Summary
On October 11, 2013, the Framingham Heart Study will celebrate 65 years since the examination of its first participant in 1948. During this period, the study has provided substantial insight into the epidemiology of cardiovascular disease and its risk factors. The origin of the study is closely linked to the cardiovascular health of President Franklin D. Roosevelt and his premature death from hypertensive heart disease and stroke in 1945. The present article describes the events leading to the founding of the Framingham Heart Study, and provides a brief historical overview of selected contributions from the study.
Introduction
Cardiovascular disease is the most common cause of mortality in developed countries.1,2 Across the globe, the incidence of death from cardiovascular and circulatory diseases has risen by one third between 1990 and 2010, such that by 2015 one in three deaths worldwide will be due to cardiovascular diseases.3 Epidemiologic studies have played an important role in elucidating the factors that predispose to cardiovascular disease and highlighting opportunities for prevention. On October 11, 2013, the Framingham Heart Study will celebrate 65 years since the examination of its first participant in 1948. The present article describes the events leading to the founding of the Framingham Heart Study, and reviews some of its important contributions to our understanding of cardiovascular disease and its risk factors.
Search strategy and selection criteria
We searched the archives of the Framingham Heart Study located at the National Heart, Lung, Blood Institute in Bethesda, Maryland. We also searched Harvard University's Widener Library collection of ‘President Franklin D. Roosevelt's Office Files, 1933-1945,’ in Cambridge, Massachusetts. Additional references between January 1947 to March 2013 were obtained using PubMed and Google Scholar by combining the search term “Framingham Heart Study” with the search term “risk factor,” “hypertension,” “coronary heart disease,” “diabetes mellitus,” “atrial fibrillation,” “cerebrovascular accident,” “stroke,” “lipids,” “cholesterol,” “triglyceride,” “LDL,” “HDL,” “obesity”, “survival,” “prognosis,” “risk profile.” We restricted our search to works published in English.
Origins of the Framingham Heart Study
By the 1940s, cardiovascular disease had become the number one cause of mortality among Americans, accounting for 1 in 2 deaths.4 Prevention and treatment were so poorly understood that most Americans accepted early death from heart disease as unavoidable. Franklin Delano Roosevelt, the United States' war-time President from 1933 to 1945, was in no way exempt from the epidemic, suffering from heart failure due to undiagnosed, and later untreated, risk factors.5 The present article will describe how medical care provided to the President preceding his sudden death while still in office in 1945 illustrates the poor state of our understanding of cardiovascular disease in the mid-20th century. These events likely played a role in prompting the creation of the Framingham Heart Study in 1948.
In 1932, candidate Roosevelt's campaign office released medical records showing his blood pressure to be 140/100 mm Hg, which did not prompt any medical intervention.6 p211 Such was the lack of understanding of cardiovascular disease that the following year the President-elect chose an ear, nose, throat specialist, Admiral Ross McIntyre, as his personal physician since it was felt that headaches and sinus problems would be the primary future health concern.7 Between 1935 to 1941, the President experienced a gradual rise in blood pressure from 136/78 to 188/105 mm Hg.6 p213 During this period, he dedicated the National Institute of Health's newly established Bethesda, Maryland campus in 1940. Despite the rising blood pressure, his personal physician insisted that the President was healthy, and his blood pressure “no more than normal for a man of his age.”6 p215 Roosevelt's physical deterioration was evident to many, and when British Prime Minister Winston Churchill visited the White House in May 1943, he asked his own physician whether he too had “… noticed that the President is a very tired man?”8
On March 27, 1944, as planning for the Allied landing at Normandy was underway, daughter Anna Roosevelt insisted on a second opinion, and the President was admitted to Bethesda Naval Hospital for dyspnea on exertion, diaphoresis, and abdominal distension.9 Cardiologist Dr. Howard G. Bruenn, one of only a few hundred such specialists in the entire nation, attended to the President. Dr. Bruenn noted that the patient appeared “slightly cyanotic,” with “blood pressure 186/108” mmHg and a chest x-ray showing an “increase in size of the cardiac shadow.” The young cardiologist gave the President his first diagnosis of “hypertension, hypertensive heart disease, and cardiac failure.”5 However, Dr. Bruenn had few therapeutic options to provide, suggesting digitalis and salt intake reduction. After at first rejecting the cardiologist's advice, the President eventually started digitalis with some symptom relief, and a follow-up chest x-ray 2 week later showed reduced cardiomegaly. A month after coming under Dr. Bruenn's care, Roosevelt's blood pressure had risen to 240/130 mmHg after unsuccessful phenobarbital treatment.
In 1945, two months before his death, Roosevelt attended the Yalta Conference with Churchill and Soviet Premier Joseph Stalin to negotiate the anticipated post-war administration of Germany, and a future United Nations.10 Lord Charles Moran, Churchill's personal physician, wrote in his diary “the President appears a very sick man. He has all the symptoms of hardening of the arteries…” and “I give him only a few months to live.” 8 p242 Critical of the unappreciated cardiovascular state, he also noted: “… the Americans here cannot bring themselves to believe that he is finished. His daughter thinks he is not really ill, and his doctor backs her up.” As predicted, President Roosevelt died a few weeks later on April 12, 1945, at the age of 63, from cerebral hemorrhage with a blood pressure of 300/190 mmHg.5 Like countless other Americans, he had succumbed to the national epidemic of cardiovascular disease.
On June 16, 1948, President Harry Truman signed into law the ‘National Heart Act.’ In it the US Congress declared “Whereas the Congress hereby finds and declares that the Nation's health is seriously threatened by diseases of the heart and circulation, including high blood pressure… These diseases are the main cause of death in the United States and more than one in every three of our people die from them…”11 The law allocated a $500,000 seed grant for a twenty-year epidemiological heart study, and also established the National Heart Institute, which today is known as the National Heart, Lung and Blood Institute.
Choosing a location
In 1947, as legislators were drafting the National Heart Act, the US Public Health Service (PHS) delegated a young officer and physician, Gilcin Meadors, to compile a proposal for the future epidemiological study (Figure 1). Although initially focusing on ischemic heart disease, Dr. Meadors set the tone for the next 65 years by proposing “to study the expression of coronary artery disease in a ‘normal’ or unselected population and to determine the factors predisposing to the development of the disease through clinical and laboratory exam and long term follow-up…”12 The initial budget request was $94,350 to cover office supplies, and even included funds to buy ashtrays for the smoking needs of the study's staff members.
Figure 1.
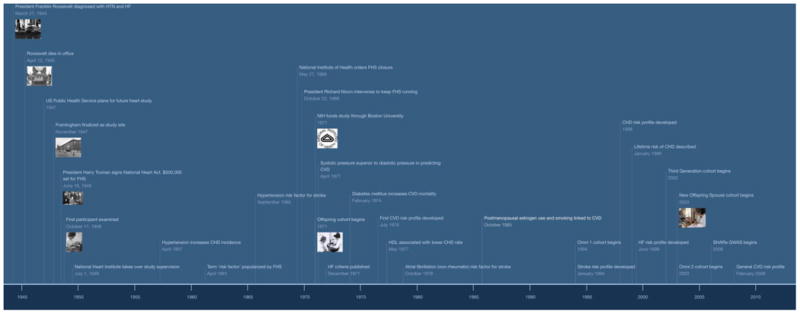
Key dates in the history of Framingham Heart Study. HTN = hypertension, HF = Heart failure, FHS = Framingham Heart Study, CHD = coronary heart disease, NIH = National Institutes of Health, CVD = cardiovascular disease, SHARe = SNP Health Association Resource, GWAS = genome wide association study
Dr. Paul Dudley White of the Massachusetts General Hospital and Dr. David Rutstein of Harvard Medical School advocated for the epidemiological study to be located at Framingham, Massachusetts. The state was considered ideal due to the enthusiastic response of area physicians (Figure 2), and Framingham prevailed over neighboring towns due to geographical proximity to the many cardiologists at Harvard Medical School.13 Framingham residents, who made decisions through a town-meeting form of government, had already participated in the Framingham Tuberculosis Demonstration Study two decades earlier.14 The one-time farming community was now a factory town of 28,000 middle-class residents of predominantly European origin producing rugs, paper products, and General Motor automobiles, and was therefore considered to be representative of the United States in the 1940s.15 p1b-5 On October 12, 1947, the US Public Health Service began funding the study in Massachusetts, in cooperation with the state's Health Department and Harvard Medical School, which would provide “overall professional and technical guidance.”16,17 Framingham was finalized as the study site in the following month.
Figure 2.

Letter from US Public Health Service to Gilcin Meadors on positive response from Massachusetts physicians regarding Framingham as a site. Courtesy: NHLBI archives. Non-Federal employees names deleted per NHLBI policy.
Gilcin Meadors, who had been given authority by the PHS to establish the study, and effectively became its first director, relocated to Boston in order to start recruiting 6,000 of the town's 10,000 adult residents. 14,18 Initially operating out of the Harvard Medical School, he hired nurse Nell McKeever and together they visited parent-teacher associations, churches, and civic groups, and brought in local volunteers to act as telemarketers calling nearly all the town phone numbers.
On September 29, 1948, the Framingham Heart Study examined its own staff members “for the purpose of testing schedules, procedures, equipment and [smoothing] out technique for interview and records completion.”19 On October 11, 1948 the study officially examined its first Framingham participant, exactly 12 months after Gilcin Meadors' arrival in Massachusetts.20 The groundwork had thus been laid for the longitudinal follow up of this cohort, for the purpose of identifying individual factors that could be related to the future development of diseases.
Early days of Framingham
The Framingham Heart Study was the first long-term study of its kind, with the exception of Sir James Mackenzie's aborted attempt to longitudinally follow the health status of St. Andrews, Scotland residents.21 As Framingham investigators were setting up their research in the late 1940s, Ancel Keys in Minnesota was also in the process of establishing a three decades long study to be called ‘Twin Cities Business and Professional Men's Study,’ as were researchers at University of California Los Angeles who would go on to follow city civil servants for a decade.22-24 Framingham investigators initially struggled between whether to be an observational study to understand heart disease, or instead focus on preventing heart disease in the local population. Ultimately, given the paucity of effective interventions, the former approach won favor. Within a few months of the first examination, the newly-established National Heart Institute assumed control of the study.15 p1c-2 Gilcin Meadors was responsible for building study infrastructure, though the National Heart Institute played a large role in ensuring the early scientific robustness of the study. Felix Moore, the institute's chief of biometrics, designed the original model for longitudinal follow up. Additionally, realizing that population prevalence of diseases required random sampling, he changed the methodology from soliciting volunteers to active recruitment of a random sample of town adults.
Organizers of the new study also had to ensure community and local physician support. An Executive Committee, composed of 15 residents representing the town's various groups, recommended that the study not break up families, and that all members aged 30-60 years be recruited.15 p1d-2 Community ownership of the study was realized through a Neighborhood Organizing Committee that reached out to residents to urge participation in the study.15 p1d-3 The Professional Advisory Committee, consisting of local physicians, asked that investigators not treat or offer advice to any participants, and instead diagnostic information was furnished to each participant's personal physician.
The original cohort was recruited between 1948 and 1952 and consisted of 5209 residents aged 28 to 62 years (Table 1).25 Women comprised more than half of all participants. The study's inclusion of women contrasted with contemporaneous epidemiological studies, which had very small numbers of women or excluded them altogether.23,24 Records were originally kept on carbon paper, an innovative system at the time (Figure 3). These approaches were developed by Dr. Thomas Dawber, who became the second director in April 1950, along with Patricia McNamara and Dr. William Kannel. Hospital admissions were recorded by daily visits to both Framingham hospitals, while deaths were noted by scanning newspapers, personal physician communications or coroner reports.
Table 1. Population cohorts for the heart study.
| Cohort | First Year | Size | % Female | Salient Features |
|---|---|---|---|---|
| Original | 1948 | 5209 | 55% | |
| Offspring | 1971 | 5124 | 52% | Children of the Original Cohort, and their spouses |
| Third Generation | 2002 | 4095 | 53% | Children of the Offspring Cohort |
| New Offspring Spouse | 2003 | 103 | 54% | Spouses of Offspring Cohort who were not initially enrolled in FHS, and whose 2 children are in Third Generation Cohort. Added to improve statistical power |
| Omni 1 | 1994 | 506 | 58% | To reflect the increasing ethnical diversity of the community. African-American, Hispanic, Asian, Indian, Pacific Islander and Native American |
| Omni 2 | 2003 | 410 | 57% | Recruited in order to achieve 10% of Third Generation Cohort size |
Figure 3.
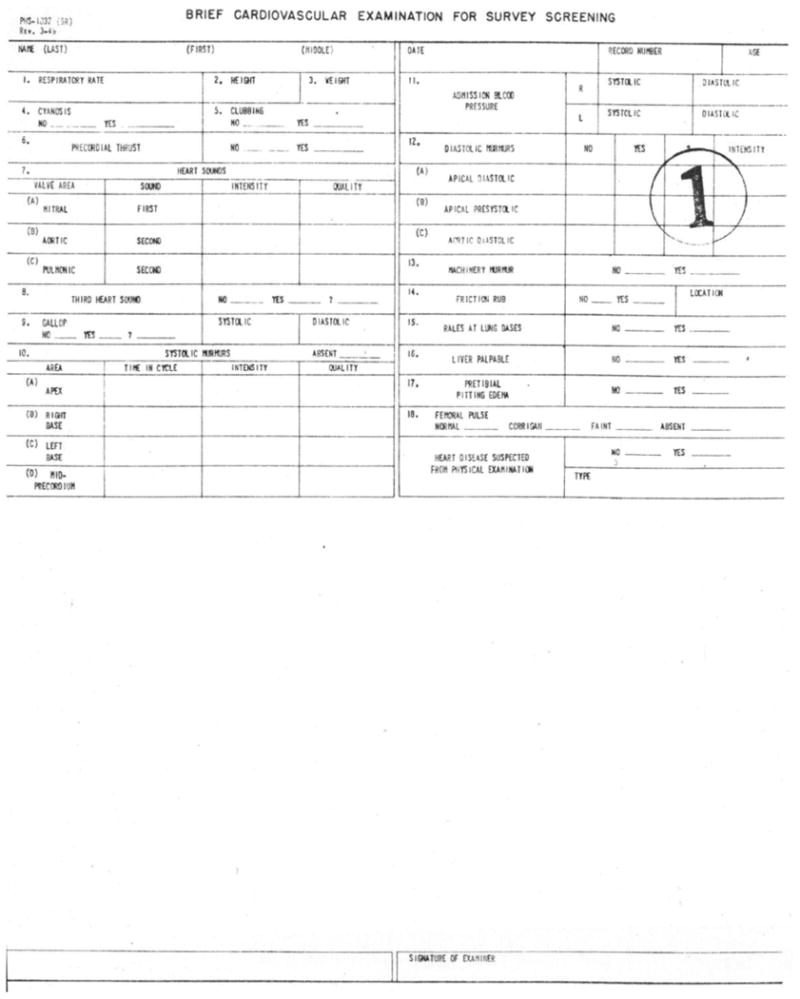
Data entry form for the Original Cohort c1947. Courtesy: NHLBI archives
The first major study findings were published in 1957, almost a decade after the initial participant was examined. Defining hypertension as a systolic blood pressure ≥160/95 mmHg, they found a nearly 4 fold increase in coronary heart disease incidence per 1000 persons among hypertensive participants.26 A few years later they noted that stroke was also a major consequence of high blood pressure.27
Despite these early reports, many still believed that a permissible systolic blood pressure was 100 plus the participant's age in millimeters of mercury.28,29 In 1964, when the hypotensive properties of the beta blocker propranolol were first studied, “normotensive” controls included patients with systolic blood pressures as high as 170mmHg.30 For those aged >70 years, some considered the acceptable upper limits of normal blood pressure to be 210 mmHg systolic and 120 mmHg diastolic.31 Adherence to these beliefs stemmed from the medical community's uncertainty regarding the validity of an epidemiological approach, as evidenced from the challenges faced during attempts to create the Council on Epidemiology at the American Heart Association in 1961.32 Critics of Framingham expressed uncertainty about whether study participants represented Americans in general and about the study's family-based approach.33
Fight for survival
In 1966, as the initial twenty-year funding commitment neared an end the National Heart Institute established a committee to evaluate the Framingham Heart Study. Sensing the possible loss of the needed $336,000 annual funding, Dr. Dawber moved to Boston University to raise private funds to continue the study.34 In his place, Dr. Kannel took over as the third director of the Framingham Heart Study.
Dr. Dawber's concern was well founded. On May 27, 1969, the National Institute of Health issued a directive ordering phasing out of the study over the next year, despite a favorable review by the expert committee. In response, Framingham investigators toured the nation to raise private funds. Donors included a large number of life insurance corporations that saw the actuarial benefits of the study. The list also included some surprising bedfellows, including the Tobacco Research Institute and the Oscar Mayer Company.35 On being notified of the impending study closure by Paul Dudley White, President Richard Nixon intervened, allowing the study to continue to fulfill its mission.18 In 1971, the National Heart Institute entered an agreement with Boston University that provided support for the Framingham Heart Study through a federal contract, thereby ending the need for private donors.
With the renewal of funding, the study began to recruit the offspring of the original Framingham participants into a new ‘Offspring cohort’ (Table 1).36 The purpose of this new cohort was to provide insight into familial clustering of disease. Since the study would also require the examination of biologically unrelated individuals, spouses of offspring participants were also invited into the study and ended up comprising nearly a third of the study sample. The creation of a family-based cohort was prescient, given the emergence of new genotyping and sequencing technologies a few decades later.
Epidemiological activism
Thomas Dawber, the second study director, noted that medical practice in the mid-20th century was directed towards caring for those who were already ill rather than prevention of disease.35 p222 Having had limited success in altering the way physicians practiced medicine despite early Framingham study findings, Dawber concluded that “attitudinal changes on the parts of physicians, although difficult, is essential [for] advances,” and that “medical education and training was basically responsible for the attitude of physicians.”35 p222 Even in the early 1970s, physician reference books such as Harrison's Principles of Internal Medicine and Cecil Loeb Textbook of Medicine reiterated that diastolic pressure was a superior measure of blood pressure, and consequently elevated systolic pressure was considered innocuous, especially in the elderly.37,38 Although some hospital based and autopsy studies had begun to challenge the presumed unimportance of systolic pressure, they were limited by selectivity bias and sample size.39,40 Having recovered from the funding crisis, Framingham investigators were now ready to adapt an approach of ‘epidemiological activism’ with a focus on hypertension.41
In 1971, Framingham investigators analyzed 14 years of follow-up data to demonstrate increased risk of coronary heart disease morbidity with rising baseline blood pressure.42 When systolic and diastolic pressures were studied for association with coronary heart disease events, systolic pressure demonstrated a stronger link than diastolic pressure (Figure 4). In two other studies, investigators found that elevated systolic blood pressure was a predictor of cerebrovascular accidents as well as heart failure, and that diastolic pressure was not superiorly associated with these events. 43,44
Figure 4.
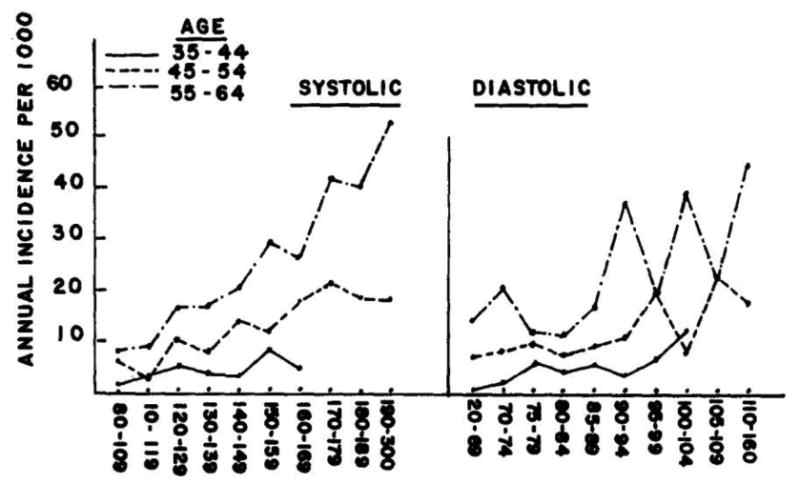
Systolic pressure as a superior marker for average annual incidence for coronary heart disease. Adapted with permission from Kannel at. al. 1971
More than two decades after President Roosevelt's death from poorly controlled blood pressure, Framingham authors commented on the “mounting evidence that many of the commonly accepted beliefs concerning hypertension and its cardiovascular consequences may be in error.”42 They challenged the existing belief “that systolic pressure is unimportant,” and that “labile hypertension is of little consequence,” pointing out that there was not only “little evidence to support these contentions,” but in fact “considerable reason to doubt them.”42
The importance of controlling blood pressure was finally embraced in practice guidelines in the first ‘Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure’ in 1977.45 The Committee still recommended diastolic blood pressure as the basis for diagnosis and treatment of hypertension, but over the next decade, the emphasis on diastolic blood pressure as the principal treatment target was dropped after randomized controlled trials demonstrated the cardiovascular benefits of systolic blood pressure reduction.46,47
Framingham Risk Scores
Thus, studies from Framingham and other epidemiological cohorts contributed to a paradigm shift in the latter half of the 20th century, from a focus on treating individuals with established cardiovascular disease to the prevention of disease in those at risk. A key element of this strategy was the ability to identify individuals most likely to have a future cardiovascular event, to enable the targeting of preventive interventions. Studies in this period shed light on what we now refer to as cardiovascular ‘risk factors,’ including hypertension, hyperlipidemia, and diabetes mellitus. Indeed, the term “risk factor” was popularized in the medical lexicon by Thomas Dawber and William Kannel by their 1961 publication, “Factors of Risk in the Development of Coronary Heart Disease.”25
The articulation of the risk factor concept laid the foundation for the development of clinical risk scores. The first attempt to create a multivariable risk function for coronary heart disease in Framingham was published by Truett, Cornfield, and Kannel in 1967.48 Prior to that, the typical approach to considering multiple risk factors simultaneously was “multiple cross-classification,” which involved creating tables with cells corresponding to combinations of the risk factors. Unfortunately, with more than a few variables, thousands of cells would be needed. The authors proposed a multivariable logistic models with 7 risk factors: age, total cholesterol, weight, ECG abnormality, hemoglobin, cigarettes smoked, and systolic blood pressure. Individuals in the top decile compared with the lowest decile had a thirty-fold (men) to seventy-fold (women) difference in the incidence of coronary heart disease.
Subsequent papers provided risk functions, or “risk profiles,” that enabled physicians to directly calculate an individual's predicted risk of having a cardiovascular event. The first risk profile was published in 1976 by Kannel and colleagues, and covered a general cardiovascular endpoint that included coronary heart disease, stroke, claudication, and heart failure.49 The variables in the model mirrored those in the Truett study, except that glucose intolerance was added, and weight was dropped.
The best known risk profile is the Framingham Risk Score for coronary heart disease, published in 1998 by Wilson and colleagues.50 This function became the basis of the risk calculator adopted by the Adult Treatment Panel of the National Cholesterol Education Program in the United States.51 Compared with previously published functions, the 1998 model substituted risk factor categories in the place of continuous values, which enabled clinicians to use lookup tables to obtain risk estimates. The 10-year risk estimates used in the 1998 score provided a convenient way to classify individuals as low, intermediate, or high risk for future coronary heart disease.
Framingham and the epidemiology of heart failure
President Roosevelt's failing health from heart failure underscores the relatively poor understanding of the clinical syndrome at the time the Framingham cohort was initiated. Until the late 1960s, research on heart failure was hampered by a lack of consistent diagnostic criteria. For instance, in a 1965 observational study to assess the prevalence of heart failure in two rural US communities, the author explained that “no attempt was made to define congestive heart failure to the assessing physician, since it was his operational diagnosis that was sought.” 52 In the absence of standard criteria, investigators faced a pathology with “an ill-defined collection of signs and symptoms,” which hampered efforts to identify factors predisposing to and influencing the course of true heart failure.53
By the end of the 8th Framingham examination cycle in 1966, investigators had noted a rising prevalence of heart failure in the cohort. They therefore developed a set of clinical criteria for heart failure (Table 2), which were applied to nearly two decades of collected data where staff had already been noting whether they clinically suspected participants of having heart failure.54,55 The first paper introducing these criteria was published in 1971 by McKee and Kannel.56 Nine ‘major’ criteria and seven ‘minor’ criteria were defined, along with one criterion that could be major or minor depending on whether the ≥4·5 Kg weight loss was due to heart failure treatment or from another possible etiology. A diagnosis of ‘Definite heart failure’ was made if the patient had two major, or one major plus two minor criteria concurrently. By focusing on clinical symptoms, the investigators understood that heart failure was a clinical syndrome. Since their development in the late 1960s, these criteria have been in continuous use, not only in Framingham but in multiple cohorts around the world. 57
Table 2. Criteria for heart failure.
| MAJOR | MINOR |
|---|---|
|
|
Minor or Major: Weight loss (≥4.5 Kg) in 5 days, in response to HF therapy.
In 1971, McKee and Kannel used the newly described criteria to show for the first time that hypertension was in fact the leading risk factor for heart failure (Figure 5).56 Examining 16 years of observational data, the investigators found that hypertension preceded three-quarters of heart failure cases, compared with coronary artery disease, which preceded heart failure in less than 40% of cases.
Figure 5.
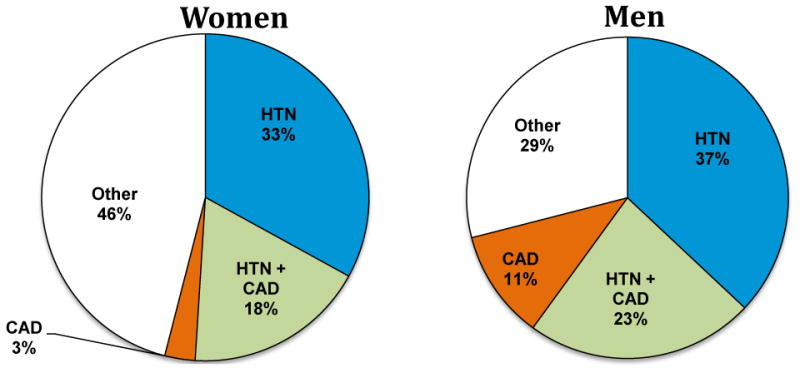
Hypertension as the dominant risk factor for heart failure. HTN = hypertensive cardiovascular disease, CAD = coronary artery disease. Adapted from McKee at. al. 1971
Years later, Levy and colleagues calculated the Population Attributable Risk (PAR) for heart failure risk factors, showing the percentage of heart failure cases that would be reduced if a causal risk factor was eliminated from the population.57 Myocardial infarction was associated with a 6-fold risk for heart failure, but the PAR was only 13% (women) and 34% (men). On the other hand, hypertension was associated with a >2 fold increased risk of heart failure, but had a PAR of 59% (women) and 39% (men) owing to its high prevalence.
Because heart failure diagnoses in Framingham have been prospectively adjudicated using a single set of diagnostic criteria since the 1960s, the study has been a valuable source of information regarding long-term trends in the epidemiology of the condition. The original 1971 publication highlighted the poor prognosis of heart failure: only 2 in 5 men were alive 5 years after diagnosis the heart failure, and only 1 in 5 survived to 10 years.56 For women, the survival experience was similar. By the 1990s, the widespread use of mortality-altering medications such as beta-blockers and ACE inhibitors had altered this prognosis. Using 50 years of follow up data, Levy and colleagues reported that the 5 year mortality declined in men from 70% in 1950 to 59% in 1999, and in women from 57% to 45%.58
Other studies from Framingham documented alterations in cardiac structure or function that preceded overt heart failure. A study in 1997 by Vasan and colleagues demonstrated that increased left ventricular end-diastolic diameter predicted incident heart failure in individuals free of myocardial infarction.59 Wang and colleagues showed that individuals with asymptomatic left ventricular systolic dysfunction had a 5-fold increased risk for heart failure, even after adjusting for conventional risk factors.60 Recent guidelines have a notable emphasis on the detection of asymptomatic ventricular dysfunction (“stage B heart failure”) as part of efforts to understand and prevent overt heart failure.61
It also became apparent that clinical manifestations of heart failure can be present in the absence of left ventricular systolic dysfunction. Interestingly, this recognition was possible because the Framingham criteria for heart failure predated the widespread availability of echocardiography, meaning that the diagnosis of heart failure did not require left ventricular systolic dysfunction. In 1999, Vasan and colleagues reported that approximately half of Framingham participants with heart failure had a normal ejection fraction (>50%) at the time of diagnosis.62 Survival of individuals with heart failure with preserved ejection fraction (or diastolic heart failure) was substantially worse compared with individuals without heart failure, though it was better compared with individuals with systolic heart failure. Studies from other cohorts yielded consistent findings, and led to increased recognition that diastolic heart failure is common in the community, particularly among older individuals and women.
Metabolic risk factors for heart disease
Framingham investigators also joined the effort of researchers worldwide to understand the links between metabolic risk factors and cardiovascular disease. In the first half of the 20th century, autopsy and hospital-based studies had noted an association between diabetes mellitus and cardiovascular disease.63,64 By the middle of the century, clinical data had emerged on the link between diabetes and vascular disease,65-67 an association also demonstrated in Framingham.68 In Framingham, cardiovascular mortality was 3 fold higher for individuals with diabetes. Diabetes was associated with substantially higher risks for heart failure and hypertensive heart disease.69,70
Cholesterol had been linked to cardiovascular disease by the early 20th century through animal and autopsy studies.71,72 Ancel Keys described elevated levels of cholesterol among coronary heart disease patients.73 In 1977, Gordon and other Framingham investigators reported an inverse relationship between HDL concentrations and coronary heart disease incidence, in contrast to the positive association between LDL concentrations and coronary heart disease incidence.74 That same year, in collaboration with other epidemiological studies in the United States, investigators reported that individuals with coronary disease had lower HDL concentrations than healthy participants across multiple ethnicities.75 Framingham researchers commented, “It is curious … that the determination of HDL cholesterol has not long since become part of standardized coronary heart disease risk profile.”74 Notably, the 1998 version of the Framingham Risk Score, adopted by the National Cholesterol Education Program, contained both total and HDL cholesterol.50
As obesity occurs concomitantly with hypertension, elevated lipids, and diabetes, the increased cardiovascular risk in obese individuals is often attributed to these co-existing risk factors. By the late 1970s, when William Castelli became the fourth director of the Framingham Heart Study, the general US population weight had been increasing for several decades.76 In 1983, he and his colleagues reported that weight gain conferred an increased risk for cardiovascular disease, which persisted despite adjustment for other risk factors.77 This risk was particularly apparent for heart failure. Framingham participants less than 50 years old had a 2 to 3-fold excess risk of heart failure from the lightest to the heaviest weight category.77 A paper by Kenchaiah and colleagues in 2002 using updated Framingham data arrived at similar conclusions.78 The PAR for heart failure due to obesity was 14% in women, and 11% in men, higher than that observed for diabetes mellitus, valvular heart disease, or left ventricular hypertrophy.
Epidemiology of stroke and atrial fibrillation
By the 1960s, stroke was still the third leading cause of death among Americans.27 In the pre-imaging era, Framingham investigators diagnosed stroke through clinical history, neurologic examination, and sometimes, lumbar puncture. Each suspected new case of stroke was confirmed by a second examiner as well as through neurological consultation. In addition to establishing the link between systolic blood pressure and stroke,27 investigators showed that the risk of stroke from hypertension was even greater than the conferred risk for coronary heart disease.43
One of the most valuable clinical contributions from Framingham was the demonstration that non-rheumatic atrial fibrillation was a potent risk factor for stroke. In a study published in 1978, Wolf and colleagues reported that chronic AF not due to rheumatic heart disease was associated with a >5-fold excess risk of stroke,79 an observation that led them to call for “controlled trials of anticoagulation or antiarrhythmic agents in persons with chronic atrial fibrillation.” Fortunately, such trials were performed and anticoagulation for individuals with atrial fibrillation became the standard of care.
Given the similarities between atrial fibrillation and heart failure, two conditions that follow adverse cardiac remodeling, it is not surprising that they share a number of epidemiological features. For instance, Benjamin and colleagues showed that the PAR for developing atrial fibrillation was highest for hypertension, despite the fact that other risk factors were associated with higher relative risks.80 Data from Framingham also emphasized the underappreciated contribution of obesity to atrial fibrillation risk.81
New cohorts
Towards the end of the 20th century, Framingham investigators identified a need to expand the knowledge regarding genetic and environmental risk factors for cardiovascular diseases.82 Thus, in 2002, they began the recruitment of a new generation of participants, the Third Generation cohort (Table 1). The cohort was comprised of children of Offspring cohort participants.82 Recognizing the power of the family-based approach, investigators gave priority to 879 large, extended families that already had multiple participants in the study.
Investigators also recognized the disadvantages of a cohort that was predominantly white of European descent. The Omni 1 cohort was recruited in 1994 and included 506 minority residents of Framingham. A decade later, an additional 410 minority participants were recruited through the Omni 2 cohort.
In 2006, the National Institutes of Health funded the SHARe project (SNP Health Association Resource), which supported genome-wide genotyping across all of the Framingham cohorts. The data enabled Framingham to contribute to the global effort to study the genetic determinants of complex diseases, which has led to the identification of hundreds of common genetic variants influencing the risk of cardiovascular diseases.83
Conclusion
It has been over sixty-five years since President Franklin Delano Roosevelt's death in 1945 after a prolonged illness that started with uncontrolled hypertension and progressed to heart failure and stroke. Years later, reflecting on President Roosevelt's premature death, his cardiologist wrote: “I have often wondered what turn the subsequent course of history might have taken if the modern methods for the control of hypertension had been available.”5 The Framingham Heart Study was the product of a bill signed into law by President Roosevelt's successor. Fittingly, it has made many contributions to the understanding of the very cardiovascular conditions that led to President Roosevelt's death.
Acknowledgments
We thank Paul Sorlie, Ph.D., Chief of the Epidemiology Branch of the National Heart, Lung, Blood Institute, for his assistance granting access to the Framingham Archives in Bethesda, Maryland. We thank Gerald Oppenheimer, Ph.D., Professor of Clinical Sociomedical Sciences at Columbia University, New York for comments on the history of Framingham Heart Study.
Footnotes
Author Contributions: SSM performed literature search, and searched the Framingham Archives at National Heart, Lung, Blood Institute in Bethesda, Maryland as well as Harvard University's Widener Library collection of ‘President Franklin D. Roosevelt's Office Files, 1933-1945.’ All authors contributed to the compilation of this review.
Conflict of Interest: We declare no conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The European health report 2012: charting the way to well-being. Copenhagen, Denmark: WHO Regional Office for Europe; 2012. [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel WB. Contribution of the Framingham Study to preventive cardiology. Journal of the American College of Cardiology. 1990;15:206–11. doi: 10.1016/0735-1097(90)90203-2. [DOI] [PubMed] [Google Scholar]
- 5.Bruenn HG. Clinical Notes on the Illness and Death of President Franklin D. Roosevelt. Annals of Internal Medicine. 1970;72:579–91. doi: 10.7326/0003-4819-72-4-579. [DOI] [PubMed] [Google Scholar]
- 6.Bumgarner J. The health of Presidents. Jefferson, N.C.: McFarland; 1994. [Google Scholar]
- 7.Goldsmith H. A Conspiracy of Silence: The Health and Death of Franklin D Roosevelt. Lincoln, NE: iUniverse, Inc.; 2007. [Google Scholar]
- 8.Moran CMW. Churchill: Taken From the Diaries of Lord Moran: The Struggle for Survival, 1940-1965. Boston, MA: Houghton Mifflin Company; 1966. [Google Scholar]
- 9.Klara R. FDR's funeral train. New York, NY: Palgrace Macmillan; 2010. [Google Scholar]
- 10.Bernstein BJ. The Uneasy Alliance: Roosevelt, Churchill, and the Atomic Bomb, 1940-1945. The Western Political Quarterly. 1976;29:202–30. [Google Scholar]
- 11.Committee on Health E, Labor and Pensions, editor. 80th U S Congress. Senate. 2. 1948. The National Heart Act of 1948. [Google Scholar]
- 12.Meadors GF. In: Justification for the budget estimate for the sub-project “Epidemiology”. Service USPH, editor. Rockville, Maryland: US Public Health Service; 1947. Jul 19, [Google Scholar]
- 13.Robbins LC. In: Letter to Gilcin F Meadors. US Public Health Service PPA, editor. Epidemiology Branch, National Heart Lung Blood Institute; Bethesda, MD: 1947. Sep 5, [Google Scholar]
- 14.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–81. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon T, Kannel WB. Framingham Study. Framingham, MA: Framingham Heart Study; 1968. Jun, [Google Scholar]
- 16.Heart Disease Epidemiology Study: Manual of operation. Framingham, MA: Heart Disease Epidemiology Study; 1949. Nov 1, [Google Scholar]
- 17.Service USPH, editor. Service UPH. Agreement on Administrative Relationships for Fiscal Year 1948. Boston, Massachusetts: Epidemiology Branch, National Heart Lung Blood Institute; Bethesda, MD: 1947. Dec, [Google Scholar]
- 18.Levy D, Brink S. A Change of Heart: How the People of Framingham, Massachusetts, Helped Unravel the Mysteries of Cardiovascular Disease: First Vintage Books. 2006 [PubMed] [Google Scholar]
- 19.Meadors GF. In: Memorandum to Dr Bert R Boone (Chief Heart Disease Control Section, US Public Health Service) Study FH, editor. Framingham, Massachusetts: Framingham Heart Study; 1948. Oct 11, [Google Scholar]
- 20.Meadors GF. In: Memorandum to Chief, Heart Disease Control Section, US Public Health Service. Study FH, editor. Framingham, MA: Framingham Heart Study; 1948. Nov 1, [Google Scholar]
- 21.Waterston D, Orr J, Cappell DF. Sir James Mackenzie's Heart. Br Heart J. 1939;1:237–48. doi: 10.1136/hrt.1.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oransky I. Ancel Keys. The Lancet. 2004;364:2174. doi: 10.1016/S0140-6736(04)17578-8. [DOI] [PubMed] [Google Scholar]
- 23.Chapman JM, Goerke L, Dixon W, Loveland DB, Phillips E. IV. The Clinical Status of a Population Group in Los Angeles Under Observation for Two to Three Years. American Journal of Public Health and the Nations Health. 1957;47:33–42. doi: 10.2105/ajph.47.4_pt_2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keys A. Longevity of man: relative weight and fatness in middle age. Annals of medicine. 1989;21:163–8. doi: 10.3109/07853898909149927. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J., 3rd Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 26.Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nations Health. 1957;47:4–24. doi: 10.2105/ajph.47.4_pt_2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannel WB, Dawber TR, Cohen ME, McNamara PM. Vascular Disease of the Brain--Epidemiologic Aspects: the Farmingham Study. Am J Public Health Nations Health. 1965;55:1355–66. doi: 10.2105/ajph.55.9.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannel WB. Fifty years of Framingham Study contributions to understanding hypertension. J Hum Hypertens. 2000;14:83–90. doi: 10.1038/sj.jhh.1000949. [DOI] [PubMed] [Google Scholar]
- 29.Mickerson JN. Heart failure in hypertensive patients. Am Heart J. 1963;65:267–74. doi: 10.1016/0002-8703(63)90161-3. [DOI] [PubMed] [Google Scholar]
- 30.Prichard BN, Gillam PM. Use of Propranolol (Inderal) in Treatment of Hypertension. Br Med J. 1964;2:725–7. doi: 10.1136/bmj.2.5411.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caird FI. Heart disease in old age. Postgraduate medical journal. 1963;39:408. doi: 10.1136/pgmj.39.453.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackburn H, Epstein FH. History of the Council on Epidemiology and Prevention, American Heart Association. The pursuit of epidemiology within the American Heart Association: prehistory and early organization. Circulation. 1995;91:1253–62. doi: 10.1161/01.cir.91.4.1253. [DOI] [PubMed] [Google Scholar]
- 33.Werkö L. Risk factors and coronary heart disease—facts or fancy? American heart journal. 1976;91:87–98. doi: 10.1016/s0002-8703(76)80439-5. [DOI] [PubMed] [Google Scholar]
- 34.Oransky I. Thomas Royle Dawber. Lancet (London, England) 2006;367:106. [Google Scholar]
- 35.Dawber TR. The Framingham Heart Study: The epidemiology of atherosclerotic disease. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- 36.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 37.Harrison TR. Principles of Internal Medicine. Fifth. New York: McGraw-Hill; 1966. [Google Scholar]
- 38.Beeson P WM. Cecil Loeb Textbook of Medicine. Fifth. Philadelphia: WB Saunders; 1963. [Google Scholar]
- 39.Gubner RS. Systolic hypertension: A pathogenetic entity: Significance and therapeutic considerations. The American Journal of Cardiology. 1962;9:773–6. doi: 10.1016/0002-9149(62)90139-x. [DOI] [PubMed] [Google Scholar]
- 40.Finenberg M. Systolic hypertension. Its relationship to atherosclerosis of the aorta and larger arteries. Am J Med Sci. 1927;173:835–43. [Google Scholar]
- 41.Oppenheimer GM. Profiling risk: The emergence of coronary heart disease epidemiology in the United States (1947–70) International Journal of Epidemiology. 2006;35:720–30. doi: 10.1093/ije/dyl014. [DOI] [PubMed] [Google Scholar]
- 42.Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease: the Framingham study. The American Journal of Cardiology. 1971;27:335–46. doi: 10.1016/0002-9149(71)90428-0. [DOI] [PubMed] [Google Scholar]
- 43.Kannel WB, Wolf PA, Verter J, McNamara PM. Epidemiologic assessment of the role of blood pressure in stroke. The Framingham study. JAMA. 1970;214:301–10. [PubMed] [Google Scholar]
- 44.Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med. 1972;287:781–7. doi: 10.1056/NEJM197210192871601. [DOI] [PubMed] [Google Scholar]
- 45.Moser M, Guyther J, Finnerty F, Richardson D, Langford H. Report of the joint national committee on detection, evaluation, and treatment of high blood pressure: A cooperative study. JAMA. 1977;237:255–61. [PubMed] [Google Scholar]
- 46.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 47.Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–64. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 48.Truett J, Cornfield J, Kannel W. A multivariate analysis of the risk of coronary heart disease in Framingham. J Chronic Dis. 1967;20:511–24. doi: 10.1016/0021-9681(67)90082-3. [DOI] [PubMed] [Google Scholar]
- 49.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 50.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 51.2002 Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): National Heart, Lung and Blood Institute. 2002 Sep; [Google Scholar]
- 52.Gibson TC, White KL, Klainer LM. The prevalence of congestive heart failure in two rural communities. J Chronic Dis. 1966;19:141–52. doi: 10.1016/0021-9681(66)90045-2. [DOI] [PubMed] [Google Scholar]
- 53.Benack RT. Etiology of congestive heart failure in clinical medicine. Ann N Y Acad Sci. 1969;156:594–602. doi: 10.1111/j.1749-6632.1969.tb16754.x. [DOI] [PubMed] [Google Scholar]
- 54.Study FH, editor. Criterion for events: Framingham Monograph. Framingham, MA: Framingham Heart Study; 1968. May 27, [Google Scholar]
- 55.McNamara PM. In: Correspondance to Tavia Gordon. Study FH, editor. Framingham, MA: Framingham Heart Study; 1967. Nov 17, [Google Scholar]
- 56.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 57.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KKL. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 58.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 59.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left venticular dilation and the risk of congestive heart failure in people without myocardial infraction. N Engl J Med. 1997;336:1350–5. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 60.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 61.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 62.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricle ejection fraction: prevalence and mortality in a population-based cohort. j am coll cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 63.Root HF, Bland EF, Gordon WH, White PD. Coronary atherosclerosis in diabetes mellitus: A postmortem study. Journal of the American Medical Association. 1939;113:27–30. [Google Scholar]
- 64.Liebow IM, Hellerstein HK. Cardiac complications of diabetes mellitus. Am J Med. 1949;7:660–70. doi: 10.1016/0002-9343(49)90388-5. [DOI] [PubMed] [Google Scholar]
- 65.Partamian JO, Bradley RF. Acute myocardial infarction in 258 cases of diabetes. Immediate mortality and five-year survival. N Engl J Med. 1965;273:455–61. doi: 10.1056/NEJM196508262730901. [DOI] [PubMed] [Google Scholar]
- 66.Liebow IM, Hellerstein HK, Miller B. Arteriosclerotic heart disease in diabetes mellitus; a clinical study of 383 patients. Am J Med. 1955;18:438–47. doi: 10.1016/0002-9343(55)90224-2. [DOI] [PubMed] [Google Scholar]
- 67.Keen H, Rose G, Pyke DA, Boyns D, Chlouverakis C, Mistry S. Blood-Sugar and Arterial Disease. The Lancet. 1965;286:505–8. doi: 10.1016/s0140-6736(65)91470-4. [DOI] [PubMed] [Google Scholar]
- 68.Garcia MJ, McNamara PM, Gordon T, Kannell WB. Morbidity and mortality in diabetics in the Framingham population: sixteen year follow-up study. Diabetes. 1974;23:105–11. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 69.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 70.Kannel W, McGee D. Diabetes and cardiovascular risk factors: the Framingham Study. Circulation. 1979;59:8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- 71.Herrick JB. Clinical features of sudden obstruction of the coronary arteries. Journal of the American Medical Association. 1912;59:2015–22. [Google Scholar]
- 72.Classics in arteriosclerosis research: On experimental cholesterin steatosis and its significance in the origin of some pathological processes by N. Anitschkow and S. Chalatow, translated by Mary Z. Pelias, 1913. Arteriosclerosis. 1983;3:178–82. [PubMed] [Google Scholar]
- 73.Keys A, Fidanza F. Serum cholesterol and relative body weight of coronary patients in different populations. Circulation. 1960;22:1091–106. doi: 10.1161/01.cir.22.6.1091. [DOI] [PubMed] [Google Scholar]
- 74.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: The Framingham study. The American Journal of Medicine. 1977;62:707–14. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 75.Castelli WP, Doyle JT, Gordon T, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977;55:767–72. doi: 10.1161/01.cir.55.5.767. [DOI] [PubMed] [Google Scholar]
- 76.DHEW US. Weight by Height and Age for Adults 18-74 Years. In: Statistics NCfH, editor. Vital and Health Statistics. 1971-74. [PubMed] [Google Scholar]
- 77.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 78.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 79.Wolf PA, Dawber TR, Thomas HE, Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–7. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 80.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: The framingham heart study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 81.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 82.Splansky GL, Corey D, Yang Q, et al. The third generation cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. American journal of epidemiology. 2007;165:1328–35. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 83.Benjamin EJ, Rice KM, Arking DE, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–81. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]


