Abstract
Background
The social and medical environments which surround people are each independently associated with their cancer course. The extent to which these characteristics may together mediate patients’ cancer care and outcomes is not known.
Methods
Using multilevel methods and data, we studied elderly breast and colorectal cancer patients (level I) within urban social (level II- ZIP code tabulation area) and health care (level III – hospital service area) contexts. We sought to determine (1) which, if any, observable social and medical contextual attributes were associated with patient cancer outcomes after controlling for observable patient attributes, and (2) the magnitude of residual variation in patient cancer outcomes at each level.
Results
Numerous patient attributes and social area attributes including poverty were associated with unfavorable patient cancer outcomes across the full clinical cancer continuum for both cancers. Health care area attributes were not associated with patient cancer outcomes. After controlling for observable covariates at all three levels, there was substantial residual variation in patient cancer outcomes at all levels.
Conclusions
After controlling for patient attributes known to confer risk of poor cancer outcomes, we find that neighborhood socioeconomic disadvantage exerts an independent and deleterious effect on residents' cancer outcomes but the area supply of the specific types of health care studied do not. Multilevel interventions targeted at cancer patients and their social areas may be useful. We also show substantial residual variation in patient outcomes across social and health care areas, a finding potentially relevant to traditional small area variation research methods.
Keywords: cancer, elderly, neighborhoods and health, small area variation
Introduction
Geographic variation in health and health care in the US has been established by nearly forty years of research from varied scientific disciplines, [1-7] but its mediators are not well understood. Social epidemiologists have identified neighborhoods factors like “material disadvantage” and “social disorder” as affecting the health and health care use of individuals living in these social geographies.[3, 4] Health services researchers have published similarly compelling research on influence of health care market characteristics, like the number of cardiac catheterization facilities, on health care use. [1]
Because these research traditions have advanced in parallel, it is not known whether neighborhood factors and health care market factors are simultaneously associated with patients’ health and health care use. Here we integrate these research traditions to acknowledge that attributes of individuals, their social geographies, and their medical geographies may each contribute simultaneously to residents’ health and health care use in urban areas. We see this question as important in two ways, (1) examining different aspects of the cancer continuum in two highly curable cancers to determine if there are certain parts of the continuum that are particularly sensitive to individual attributes, neighborhood attributes and/or area health care supply; and (2) integrating two thus far parallel research traditions in the domain of “place and health”, we minimize confounding between attributes of individuals’ and their cancer-related health and thus seek to better understand potential mediators of racial and economic-based disparities in patients cancer outcomes in a novel manner.
To do this, we apply multi-level modeling techniques to data from elderly Medicare patients with breast and colorectal cancer (CRC) living in urban areas to identify attributes of residents, their social geography, and their medical geography associated with unfavorable cancer outcomes (i.e., explainable variation) and determine the extent to which unmeasured area social and health care characteristics may affect individuals’ cancer outcomes (i.e., residual variation). Based on results of recent research showing no association between unfavorable social characteristics and health care supply in urban areas [8], we hypothesize that after adjusting for patient attributes and health care area attributes, attributes of patients’ social areas will be strongly and significantly associated with their outcomes across the full clinical course of breast and CRC, but not their local supply of health care.
Methods
Data Sources and Cohort Development
As depicted in Figure 1, we assembled a three-level data set clustering elderly Medicare patients with breast cancer and CRC within their neighborhoods defined by urban ZIP code tabulation area (ZCTA) of residence and then nesting those within spatially larger Dartmouth-defined hospital service areas (HSAs). [8, 9] We used SEER (Surveillance, Epidemiology, and Endpoint Results)-Medicare data to select those patients who were diagnosed at or after age 66 with breast or CRC and to define patients’ demographic and premorbid health attributes and their cancer outcomes of interest; US Census 2000 Summary File 3 (SF3) to define the neighborhood attributes; and American Hospital Association (AHA), American Medical Association (AMA), and Federal Drug Administration (FDA) data to define their HSA-level health care supply that is relevant to provision of guideline-recommended care across the cancer continuum for breast and CRC cancer.
Figure 1.
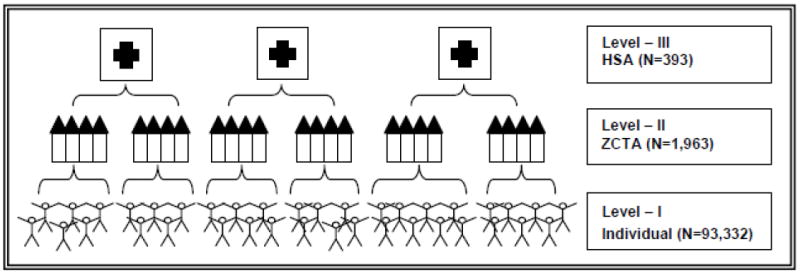
SEER-Medicare is an NCI-sponsored patient-level linkage of cancer registry data from SEER to health care claims from Medicare. [10] Tumor registries document the occurrence and fatality of new cases of cancer within specified areas. The SEER consortium collects information from geographically disparate tumor registries representing roughly a quarter of the American population with cancer. [11, 12] Patients in SEER are demographically representative of the general population. [13] SEER collects detailed information about initial presentation and treatment, including date of diagnosis; reporting source; tumor site, histology, and stage; treatment modalities; date of death; and socio-demographic information including marital status. SEER conducts annual audits to ensure data quality and completeness, holding the standard of ascertainment at 98%. [14]
Medicare is a federally sponsored health insurance program administered by the Centers for Medicare and Medicaid Services (CMS), whose beneficiaries include more than 96% of all US citizens aged 65 and older. [15] CMS maintains records of outpatient, inpatient, and other claims for all beneficiaries not enrolled in Medicare Advantage contracts. CMS provides the claims for SEER individuals to the NCI for linkage. We use the Medicare “Denominator File” to ascertain the age, sex, race, marital status, and poverty status of each patient. We created a proxy measure of poverty status that was equal to one for Medicare beneficiaries who received state health insurance supplements in the year prior to diagnosis. [16-23] Hospital and ambulatory medical care is catalogued in the MEDPAR and OUTPT and NCH files respectively. These files contain patient diagnostic and procedure codes and are associated with dates of service. We use the data to estimate patients’ medical comorbidity (i.e., non-cancer co-occurring illnesses) prior to cancer diagnosis using conventional metrics [24] and to develop the dependent variables describing patients’ clinical cancer course.
Because the SEER-Medicare data included patients diagnosed between 1993 and 2007, we chose to study data from the year 2000 for the data pertaining to social and health care areas which is the midpoint between 1993 and 2007. From the US Census 2000 Summary File 3 (SF3), we chose explanatory variables in which we had substantive interest (e.g., % residents who meet the federal definition of impoverished, % residents who are black, % residents who are college-educated). We also included in models Census-based composite variables representing area “socioeconomic disadvantage” and “ethnic isolation,” which we developed previously through factor analysis of Census 2000 data using the method of Sampson. [8, 25]
We characterize health care supply at the level of the HSA using data on local hospitals including hospital accreditation status, hospital type, capacity, and other aspects of services provided by hospitals in 2000 through the American Hospital Association (AHA) survey. [26] We obtained counts of local physician specialty work force in 2000 from American Medical Association (AMA) data. Finally, we obtained counts of area Federal Drug Administration (FDA) approved mammogram facilities in 2000 from the FDA. All of these data sources are indexed at the ZIP-code level and were aggregated up to the HSA level using a ZIP code to HSA cross-walk file provided by the Dartmouth group. [27] We then re-scaled the health care supply variables into counts per 100,000 residents in the HSA using ZIP population estimates. For counts of FDA-approved mammogram facilities, we restricted the population denominator to women aged 40 or older in the HSA. We chose to study health care at the HSA level, rather than the HRR, as the HSA represents the more immediate health care market.
Predictor Variables
Table 1 describes the predictor and outcome variables, the component data sources, and the geographical levels at which attributes were measured. Patient-level predictor variables included demographic variables, marital status, receipt of supplemental insurance (a proxy for individual poverty), and disease variables including medical comorbidity, tumor site, and stage. Social area variables were percentage of residents within the ZCTA who were impoverished, black, and college educated. Additionally, we included two composite variables to measure material disadvantage and ethnic isolation. [8] At the hospital service area, we measured supply per capita of: mammography facilities with FDA data, Joint Commission on the Accreditation of Healthcare Organizations (JCAHO) hospitals, hospital beds with AHA data, number of cancer screening physicians, gastroenterology physicians, medical oncologists, radiation oncologists and surgeons with AMA data.
Table 1.
Linked Analytic Dataset (N=93,332)
| Analytic Level | Identifier | Data Source | Data Files | Variables |
|---|---|---|---|---|
| I (Patient) | Unique Patient Identifier | NCI | SEER | Tumor site and extent |
| Tumor directed treatment | ||||
| SEER-Medicare | Year of diagnosis | |||
| Marital status | ||||
| Census tract of residence | ||||
| ZIP code of residence | ||||
|
| ||||
| I (Patients) | Unique Patient Identifier | CMS | Medicare Claims | Medicare entitlement type and duration |
| SEER-Medicare | HMO membership | |||
| Age | ||||
| Sex | ||||
| Race | ||||
| Poverty indicator | ||||
| Comorbidity | ||||
| Tumor directed treatment | ||||
| Post-tx tumor surveillance | ||||
| Survival at five years post-dx | ||||
|
| ||||
| II (Neighborhood) | Unique ZCTA | US Census | Census 2000 | % College Educated |
| Summary File 3 | % Black | |||
| Socioeconomic Disadvantage Score [1] | ||||
| Ethnic Isolation Score [2] | ||||
|
| ||||
| III (Medical Market) | Unique HSA | Various | ||
| AMA | # medical oncologists/capita | |||
| # surgeons/capita | ||||
| # radiation oncologists/capita | ||||
| # cancer screening physicians/capita | ||||
| # gastroenterologists/capita | ||||
| FDA | # mammography facilities/capita | |||
| AHA | # acute care beds/capita | |||
| # JCAHO accredited hospitals/capita | ||||
Legend: NCI=National Cancer Institute; SEER=Surveillance Epidemiology and End Results; CMS Centers for Medicare and Medicaid Services; ICD =International Diagnostic Code; HCPC=Health Care Financing Administration Common Procedure Codes; CPT=Common Procedure Terminally; HMO= health maintenance organization; ZCTA= ZIP Code Tabulation Area; AHA= American Hospital Association; AMA=American Medical Association; FDA = Food and Drug Administration; HAS = hospital service area; JCAHO=Joint Committee on Accreditation of Health Care Organizations; Tx= treatment; Dx= diagnosis
Outcome Variables
We were interested in outcomes across the full clinical cancer course which we operationalized with five types of variables which all came from the SEER-Medicare data (1) stage at diagnosis, followed by (2) local cancer control (i.e., surgery +/-radiation therapy), followed by (3) post-operative adjuvant chemotherapy (4) post-treatment tumor surveillance, and ending with (5) death.
Analytic Sample
In aggregate, there were 356,435 distinct elderly Medicare patients with breast, colon, or rectal cancer who were diagnosed alive at or after age 66 years in 16 SEER areas from 1993-2007. After excluding the 8.4% of patients who had a history of prior cancers, the 30.5% who were enrolled in an HMO in the year prior to diagnosis through death or fixed right censoring at last claim, the 6.0% who did not have continuous enrollment in Medicare parts A and B in the year prior to diagnosis through death or fixed right censoring at last claim, the 0.6% with claims for chemotherapy in the year prior to diagnosis, the 0.2% with missing values for race, and the 1.3% who were missing dates of death, we had a sample of 227,804(breast=106,739, colon=92,357, and rectal=28,708). Of note, none of these patients had codes indicating treatment on clinical trials. These patients resided at the time of diagnosis in 9,523 distinct ZIP codes and 1,750 distinct HSAs. We were able to assign 9,251 (97.1%) of ZIP codes to 1,750 unique HSAs. We had 2000 AMA data for 99.4% (1740/1750) of HSAs, 2000 AHA data for 97.3% (1703/1750) of HSAs, and 2000 FDA mammography data for 95.4% (1670/1750) of HSAs. We then restricted our sample to patients living in wholly urban areas during the month of their cancer diagnosis using the US Department of Agriculture Rural Urban Commuting Areas (RUCA) algorithm. [9, 28, 29, 30] This resulted in a final analytic sample of 93,332 distinct elderly Medicare cancer patients (breast=43,830, colon=37,807, and rectal = 11,695) nested within 1963 distinct ZCTAs, which were nested in 393 distinct urban HSAs.
Determination of Urban Geography
We restricted our study to patients residing in urban areas at the time of their cancer diagnosis for two reasons. First, prior work suggests that area “social disadvantage” in rural settings may manifest differently than in urban settings [3-33] Second, rural areas of the US have fewer health services/capita including those health services related to cancer. [34-42] Consequently, conflating the urban and rural areas might yield results that are not generalizable to either setting. We identified strictly urban ZIP codes as defined by the Department of Agriculture through the Rural Urban Commuting Areas (RUCA) algorithm [9, 28, 29, 30] (N=16,528) and selected the subset of ZIP codes that formed fully urban HSAs (5102/16528). The excluded areas represented ZIP codes and corresponding HSAs on the margin of urban areas. Such HSAs may represent an indeterminate combination of urban, suburban, and rural areas. Finally, we used a cross-walk file developed by Dartmouth investigators to assign each unique ZIP code (N=5102) to its corresponding ZCTA (N=1963). [1] Our parent data structure ultimately consisted of 93,332 distinct elderly Medicare cancer patients (breast=43,830, colon=37,807, and rectal = 11,695) nested within 1963 distinct ZCTAs, which were nested in 393 distinct HSAs.
Statistical Analyses
Consistent with the nested structure of our data, patient, social variables, and health care supply variables were predictors in the models and patient-level process and outcome variables were modeled as outcomes. Our three-level random-effects model is specified by the following equation
Where i,j,k index the patient, neighborhood (ZCTA), and HSA levels, respectively. We used a logistic model for a binary outcome yijk (e.g., whether breast cancer patient has received adjuvant chemotherapy). We include patient-level covariate Xijk, ZCTA-level covariate Zjk, and HSA-level covariate Wk, with fixed-effects coefficients β1, β2, β3. We also include random effects to account for unexplained heterogeneity at both ZCTA-level ujk and HSA-level vk, assuming that. . The estimated the random-effects variances quantify the geographical variations separately exhibited at neighborhood (ZCTA) and health care (HSA) level. All coefficients reported in the text were significant below the 0.05 level (2-sided).
The research was approved by the Harvard Medical School Committee on Human Subjects. All analyses were performed using SAS version 9.2 statistical software.
Results
Explained Variation in Patient Outcomes
Table 2A describes the attributes of the 93,332 elderly Medicare patients we studied according to tumor site and Table 2B describes the attributes of their social and health care areas. Appendices A-E contain adjusted associations between patients’ social and health care area attributes (predictors) and patients’ outcomes by tumor site. Fairly consistently for each tumor type and across the cancer control continuum, the patient (level I) attributes associated with unfavorable outcomes were advanced age, male sex, black race (compared to white race), poverty (i.e., receipt of supplemental health insurance from the state in the year prior to diagnosis), and a lack of Medicare financed medical care in the year prior to diagnosis. For all three tumor sites, married patients had more favorable outcomes than unmarried patients across the full cancer control continuum. For example, compared to married women with breast cancer, widowed women with breast cancer were less likely to be diagnosed with early stage disease (OR 0.85), less likely to receive guideline-recommended local tumor control (OR 0.83), less likely to receive adjuvant chemotherapy in the setting of regionally advanced disease (OR 0.85), and less likely to undergo surveillance mammography following curative surgery (OR 0.88). Not surprisingly, compared to married women, widowed women were less likely to be alive at five years following diagnosis (OR 0.84). Similar patterns were apparent among widows and widowers with CRC. Patients’ comorbid disease burden was associated with lower stages at diagnoses for all three tumor sites, but also with less treatment following diagnosis of curable disease (i.e., local control and adjuvant chemotherapy), less surveillance after curative surgery and lower overall survival rates at five years.
Table 2.
A. Attributes of the Cohort, their Neighborhoods, and Surrounding Health Care Supply (N=93,332)
| Breast | Colon | Rectal | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | Proportion | N | Proportion | N | Proportion | N | |||
| Individual-level | 43,830 | 37,807 | 11,695 | ||||||
| Demographic Variables | |||||||||
| Age in decades (mean, range) | 7.66 | (6.60-10.80) | 7.86 | (6.60-10.80) | 7.74 | (6.60-10.20) | |||
| Sex | |||||||||
| Female | 1.00 | 0.58 | 0.49 | ||||||
| Male | 0.00 | 0.42 | 0.51 | ||||||
| Race | |||||||||
| White | 0.85 | 0.80 | 0.82 | ||||||
| Black | 0.10 | 0.13 | 0.10 | ||||||
| Other Race | 0.05 | 0.07 | 0.08 | ||||||
| Marital Status | |||||||||
| Married | 0.39 | 0.45 | 0.47 | ||||||
| Widowed | 0.41 | 0.36 | 0.32 | ||||||
| Single | 0.09 | 0.10 | 0.10 | ||||||
| Separated | 0.01 | 0.01 | 0.01 | ||||||
| Divorced | 0.06 | 0.05 | 0.06 | ||||||
| Unknown | 0.04 | 0.03 | 0.04 | ||||||
| Poverty Status (0/1) | 0.12 | 0.15 | 0.14 | ||||||
| Disease Variables | |||||||||
| Charlson Comorbidity Score | |||||||||
| 0 | 0.41 | 0.32 | 0.36 | ||||||
| 1 | 0.26 | 0.25 | 0.25 | ||||||
| 2 | 0.14 | 0.16 | 0.14 | ||||||
| ≥3 | 0.16 | 0.23 | 0.19 | ||||||
| No look-back claims | 0.03 | 0.04 | 0.06 | ||||||
| Cancer Stage at Diagnosis | |||||||||
| Local | 0.64 | 0.38 | 0.42 | ||||||
| Regional | 0.26 | 0.37 | 0.34 | ||||||
| Metastasis | 0.07 | 0.20 | 0.16 | ||||||
| Unstaged | 0.03 | 0.05 | 0.08 | ||||||
| Treatment Variables | |||||||||
| Local Control# | 39,229 | 28,173 | 8,879 | ||||||
| Yes | 0.78 | 0.83 | 0.68 | ||||||
| No | 0.22 | 0.17 | 0.32 | ||||||
| Adjuvant ChemotherapyΔ | 4,326 | 5,316 | 1,887 | ||||||
| Yes | 0.10 | 0.14 | 0.16 | ||||||
| No | 0.90 | 0.86 | 0.84 | ||||||
| Any Chemotherapy | 43,832 | 37,807 | 11,695 | ||||||
| Yes | 0.17 | 0.24 | 0.31 | ||||||
| No | 0.83 | 0.76 | 0.69 | ||||||
| Post-Op Mammogram/Colonoscopy * | 29,482 | 18,333 | 5,085 | ||||||
| Yes | 0.67 | 0.48 | 0.43 | ||||||
| No | 0.33 | 0.52 | 0.57 | ||||||
| Mortality Variables | |||||||||
| Alive 5 years after diagnosis | 43,830 | 37,807 | 11,695 | ||||||
| Yes | 0.50 | 0.31 | 0.31 | ||||||
| No | 0.50 | 0.69 | 0.69 | ||||||
| B. Attributes of the Neighborhoods (N=1,963) and Surrounding Health Care Areas (N=393) | |||||||||
|
| |||||||||
| Breast | Colon | Rectal | |||||||
|
| |||||||||
| Variable | Proportion | N | Proportion | N | Proportion | N | |||
|
| |||||||||
| ZCTA-level | 1,561 | 1,539 | 1,285 | ||||||
| Black (%/ZCTA) (mean, range) | 13.27 | (0.00-98.12) | 14.93 | (0.00-98.12) | 13.87 | (0.00-98.12) | |||
| Socioeconomic disadvantage Score (mean) | -0.01 | (-1.18-4.69) | 0.10 | (-1.15-4.69) | 0.10 | (-1.05-4.69) | |||
| Ethnic Isolation Score (mean) | 0.12 | (-0.86-6.08) | 0.17 | (-0.86-6.08) | 0.20 | (-0.86-6.08) | |||
| College Educated (%/ZCTA) (mean) | 0.32 | (0.02-0.94) | 0.30 | (0.02-0.94) | 0.29 | (0.02-0.94) | |||
| HSA-level (per 100K individuals in HSA) | 323 | 333 | 260 | ||||||
| Hospital Beds (mean) | 148.11 | (0.00-4040.39) | 99.26 | (0.00-4040.39) | 149.11 | (0.00-4040.39) | |||
| JCAHO Accredited Hospitals (mean) | 0.54 | (0.00-6.32) | 0.53 | (0.00-6.32) | 0.54 | (0.00-6.32) | |||
| Screening MDs (mean) | 73.96 | (14.30-424.68) | 73.29 | (14.30-424.68) | 72.08 | (14.30-424.68) | |||
| Mammogram Facilities (mean) | 14.50 | (0.00-81.40) | - | - | |||||
| Gastroenterologists (mean) | - | - | 4.57 | (0.00-33.09) | 4.46 | (0.00-33.09) | |||
| Surgeons (mean) | 10.12 | (0.00-74.46) | 10.13 | (0.00-74.46) | 9.91 | (0.00-74.46) | |||
| Medical Oncologists (mean) | 10.32 | (0.00-95.84) | 10.24 | (0.00-95.84) | 37,808 | 9.94 | (0.00-95.84) | 11,695 | |
| Radiation Oncologists (mean) | 1.53 | (0.00-16.26) | 1.51 | (0.00-16.26) | 37,808 | 1.45 | (0.00-16.26) | 11,695 | |
| US Census Region | 4 | 4 | 4 | ||||||
| Northeast | 0.30 | 0.33 | 0.34 | ||||||
| South | 0.18 | 0.19 | 0.20 | ||||||
| West | 0.09 | 0.08 | 0.07 | ||||||
| Midwest | 0.42 | 0.40 | 0.39 | ||||||
Sub-cohort of patients diagnosed with local or regional stage disease
Sub-cohort of regional stage patients s/p resection and alive at 6 month following diagnosis
Sub-cohort of regional stage patients s/p resection and alive at 18 months following surgery
Legend: Univariate description of variables within the three-level data source
Table 3 describes associations of particular interest from Appendices C-G, those between individual patient (level I) attributes (i.e., race, poverty, and no Medicare financed health care in the year prior to diagnosis) and patient outcomes, controlling for social and health care contextual variables. Compared to white patients, black patients with breast cancer or colon cancer were less likely to be diagnosed with early stage disease (ORs 0.80 and 0.90 respectively), black patients with breast cancer were less likely to receive guideline-recommended local tumor control (OR 0.81), and black patients with breast cancer or colon cancer were less likely to receive post-operative surveillance (ORs 0.76 and 0.72 respectively) The patient-level poverty indicator was strongly negatively associated with nearly all outcomes for all three tumors. Compared to more affluent patients, impoverished breast cancer and colon cancer patients were less likely to be diagnosed with early stage disease (ORs 0.78 and 0.89 respectively); less likely to undergo local tumor control (ORs 0.78 and 0.89 respectively); less likely to undergo adjuvant chemotherapy (ORs 0.69 and 0.69 respectively); and less likely to survive five years beyond the date of their cancer diagnoses (ORs 0.69 and 0.78 respectively). Compared to breast cancer and CRC patients with Medicare claims preceding their cancer diagnoses, those breast cancer and CRC patients with no evidence of Medicare paid services in the year prior to diagnosis almost uniformly had substantially poorer outcomes across the cancer continuum for each tumor.
Table 3.
Multi-Level Adjusted Associations Between Select Patient Attributes and All Cancer Outcomes (N=93,332)
| Patient-Level Attributes | ||||||
|---|---|---|---|---|---|---|
| Breast Outcomes | Black vs. White | 95% CI | Poverty | 95% CI | No Prior Claims | 95% CI |
| Stage local vs. regional/metastatic | 0.80* | 0.75-0.87 | 0.78* | 0.78-0.89 | 0.59* | 0.56-0.62 |
| Stage regional vs. metastatic | 0.98 | 0.85-1.12 | 0.78* | 0.69-0.89 | 0.44* | 0.40-0.48 |
| Local Control | 0.81* | 0.71-0.92 | 0.78* | 0.69-0.78 | 0.52* | 0.43-0.63 |
| Adjuvant Chemotherapy | 1.09 | 0.88-1.35 | 0.69* | 0.54-0.78 | 0.88 | 0.69-1.13 |
| Post-Operative Surveillance | 0.76* | 0.66-0.88 | 0.61* | 0.54-0.69 | 0.28* | 0.23-0.34 |
| Five Year Survival | 0.92 | 0.82-1.04 | 0.69* | 0.69-0.78 | 0.48* | 0.41-0.56 |
| Patient-Level Attributes | ||||||
| Colon Outcomes | Black vs. White | 95% CI | Poverty | 95% CI | No Prior Claims | 95% CI |
| Stage local vs. regional/metastatic | 0.90* | 0.83-0.97 | 0.89* | 0.89-1.00 | 0.78 | 0.74-0.83 |
| Stage regional vs. metastatic | 0.76* | 0.69-0.83 | 0.89* | 0.89-1.00 | 0.71* | 0.66-0.76 |
| Local Control | 0.87 | 0.71-1.06 | 0.89* | 0.69-1.00 | 0.88 | 0.65-1.19 |
| Adjuvant Chemotherapy | 0.82* | 0.68-1.00 | 0.69* | 0.61-0.78 | 0.42* | 0.34-0.53 |
| Post-Operative Surveillance | 0.72* | 0.59-0.87 | 1.13* | 1.00-1.43 | 0.27* | 0.23-0.33 |
| Five Year Survival | 0.92 | 0.82-1.04 | 0.78* | 0.78-0.89 | 0.45* | 0.38-0.52 |
| Patient-Level Attributes | ||||||
| Rectal Outcomes | Black vs. White | 95% CI | Poverty | 95% CI | No Prior Claims | 95% CI |
| Stage local vs. regional/metastatic | 0.99 | 0.85-1.14 | 0.89* | 0.78-1.00 | 0.77* | 0.70-0.85 |
| Stage regional vs. metastatic | 1.11 | 0.91-1.36 | 0.78* | 0.69-1.00 | 0.65* | 0.57-0.74 |
| Local Control | 0.81 | 0.63-1.05 | 0.89 | 0.69-1.00 | 0.62* | 0.47-0.81 |
| Adjuvant Chemotherapy | 0.56* | 0.39-0.81 | 0.78 | 0.61-1.13 | 0.39* | 0.27-0.56 |
| Post-Operative Surveillance | 0.76 | 0.52-1.11 | 1.00 | 0.69-1.27 | 0.35* | 0.26-0.49 |
| Five Year Survival | 0.76* | 0.60-0.97 | 0.89 | 0.78-1.00 | 0.44* | 0.34-0.55 |
Legend: Patient-level ORs from three-level multivariable logistic regressions of SEER-Medicare patients with breast cancer or colorectal cancer. Estimates are adjusted for the following other patient (level I) attributes: age, sex, marital status, comorbidity, and social (Level II) attributes: ZCTA % black and college educated and, ethnic isolation and deprivation scores; and health care (Level III) attributes: hospital beds per 100K people, JCAHO-accredited hospitals per 100K people, screening MDs per 100K people, CRC screening per 100K people, medical oncologists per 100K people, surgeons per 100k people, and radiation oncologists per 100k people; and Census Region. Asterisk (*) indicates statistical significance to p<0.05.
Table 4 describes associations of particular interest (from Appendices C-G) between select social area (level II) attributes and patient outcomes controlled for patient attributes and area health care supply. For all three tumor sites, the percent of residents in the area who were college educated was generally associated with favorable outcomes across the clinical course of cancer. For example, with each 10% increase in college educated residents within a ZCTA, breast cancer, colon cancer, and rectal cancer patients were more likely to be diagnosed with local vs. regional or metastatic stage cancer (ORs 1.02, 1.02 and 1.03 respectively) and more likely to survive five years following diagnosis (ORs 1.06, 1.06, and 1.09 respectively). Controlling for the race of the individual, the percent of black residents in the area was not associated, either favorably or unfavorably, with any of the five outcomes. Associations between area ethnic isolation and outcomes were inconsistent.
Table 4.
Multi-Level Adjusted Associations Between Select Social Area Attributes and All Cancer Outcomes (N=93,332)
| ZCTA-Level Attributes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Breast Outcomes | % Black | 95% CI | Disadvantage | 95% CI | Ethnic Isolation | 95% CI | % College-Educated | 95% CI |
| Stage local vs. regional/metastatic | 1.00 | 1.00-1.00 | 0.97 | 0.93-1.01 | 0.95* | 0.93-0.98 | 1.02* | 1.00-1.04 |
| Stage regional vs. metastatic | 1.00 | 1.00-1.00 | 0.99 | 0.92-1.07 | 0.99 | 0.94-1.04 | 1.05* | 1.02-1.08 |
| Local Control | 1.00 | 1.00-1.00 | 0.88* | 0.81-0.94 | 1.01 | 0.96-1.07 | 0.93* | 0.90-0.95 |
| Adjuvant Chemotherapy | 1.00 | 1.00-1.01 | 0.83* | 0.73-0.94 | 1.05 | 0.97-1.14 | 0.92* | 0.88-0.96 |
| Post-Operative Surveillance | 1.00 | 1.00-1.01 | 0.96 | 0.87-1.05 | 0.97 | 0.91-1.03 | 1.02 | 0.98-1.05 |
| Five Year Survival | 1.00 | 1.00-1.00 | 0.96 | 0.90-1.02 | 1.03 | 0.99-1.08 | 1.06 | 1.03-1.08 |
| ZCTA-Level Attributes | ||||||||
| Colon Outcomes | % Black | 95% CI | Disadvantage | 95% CI | Ethnic Isolation | 95% CI | % College-Educated | 95% CI |
| Stage local vs. regional/metastatic | 1.00* | 1.00-1.01 | 0.93* | 0.89-0.98 | 1.03* | 1.00-1.07 | 1.02* | 1.00-1.04 |
| Stage regional vs. metastatic | 1.00 | 1.00-1.00 | 0.97 | 0.91-1.02 | 1.00 | 0.96-1.04 | 1.00 | 0.98-1.03 |
| Local Control | 1.00 | 1.00-1.00 | 0.98 | 0.88-1.10 | 0.96 | 0.89-1.04 | 1.03 | 0.98-1.08 |
| Adjuvant Chemotherapy | 1.00 | 1.00-1.00 | 0.90 | 0.80-1.00 | 1.09* | 1.01-1.18 | 0.96 | 0.92-1.01 |
| Post-Operative Surveillance | 1.00 | 0.99-1.00 | 1.00 | 0.89-1.12 | 0.97 | 0.90-1.05 | 0.99 | 0.95-1.04 |
| Five Year Survival | 1.00 | 1.00-1.00 | 0.98 | 0.92-1.05 | 1.04 | 1.00-1.09 | 1.06* | 1.03-1.09 |
| ZCTA-Level Attributes | ||||||||
| Rectal Outcomes | % Black | 95% CI | Disadvantage | 95% CI | Ethnic Isolation | 95% CI | % College-Educated | 95% CI |
| Stage local vs. regional/metastatic | 1.00 | 1.00-1.00 | 0.97 | 0.89-1.05 | 0.97 | 0.92-1.02 | 1.03* | 1.00-1.07 |
| Stage regional vs. metastatic | 1.00 | 1.00-1.01 | 0.86* | 0.77-0.95 | 1.03 | 0.96-1.11 | 1.00 | 0.96-1.05 |
| Local Control | 1.00 | 0.99-1.00 | 0.96 | 0.84-1.10 | 1.17* | 1.07-1.29 | 1.00 | 0.95-1.06 |
| Adjuvant Chemotherapy | 1.00 | 0.99-1.00 | 0.93 | 0.76-1.15 | 0.91 | 0.79-1.04 | 0.94 | 0.87-1.02 |
| Post-Operative Surveillance | 1.00 | 0.99-1.00 | 1.03 | 0.83-1.27 | 1.04 | 0.91-1.20 | 1.09* | 1.01-1.18 |
| Five Year Survival | 1.00 | 0.99-1.00 | 1.04 | 0.91-1.18 | 0.97 | 0.89-1.05 | 1.09 | 1.04-1.15 |
Area health care (level III) supply was not associated with any of the five outcomes for any of the three tumor sites (91 associations evaluated) with only one exception. The number of JCAHO accredited hospitals per capita in an HSA was negatively associated with the likelihood of breast cancer patients in the area living at least five years following diagnosis. While Census regions differed significantly in a variety of outcomes, no consistent pattern of associations emerged.
Unexplained Variation in Patient Outcomes
To further assess the importance of social context to small area variation, we studied residual variation in outcomes at the two model levels. For most outcomes there was substantial residual variance at both levels even after accounting for observed characteristics of the areas (Appendix F). For example the odds of receiving adjuvant chemotherapy for LN+ colon cancer was 1.6 times higher for a patients living in a ZTCA at approximately one standard deviation above the mean compared to those living in an area one standard deviation below the mean. While in most cases the larger component of residual variance was at the HSA level (approximately 66%), ZCTA accounted for approximately 33% of supra-individual residual variance on average across the outcomes (Appendix H). Finally, comparing hierarchical models with and without fixed covariates we find that observed characteristics diminish the magnitude of the unobserved variation at levels II and III and account for approximately a quarter to a third of ZTCA and HSA variation (Appendix G). The fact that residual variation in patient outcomes that are attributable to these geospatial levels remain, suggests that there are important covariates that are associated with patient outcomes at these levels that are not included in our models.
Discussion
Studying urban elderly Medicare patients with breast cancer or CRC, their neighborhoods of residence and their local health care areas, we found that with rare exception patients’ advanced age, black race, poverty, comorbid illness, and lack of prior Medicare utilization largely remain important predictors of their unfavorable outcomes for all three cancers across the full clinical cancer course from stage at diagnosis through death even after accounting for neighborhood disadvantage and health care supply. We also found that after accounting for all of these patient features and area health care supply, individuals who live in poor neighborhoods have a greater risk of experiencing poor cancer outcomes than those living in less materially disadvantaged neighborhoods, independent of their individual attributes like race and poverty status, a finding which supports “neighborhoods and health”. [3, 4] Our work identifies two targets for policy directed interventions to improve breast and CRC outcomes: individuals and neighborhoods.
We did not find an independent deleterious effect of neighborhood racial composition on residents’ cancer outcomes after controlling for individual race. Previously reported associations between neighborhood racial composition and individuals' cancer outcomes may have been confounded by unmeasured individual and contextual factors, chiefly individual and area poverty. [43-46] We also found that net of patient compositional factors and social contextual factors, low supplies of area health care in urban areas does not appear to impact patients’ cancer outcomes.
Our results suggest that patient and social geospatial attributes are significant barriers to cancer care across the full clinical course of breast and CRC for elderly Medicare patients in urban areas. To address these barriers, health policy interventions should address barriers to care for individuals with low incomes, who are minorities, and who are under-insured but should also focus, as articulated above, on areas with concentrations of these characteristics especially poverty and low rates of college education. We did not find that greater levels of health care supply at the HSA level would remove these barriers, although we did find substantial unexplained variation at the HSA level, which may reflect unmeasured health care factors that affect access to care. For example, it may not be enough for a hospital to provide cancer screening without also providing transportation for local residents to come to be screened. Questions about medical facilities provision of “outreach or social services” like transportation are asked routinely in the AHA survey but in our data these variables often have so many missing values that they could not be studied.
There are further limitations to this work. Variables on social and health services were limited. The SEER-Medicare data lack important patient variables including patient income, performance status, patient-physician decision-making, and social support. Receipt of supplemental state financed insurance was proxy, not a direct measure, for individual poverty. Neither mammography facility capacity nor JCAHO hospital capacity were measured. Restriction to the urban setting limits the scope and generalizability of our findings to similar areas and may explain the lack of association between health care supply and patient outcomes. (Prior work by the Dartmouth group included rural areas.) Finally, we interpret these models as descriptive of associations but not causal.
In conclusion, we confirm that previously described specific patient demographic and disease attributes confer risk of poor cancer outcomes across the cancer course among elderly breast and CRC patients from the urban United States (US) even after adjusting for area socioeconomic disadvantage and are health care supply; that neighborhood socioeconomic disadvantage exerts an independent and deleterious effect on residents’ cancer outcomes but racial composition does not; and that the supply of the specific types of health care studied do not appear to be salient determinants of individuals’ cancer outcomes. We also show substantial residual variation in patient clinical outcomes across urban social and health care areas that are not explained by the observed patient, patient, neighborhood, or health care attributes we evaluated. This latter finding suggests that traditional small area variation research methods may be insufficient in omitting such variables.
Supplementary Material
Acknowledgments
Funding: P01 AG031093 and R21 AG030607
References
- 1.The Dartmouth Institute for Health Policy and Clinical Practice. Robert Wood Johnson Foundation: The Dartmouth Atlas of Health Care; http://www.dartmouthatlas.org/tools/downloads.aspx. [Google Scholar]
- 2.Wennberg J, Gittelsohn A. Small area variations in health care delivery. Science. 1973;182(4117):1102–8. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 3.Kawachi I, Berkman LF, editors. Neighborhoods and Health. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 4.Berkman lF, Kawachi I., editors. Social Epidemiology. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 5.Baicker K, Chandra A, Skinner JS, Wennberg JE. Who you are and where you live: how race and geography affect the treatment of medicare beneficiaries. Health Aff (Millwood) 2004:VAR33–44. doi: 10.1377/hlthaff.var.33. Suppl Variation. [DOI] [PubMed] [Google Scholar]
- 6.Fowler FJ, Gallagher PM, Anthony DL, Larsen K, Skinner JS. Relationship between regional per capita Medicare expenditures and patient perceptions of quality of care. JAMA. 2008;299(20):2406–12. doi: 10.1001/jama.299.20.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keyshani S, Falk R, Bishop T, Howell E, Korenstein D. The relationship between geographic variations and overuse of healthcare services: a systematic review. Med Care. 2012;50(3):257–61. doi: 10.1097/MLR.0b013e3182422b0f. [DOI] [PubMed] [Google Scholar]
- 8.Lamont EB, He Y, Subramanian SV, Zaslavsky AM. Do socially deprived areas have lesser supplies of cancer care services? J ClinOncol. 2012;30(26):3250–3257. doi: 10.1200/JCO.2011.40.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Center of the Evaluative Clinicial Sciences, Dartmouth Medical School. The Dartmouth Atlas of Health Care. Chicago, IL: AHA Press; 1999. [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability of the US elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. 8 suppl. [DOI] [PubMed] [Google Scholar]
- 11.Ries LAG, Kosary CL, Hankey BF, et al. SEER cancer statistics review, 1973-1994. Bethesda, MD: National Cancer Institute; 1997. NIH publication 97-2789. [Google Scholar]
- 12.http://seer.cancer.gov/
- 13.Nattinger AB, McAuliffe TL, Schapira MM. Generalizability of the Surveillance, Epidemiology, and End Results registry population: factors relevant to epidemiologic and health care research. Journal Clinical Epidemiology. 1997;50(8):939–945. doi: 10.1016/s0895-4356(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 14.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER program of the National Cancer Institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Hatten J. Medicare's common denominator: the covered population. Health Care Financing Review. 1980:53–64. [PMC free article] [PubMed] [Google Scholar]
- 16.Carpenter L. Evolution of Medicaid coverage of Medicare cost sharing. Health Care Financing Review. 1998;20:11–18. [PMC free article] [PubMed] [Google Scholar]
- 17.Clark WD, Hulbert MM. Research issues: Dually eligible Medicare and Medicaid beneficiaries, challenges and opportunities. Health Care Financing Review. 1998;20:1–10. [PMC free article] [PubMed] [Google Scholar]
- 18.Escarce JJ, Epstein KR, Colby DC, Schwartz JS. Racial differences in the elderly's use of medical procedures and diagnostic tests. American Journal of Public Health. 1993;83:948–954. doi: 10.2105/ajph.83.7.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettner SL. Inpatient psychiatric care of Medicare beneficiaries with state buy-in coverage. Health Care Financing Review. 1998;20:55–69. [PMC free article] [PubMed] [Google Scholar]
- 20.Khandker RK, McCormack LA. Medicare spending by beneficiaries with various type of supplemental insurance. Medical Care Research and Review. 1999;56:137–155. doi: 10.1177/107755879905600202. [DOI] [PubMed] [Google Scholar]
- 21.Liu K, Long SK, Aragon C. Does health status explain higher Medicare costs of Medicaid enrollees? Health Care Financing Review. 1998;20:39–54. [PMC free article] [PubMed] [Google Scholar]
- 22.Parente ST, Evans WN. Effect of low-income elderly insurance copayment subsidies. Health Care Financing Review. 1998;20:19–38. [PMC free article] [PubMed] [Google Scholar]
- 23.Pope Gregory C, Adamache Killard W, Walsh Edith G, Khandker Rezaul K. Evaluating alternative risk adjusters for Medicare. Health Care Financing Review. 1998;20:109–129. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for Charlson Comorbidity adjustment in Medicare claims. Medical Care. 1999;37(11):1128–1139. doi: 10.1097/00005650-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 26. [8.24.11]; http://www.ahadata.com/ahadata/html/AHASurvey.html.
- 27. [7.13.11]; http://www.dartmouthatlas.org/tools/downloads.aspx.
- 28.US Census Bureau, Geography Division. Geographic Standards and Criteria Branch: ZIP code tabulation areas (ZCTAs). http://www.census.gov/geo/ZCTA/zcta.html.
- 29.Thomas AJ, Eberly LE, Davey Smith G, et al. ZIP-code-based versus tract-based income measures as long-term risk-adjusted mortality predictors. Am J Epidemiol. 2006;164:586–590. doi: 10.1093/aje/kwj234. [DOI] [PubMed] [Google Scholar]
- 30.Krieger N, Chen JT, Waterman PD, et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: Does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–82. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 31.Arcury TA, Preisser JS, Gesler WM, Powers JM. Access to transportation and the health care utilization in a rural region. J Rural Health. 2005;21(1):31–38. doi: 10.1111/j.1748-0361.2005.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 32.Probst JC, Laditka SB, Wang JY, Johnson AO. Effects of residence and race on burden of travel for care: cross-sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res. 2007;7:40. doi: 10.1186/1472-6963-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoenberg NE, Coward TE. Residential differences in attitudes about barriers to using community-based services among older adults. J Rural Health. 1998;14(4):295–304. doi: 10.1111/j.1748-0361.1998.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 34.Ricketts T. The rural patient. In: Geyman JP, Norris TE, Hart LG, editors. Texbook of Rural Medicine. NY, NY: McGraw Hill; 2001. pp. 15–26. [Google Scholar]
- 35.Gatz JL, Rowles GD, Tyas SL. Health disparities in rural Appalachia. In: Glasgow N, Morton LW, Johnson NE, editors. Critical Issues in Rural Health. Ames, Iowa: Blackwell Publishing; 2004. pp. 188–190. [Google Scholar]
- 36.Coughlin SS, Thompson TD. Colorectal cancer screening practices among men and women in rural and nonrural areas of the US. J Rural Health. 2004;20(2):118–124. doi: 10.1111/j.1748-0361.2004.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; revisions to payment policies under the physician fee schedule for calendar year 2003 and inclusion of registered nurses in the personnel provision of the critical access hospital emergency services requirement for frontier areas and remote locations. Final rule with comment period. Fed Regist. 2002;67(251):79965–80184. [PubMed] [Google Scholar]
- 38.Goodman M, Almon L, Bayakly R, Butler S, Crosby C, DiIorio C, Ekwueme D, Fletcher D, Fowler J, Gillespie T, Glanz K, Hall I, Lee J, Liff J, Lipscomb J, Pollack LA, Richardson LC, Roberts P, Steenland K, Ward K. Cancer outcomes research in a rural area: a multi-institution partnership model. J Community Health. 2009;34(1):23–32. doi: 10.1007/s10900-008-9123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coughlin SS, Thompson TD, Seef L, Richards Stallings F. Breast, cervical, and colorectal carcinoma screening in a demographically defined region of the southern US. Cancer. 2002;95(10):2211–22. doi: 10.1002/cncr.10933. [DOI] [PubMed] [Google Scholar]
- 40.Beyer KM, Comstock S, Seagren R, Rushton G. Explaining place-based colorectal cancer health disparities: evidence from a rural context. SocSci Med. 2011;72(3):373–382. doi: 10.1016/j.socscimed.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev OncolHematol. 2001;40(2):131–138. doi: 10.1016/s1040-8428(01)00139-1. [DOI] [PubMed] [Google Scholar]
- 42.Elting LS, Cooksley CD, Bekele BN, Giordano SH, et al. Mammography capacity impact on screening rates and breast cancer stage. Am J Prev Med. 2009;37(2):102–8. doi: 10.1016/j.amepre.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Haas JS, Earle CC, Orav JE, Brawarsky P, Keohane M, Neville BA, Williams DR. Racial segregation and disparities in breast cancer care and mortality. Cancer. 2008;113(8):2166–2172. doi: 10.1002/cncr.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao Y, Landrine H, Smith T, Kaw C, Corral I, Stein K. Residential segregation and disparities in health-related quality of life among Black and White cancer survivors. Health Psychol. 2011;30(2):137–144. doi: 10.1037/a0022096. [DOI] [PubMed] [Google Scholar]
- 45.Tian N, Wilson JG, Zhan FB. Spatial association of racial/ethnic disparities between late-stage diagnosis and mortality for female breast cancer: where to intervene? Int J Health Geogr. 2011;10:24. doi: 10.1186/1476-072X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schootman M, Jeffe DB, Lain M, Gillanders WE, Aft R. The role of poverty rate and racial distribution in the geographic clustering of breast cancer survival among older women: A geographic and multilevel analysis. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


