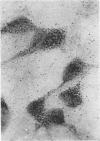
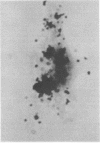
Abstract
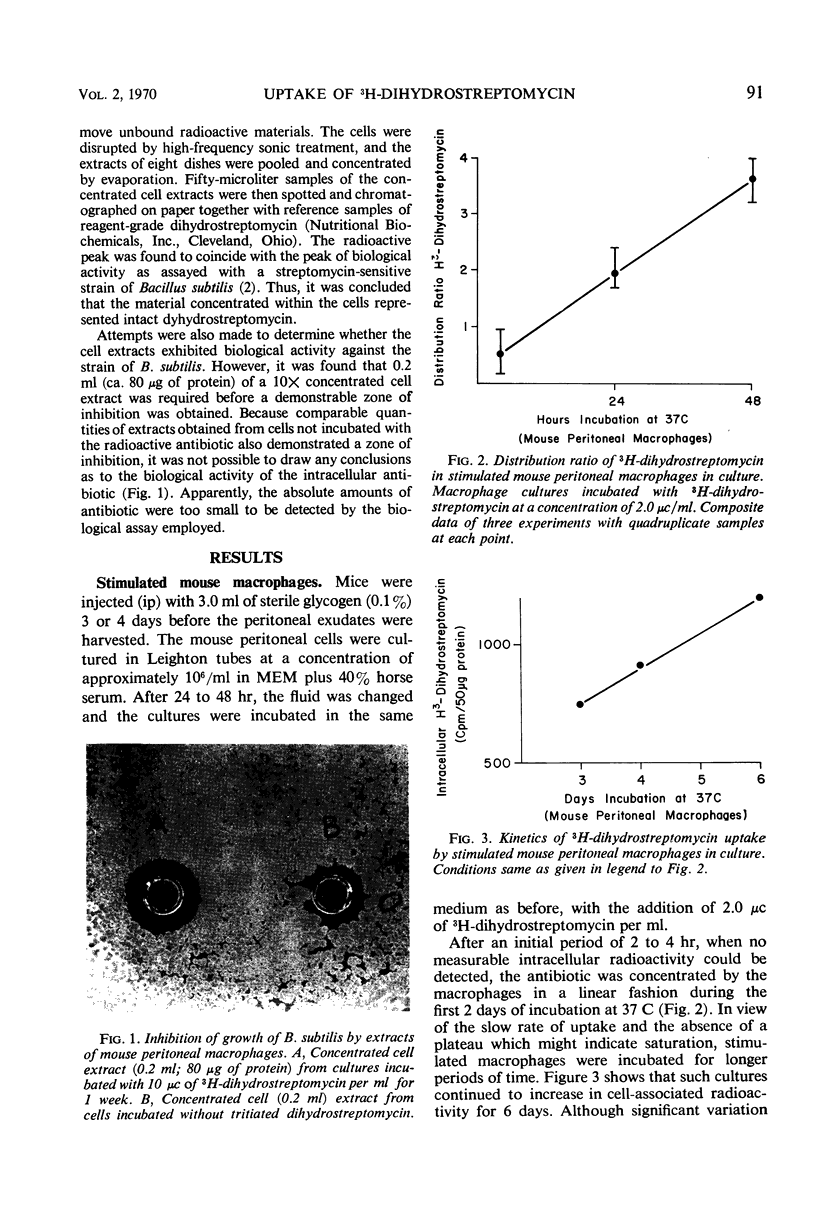
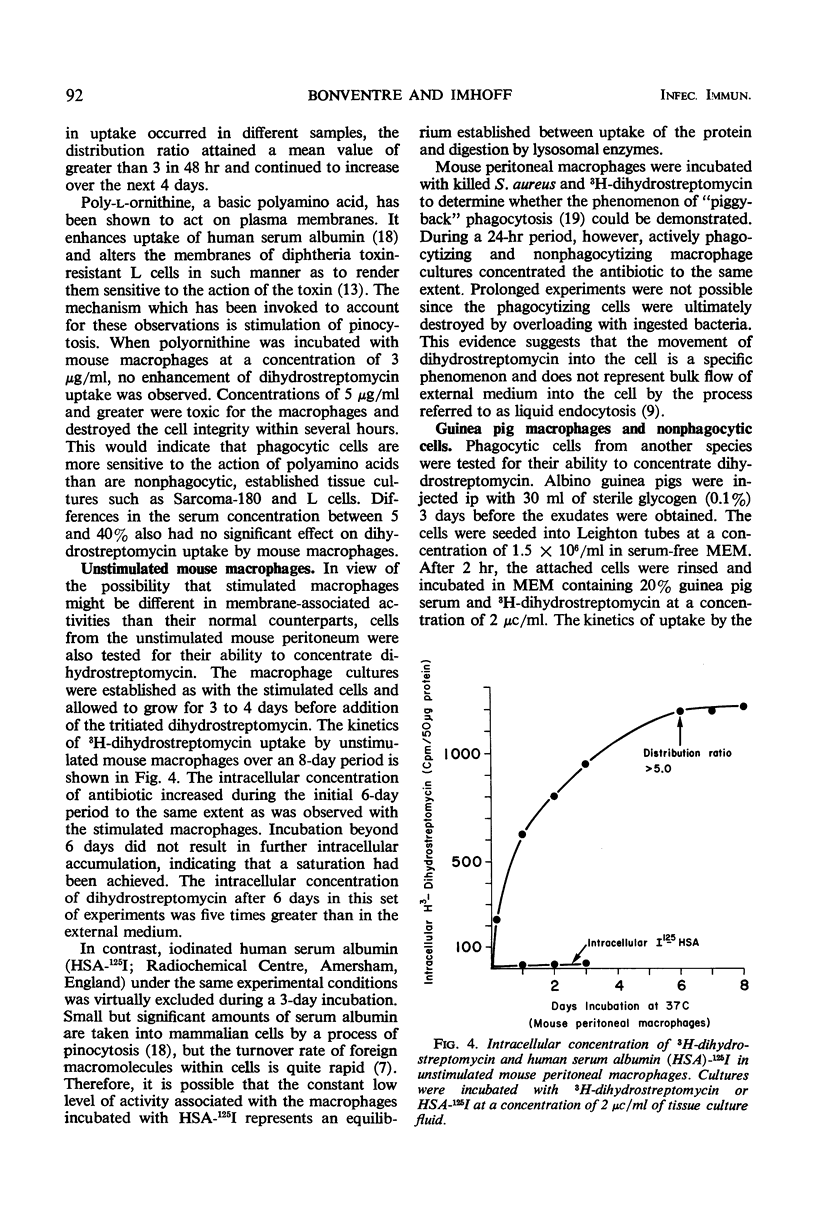
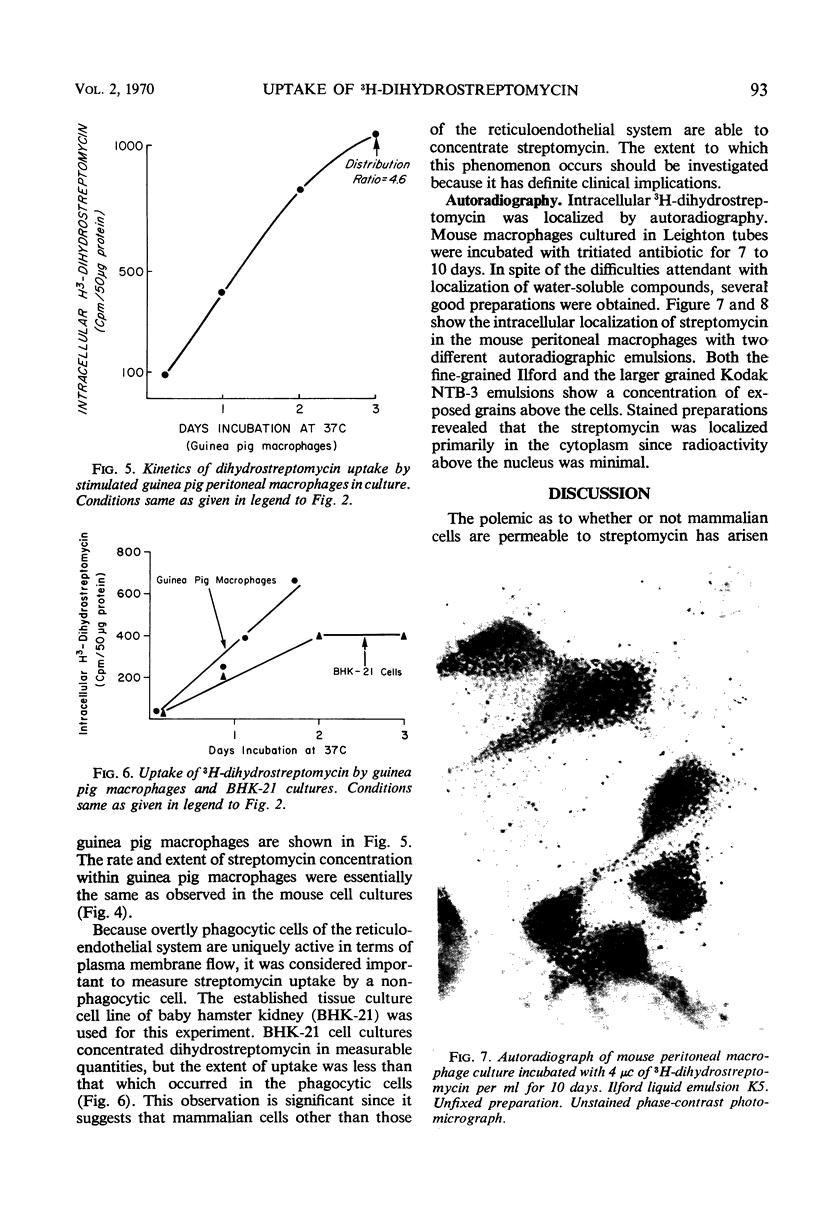
Mouse peritoneal macrophages, in culture, concentrate significant amounts of 3H-dihydrostreptomycin, provided that the incubation period is sufficiently extended. Macrophages cultured in vitro from both stimulated and unstimulated animals concentrate the antibiotic from growth or maintenance media. The increase in cell-associated radioactivity is linear for almost a week before a plateau is reached. Calculations based on intracellular volumes of the cells indicate that the intracellular concentration of dihydrostreptomycin may attain levels greater than five times that of the external milieu. No uptake is measurable at 4 C, suggesting an active mechanism of transport into the cell. Phagocytosis of killed bacteria during incubation did not increase uptake of the antibiotic nor did the addition of poly-l-ornithine to the medium augment uptake. A nonphagocytic cell line (BHK-21) concentrated 3H-dihydrostreptomycin to a lesser extent than the macrophages. These observations suggest that a wide variety of mammalian cells may be permeable to the antibiotic, and thus potential bactericidal action on intracellular bacteria cannot be ignored.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett B. Isolation and cultivation in vitro of macrophages from various sources in the mouse. Am J Pathol. 1966 Jan;48(1):165–181. [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Hayes R., Imhoff J. Autoradiographic evidence for the impermeability of mouse peritoneal macrophages to tritiated streptomycin. J Bacteriol. 1967 Jan;93(1):445–450. doi: 10.1128/jb.93.1.445-450.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. T. Suppressive activity of streptomycin on the growth of Mycobacterium lepraemurium in macrophage cultures. Appl Microbiol. 1969 May;17(5):750–754. doi: 10.1128/am.17.5.750-754.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J. Uptake of biologically active substances by lysosomes. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):85–93. doi: 10.1098/rspb.1969.0040. [DOI] [PubMed] [Google Scholar]
- FREEMAN B. A., VANA L. R. Host-parasite relationships in brucellosis. I. Infection of normal guinea pig macrophages in tissue culture. J Infect Dis. 1958 May-Jun;102(3):258–267. doi: 10.1093/infdis/102.3.258. [DOI] [PubMed] [Google Scholar]
- Gabathuler M. P., Ryser H. J. The digestive function of lysosomes as studies by the turnover of ingested foreign macromolecules. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):95–98. doi: 10.1098/rspb.1969.0041. [DOI] [PubMed] [Google Scholar]
- Hart P. D. Mycobacterium tuberculosis in macrophages: effect of certain surfactants and other membrane-active compounds. Science. 1968 Nov 8;162(3854):686–689. doi: 10.1126/science.162.3854.686. [DOI] [PubMed] [Google Scholar]
- JENKIN C., BENACERRAF B. In vitro studies on the interaction between mouse peritoneal macrophages and strains of Salmonella and Escherichia coli. J Exp Med. 1960 Aug 1;112:403–417. doi: 10.1084/jem.112.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCHAN I., SMITH L. ANTIMYCOBACTERIAL ACTIVITY OF TUBERCULOSTATIC FACTOR ON INTRACELLULAR BACILLI. J Immunol. 1965 Feb;94:220–227. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. The response of cultured mammalian cells to diphtheria toxin. II. The resistant cell: enhancement of toxin action by poly-L-ornithine. J Exp Med. 1968 Mar 1;127(3):541–554. doi: 10.1084/jem.127.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Oxman E., Bonventre P. F. Facilitated uptake of streptomycin by Kupffer cells during phagocytosis. Nature. 1967 Jan 21;213(5073):294–295. doi: 10.1038/213294a0. [DOI] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Multiplication of Mycobacterium tuberculosis Within Normal and "Immune" Mouse Macrophages Cultivated With and Without Streptomycin. Infect Immun. 1970 Jan;1(1):30–40. doi: 10.1128/iai.1.1.30-40.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser H. J. Uptake of protein by mammalian cells: an underdeveloped area. The penetration of foreign proteins into mammalian cells can be measured and their functions explored. Science. 1968 Jan 26;159(3813):390–396. doi: 10.1126/science.159.3813.390. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., SHIRLEY W., BARDAWIL W. A. 'Piggy-back' phagocytosis. Nature. 1962 Apr 21;194:255–256. doi: 10.1038/194255a0. [DOI] [PubMed] [Google Scholar]
- SHEPARD C. C. Use of HeLa cells infected with tubercle bacilli for the study of antituberculous drugs. J Bacteriol. 1957 Apr;73(4):494–498. doi: 10.1128/jb.73.4.494-498.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. The multiplication of tubercle bacilli within normal phagocytes in tissue culture. J Exp Med. 1952 Aug;96(2):137–150. doi: 10.1084/jem.96.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]