Opinion Statement
The epidemic of childhood obesity is becoming a major predictor for risk of cardiovascular diseases (CVD) and mortality during adulthood. Alterations in the morphology of the heart due to obesity could be a predictor for the dysfunction of cardiac autonomic modulation (CAM). A number of epidemiological studies have evaluated the effect of obesity and CAM in children, finding that obesity impaired the balance of CAM towards a sympathetic overflow and reduced parasympathetic modulation, a significant predictor of CVD morbidity and mortality in adults. Lifestyle modifications, for example long-term exercise programs, have been shown to improve CAM in the obese. This review discusses the recent evidence on childhood and adolescent obesity and its impact on CAM, as well as how early lifestyle changes could help improve CAM, which may in turn reduce the burden of CVD in adults.
Keywords: Obesity, Children, Cardiac Autonomic Modulation, Heart Rate Variability, Physical Activity, Nutrition
Introduction
Childhood obesity has become a major public health concern worldwide. The World Health Organization (WHO) calls it “one of the most serious public health challenges of the 21st century” [1]. The largest set of data ever-available accessing trends of overweight and obesity worldwide, including 144 developing and developed countries, suggest that in 2010, 43 million preschool children (age ranged from birth to 5 years old) are overweight and obese [2]. In addition, in a 20 years period, the global prevalence of overweight and obesity in preschool children increased 2.5%, from 4.2% in 1990 to 6.7% in 2010. This trend is expected to increase to a global prevalence of 9.1%, or 60 million, by the year of 2020. In the United State alone, during the past 30 years (from 1980 to 2010), the prevalence of childhood obesity has more than doubled in children (from 7% nearly 18%) and tripled in adolescents (from 5% to 18%) [3]. In 2009–2010, 16.9% of the United State children and adolescents from 2 to 19 years of age were obese [3]. In general, these numbers on childhood obesity are alarming and concerning, especially since obese children are more likely to stay obese and become obese adults increasing their risk of developing obesity related diseases, such as CVD [4, 5].
Children today, compared with children from the years of 1986 to 1989, have a higher predicted cardiovascular risk in childhood, as well as a higher predicted risk of heart disease in adulthood [6]. CVD in children are becoming more prevalent in conjunction with the rise in childhood obesity [6]. In fact, by the year of 2035 the predicted number of additional cardiovascular events attributable to excess weight in adolescence is expected to be >100,000 [7]. In addition, several reports have shown that childhood obesity is often accompanied by concurrent cardiovascular abnormalities, suggesting immediate attention to prevent progressive cardiovascular damage at such a young age [8–11].
A recent systematic review on the associations between childhood and adolescent obesity and risk of premature mortality and physical morbidity in adulthood, presented 11 studies reporting that overweight and obesity were associated with significantly increased risk of later cardiometabolic morbidity in adult life [12]. In addition, 4 out of 5 studies examined the associations between overweight and/or obesity, and premature mortality found significantly increased risk of premature mortality with children and adolescent overweight or obesity [12]. Moreover, a recent study reported that cardiovascular risk factors, such as hypertension, obesity, and glucose intolerance during childhood, are strongly associated with increase death during early adulthood [13].
Numerous studies have evaluated the effect of obesity and cardiac autonomic modulation (CAM) in children [14–28]. The majority of these studies have found that obesity impaired the balance of CAM towards a sympathetic overflow and reduced parasympathetic modulation in obese children. Thus, the goal of this paper is to provide a review of the recent evidence on how childhood and adolescent obesity impaired CAM, and how implementing an exercise routine and/or a well-balanced diet could help reverse this association in obese children.
Cardiac Autonomic Modulation (CAM) and Heart Rate Variability (HRV)
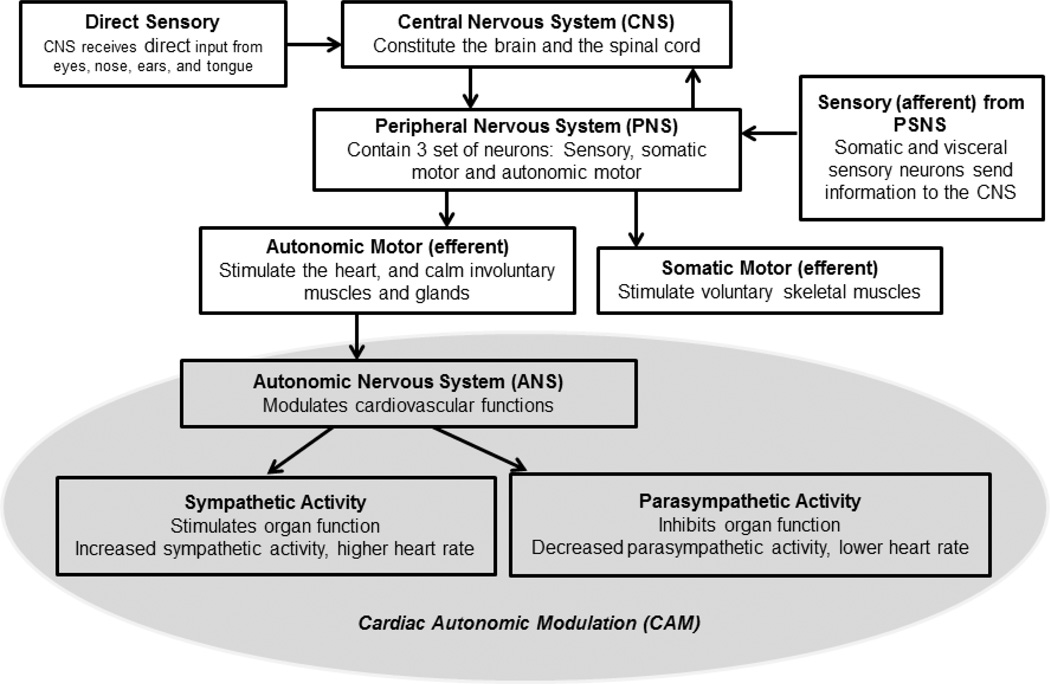
In order to understand how CAM functions, it is important to understand how the central nervous system (CNS) is involved. Figure 1 shows a schematic diagram on how the CNS operates. The autonomic motor neurons, which stimulates the heart and calm involuntary muscles and gland such as those around the intestine, stomach, and liver. This part of the CNS is known as the autonomic nervous system (ANS). In addition to the involvement on vessel and gastrointestinal tract, there is substantial literature demonstrating that the ANS is also involved in the modulation of metabolism, inflammation and immune system [29, 30]. One of the main functions of the ANS is to regulate the modulation of cardiovascular activity. This is when the term CAM comes to play. The sympathetic nervous system (SNS) and the parasympathetic nervous system (PSNS) are the two branches of the ANS. In the heart, SNS innervate organ functioning. The sympathetic nerve fibers stimulate the sinus node, the atrioventricular conducting pathway and the arterial and ventricular myocardium; increased sympathetic activity increases the heart rate (HR). The parasympathetic nerves decrease the function of the sinus node and the atrioventricular pathway and the atrial muscle. In contrast to the SNS, the increased parasympathetic activity decreases the HR. Under physiological or physical changes, such as exercising, the regulation of the cardiovascular and other visceral functions is based on the withdrawal of one branch, for example the PSNS, and the activation of the other, in this case the SNS, and vice versa; this dynamic physiological process is called the “sympatho-vagal balance” [31, 32].
Figure 1.
Schematic diagram of the Central Nervous System (CNS) involvement in the Cardiac Autonomic Modulation (CAM)
Due to the importance of the SNS and PSNS on CAM, several invasive and non-invasive approaches to assess ANS have been applied over the last decades. From invasive techniques that once were designed to only measure sympathetic activity, i.e, levels of catecholamines in the plasma and urine [33, 34] and the muscle sympathetic nerve activity [35], to the most utilized and reliable non-invasive technique, the Heart Rate Variability (HRV) [36].
HRV is used as a non-invasive measurement of CAM [37]. In the past 30 years, HRV has been widely accepted as a noninvasive method, from short or long-term electrocardiograph recordings, to measure the balance of the cardiac sympathetic and parasympathetic modulation. Following the standards set by the Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology, 1996, two major approaches for the calculation of HRV have been developed: the time-domain, and the frequency-domain analysis. The time domain method uses various summary statistics to measure the variations of beat-to-beat RR intervals. Whereas, the frequency-domain method decomposes beat-to-beat RR interval variations into powers at predefined frequency ranges to calculate: (1) power in the low frequency range (0.04–0.15 Hz, LF), which is often considered to be influenced by both sympathetic and/or parasympathetic modulations, depending on the study protocol [31, 38]; (2) power in the high frequency range (0.15–0.40 Hz, HF), a well-accepted marker for parasympathetic activity [39]; and (3) the ratio of LF to HF (LF/HF), a marker of sympathovagal balance [40]. For the time-domain method, the following HRV indices can be calculated: (1) standard deviation of all RR intervals (SDNN, ms), another marker for sympathetic activity; and (2) the square root of the mean of the sum of the squares of differences between adjacent RR intervals (RMSSD, ms), a marker for parasympathetic activity [41]. In addition, in the last several years non-lineal approaches have been developed, i.e., entropy-derived measures, such as reflexes circuits like the baroreflex and chemoreflex control [42].
In addition to analyze the HRV indices as the overall mean levels, which is the most frequently used approach by the investigators, another way to analyze HRV is to examine the circadian patterns of HRV indices. This method has been utilized more frequently in adults [43–47] than in children [48, 49]. Lack of circadian variation of HRV has been associated with increased risk to cardiovascular events in adults [46]. The circadian patterns of HRV can be quantified using a cosine periodic regression model consisting of three cosine function parameters: mean, measures the overall average of a HRV index; amplitude, measures the amplitude of the oscillation of a HRV index; and acrophase, measures the clock time when the highest amplitude is reached. The majority of the articles on childhood obesity and HRV available only examined the effect of obesity on the overall mean levels of HRV. Using the circadian pattern of CAM, one can first fit a cosine periodic regression model based on HRV indices calculated from 24-hour beat-to-beat RR interval data to estimate the individual-level mean, amplitude, and crescent time. One can then systematically assess the impact of various factors that may affect not only the mean levels, but also may affect the amplitude and the acrophase time of HRV indices.
Childhood Obesity and impairment of CAM
Several methods have been used to define obesity in children and adolescents: from age- and gender-specific body mass index (BMI) [15, 17], to the most recent use of dual-energy x-ray absorptiometry to measure central obesity [21, 24, 50]. Out of these two methods, having BMI at ≥95th percentile of the national age and gender-specific distribution has been more widely utilized as the preferred measure for detecting obesity in children and adolescents, because it is practical, trustworthy, and follows adults obesity measures [51, 52]. However, previous studies in adults and children have suggested that central obesity, compared to peripheral obesity, is more closely associated with CVD risk [21, 53].
In general, children and adults obesity is known to increase amounts of adipose tissue and lean mass through the body, and subsequently increase blood flow demand. Therefore, the heart has to work harder to pump the extra blood out to the rest of the body. The heart gets bigger and thicker, making it difficult to compress and relax during each heartbeat [14, 54]. These alterations in the morphology of the heart due to obesity could be the precursor to the impairment of CAM. In adults, impaired CAM has been related to all-cause mortality and unexpected cardiac events [14, 55]. More importantly, a reduction in the parasympathetic activity has been related to morbidity of CVD and mortality [14, 56]. Therefore, early identification of impairment of CAM in obese children could be an important clinical tool in the prevention of CVD in later life [14].
In recent years, several epidemiological studies have reported conflicting evidence of cardiac autonomic dysfunction in obese children [14–28]. The reduction of the vagal parasympathetic activity [15, 17–20, 21, 23–25, 28, 56], with the overflow or increase in the sympathetic activity of HRV have been most often reported [17, 20, 21, 23, 24, 28, 58]. In addition, some studies have shown an association between childhood obesity and increased LF/HF ratio, a marker for sympatho-vagal balance [17, 18, 20,21,23, 25, 58, 59]. Tascilar and coworkers conducted a casecontrol study of the association between childhood obesity and HRV; finding that obese children (BMI >97th percentile for age and sex) have lower HF, SDNN, RMSSD, but higher LF and LF/HF ratio than controls (BMI <85th percentile for age and sex) [20]. Similarly, in another case-control study, Kaufman and coworkers demonstrated that obese children (BMI >95th percentile for age and sex) have lower HF, and higher LF and LF/HF ratio than normal weight children [24]. In addition, Soares-Miranda et al., evaluated the relationship between central fat and CAM in obese and overweight girls (central fat > 50%) combined. They were able to find that being overweight and obese was associated with decreased parasympathetic (lower HF) and increased sympathetic modulation (higher LF and LF/HF ratio) [21]. In contrast, only few reports have shown that obesity in children cause a reduction [22, 60] or no effect [18, 19, 25] in the sympathetic activity of the heart. For example, Vanderlei and coworkers studied 56 obese children and found that these obese children have decreased sympathetic activity (low LF) than lean children [22]. Furthermore, Birch et al., found that overweight/obese children have lower parasympathetic activity (low HF, RMSSD), no difference were found in the sympathetic activity (LF and LF/HF ratio), than children of normal weight [19]. In addition, some other studies have demonstrated a reduction of cardiac baroreflex sensitivity in obese children [16, 26, 27, 57]. Dangardt et al. conducted a large study measuring baroreflex sensitivity on 311 children. They reported that 120 obese children had reduced cardiac baroreflex sensitivity compared to 148 lean children [16]. Although puberty has been associated with change in autonomic function, this group controlled for pubertal status in their analysis, finding that the impact of obesity on baroreflex function is independent of age and sex [14, 16]. Similar to Dangardt et al., Lazarova and coworkers conducted a study on adolescents and found similar results that decreased baroreflex sensitivity in obese adolescents [57]. Additionally, two other studies reported that overweight and obese children had a reduction in baroreflex sensitivity and increased values of LF and LF/HF ratio [26, 27]. One study in particular, studying the effects of age, sex, race, BMI, and Tanner’s stage on short-term and 24-hour HRV in a sample of healthy adolescents, did not find any linkage between BMI and HRV [61]. One possible explanation to why no association was found is that the study population was adolescent with type 1 diabetes, who tend not to be overweight. When compared with healthy controls, it limited the variation of weight distribution in the sample.
Despite these findings, more epidemiological studies, especially longitudinal studies, evaluating the association between childhood obesity and CAM are needed. Also, future research should implement the HRV circadian rhythm following the cosine periodic function, which can be quantified with three parameters—the mean, the amplitude, and the acrophase time.
Childhood Obesity Co-morbidities and HRV
Some major predictors of CVD like metabolic syndrome, hypertension, type 2 diabetes, and insulin resistance are also commonly known co-morbid conditions related to obesity and HRV in children and adolescents. The association between each individual condition and HRV indices in children has been evaluated elsewhere [17, 20, 21, 24, 62–65]. Previously published data from our group on a healthy population-based sample of children showed that a one standard deviation increase in waist circumference was significantly associated with lower HF, LF, RMSSD, SDNN, and higher LF/HF ratio and HR [17]. In addition, Soares-Miranda and colleagues studied a sample of 16 overweight/obese girls 8–16 years of age and found an association between increase of central fat and lower HF and higher LF/HF ratio [21]. These two studies agreed that having a high amount of central obesity is associated with impairment of the parasympathetic and overflow of the sympathetic activity of CAM. Numerous studies have examined the association between blood pressure and HRV [20, 73–75]. In a research performed on 9–11 years old healthy children studying the association between blood pressure and HRV, investigators found that children with high systolic blood pressure displayed significant lower HF, and RMSSD [62]. Fitzgibbon et al. found that children with high blood pressure have significantly lower HF, LF, and higher LF/HF ratio than those with normal blood pressure levels [63]. In another study, high blood pressure in children 15–16 years of age also correlated with a reduced HF[65]. The relationship between insulin resistance and CAM has been studied in children [20]. A study on children classified by their homeostasis model assessment of insulin resistance (HOMA-IR) values demonstrated that those in the insulin-resistance group had lower HF and higher LF/HF ratio than the non-resistant group [20]. No data in children studying the association between triglycerides and HRV is available. However, a study in 18–21 year old adults, demonstrated that increased levels of triglycerides are associated with lower HF, and higher LF/HF ratio and HR [62].
Lifestyle modifications for Childhood Obesity and HRV
In contrast to other chronic diseases, where no treatment or intervention is available, obesity is a modifiable condition. By making some early lifestyle changes, one could improve his health status; therefore, decrease the likelihood of developing CVD as an adult. The US Preventive Service Task Force recommends that children aged 6 years and older should be screened for obesity and should be offered or referred to comprehensive, moderate-to high-intense behavioral interventions to promote improvement in weight status [66]. After reviewing many different clinical studies on childhood obesity, they concluded that short-term (6–12 months) comprehensive interventions, that include regular moderate to vigorous physical activity, well-balance healthy diet, and behavioral counselling, were effective in reducing the BMI in obese children. For the purpose of this review, we will be discussing the effect of physical activity (PA) and nutrition on HRV in childhood obesity.
Physical Activity (PA)
Currently, the Centers for Disease Control and Prevention (CDC) recommend that every child 6 to 17 years of age perform ≥60 minutes of PA each day [67]. PA is defined as moderate-intensity aerobic activity, like walking, or vigorous-intensity activity, like running. Vigorous-intensity aerobic activity, muscle strengthening activities, and bone strengthening activities should be included at least 3 days per week as part of the 60 minutes daily physical activity.
A number of epidemiological studies have evaluated the effect of exercise alone on HRV [60, 68–75]. Table 1 summarizes several publications studying the effect of PA on CAM in children. The first study to investigate the effect of endurance training on HRV in healthy children was conducted by Mandigout et al [73]. They evaluated the effect of a 13 weeks endurance training program on HRV in pre-pubertal healthy children and determined the relationships between HRV components and training-induced cardiac adaptation. An increase in the majority of frequency (VLF, LF, HF) and time (SDNNIDX, SDNN, RMSSD, PNN50) domains of HRV was found in healthy children. In addition, Nagai et al., examined, for the first time, the effect of long-term (1-year exercise training program) moderate training on cardiac ANS activity in a large number of healthy children. They found that even during short duration (20 min/day) and mild intensity physical activity, there was an increase in CAM activity even in children who initially possessed low HRV [72]. Gutin et al. studied the effect of moderate-vigorous physical activity (MVPA) in healthy adolescents, and found that higher MVPA was associated with higher RMSSD (marker of parasympathetic activity) and lower LF/HF ratio [74]. A group of investigators evaluated the association of participation in 210min/week of moderate intensity PA or 60min/week of high intensity activity on HRV; they found that habitual participation in activities of high intensity for 1 hour a week is necessary to observe favorable alternation in HRV [70]. Triposkiadis et al. demonstrated that highly trained (12–14 h weekly for at least 4 years) prepubertal swimmers had higher HF, RMSSD, and lower LF/HF ratio than non-training normal children. [75]. Different from these findings, few studies have found no differences between exercise intensity and HRV [68–71]. Vinet et al. compared the HRV parameters in highly trained swimmer and untrained boys, finding that neither time nor frequency domains of HRV variables were significantly different between the two groups [71]. Also, Leicht et al. found no significant alterations of HR and frequency domain measures of HRV during an 8 weeks rest and light to moderate exercise training in pre-pubertal children [69]. Similarly, Gamelin et al. found no significant effect on the autonomic cardiovascular control, as measured by HRV, from 7 weeks of high intensity intermittent exercise training programs [68].
Table 1.
Studies investigating the effects of physical activity on CAM in healthy and obese children
| First author, Year[Ref.] | n | Population (age range) | Physical Activity | HRV Indices | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Obese | Healthy | Intensity | Duration | HF | LF |
LF/H F |
SDNN | RMSSD | HR | ||
| Farah 2013[76] | 5 | 13–18 | high | 6-month† | ↑ | = | = | = | ↓ | ||
| Gutin 2000[50] | 70 | 7–11 | high | 4-month† | ↑ | ↓ | |||||
| Gutin 1997[77] | 17 | 7–11 | high | 4-month† | ↓ | ↓ | ↑ | ↓ | |||
| Gamelin 2009[68] | 22 | 8–10 | high | 7-week† | = | = | = | = | ↓ | ||
| Gutin 2005[74] | 304 | 14–18 | MVPA | 1-week‡ | ↓ | ↑ | ↓ | ||||
| Nagai 2004[72] | 200 | 6–11 | high | 12-month† | ↑ | ↑ | ↓ | ||||
| Mandigout 2002[73] | 12 | 10–11 | high | 13-week† | ↑ | ↑ | = | ↑ | ↑ | ||
Abbreviations: HF, high frequency power; HR, heart rate; MVPA, moderate-vigorous physical activity; LF, low frequency power; RMSSD, the square root of the mean of the sum of the squares of differences between adjacent RR intervals; and SDNN, standard deviation of all RR.
exercise training intervention;
free-living physical activity measured by an accelerometer; ↑, increase; ↓, decrease; =, non-significant effect.
Moreover, only a few intervention studies on the effect of exercise on HRV in childhood obesity have been published [50, 76, 77] (Table 1). Farah et al., compared the effects of high-intensity aerobic exercise training and aerobic exercise training on HR and HRV in obese adolescents [76]. They found that those obese adolescents in the high-intensity group showed improvements on HR and HRV; suggesting a shift towards vagal modulation of the heart, imparting better cardiovascular health than those in the low-intensity group. Gutin et al., studied the relationship between baseline parasympathetic activity to body composition, and the responsivity of parasympathetic activity to physical training [50]. They showed that, in obese children, the RMSSD increased during the 4-month of physical training engagement, but declined in the 4- month period following cessation of physical training. This same group of investigators in a different study demonstrated that controlled physical training can shift heart period variability of CAM towards less sympathetic activity (declined LF and the LF/HF ratio) and greater parasympathetic activity (increase in the RMSSD), suggesting that the sympathetic/parasympathetic balance was improved by the training [77]. These studies demonstrated that high-intense exercise training can improve CAM in obese children. However, more studies are needed, especially longitudinal studies.
Nutrition
Changing to a well-balance diet, that includes higher consumption of fruit, vegetables, and oily fish, is a good approach to reduce the risk of CVD [78]. Numerous amounts of studies in adults have shown that high intake of fruit, vegetables, and fish are associated with lower rates of allcause, cancer, and CVD mortality [79–80]. Also, intake of vitamin C and carotenoids found in vegetables and fruits have been associated with reducing the risk of CVD [81, 82]. Park et al., demonstrated that after adjustment for healthy lifestyle factors, such as PA and use of multivitamins, the consumption of green leafy vegetables was positively associated with normalized HF power and inversely associated with LF power [83]. Several epidemiological studies have evaluated the effect of a well-balance diet on HRV in overweight and obese adults [84–88]. De Jonge and coworkers evaluated the effect of different approaches of caloric restriction on autonomic function in 28 overweight adults from a long-term low intake of energy trial. They found that, in general, caloric restriction decreases sympathetic activity and increases parasympathetic activity, indicating an improvement of the sympathetic and parasympathetic balance [84]. This finding is consistent with the results from Dietrich et al., where a caloric restriction program in obese adults was associated with decreased sympathetic activity and an increase in the parasympathetic activity [85]. Another study performed by Akehi Y et al., on 16 obese patients treated with the very-low-calorie conventional Japanese diet therapy combined with behavior therapy, demonstrated that weight reduction increased HF, but decreased LF/HF ratio [86]. A case-control study evaluating the effects of exercise and mild calorie restriction on HRV in 12 mildly obese, normotensive Japanese women demonstrated a significant increase in HF after the intervention when compared controls [87]. In addition, Poirier et al., determined the impact of diet-induced weight loss on CAM, they found that weight loss is associated with significant improvement in CAM through enhancement of parasympathetic modulation, and decreased HR [88]. All of this extensive data in obese adults demonstrates that the implementation of a well-balance diet could improve CAM. However, there is very limited data available in children and adolescents.
The CAM response to eating has been largely investigated in healthy and obese adults, but comparable data in childhood obesity is needed. We were able to find only one related study, which was conducted by Pivik and coworkers [89]. They initially evaluated HR and HRV in preadolescents who maintained overnight fasting, and again after the participants either ate a standardized breakfast or continued fasting. Their findings indicated that continuation of overnight fasting is associated with a significant increase in parasympathetic activity, but this was attenuated after eating breakfast.
Conclusion
This review of current evidence suggest that there is sufficient data showing that childhood obesity is associated with impairment of CAM, in the direction towards sympathetic overflow and reduced parasympathetic modulation. Since it has been consistently demonstrated that lower HRV is associated with the development of coronary heart disease, hypertension, metabolic syndrome, and all-cause mortality [90–94] in the adults, it is highly plausible that obesity in childhood and adolescents affect the CAM first, and if the adverse impact of obesity on CAM continues, these individuals will be at much higher risk when they become adults. However, longitudinal studies are needed to further support this elucidation. More studies examining the circadian pattern of HRV are needed. The evidences are emerging to suggest that the implementation of long-term regular PA programs and a well-balanced diet can help obese children reduce their weight, improve their CAM, and therefore, reduce the risk of future CVD in adulthood.
Acknowledgments
National Institutes of Health (NIH) grants: 1 R01 HL 097165, R01 HL63772, R21HL087858, and the Penn State CTSI Grant UL Tr000127. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest
Dr. Duanping Liao, Dr. Sol M. Rodriguez-Colon, Dr. Fan He, and Dr. Edward O. Bixler each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Duanping Liao, Email: dliao@phs.psu.edu.
Sol M. Rodriguez-Colon, Email: srodrigu@phs.psu.edu.
Fan He, Email: fhe@phs.psu.edu.
Edward O. Bixler, Email: ebixler@hmc.psu.edu.
References
References of particular importance, published recently, have been highlighted as:
• Of importance
•• Of outstanding importance
- 1.World Health Organization. [Accessed 08 Apr 2014];Global strategy on diet, physical activity, and health: childhood overweight and obesity. http://www.who.int/dietphysicalactivity/childhood/en/
- 2.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents: 1999–2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 5.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 6.Crowley DI, Khoury PR, Urbina EM, Ippisch HM, Kimball TR. Cardiovascular impact of the pediatric obesity epidemic: higher left ventricular mass is related to higher body mass index. J Pediatr. 2011;158:709–714. doi: 10.1016/j.jpeds.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357:2371–2379. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SK, Richards N, Lorber R, Rosenthal GL. Abdominal obesity, waist circumference, body mass index, and echocardiographic measures in children and dolescents. Congenit Heart Dis. 2009;4:338–347. doi: 10.1111/j.1747-0803.2009.00330.x. [DOI] [PubMed] [Google Scholar]
- 9.Koopman LP, McCrindle BW, Slorach C, et al. Interaction between myocardial and vascular changes in obese children: a pilot study. J Am Soc Echocardiogr. 2012;25:401–410. doi: 10.1016/j.echo.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Ozdemir O, Hizli S, Abaci A, Agladioglu K, Aksoy S. Echocardiographic measurement of epicardial adipose tissue in obese children. Pediatr Cardiol. 2010;31:853–860. doi: 10.1007/s00246-010-9720-y. [DOI] [PubMed] [Google Scholar]
- 11.Atabek ME, Akyuz E, Selver Eklioglu B, Cimen D. The relationship between metabolic syndrome and left ventricular mass index in obese children. J Clin Res Pediatr Endocrinol. 2011;3:132–138. doi: 10.4274/jcrpe.v3i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2011;35(7):891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 13.Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol; 2013;62(15):1309–1319. doi: 10.1016/j.jacc.2013.07.042. Recap of resent research on cardiovascular abnormalities in children with obesity.
- 15.Vanderlei LC, Pastre CM, Freitas IF, Jr, Godoy MF. Analysis of cardiac autonomic modulation in obese and eutrophic children. Clinics. 2010;65:789–792. doi: 10.1590/S1807-5932201000080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dangardt F, Volkmann R, Chen Y, Osika W, Marild S, Friberg P. Reduced cardiac vagal activity in obese children and adolescents. Clin Physiol Funct Imaging. 2011;31:108–113. doi: 10.1111/j.1475-097X.2010.00985.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Colón SM, Bixler EO, Li X, Vgontzas AN, Liao D. Obesity is associated with impaired cardiac autonomic modulation in children. Int J Pediatr Obes. 2011;6:128–134. doi: 10.3109/17477166.2010.490265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum P, Petroff D, Classen J, Kiess W, Blüher S. Dysfunction of autonomic nervous system in childhood obesity: a cross-sectional study. PLoS One. 2013 doi: 10.1371/journal.pone.0054546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birch SL, Duncan MJ, Franklin C. Overweight and reduced heart rate variability in British children: an exploratory study. Prev Med. 2012;55(5):430–432. doi: 10.1016/j.ypmed.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Taşçılar ME, Yokuşoğlu M, Boyraz M, Baysan O, Köz C, Dündaröz R. Cardiac autonomic functions in obese children. J Clin Res Pediatr Endocrinol. 2011;3(2):60–64. doi: 10.4274/jcrpe.v3i2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soares-Miranda L, Alves AJ, Vale S, et al. Central fat influences cardiac autonomic function in obese and overweight girls. Pediatr Cardiol. 2011;32(7):924–928. doi: 10.1007/s00246-011-0015-8. [DOI] [PubMed] [Google Scholar]
- 22.Vanderlei LC, Pastre CM, Freitas IF, Jr, Godoy MF. Geometric indexes of heart rate variability in obese and eutrophic children. Arq Bras Cardiol. 2010;95(1):35–40. doi: 10.1590/s0066-782x2010005000082. [DOI] [PubMed] [Google Scholar]
- 23.Rabbone I, Bobbio A, Rabbia F, Bertello MC, Ignaccoldo MG, Saglio E, et al. Early cardiovascular autonomic dysfunction, beta cell function and insulin resistance in obese adolescents. Acta Biomed. 2009;80(1):29–35. [PubMed] [Google Scholar]
- 24.Kaufman CL, Kaiser DR, Steinberger J, Dengel DR. Relationships between heart rate variability, vascular function, and adiposity in children. Clin Auton Res. 2007;17(3):165–171. doi: 10.1007/s10286-007-0411-6. [DOI] [PubMed] [Google Scholar]
- 25.Rabbia F, Silke B, Conterno A, et al. Assessment of cardiac autonomic modulation during adolescent obesity. Obes Res. 2003;11(4):541–548. doi: 10.1038/oby.2003.76. [DOI] [PubMed] [Google Scholar]
- 26.Latchman PL, Mathur M, Bartels MN, Axtell RS, De Meersman RE. Impaired autonomic function in normotensive obese children. Clin Auton Res. 2011;21(5):319–323. doi: 10.1007/s10286-011-0116-8. [DOI] [PubMed] [Google Scholar]
- 27.Lucini D, de Giacomi G, Tosi F, Malacarne M, Respizzi S, Pagani M. Altered cardiovascular autonomic regulation in overweight children engaged in regular physical activity. Heart. 2013;99(6):376–381. doi: 10.1136/heartjnl-2012-302616. [DOI] [PubMed] [Google Scholar]
- 28.Paschoal MA, Trevizan PF, Scodeler NF. Heart rate variability, blood lipids and physical capacity of obese and non-obese children. Arq Bras Cardiol. 2009;93(3):239–246. doi: 10.1590/s0066-782x2009000900007. [DOI] [PubMed] [Google Scholar]
- 29.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grassi G, Quarti-Trevano F, Seravalle G, Dell’Oro R. Cardiovascular risk and adrenergic overdrive in the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2007;17:473–481. doi: 10.1016/j.numecd.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Malliani A, Pagani M, Montano N, Mela GS. Sympatho vagal balance: areappraisal. Circulation. 1998;98:2640–2643. [PubMed] [Google Scholar]
- 32.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiacautonomic control: vago-sympathetic interactions revisited. BrainRes Rev. 2005;49:555–565. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein DS, McCarty R, Polinsky RJ, Kopin IJ. Relationship between plasman or e- pinephrine and sympathetic neural activity. Hypertension. 1983;5:552–559. doi: 10.1161/01.hyp.5.4.552. [DOI] [PubMed] [Google Scholar]
- 34.Esler M. Clinical application of nor adrenaline spill over methodology: delineation of regional human sympathetic nervous responses. Pharmacol Toxicol. 1993;73:243–253. doi: 10.1111/j.1600-0773.1993.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 35.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 36.Hyndman BW, Kitney RI, Sayers BM. Spontaneous rhythms in physiological control systems. Nature. 1971;233:339–341. doi: 10.1038/233339a0. [DOI] [PubMed] [Google Scholar]
- 37.Kleiger RE, Stein PK, Bigger JT. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billman GE. Heart rate variability a historical perspective. Front Physiol. 2011;2:86. doi: 10.3389/fphys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thayler JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int.J.Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 40.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 41.Otzenberger H, Gronfier C, Simon C, et al. Dynamic heart rate variability: A tool for exploring sympathovagal balance continuously during sleep in men. Am J Physiol. 1998;275(3):946–950. doi: 10.1152/ajpheart.1998.275.3.H946. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan DT, Furman MI, Pincus SM, Ryan SM, Lipsitz LA, Goldberger AL. Aging and the complexity of cardiovascular dynamics. Biophys J. 1991;59:945–949. doi: 10.1016/S0006-3495(91)82309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malpas SC, Purdie GL. Circadian variation of heart rate variability. Cardiovasc Res. 1990;24:210–213. doi: 10.1093/cvr/24.3.210. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Shaffer ML, Rodríguez-Colón SM, et al. Systemic inflammation and circadian rhythm of cardiac autonomic modulation. Auton Neurosci. 2011;162(1–2):72–76. doi: 10.1016/j.autneu.2011.03.002. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez-Colón SM, Li X, Shaffer ML, et al. Insulin resistance and circadian rhythm of cardiac autonomic modulation. Cardiovasc Diabetol. 2010;6(9):85. doi: 10.1186/1475-2840-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kardelen F, Akcurin G, Ertug H, Akcurin S, Bircan I. Heart rate variability and circadian variations in type 1 diabetes mellitus. Pediatr Diabetes. 2006;7:45–50. doi: 10.1111/j.1399-543X.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Shaffer ML, Rodríguez-Colón S, et al. The circadian pattern of cardiac autonomic modulation in a middle-aged population. Clin Auton Res. 2011;21(3):143–150. doi: 10.1007/s10286-010-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massin MM, Maeyns K, Withofs N, Ravet F, Gerard P. Circadian rhythm of heart rate and heart rate variability. Arch Dis Child. 2000;83:179–182. doi: 10.1136/adc.83.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bilan A, Witczak A, Palusiński R, Myśliński W, Hanzlik J. Circadian rhythm of spectral indices of heart rate variability in healthy subjects. J Electrocardiol. 2005;38(3):239–243. doi: 10.1016/j.jelectrocard.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Gutin B, Barbeau P, Litaker MS, Ferguson M, Owens S. Heart rate variability in obese children: relations to total body and visceral adiposity, and changes with physical training and detraining. Obes Res. 2000;8(1):12–19. doi: 10.1038/oby.2000.3. [DOI] [PubMed] [Google Scholar]
- 51.Barlow SE Expert Committee. Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(4):164–192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 52.Raj M. Obesity and cardiovascular risk in children and adolescents. Indian J Endocrinol Metab. 2012;16(1):13–19. doi: 10.4103/2230-8210.91176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Despres JP, Lemieux I, Prud’homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. Br Med J. 2001;322:716–720. doi: 10.1136/bmj.322.7288.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 56.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Lazarova Z, Tonhajzerova I, Trunkvalterova Z, et al. Baroreflex sensitivity is reduced in obese normotensive children and adolescents. Can J Physiol Pharmacol. 2009;87:565–571. doi: 10.1139/y09-041. [DOI] [PubMed] [Google Scholar]
- 58.Riva P, Martini G, Rabbia F, et al. Obesity and autonomic function in adolescence. Clin Exp Hypertens. 2001;23(1–2):57–67. doi: 10.1081/ceh-100001197. [DOI] [PubMed] [Google Scholar]
- 59.Guízar JM, Ahuatzin R, Amador N, Sánchez G, Romer G. Heart autonomic function in overweight adolescents. Indian Pediatr. 2005;42(5):464–469. [PubMed] [Google Scholar]
- 60.Nagai N, Moritani T. Effect of physical activity on autonomic nervous system function in lean and obese children. Int J Obes Relat Metab Disord. 2004;28(1):27–33. doi: 10.1038/sj.ijo.0802470. [DOI] [PubMed] [Google Scholar]
- 61.Faulkner MS, Hathaway D, Tolley B. Cardiovascular autonomic function in healthy adolescents. Heart Lung. 2003;32(1):10–22. doi: 10.1067/mhl.2003.6. [DOI] [PubMed] [Google Scholar]
- 62. Soares-Miranda L, Sandercock G, Vale S, et al. Metabolic syndrome, ` physical activity and cardiac autonomic function. Diabetes Metab Res Rev. 2012;28(4):363–369. doi: 10.1002/dmrr.2281. They demonstrated that increasing in central fat, measured dual-energy X-ray absorptiometry, is associated with impairment of CAM in overweight and obese children.
- 63.Gui-Ling X, Jing-Hua W, Yan Z, Hui X, Jing-Hui S, Si-Rui Y. Association of high blood pressure with heart rate variability in children. Iran J Pediatr. 2013;23(1):37–44. [PMC free article] [PubMed] [Google Scholar]
- 64.Fitzgibbon LK, Coverdale NS, Phillips AA, et al. The association between baroreflex sensitivity and blood pressure in children. Appl Physiol Nutr Metab. 2012;37(2):301–307. doi: 10.1139/h11-163. [DOI] [PubMed] [Google Scholar]
- 65.Pankova NB, Alchinova IB, Afanas'eva EV, Karganov MIu. Functional characteristics of cardiovascular system in adolescents with prehypertension. Fiziol Cheloveka. 2010;36(3):82–89. [PubMed] [Google Scholar]
- 66.Barton M. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125:361–367. doi: 10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- 67.Center for Disease Control and Prevention. [Accessed 08 Apr 2014];Division of Nutrition, Physical Activity, and Obesity. How much physical activity do children need? http://www.cdc.gov/physicalactivity/everyone/guidelines/children.html.
- 68.Gamelin FX, Baquet G, Berthoin S, et al. Effect of high intensity intermittent training on heart rate variability in prepubescent children. Eur J Appl Physiol. 2009;105(5):731–738. doi: 10.1007/s00421-008-0955-8. [DOI] [PubMed] [Google Scholar]
- 69.Leicht AS, Allen GD. Moderate-term reproducibility of heart rate variability during rest and light to moderate exercise in children. Braz J Med Biol Res. 2008;41(7):627–633. doi: 10.1590/s0100-879x2008000700013. [DOI] [PubMed] [Google Scholar]
- 70.Buchheit M, Platat C, Oujaa M, Simon C. Habitual physical activity, physical fitness and heart rate variability in preadolescents. Int J Sports Med. 2007;28(3):204–210. doi: 10.1055/s-2006-924296. [DOI] [PubMed] [Google Scholar]
- 71.Vinet A, Beck L, Nottin S, Obert P. Effect of intensive training on heart rate variability in prepubertal swimmers. Eur J Clin Invest. 2005;35(10):610–614. doi: 10.1111/j.1365-2362.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 72.Nagai N, Hamada T, Kimura T, Toshio Moritani. Moderate physical exercise increases cardiac autonomic nervous system activity in children with low heart rate variability. Childs Nerv Syst. 2004;20(4):209–214. doi: 10.1007/s00381-004-0915-5. [DOI] [PubMed] [Google Scholar]
- 73.Mandigout S, Melin A, Fauchier L, N'Guyen LD, Courteix D, Obert P. Physical training increases heart rate variability in healthy prepubertal children. Eur J Clin Invest. 2002;32(7):479–487. doi: 10.1046/j.1365-2362.2002.01017.x. [DOI] [PubMed] [Google Scholar]
- 74.Gutin B, Howe C, Johnson MH, Humphries MC, Snieder H, Barbeau P. Heart rate variability in adolescents: relations to physical activity, fitness, and adiposity. Med Sci Sports Exerc. 2005;37(11):1856–1863. doi: 10.1249/01.mss.0000175867.98628.27. [DOI] [PubMed] [Google Scholar]
- 75.Triposkiadis F, Ghiokas S, Skoularigis I, Kotsakis A, Giannakoulis I, Thanopoulos V. Cardiac adaptation to intensive training in prepubertal swimmers. Eur J Clin Invest. 2002;32:16–23. doi: 10.1046/j.0014-2972.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 76. Farah BQ, Ritti-Dias RM, Balagopal PB, Hill JO, Prado WL. Does exercise intensity affect blood pressure and heart rate in obese adolescents? A 6-month multidisciplinary randomized intervention study. Pediatr Obes. 2014;9(2):111–120. doi: 10.1111/j.2047-6310.2012.00145.x. An intervention trial in obese adolescents, demonstrating that high-intensity exercise training can enhance HRV, therefore improving CAM.
- 77.Gutin B, Owens S, Slavens G, Riggs S, Treiber F. Effect of physical training on heart-period variability in obese children. J Pediatr. 1997;130(6):938–943. doi: 10.1016/s0022-3476(97)70280-4. [DOI] [PubMed] [Google Scholar]
- 78.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 79.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004;160:1223–1233. doi: 10.1093/aje/kwh339. [DOI] [PubMed] [Google Scholar]
- 80.Bazzano LA, He J, Ogden LG, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 81.Knekt P, Ritz J, Pereira MA, et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80:1508–1520. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- 82.Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr. 2006;83:1265–1271. doi: 10.1093/ajcn/83.6.1265. [DOI] [PubMed] [Google Scholar]
- 83.Park SK, Tucker KL, O'Neill MS, et al. Fruit, vegetable, and fish consumption and heart rate variability: the Veterans Administration Normative Aging Study. Am J Clin Nutr. 2009;89(3):778–786. doi: 10.3945/ajcn.2008.26849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Jonge L, Moreira EA, Martin CK, Ravussin E Pennington CALERIE Team. Impact of 6-month caloric restriction on autonomic nervous system activity in healthy, overweight, individuals. Obesity. 2010;18(2):414–416. doi: 10.1038/oby.2009.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dietrich DF, Schindler C, Schwartz J, et al. Heart rate variability in an aging population and it association with lifestyle and cardiovascular risk factors: Results of the SAPALDIA study. Europace. 2006;8:521–529. doi: 10.1093/europace/eul063. [DOI] [PubMed] [Google Scholar]
- 86.Akehi Y, Yoshimatsu H, Kurokawa M, et al. VLCD-induced weight loss improves heart rate variability in moderately obese Japanese. Exp Biol Med. 2001;226(5):440–445. doi: 10.1177/153537020122600508. [DOI] [PubMed] [Google Scholar]
- 87.Ito H, Ohshima A, Tsuzuki M, Ohto N, et al. Effects of increased physical activity and mild calorie restriction on heart rate variability in obese women. Jpn Heart J. 2001;42(4):459–469. doi: 10.1536/jhj.42.459. [DOI] [PubMed] [Google Scholar]
- 88.Ito H, Ohshima A, Tsuzuki M, Ohto N, et al. Effects of increased physical activity and mild calorie restriction on heart rate variability in obese women. Jpn Heart J. 2001;42(4):459–469. doi: 10.1536/jhj.42.459. [DOI] [PubMed] [Google Scholar]
- 89.Pivik RT, Dykman RA. Cardiovascular effects of morning nutrition in preadolescents. Physiol Behav. 2004;82(2–3):295–302. doi: 10.1016/j.physbeh.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 90.Liao D, Cai Jianwen, Barnes RW, et al. Cardiac autonomic function and the development of hypertension -- The ARIC Study. Am J Hypertens. 1996;9:1147–1156. doi: 10.1016/s0895-7061(96)00249-x. [DOI] [PubMed] [Google Scholar]
- 91.Liao D, Cai J, Rosamond W, et al. Cardiac autonomic function and incident CHD: a population based case-cohort study -- The ARIC Study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 92.Liao D, Sloan RP, Cascio WE, et al. The multiple metabolic syndrome is associated with lower heart rate variability -- The ARIC Study. Diabetes Care. 1998;21:2116–2122. doi: 10.2337/diacare.21.12.2116. [DOI] [PubMed] [Google Scholar]
- 93.Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a two minute rhythm strip predicts risk of coronary heart disease and mortality from several causes -- The ARIC Study. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 94.Liao D, Carnethon MR, Evans GW, Cascio WE, Heiss G. Lower Heart Rate Variability Is Associated with the Development of Coronary Heart Disease in Individuals with Diabetes - The ARIC Study. Diabetes. 2002;51:3524–3531. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]