872-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 160.Choi HK, Ford ES. Haemoglobin A1c, fasting glucose, serum C-peptide and insulin resistance in relation to serum uric acid levels--the Third National Health and Nutrition Examination Survey. Rheumatology (Oxford, England) 2008;47:713–7. doi: 10.1093/rheumatology/ken066. [DOI] [PubMed] [Google Scholar]
- 161.Rodriguez G, Soriano LC, Choi HK. Impact of diabetes against the future risk of developing gout. Annals of the rheumatic diseases. 2010;69:2090–4. doi: 10.1136/ard.2010.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bandaru P, Shankar A. Association between Serum Uric Acid Levels and Diabetes Mellitus. International journal of endocrinology. 2011;2011:604715. doi: 10.1155/2011/604715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ioachimescu AG, Brennan DM, Hoar BM, et al. Serum uric acid, mortality and glucose control in patients with Type 2 diabetes mellitus: a PreCIS database study. Diabetic medicine: a journal of the British Diabetic Association. 2007;24:1369–74. doi: 10.1111/j.1464-5491.2007.02302.x. [DOI] [PubMed] [Google Scholar]
- 164.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. The American journal of medicine. 2007;120:442–7. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 165.Li Q, Yang Z, Lu B, et al. Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovascular diabetology. 2011;10:72. doi: 10.1186/1475-2840-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu PW, Chang TY, Chen JD. Serum uric acid and metabolic syndrome in Taiwanese adults. Metabolism: clinical and experimental. 2010;59:802–7. doi: 10.1016/j.metabol.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 167.Ford ES, Li C, Cook S, et al. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–32. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 168.Hjortnaes J, Algra A, Olijhoek J, et al. Serum uric acid levels and risk for vascular diseases in patients with metabolic syndrome. The Journal of rheumatology. 2007;34:1882–7. [PubMed] [Google Scholar]
- 169.Lee JM, Kim HC, Cho HM, et al. Association between serum uric acid level and metabolic syndrome. Journal of preventive medicine and public health = Yebang Uihakhoe chi. 2012;45:181–7. doi: 10.3961/jpmph.2012.45.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chiou WK, Huang DH, Wang MH, et al. Significance and association of serum uric acid (UA) levels with components of metabolic syndrome (MS) in the elderly. Archives of gerontology and geriatrics. 2012;55:724–8. doi: 10.1016/j.archger.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 171.Ioachimescu AG, Brennan DM, Hoar BM, et al. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis and rheumatism. 2008;58:623–30. doi: 10.1002/art.23121. [DOI] [PubMed] [Google Scholar]
- 172.Puig JG, Martinez MA, Mora M, et al. Serum urate, metabolic syndrome, and cardiovascular risk factors. A population-based study. Nucleosides, nucleotides & nucleic acids. 2008;27:620–3. doi: 10.1080/15257770802138582. [DOI] [PubMed] [Google Scholar]
- 173.Juraschek SP, Miller ER, 3rd, Gelber AC. Body mass index, obesity, and prevalent gout in the United States in 1988–1994 and 2007–2010. Arthritis care & research. 2013;65:127–32. doi: 10.1002/acr.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Simao AN, Lozovoy MA, Simao TN, et al. Adiponectinemia is associated with uricemia but not with proinflammatory status in women with metabolic syndrome. Journal of nutrition and metabolism. 2012;2012:418094. doi: 10.1155/2012/418094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen JH, Pan WH, Hsu CC, et al. Impact of obesity and hypertriglyceridemia on gout development with or without hyperuricemia: a prospective study. Arthritis care & research. 2013;65:133–40. doi: 10.1002/acr.21824. [DOI] [PubMed] [Google Scholar]
- 176.Cipriani S, Chen X, Schwarzschild MA. Urate: a novel biomarker of Parkinson’s disease risk, diagnosis and prognosis. Biomarkers in medicine. 2010;4:701–12. doi: 10.2217/bmm.10.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: a danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: evolutionary considerations. Seminars in nephrology. 2011;31:394–9. doi: 10.1016/j.semnephrol.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Orowan E. The origin of man. Nature. 1955;175:683–4. doi: 10.1038/175683a0. [DOI] [PubMed] [Google Scholar]
- 179.Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Whiteman M, Ketsawatsakul U, Halliwell B. A reassessment of the peroxynitrite scavenging activity of uric acid. Annals of the New York Academy of Sciences. 2002;962:242–59. doi: 10.1111/j.1749-6632.2002.tb04072.x. [DOI] [PubMed] [Google Scholar]
- 181.Regoli F, Winston GW. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite, peroxyl radicals, and hydroxyl radicals. Toxicology and applied pharmacology. 1999;156:96–105. doi: 10.1006/taap.1999.8637. [DOI] [PubMed] [Google Scholar]
- 182.Cohen AM, Aberdroth RE, Hochstein P. Inhibition of free radical-induced DNA damage by uric acid. FEBS letters. 1984;174:147–50. doi: 10.1016/0014-5793(84)81094-7. [DOI] [PubMed] [Google Scholar]
- 183.Duan W, Ladenheim B, Cutler RG, et al. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. Journal of neurochemistry. 2002;80:101–10. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 184.Guerreiro S, Ponceau A, Toulorge D, et al. Protection of midbrain dopaminergic neurons by the end-product of purine metabolism uric acid: potentiation by low-level depolarization. Journal of neurochemistry. 2009;109:1118–28. doi: 10.1111/j.1471-4159.2009.06040.x. [DOI] [PubMed] [Google Scholar]
- 185.Church WH, Ward VL. Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain research bulletin. 1994;33:419–25. doi: 10.1016/0361-9230(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 186.Weisskopf MG, O’Reilly E, Chen H, et al. Plasma urate and risk of Parkinson’s disease. American journal of epidemiology. 2007;166:561–7. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen H, Mosley TH, Alonso A, et al. Plasma urate and Parkinson’s disease in the Atherosclerosis Risk in Communities (ARIC) study. American journal of epidemiology. 2009;169:1064–9. doi: 10.1093/aje/kwp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alonso A, Rodriguez LA, Logroscino G, et al. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007;69:1696–700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- 189.De Vera M, Rahman MM, Rankin J, et al. Gout and the risk of Parkinson’s disease: a cohort study. Arthritis and rheumatism. 2008;59:1549–54. doi: 10.1002/art.24193. [DOI] [PubMed] [Google Scholar]
- 190.Toncev G, Milicic B, Toncev S, et al. Serum uric acid levels in multiple sclerosis patients correlate with activity of disease and blood-brain barrier dysfunction. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2002;9:221–6. doi: 10.1046/j.1468-1331.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 191.Auinger P, Kieburtz K, McDermott MP. The relationship between uric acid levels and Huntington’s disease progression. Movement disorders: official journal of the Movement Disorder Society. 2010;25:224–8. doi: 10.1002/mds.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Spitsin S, Hooper DC, Leist T, et al. Inactivation of peroxynitrite in multiple sclerosis patients after oral administration of inosine may suggest possible approaches to therapy of the disease. Multiple sclerosis (Houndmills, Basingstoke, England) 2001;7:313–9. doi: 10.1177/135245850100700507. [DOI] [PubMed] [Google Scholar]
- 193.Macmullan P, McCarthy G. Treatment and management of pseudogout: insights for the clinician. Therapeutic advances in musculoskeletal disease. 2012;4:121–31. doi: 10.1177/1759720X11432559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Salaffi F, Carotti M, Guglielmi G, et al. The crowned dens syndrome as a cause of neck pain: clinical and computed tomography study in patients with calcium pyrophosphate dihydrate deposition disease. Clinical and experimental rheumatology. 2008;26:1040–6. [PubMed] [Google Scholar]
- 195.Felson DT, Anderson JJ, Naimark A, et al. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. The Journal of rheumatology. 1989;16:1241–5. [PubMed] [Google Scholar]
- 196.Sanmarti R, Panella D, Brancos MA, et al. Prevalence of articular chondrocalcinosis in elderly subjects in a rural area of Catalonia. Annals of the rheumatic diseases. 1993;52:418–22. doi: 10.1136/ard.52.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Neame RL, Carr AJ, Muir K, et al. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Annals of the rheumatic diseases. 2003;62:513–8. doi: 10.1136/ard.62.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ramonda R, Musacchio E, Perissinotto E, et al. Prevalence of chondrocalcinosis in Italian subjects from northeastern Italy. The Pro.V.A (PROgetto Veneto Anziani) study. Clinical and experimental rheumatology. 2009;27:981–4. [PubMed] [Google Scholar]
- 199*.Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Annals of the rheumatic diseases. 2011;70:563–70. doi: 10.1136/ard.2010.139105. [DOI] [PubMed] [Google Scholar]
- 200.Ciancio G, Bortoluzzi A, Govoni M. Epidemiology of gout and chondrocalcinosis. Reumatismo. 2011;63:207–20. doi: 10.4081/reumatismo.2011.207. [DOI] [PubMed] [Google Scholar]
- 201.Nguyen C, Lieberherr M, Bordat C, et al. Intracellular calcium oscillations in articular chondrocytes induced by basic calcium phosphate crystals lead to cartilage degradation. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2012;20:1399–408. doi: 10.1016/j.joca.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 202.Rosenthal AK. Crystals, inflammation, and osteoarthritis. Current opinion in rheumatology. 2011;23:170–3. doi: 10.1097/BOR.0b013e3283432d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Molloy ES, McCarthy GM. Basic calcium phosphate crystals: pathways to joint degeneration. Current opinion in rheumatology. 2006;18:187–92. doi: 10.1097/01.bor.0000209433.43978.a8. [DOI] [PubMed] [Google Scholar]
- 204.Netter P, Bardin T, Bianchi A, et al. The ANKH gene and familial calcium pyrophosphate dihydrate deposition disease. Joint, bone, spine: revue du rhumatisme. 2004;71:365–8. doi: 10.1016/j.jbspin.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 205.Sun Y, Mauerhan DR, Honeycutt PR, et al. Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis research & therapy. 2010;12:R56. doi: 10.1186/ar2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Neogi T, Nevitt M, Niu J, et al. Lack of association between chondrocalcinosis and increased risk of cartilage loss in knees with osteoarthritis: results of two prospective longitudinal magnetic resonance imaging studies. Arthritis and rheumatism. 2006;54:1822–8. doi: 10.1002/art.21903. [DOI] [PubMed] [Google Scholar]
- 207.Pawlotsky Y, Le Dantec P, Moirand R, et al. Elevated parathyroid hormone 44–68 and osteoarticular changes in patients with genetic hemochromatosis. Arthritis and rheumatism. 1999;42:799–806. doi: 10.1002/1529-0131(199904)42:4<799::AID-ANR25>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 208.Dymock IW, Hamilton EB, Laws JW, et al. Arthropathy of haemochromatosis. Clinical and radiological analysis of 63 patients with iron overload. Annals of the rheumatic diseases. 1970;29:469–76. doi: 10.1136/ard.29.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Timms AE, Sathananthan R, Bradbury L, et al. Genetic testing for haemochromatosis in patients with chondrocalcinosis. Annals of the rheumatic diseases. 2002;61:745–7. doi: 10.1136/ard.61.8.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yashiro T, Okamoto T, Tanaka R, et al. Prevalence of chondrocalcinosis in patients with primary hyperparathyroidism in Japan. Endocrinologia japonica. 1991;38:457–64. doi: 10.1507/endocrj1954.38.457. [DOI] [PubMed] [Google Scholar]
- 211.Rubin MR, Silverberg SJ. Rheumatic manifestations of primary hyperparathyroidism and parathyroid hormone therapy. Current rheumatology reports. 2002;4:179–85. doi: 10.1007/s11926-002-0014-0. [DOI] [PubMed] [Google Scholar]
- 212.Richette P, Ayoub G, Lahalle S, et al. Hypomagnesemia associated with chondrocalcinosis: a cross-sectional study. Arthritis and rheumatism. 2007;57:1496–501. doi: 10.1002/art.23106. [DOI] [PubMed] [Google Scholar]
- 213.Cobeta-Garcia JC, Gascon A, Iglesias E, et al. Chondrocalcinosis and Gitelman’s syndrome. A new association? Annals of the rheumatic diseases. 1998;57:748–9. doi: 10.1136/ard.57.12.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Punzi L, Calo L, Schiavon F, et al. Chondrocalcinosis is a feature of Gitelman’s variant of Bartter’s syndrome. A new look at the hypomagnesemia associated with calcium pyrophosphate dihydrate crystal deposition disease. Revue du rhumatisme (English ed) 1998;65:571–4. [PubMed] [Google Scholar]
- 215.Favero M, Calo LA, Schiavon F, et al. Miscellaneous non-inflammatory musculoskeletal conditions. Bartter’s and Gitelman’s diseases. Best practice & research Clinical rheumatology. 2011;25:637–48. doi: 10.1016/j.berh.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 216.Jones AC, Chuck AJ, Arie EA, et al. Diseases associated with calcium pyrophosphate deposition disease. Seminars in arthritis and rheumatism. 1992;22:188–202. doi: 10.1016/0049-0172(92)90019-a. [DOI] [PubMed] [Google Scholar]
- 217.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. The New England journal of medicine. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]