Abstract
Background
Cohort studies have demonstrated greater risk of myocardial infarction (MI) associated with specific antiretroviral use, while meta-analyses of randomized controlled trials have not. These differences may be due to inherent biases in the observational study design or to the limited duration of randomized trials. We conducted a new-user, active-comparator cohort study emulating a randomized controlled trial comparing initiation of several antiretrovirals as part of combination antiretroviral therapy (cART) and MI.
Methods
We included North Carolina (NC) Medicaid beneficiaries infected with HIV between 2002 and 2008 who were previously untreated with cART. We compared hazard ratios (HRs) and 95% confidence intervals (CIs) of MI between abacavir and tenofovir recipients, and lopinavir-ritonavir or atazanavir recipients and non-nucleoside-reverse-transcriptase-inhibitor (NNRTI) recipients. We adjusted for confounding through inverse-probability-weighting methods.
Results
There were 3,481 NC Medicaid new cART recipients who contributed 6,399 person-years and experienced 38 MI events. Receiving abacavir compared with tenofovir as part of cART was associated with an increased rate of MI unadjusted (HR= 2.70 [95% CI= 1.24 - 5.91]; HR= 2.05 [0.72 - 5.86]). Point estimates also suggest a relationship between receipt of atazanavir or lopinavir-ritonavir compared with an NNRTI and MI, although, estimates were imprecise.
Conclusions
We found an increased rate of MI among patients initiating abacavir compared with tenofovir although the association was decreased after confounding adjustment. Without a very large prospective comparative clinical trial, a much larger observational study of patients initiating cART would be needed to better define this apparent association.
The burden of disease among patients with Human Immunodeficiency Virus (HIV) infection has changed since the development of potent combination antiretroviral therapy (cART). With these important new therapies, conditions not related to-Acquired Immune Deficiency Syndrome (AIDS) are replacing AIDS-defining conditions as major causes of morbidity and mortality in HIV-infected patients.1 In this context, comparative effects of specific antiretroviral medications on cardiovascular disease, specifically myocardial infarction (MI) have been intensively evaluated. Results from two large cohort studies (Data Collection on Adverse Events of Anti-HIV Drugs and the Strategies for Management of Antiretroviral Therapy) suggest an increased risk of MI with current or recent, but not cumulative, use of abacavir.2,3 More recent observational studies have also shown an increased risk of MI associated with abacavir, 4-7 while others have not. 7,8 In contrast, meta-analyses of randomized controlled trials (RCTs) have not shown the same increased risk.9-11 Furthermore, cohort studies have demonstrated an association between cumulative exposure to first-generation protease inhibitors and MI-likely related to effects these medications have on lipid profiles.12,13
Some of the observed increased risk for MI among patients exposed to abacavir in observational studies may be attributed to confounding; patients prescribed abacavir were at a higher baseline risk for co-morbid conditions that increase the risk of cardiovascular disease.7 Many of the studies demonstrating an increased risk include prevalent users of antiretroviral medications. Inclusion of prevalent users makes it difficult to distinguish true confounders from clinical conditions affected by prior treatment and the under-ascertainment of events, particularly if the events occur early in treatment. 14 Furthermore, these observational studies used different comparison groups rendering it difficult to compare the results. While RCTs may not be subject to the same biases as observational studies, the shorter cumulative follow-up times and younger, healthier, populations may a reduce power to detect a difference between treatment groups.
To address the discrepancy between observational studies and meta-analyses of RCTs, it is important to design an observational study that would mimic a RCT.15 The use of a first-treatment-carried-forward (intention-to-treat in an RCT), new-user, active-comparator design attempts to define study cohorts with treatment equipoise, thus reducing the potential for confounding and selection bias. We used this type of cohort study design to examine the effects of initiating specific antiretroviral therapies on the risk for MI among previously untreated HIV-infected patients receiving combination antiretroviral therapy. Our study included three comparison groups (study arms): (1) tenofovir compared with abacavir (2) atazanavir compared with NNRTIs and (3) lopinavir compared with NNRTIs (Figures 1A and 1B).
Figure 1.
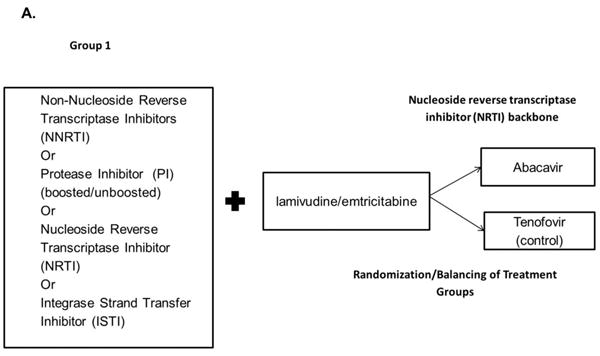
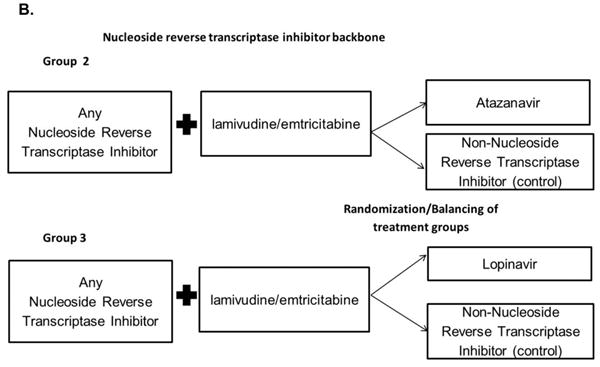
Active-comparator, new-user study design. HIV-positive patients who were initiators of combination antiretroviral therapy (cART) in the North Carolina Medicaid program between 2002 and 2008.
A.) Comparisons of the nucleoside reverse transcriptase inhibitors abacavir and tenofovir with any anchor antiretroviral plus lamivudine/emtricitabine (standard of care; Group 1).
B.) Comparisons of specific anchor antiretrovirals (the protease inhibitors atazanavir [Group 1] or lopinavir-ritonavir [Group 2] vs. any non-nucleoside reverse transcriptase inhibitor) plus lamivudine/emtricitabine and any nucleoside reverse transcriptase inhibitor.
Methods
Data source and Study Population
We implemented this cohort study using North Carolina (NC) Medicaid administrative data obtained for the years 2002-2008. The Medicaid program is state-and federally funded to provide health care benefits to persons with low income. The data contain health-care-service-reimbursement information, including that which is recorded for beneficiaries who are also eligible for Medicare.16
To be eligible for this study, patients had to (1) be ≥ 18 years of age (2) be HIV-infected, based on administrative criteria (ICD-9 code 042.xx or a claim for one of the 26 FDA-approved antiretroviral medications), (3) have at least 180 days of Medicaid eligibility prior to study entry, and (4) be new recipients of a cART regimen. A regimen was defined as a group of antiretrovirals dispensed on the same day. A cART regimen contains (1) two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) as a backbone, and (2) an anchor antiretroviral that is either an NNRTI, a protein inhibitor [PI], boosted or un-boosted with ritonavir (an integrase strand transfer inhibitor [ISTI] or an additional NRTI. For this study, we considered only cART regimens containing lamivudine or emtricitabine as one of the two NRTIs in the backbone, given that all preferred initial treatment regimens include one of these agents.17 The second NRTI was either abacavir or tenofovir. A new cART regimen recipient was defined as a patient receiving a cART regimen with all medications started on the same day, without a prescription filled for any antiretroviral in the 180 days prior to study entry, with the exception of < 30 days of a non-cART regimen (e.g. monotherapy or dual therapy). We excluded new recipients with a regimen dispensed for < 30 days followed by a non-standard cART regimen and patients with any claims for MI (acute or chronic), coronary artery bypass graft or percutaneous transluminal coronary angioplasty in the 180 days prior to cART initiation. For all analyses, we excluded patients on regimens that contained both the exposed (treated) and active comparator antiretroviral (e.g. abacavir and tenofovir).
Exposure and Outcome Definitions
Our primary outcome was myocardial infarction, defined in the Medicaid data by a diagnosis code of 410.xx in any position and a length of stay ≥ 1 day. This algorithm was previously validated in the NC Medicaid population (sensitivity=0.765 [95% confidence interval (CI)=0.501 - 0.932]; specificity=0.989 [0.980 - 0.994]).18 We first considered patients who had not previously received the most common NRTIs, abacavir and tenofovir. Next we considered patients who had not previously received atazanavir (boosted and unboosted with ritonavir) or lopinavir-ritonavir separately from NNRTIs.
Confounder and Covariates
We identified potential confounders of the antiretroviral use-MI relationship based on expert knowledge about the relationship of these factors with the exposure and the outcome. We obtained data on potential confounders from the Medicaid data in the 180 days prior to cART initiation. We included age at study entry, sex, race, calendar year of antiretroviral initiation (6 indicator variables for calendar year), concomitant cardiovascular medication use (angiotensin converting enzyme [ACE] receptor inhibitors, angiotensin receptor blocking agents, beta receptor blocking agents, calcium channel receptor blocking agents and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors), comorbidities in the 180 days prior to cART initiation (based on ICD-9 codes from the Deyo implementation of the Charlson comorbidity score,19 used separately, i.e., not as a score), number of hospitalizations in the 180 days prior to cART initiation (0, 1-2, >2 hospitalizations) and number of medication claims in the 180 days prior to cART initiation (0, 1-15, 15-20, >20 medications). For the NRTI comparative analysis, we considered regimen type based on anchor antiretroviral (ritonavir boosted PI- or ISTI -based, NNRTI-based, ritonavir unboosted PI-based, triple NRTI-based).
Statistical Analysis
To account for baseline differences in treatment, we utilized a form of inverse-probability-weighting methodology. Inverse probability weighting relies on the propensity score, calculated as the conditional probability of receiving active treatment. As we were interested in estimating the average treatment effect in three active treatment populations, we weighted the data to create a pseudo-population of patients with the same distribution of patient characteristics as patients initiating tenofovir for the NRTI comparison and the same as patients initiating atazanavir or lopinavir-ritonavir for the NNRTI/PI comparisons. Patients receiving tenofovir, atazanavir or lopinavir-ritonavir received a weight of 1 and patients receiving abacavir or an NNRTI received a weight defined as ê/(X)/(1 − ê(X)) where ê(X) is the propensity score.20,21 Before creating the weighted pseudo-populations, we trimmed non-overlapping regions of the propensity score distributions to exclude patients with characteristics that had a zero probability of initiating one of the drugs compared (non-positivity).
Follow-up started on the day of the collection of claims for the new cART regimen and continued until the occurrence of (1) MI, 2) discontinuation of Medicaid eligibility or, 3) end of study period (31 December 2008), whichever came first. We calculated overall unadjusted incidence rates for MI using Poisson regression. We then used inverse probability weights to create adjusted Kaplan-Meier curves for each of the study comparison groups. Finally, we created Cox-proportional-hazard regression models to examine unadjusted and inverse probability weighted hazard ratios (HR) and corresponding 95% CIs to evaluate the effect of each active treatment in the treated populations. For weighted analyses we used robust variance estimation. Point estimates and associated 95% CIs for risk differences were derived from 500 bootstraps of weighted data. This study was approved by the University of North Carolina IRB. Analyses were conducted using SAS, Version 9.2 Copyright, SAS institute Inc. or Stata Statistical Software Release 11, College Station, Tx, StataCorp LP. SAS and all other SAS institute Inc product or service names are registered trademarks of SAS institute Inc, Cary, NC USA.
Sensitivity Analyses
We conducted sensitivity analyses to address treatment switch or discontinuation and unmeasured confounding. We censored patients (1) at the first MI, (2) stopping or switching antiretrovirals, or (3) subject to administrative censoring. We also evaluated Kaplan-Meier curves for time-to-treatment switch or discontinuation, stratified by treatment. We used established methodology to assess the role of unmeasured confounding on our results.22
Results
Study Population and Descriptive Statistics
Between 1 January 2002 and 31 December 2008, 13,006 HIV-positive beneficiaries enrolled in NC Medicaid. Of these, 3,500 beneficiaries were new recipients of a qualifying cART regimen (Figure 2). Overall, the distribution of patient characteristics receiving an initial cART regimen was similar to those of the overall HIV patient population; however, the Medicaid population represents a larger proportion of HIV-infected women. Of the 10,082 patients prescribed antiretrovirals, 18% received regimens containing two NRTIs and an NNRTI. This also was the predominant regimen type among initial cART recipients (33%).
Figure 2.
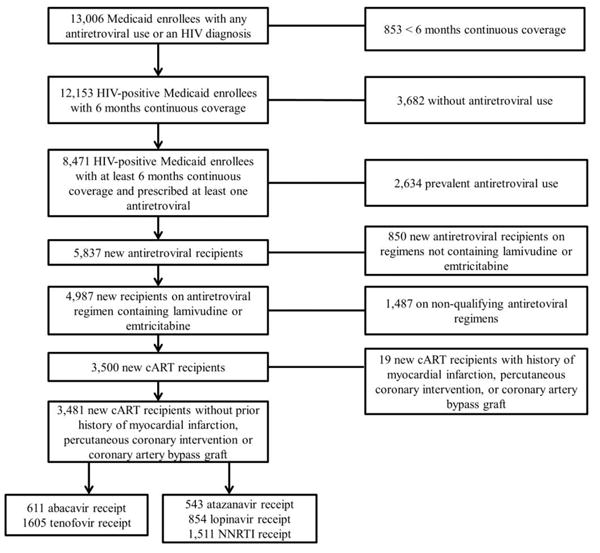
Assembly of the combination antiretroviral therapy initiator cohort.
The distribution of patient characteristics among new recipients of cART was generally similar among recipients of specific antiretrovirals. We noted differences in comorbidities, regimen type and year of antiretroviral initiation among recipients of abacavir or tenofovir (Table 1). Based on ICD-9 codes, a greater proportion of abacavir recipients had renal disease at baseline (5% vs. 2%). Conversely, a larger proportion of tenofovir recipients had mild liver disease (4% vs.2%) and a diagnosis of cancer (5% vs. 4%). Most abacavir recipients initiated cART before 2006 (60%) while the majority of tenofovir recipients initiated cART during or after 2006 (64%) (Table 1). Baseline characteristics of patients receiving atazanavir, lopinavir, or NNRTIs were generally similar (Table 2).
Table 1. Baseline characteristics of HIV-infected North Carolina Medicaid patients initiating abacavir or tenofovir as a part of a new combination antiretroviral therapy (cART) regimen before and after inverse probability weighting.
| New cART Recipients | IP Weighteda | |||
|---|---|---|---|---|
| abacavir (n=611) No. (%) |
tenofovir (n=1,605) No. (%) |
abacavir (n=1582 ) No. (%) |
tenofovir (n=1557) No. (%) |
|
| Sex | ||||
| Female | 300 (49) | 758 (47) | 777 (49) | 744 (48) |
| Age (years) | ||||
| <40 | 247 (40) | 364 (60) | 684 (43) | 674 (43) |
| 40-50 | 373 (61) | 238 (39) | 630 (40) | 602 (39) |
| >50 | 485 (27) | 126 (21) | 269 (17) | 281 (18) |
| Race | ||||
| Black | 450 (74) | 1221 (75) | 1195 (76) | 1182 (76) |
| White | 113 (18) | 287 (18) | 296 (19) | 280 (18) |
| Asian | NAb | NAb | NAb | NAb |
| Native American/Pacific Islander | NAb | 24 (2) | 26 (2) | 22 (1) |
| Unknown | 41 (7) | 68 (4) | 63 (4) | 68 (4) |
| Comorbidity at baselinec | ||||
| Heart Failure | 32 (5) | 66 (5) | 64 (4) | 74 (5) |
| Peripheral Vascular Disease | NAb | 14 (1) | 12 (1) | NAb |
| Cerebrovascular Disease | 23 (4) | 45 (3) | 41 (3) | 45 (3) |
| Mild Liver Disease | 13 (2) | 65 (4) | 52 (3) | 52 (3) |
| Renal Disease | 29 (5) | 25 (2) | 25 (0.4) | 25(2) |
| Diabetes (uncomplicated) | 39 (6) | 96 (6) | 99 (6) | 94 (6) |
| Cancer | 24 (4) | 83 (5) | 80 (5) | 77 (5) |
| Chronic Pulmonary Disease | 44 (7) | 121 (8) | 115 (7) | 117 (8) |
| Prior Medications Used (180 days before entering study)d | ||||
| HMG-CoA Reductase Inhibitors | 53 (9) | 113 (7) | 116 (7) | 101 (6) |
| Calcium Channel Blockers | NAb | 31 (2) | 31 (2) | 31 (2) |
| Beta Blocking agents | 14 (2) | 55 (3) | 61 (4) | 43 (3) |
| Angiotensin Converting Enzyme Inhibitors (ACE-I) | 40 (7) | 97 (6) | 102 (6) | 85 (5) |
| No. Prior Medications Used (180 days before entering study) | ||||
| 0 | 96 (16) | 156 (10) | 161 (10) | 155 (10) |
| 1-15 | 412 (67) | 1,126 (70) | 1081 (68) | 1101 (71) |
| 15-20 | 49 (8) | 183 (11) | 191 (12) | 162 (10) |
| >20 | 54 (9) | 140 (9) | 150 (9) | 139 (9) |
| No. Hospitalizations (180 days before entering study) | ||||
| 0 | 377 (62) | 989 (62) | 963 (61) | 975 (63) |
| 0-2 | 121 (20) | 316 (20) | 302 (19) | 312 (20) |
| >2 | 113 (18) | 300 (19) | 317 (20) | 270 (17) |
| First Antiretroviral Regimene | ||||
| 2 NRTIs+boosted PI/ISTI | 165 (27) | 644 (40) | 684 (43) | 639 (41) |
| 2 NRTIs+NNRTI | 139 (23) | 805 (50) | 739 (47) | 762 (49) |
| 2 NRTIs+ unboosted PI | 111 (18) | 165 (27) | 132 (8) | 129 (8) |
| Triple NRTI | 196 (32) | 27 (2) | 27 (1.7) | 27 (2) |
| Year of Antiretroviral Initiation | ||||
| 2002 | 39 (6) | 31 (2) | 27 (1.7) | 31 (2) |
| 2003 | 100 (16) | 101 (6) | 99 (6) | 101 (6) |
| 2004 | 79 (13) | 146 (9) | 155 (10) | 146 (9) |
| 2005 | 150 (25) | 305 (19) | 329 (21) | 305 (20) |
| 2006 | 107 (18) | 346 (22) | 361 (23) | 342 (22) |
| 2007 | 37 (6) | 132 (8) | 137 (9) | 130 (8) |
| 2008 | 99 (16) | 2008 (34) | 474 (9) | 502 (32) |
Propensity score based on the following characteristics: age, race, sex, comorbidities, drug use in the 180 days prior to antiretroviral initiation, cardiovascular drug use in the 180 days prior to antiretroviral initiation, hospitalization in the 180 days prior to antiretroviral initiation, regimen type, year of initiation (6 indicator variables for year). The median propensity scores for the receipt of abacavir or tenofovir were 0.60 (IQR: 0.15, 0.82; Full Range: 0.02, 0.92) and 0.84 (IQR: 0.78, 0.88; Full Range: 0.15, 0.97) respectively. 49 patients who had characteristics that were always associated with abacavir (n=1) or tenofovir (n=48) initiation were excluded from weighted analysis. After trimming of non-overlap in the propensity score distributions, IP weights used to estimate the effect of initiation of cART regimens containing abacavir compared with tenofovir ranged from 0.02 to 12.0.
Numbers in cell < 11 (cannot be presented based on data use agreement with North Carolina Medicaid). Cells < 11 presented for pseudo-population as persons could be represented more than once.
Comorbidities include: Heart failure, peripheral vascular disease, cerebrovascular disease, mild liver disease, moderate/severe liver disease, renal disease, diabetes (uncomplicated), diabetes (complicated), cancer, metastatic carcinoma, connective tissue disease, chronic pulmonary disease, dementia. Comorbidities with > 11 subjects in at least one cell of the baseline population presented.
Angiotensin receptor blocking agent percentages not presented as there was at least one cell in the baseline population that had < 11 subjects.
NA indicates not available; NRTI, Nucleoside Reverse Transcriptase Inhibitor; NNRTI, Non-Nucleoside Reverse Transcriptase Inhibitor; PI, Protease Inhibitor. HMG-CoA, 3-hydroxy-3-methyl-glutaryl-CoA
Table 2.
Baseline characteristics of HIV-infected North Carolina Medicaid patients receiving atazanavir, lopinavir-ritonavir or a non-nucleoside reverse transcriptase inhibitor (NNRTI) as a part of a new combination antiretroviral therapy (cART) regimen before and after inverse probability weighting
| New cART recipients | Inverse Probability Weighteda |
Inverse Probability Weighteda |
|||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| atazanavir (n=543) No. (%) |
lopinavir-ritonavir (n=654) No. (%) |
NNRTI (n= 1,511) No. (%) |
atazanavir (n=537) No. (%) |
NNRTI (n=540) No. (%) |
lopinavir-ritonavir (n=652) No. (%) |
NNRTI (n=655) No. (%) |
|
| Sex | |||||||
| Female | 260 (48) | 296 (45) | 689 (46) | 257 (48) | 255 (47) | 295 (45) | 294 (45) |
| Age, years | |||||||
| <40 | 242 (45) | 303 (46) | 614 (41) | 239 (45) | 253 (48) | 301 (46) | 304 (46) |
| 40-50 | 216 (40) | 241 (37) | 580 (38) | 214 (40) | 199 (37) | 241 (37) | 240 (37) |
| >50 | 85 (16) | 110 (17) | 317 (21) | 84 (16) | 88 (16) | 110 (17) | 111 (17) |
| Race | |||||||
| Black | 396 (73) | 501 (77) | 1,142 (76) | 396 (74) | 397 (74) | 501 (77) | 503 (77) |
| White | 117 (22) | 105 (16) | 278 (18) | 111 (21) | 115 (21) | 105 (16) | 101 (15) |
| Asian | NAb | NAb | NAb | NAb | NAb | NAb | NAb |
| Native American/Pacific Islander | NAb | NAb | NAb | NAb | NAb | NAb | NAb |
| Unknown | 21 (4) | 36 (6) | 69 (5) | 21 (4) | 19 (4) | 35 (5) | 38 (6) |
| Comorbidity at baselinec | |||||||
| Heart Failure | 28 (5) | 25 (4) | 56 (4) | 27 (5) | 27 (5) | 25 (4) | 25 (4) |
| Peripheral Vascular Disease | NAb | NAb | 16 (1) | NAb | NAb | NAb | NAb |
| Cerebrovascular Disease | 15 (3) | 17 (3) | 48 (3) | 15 (3) | 15 (3) | 17 (3) | 16 (2) |
| Mild Liver Disease | 19 (4) | 18 (3) | 50 (3) | 19 (4) | 19 (4) | 18 (3) | 20 (3) |
| Renal Disease | 14 (3) | 16 (2) | 49 (3) | 24 (3) | 13 (2) | 16 (2) | 15 (2) |
| Diabetes (uncomplicated) | 41 (8) | 27 (4) | 107 (7) | 41 (8) | 40 (7) | 27 (4) | 27 (4) |
| Cancer | 22 (4) | 31 (5) | 82 (5) | 22 (4) | 23 (4) | 30 (5) | 31 (5) |
| Chronic Pulmonary Disease | 45 (8) | 40 (6) | 119 (8) | 45 (8) | 46 (9) | 40 (6) | 40 (6) |
| No. Prior Medications Used (180 days before entering studyd | |||||||
| HMG-CoA Reductase Inhibitors | 45 (8) | 51 (8) | 112 (7) | 43 (8) | 45 (8) | 51 (8) | 53 (8) |
| Calcium Channel Blockers | NAb | 14 (2) | 29 (2) | NAb | NAb | 14 (2) | 15 (2) |
| Beta Blockers | 17 (3) | 23 (4) | 47 (3) | 17 (3) | 18 (3) | 23 (4) | 24 (4) |
| Angiotensin Converting Enzyme Inhibitors (ACE-I) | 40 (7) | 43 (7) | 92 (6) | 39 (7) | 39 (7) | 43 (7) | 45 (7) |
| No. Prior Medications Used (180 days before entering study) | |||||||
| 0 | 45 (8) | 116 (18) | 169 (11) | 44 (8) | 46 (9) | 116 (18) | 119 (18) |
| 1-15 | 362 (67) | 414 (63) | 1,078 (71) | 361 (67) | 362 (67) | 413 (63) | 412 (63) |
| 15-20 | 68 (13) | 67 (10) | 152 (10) | 68 (13) | 68 (13) | 67 (10) | 68 (10) |
| >20 | 68 (13) | 57 (9) | 112 (7) | 64 (12) | 65 (12) | 56 (9) | 56 (9) |
| No. Hospitalizations (180 days before entering study) | |||||||
| 0 | 349 (64) | 364 (56) | 952 (63) | 346 (64) | 346 (64) | 364 (56) | 365 (56) |
| 0-2 | 110 (20) | 129 (20) | 289 (19) | 108 (20) | 112 (21) | 129 (20) | 130 (20) |
| >2 | 84 (15) | 161 (25) | 270 (18) | 83 (15) | 82 (15) | 159 (24) | 160 (24) |
| Year of Antiretroviral Initiation | |||||||
| 2002 | NAb | 55 (8) | 40 (3) | NAb | NAb | 53 (8) | 56 (9) |
| 2003 | NAb | 110 (17) | 144 (10) | NAb | NAb | 110 (17) | 111 (17) |
| 2004 | 48 (9) | 100 (15) | 197 (13) | 48 (9) | 47 (9) | 100 (15) | 100 (15) |
| 2005 | 121 (22) | 88 (13) | 399 (26) | 121 (23) | 120 (22) | 88 (14) | 85 (13) |
| 2006 | 148 (27) | 93 (14) | 290 (19) | 146 (27) | 148 (27) | 93 (14) | 92 (14) |
| 2007 | 43 (8) | 59 (9) | 87 (6) | 42 (8) | 43 (8) | 59 (9) | 60 (9) |
| 2008 | 177 (33) | 149 (23) | 354 (23) | 175 (33) | 177 (33) | 149 (23) | 150 (23) |
Propensity scores based on the following characteristics: age, race, sex, comorbidities, drug use in the 180 days prior to antiretroviral initiation, cardiovascular drug use in the 180 days prior to antiretroviral initiation, hospitalization in the 180 days prior to antiretroviral initiation, year of initiation (indicators for year of initiation). The median propensity scores for the receipt of atazanavir or an NNRTI were 0.30 (IQR: 0.25, 0.37; Full Range: 0.02, 0.65) and 0.25 (IQR: 0.18, 0.33; Full Range: 1×10-7, 0.60). Of patients that received either atazanavir or an NNRTI, we trimmed 54 patients who had characteristics that were always associated with initiation of atazanavir (N=6) or NNRTI (N=48). Inverse probability weights used to estimate the effect of initiation of cART regimens containing atazanavir or an NNRTI and MI ranged from 0.02 to 1.836. The median propensity scores for the receipt of lopinavir-ritonavir or an NNRTI were 0.34 (IQR: 0.25, 0.34; Full Range: 0.10, 0.75) and 0.27 (IQR: 0.20, 0.36; Full Range: 0.06, 0.70). We trimmed 24 patients who had characteristics that were always associated with initiation of lopinavir-ritonavir (n=2) or an NNRTI (n=22). Inverse probability weights used to estimate the effect of initiation of cART regimens containing lopinavir-ritonavir or an NNRTI and MI ranged from 0.11 to 2.35.
Numbers in cell < 11 (cannot be presented based on data use agreement with NC Medicaid). Cells < 11 presented for pseudo-population as persons could be represented more than once.
Comorbidities include: Heart failure, peripheral vascular disease, cerebrovascular disease, mild liver disease, moderate/severe liver disease, renal disease, diabetes (uncomplicated), diabetes (complicated), cancer, metastatic carcinoma, connective tissue disease, chronic pulmonary disease, dementia. Comorbidities with ≥ 11 subjects in at least one cell presented.
Angiotensin receptor blocking agent percentages not presented as all cells had < 11 subjects.
NA indicates not available; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-CoA
Comparative Safety Results
Overall, patients contributed 6,399 person-years and experienced 38 MI events. The unadjusted incidence rate of MI for the entire new cART population was 5.9 (95% CI= 4.3 - 8.2) per 1000 person-years of follow-up. Unadjusted incidence rates for each of the study groups are displayed in Table 3. Patients initiating abacavir or tenofovir had an unadjusted incidence rate of 11.9 (95% CI= 7.2 - 19.7) and 4.5 (2.5 - 8.2) per 1000 person-years of follow-up (Table 3).
Table 3. Unadjusted and inverse probability weighted incidence rates (IRs) per 1000 person-years and hazard ratios (HRs) for the rate of myocardial infarction events among HIV-infected North Carolina Medicaid patients receiving new combination antiretroviral therapy regimens.
| No. | Myocardial Infarction Events No. | Person-Time at Risk (years) | Unadjusted | Inverse Probabilty Weighted | |||
|---|---|---|---|---|---|---|---|
| IR (95% CI) | HR (95% CI) | IR (95% CI*)a | HR (95% CI*)a | ||||
| Backbone: Nucleoside Reverse Transcriptase Inhibitorsb | |||||||
| Abacavir | 611 | 15 | 1,263.8 | 11.9 (7.2; 19.7) | 2.70 (1.24; 5.91) | 9.4 (3.9; 22.5) | 2.05 (0.72; 5.86) |
| Tenofovirc | 1,605 | 11 | 2,433.4 | 4.5 (2.5; 8.2) | 1.0 | 4.6 (2.5; 8.3) | 1.0 |
| Anchor Antiretrovirals: Protease Inhibitors/Non-Nucleoside Reverse Transcriptase Inhibitorsd | |||||||
| Atazanavir | 543 | <11 | 786.5 | 5.1 (1.9; 13.6) | 1.13 (0.36; 3.51) | 5.1 (1.9; 13.7) | 1.12 (0.35; 3.62) |
| NNRTIc | 1,511 | 13 | 2,603.9 | 5.0 (2.9; 8.6) | 1.0 | 4.7 (2.5; 8.8) | 1.0 |
| Lopinavir-ritonavir | 654 | <11 | 1,325.4 | 3.8 (1.6; 9.1) | 0.77 (0.27; 2.18) | 3.8 (1.6; 9.1) | 0.92 (0.26; 3.22) |
| NNRTIc | 1,511 | 13 | 2,603.9 | 5.0 (2.9; 8.6) | 1.0 | 4.3 (1.8; 10.1) | 1.0 |
Robust variance estimator used to calculate variance for inverse probability weighted data
Propensity score used to create IP weights to estimate the treatment effect in the three treatment groups based on the following characteristics: age, race, sex, comorbidities, drug use in the 180 days prior to antiretroviral initiation, cardiovascular drug use in the 180 days prior to antiretroviral initiation(statin calcium channel blocker, beta-blocker, ace-inhibitor), hospitalization in the 180 days prior to antiretroviral initiation, regimen type (NNRTI, boosted PI/Integrase strand transfer inhibitor, unboosted PI, triple NRTI), year of initiation (6 indicator variables for year).
Reference category
Propensity score used to create IP weights based on the following characteristics: age, race, sex, comorbidities, drug use in the 180 days prior to antiretroviral initiation (0, 1-15, 15-20, >20), cardiovascular drug use in the 180 days prior to antiretroviral initiation (statin, calcium channel blocker, beta-blocker, ace-inhibitor), hospitalization in the 180 days prior to antiretroviral initiation (0, 0-2, >2), year of initiation (6 indicator variables for year).
Unadjusted Cox proportional hazard regression models showed an increased HR of MI among recipients of abacavir compared with tenofovir (HR= 2.70 [95% CI= 1.24 - 5.91]). If all those who actually initiated tenofovir had initiated abacavir instead, these patients would have been twice as likely to have an MI. However, the HR point estimate was closer to the null and less precise when compared with the crude (HR= 2.05 [0.72, 5.86]). Figures 3 and 4 display Kaplan-Meier curves for the inverse probability weighted pseudo-populations for each of the study groups stratified by active or comparator antiretroviral. The absolute difference in MI risk at 5 years after cART initiation for those receiving abacavir compared with tenofovir was 3.5 per 100 (95% CI= -1.6 to 8.6). Unadjusted and inverse probability weighted models did not demonstrate clinically meaningful differences in HRs of MI among the other comparison groups (Table 3).
Figure 3.
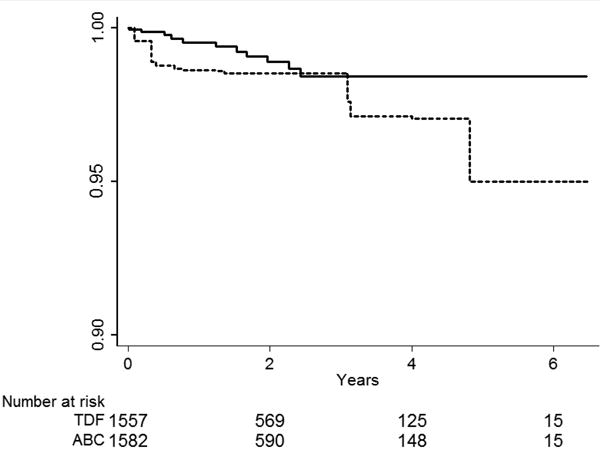
Inverse probability weighted Kaplan-Meier curves for the time to a myocardial infarction event among HIV-positive persons (identified by ICD-9 code and antiretroviral use in administrative claim) initiating nucleoside reverse transcriptase inhibitors as part of a new combination antiretroviral therapy (cART) regimen. Abacavir compared with tenofovir.
Figures 4a and b.
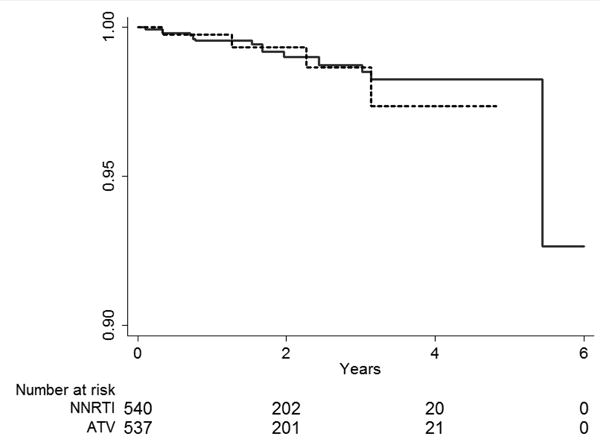
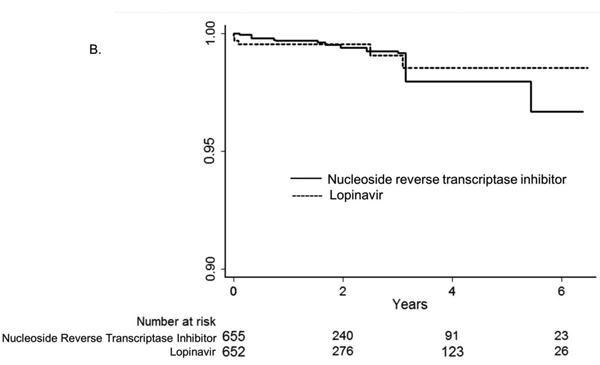
Inverse probability weighted Kaplan-Meier curves for the time to myocardial infarction event among HIV-positive persons (identified by ICD-9 code and antiretroviral use in administrative claim) initiating protease inhibitors or non-nucleoside reverse transcriptase inhibitors (NNRTI) A. atazanavir alone or in combination with ritonavir compared with non-nucleoside reverse transcriptase inhibitors (efavirenz or nevirapine) B. lopinavir-ritonavir compared with non-nucleoside reverse transcriptase inhibitors (efavirenz or nevirapine). Note: axes focused on 90% to 100% surviving.
Sensitivity Analyses
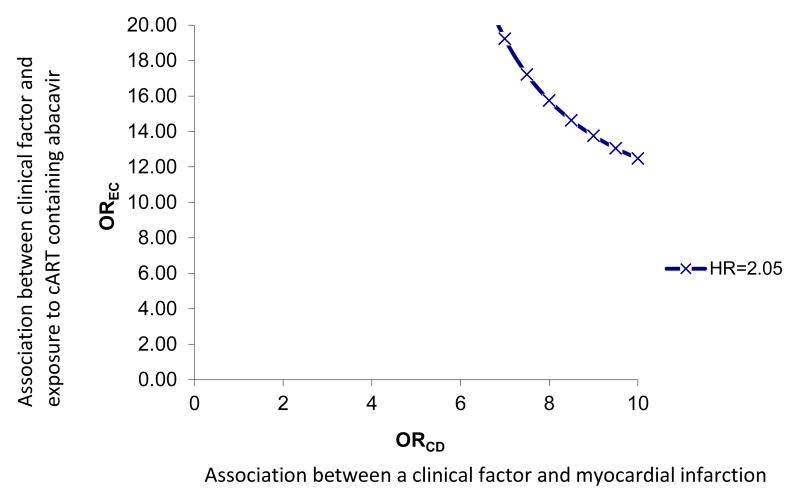
To address treatment switch or discontinuation, we attempted an analysis similar to a per-protocol analysis in an RCT; however, we did not have an adequate number of events to address this question. Among patients receiving cART regimens containing abacavir, the median time to regimen switch or discontinuation was 34 days compared with 58 days among patients receiving cART containing tenofovir (log-rank p-value=0.01). Patients initiating cART containing an NNRTI remained on their initial cART regimen longer than those initiating cART containing atazanavir or lopinavir-ritonavir (p-value all < 0.01) (Figures 5A, 5B, 5C). A sensitivity analysis evaluating the potential impact of unmeasured confounding demonstrated that the magnitude of an association between an unmeasured confounder and initiation of specific cART, as well as the association between the same unmeasured confounder and MI, would need to be very large to reduce the observed HR from 2.05 to 1.0 (eFigure 1).
Figures 5a, 5b, and 5c.
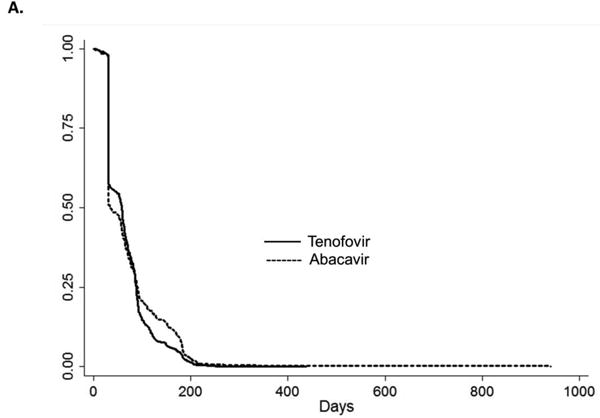
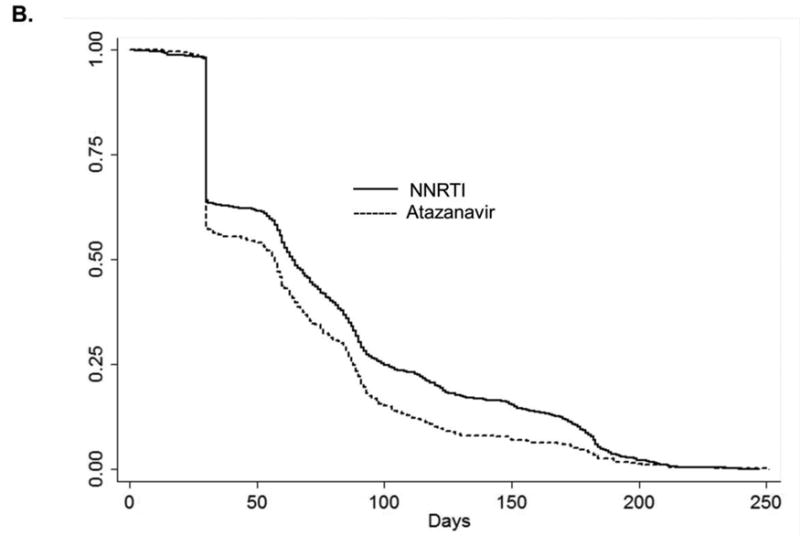
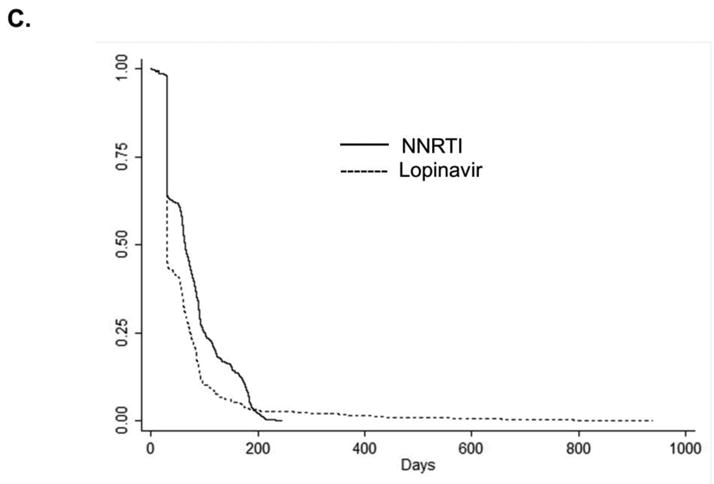
Kaplan-Meier curves for the time to treatment switch or discontinuation among HIV-positive persons (identified by ICD-9 code and antiretroviral use in administrative claim) initiating combination antiretroviral therapy. A. Abacavir compared with tenofovir B. atazanavir alone or in combination with ritonavir compared with non-nucleoside reverse transcriptase inhibitors (efavirenz or nevirapine) C. lopinavir-ritonavir compared with non-nucleoside reverse transcriptase inhibitors (efavirenz or nevirapine).
Note: different time scale for part B. compared with parts A. and C.
Discussion
We simulated three active comparison RCTs by including patients who were initiating specific antiretroviral medications as a part of guideline-recommended cART. This study design allowed us to evaluate the effects of initial treatment with specific antiretroviral medications on the risk for MI. We found that patients treated initially with abacavir as part of a new cART regimen – but not atazanavir or lopinavir – had an increased rate of MI when compared with patients treated initially with tenofovir or an NNRTI.
There are proposed biological mechanisms for an increased rate of MI among patients exposed to abacavir, although the exact underlying pathophysiology remains unclear. HIV infection influences factors related to inflammation and endothelial function, 23-26 and initiation of antiretroviral therapy generally improves these factors. 26-28 Conversely, treatment with abacavir may impair endothelial function and increase inflammation. However, results are conflicting.29-31 More recent evidence suggests that abacavir increases platelet reactivity, thus increasing MI risk. 32,33
The risk of MI over the five-year period observed in our data was 3%. Kowalska and colleagues34 calculated five-year predicted MI risks for varying risk categories using the Data Collected on Adverse Events of Anti-HIV Drugs study. Patients who smoked and had high lipid levels had a predicted risk of MI at 5 years, similar to that observed in our study. There are more men in the Kowalska et al study than in the HIV-infected Medicaid population, however. The age-and sex-standardized five-year MI risk for persons who smoke and have a high cholesterol ratio in the Framingham study is lower (0.8%) than we observed.35 We would expect a higher risk of MI in our population than in the general population. It is plausible that the risk to patients enrolled in the Adverse Events study is similar to that in our own study, given that our Medicaid cohort, which (despite having a higher proportion of women), would reflect the increased risk that comes with low socioeconomic status. 36
The observed HRs point estimates comparing the use of abacavir to tenofovir are consistent with (although slightly higher than) results from other observational studies on abacavir and MI.2,4-6 Our results do not concur with results from clinical trials. The 95% CI for the HR in our study overlaps the null and we thus cannot exclude chance as an alternative explanation. Nonetheless, the magnitude of the adjusted HR speaks against residual and unmeasured confounding as alternative explanations. We did observe that trimming the upper tails of the propensity score distribution for abacavir vs tenofovir treatment attenuated the HR, which may suggest that treatment heterogeneity may be present, with the potential to influence our results.
In general, RCTs aim to establish efficacy whereas observational studies evaluate effectiveness and safety; the mere enrollment and follow-up in a RCT may result in different outcomes than would occur among patients followed in a general clinical setting. Patients enrolled in RCTs are often healthier and have a lower risk of comorbidities.37,38 Disparate conclusions from meta-analyses and observational studies may be due to differing lengths of follow-up and overall characteristics of the study population. The average length of follow-up in the RCTs was much longer than in our study (591 days vs. 38 days).11However, the total person-time of follow-up in our study was greater; patients included in the abacavir-tenofovir analysis contributed a combined 3,697 person-years of follow-up compared with an average 719 person-years among the collective RCTs in the FDA-sponsored meta-analysis. 11This meta-analysis did not consider length of follow-up when calculating the summarized risk difference, making the interpretation of those results more difficult.
Some observational studies evaluating the risk of MI among patients prescribed abacavir demonstrated evidence of confounding and effect modification.6 Using a large cohort of military veterans, Bedimo et al.8 showed that the observed relationship between abacavir use and MI may be due to differential prescribing. Patients with baseline comorbidities that increase the risk of MI, such as chronic kidney disease, were more likely to receive abacavir. However, an observational study conducted using the same veterans database found an association between abacavir and MI, with less evidence of confounding due to baseline kidney dysfunction. 7 We also noted baseline differences in comorbidities between antiretroviral exposure groups. We addressed these known baseline clinical differences through inverse probability weighting and showed that this approach successfully balanced differences in cardiovascular risk factors within each of the treatment groups.
One main concern regarding the use of administrative data is the inability to obtain information on potentially important confounding variables (such as CD4 count, HIV RNA, LDL cholesterol and history of smoking) that may be related to treatment assignment and MI. Therefore, it is possible that our findings could be subject to unmeasured confounding. Our study design limits the potential for unmeasured confounding by both indication (likely similar for the treatment regimens compared) and frailty.39-41 Using the available variables in the data to create inverse probability weights, we anticipate that these unmeasured factors are likely balanced. However, we cannot confirm this. Research on frailty has shown that older or terminally ill patients with substantial comorbidity may be under-treated due to the expectation of shortened life expectancy.41,42
We considered only specific cART regimens that contained lamivudine or emtricitabine, which may have reduced our sample size. Further, follow-up time was relatively short, resulting in reduced numbers of MI events and low precision of estimates. A limited sample of patients initiating atazanavir and lopinavir constrained our ability to detect a difference between these groups. Finally, due to inadequate sample size, we were unable to conduct an analysis of treatment stop and discontinuation. This type of analysis would allow us to avoid bias from treatment changes during follow-up but at the price of introducing the potential for selection bias – which we avoid in our analysis of first treatment carried forward. With adequate sample size, both a first-treatment-carried-forward analysis and an analysis of treatment-stop and discontinuation would give us the ability to assess the impact of selection and attrition bias on our results.
Another concern with administrative data is the potential for medication exposure misclassification. Recently, Medicaid agencies in New York, Florida, and Pennsylvania reported that some patients sold antiretrovirals and other chronic-disease medications received through Medicaid on the black market. 43 If this had occurred in NC during our study period, we would expect the misclassification to be non-differential. However, to our knowledge, this activity was not prevalent in this state. It should also be noted that, while those included in this study had not received antiretrovirals in the previous six months via Medicaid (which we used this as a surrogate for antiretroviral naïve status), some patients may have had unobserved previous antiretroviral exposures. We do not believe that the inclusion of non-naïve patients would have a substantial impact on our results. Nonetheless, the concern warrants further investigation, either by expanding the period of required Medicaid eligibility prior to antiretroviral initiation or by linking the administrative data to a more comprehensive clinical record. Further, we were unable to determine reasons for lack of eligibility and therefore unable to discern losses to follow-up and death, as both of these instances would result in loss of Medicaid eligibility.
The new-user design is often used for comparative safety and effectiveness research, as it allows for the ascertainment of events that may have occurred early after treatment initiation, and limits the potential for confounding. 14,39 Preferential prescribing of one cART regimen or another regimen based on comorbidities (as described above) is unlikely to be as important when considering patients receiving second or third line treatments, which are often included in a prevalent-user study. However, the choice of initial cART is likely linked to future treatment decisions, rendering the new-user design relevant when assessing antiretroviral treatment outcomes. Finally, this study design allows for the assessment of confounders at the time of antiretroviral initiation, thus reducing the influence of time-dependent confounders on the causal pathway.15
We used an active-comparator, new-user design in combination with a first-treatment-carried-forward analysis and a validated algorithm for identifying myocardial infarction. To our knowledge, this type of study design has not previously been used to examine the relationship between antiretroviral use and MI. Studies completed to date have defined exposure to specific antiretrovirals as “any/recent/cumulative” use and compared these definitions to no use of the antiretroviral in question.2,4-6,8 While important, these types of comparisons make it difficult to compare across studies, particularly studies relating to HIV, as “no-use” is likely to equate to use of some other antiretroviral that differs by study. This heterogeneity of comparison group makes generalization across populations difficult, as treatment patterns may differ. While the Data Collection on Adverse Events of Anti-HIV Drugs study 2 and other clinical cohort collaborations have superior sample size and follow-up, our study design is unique and our results are potentially more generalizable to patients receiving regular clinical care in the United States. Additionally, almost half of our study population were women, whereas other observational studies have included between 2% and 27% women.2-5, 8, 10-12 This further improves the generalizability of our results.
Despite the limitations of our study and those inherent in all observational studies, such as unmeasured confounding, our results demonstrate an increased risk for MI among patients initiating abacavir compared with tenofovir as part of a standard cART regimen. To confirm these findings, future studies should use active-comparison designs, and include more HIV-infected patients initiating cART as well as information on important confounding factors not available in administrative data.
Acknowledgments
We thank M. Alan Brookhart for his contributions to the study design and Daniela Moga and Karen Blumenschein for their helpful comments on this manuscript.
Conflicts of Interest and Sources of Funding: This project was supported in part by grants M01RR00046 and UL1RR025747 from the National Center of Research Resources, National Institutes of Health. The project was also supported by National Institutes of Health grant P30 AI590410, R01 AG023178, and the Agency for Healthcare Research and Quality grant R01 HS018731. This publication was also supported by Grant Number K12 DA035150 from the Office of Women's Health Research and the National Institute on Drug Abuse at the National Institutes of Health (NIH). Dr. Til Stürmer receives investigator-initiated research funding and support as Principal Investigator (RO1 AG023178) from the National Institute on Aging at the National Institutes of Health. He also receives research funding as Principal Investigator of the UNC-DEcIDE center from the Agency for Healthcare Research and Quality. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company, though he receives salary support from the UNC Center of Excellence in Pharmacoepidemiology and Public Health and from unrestricted research grants from pharmaceutical companies to UNC. Dr. Napravnik received grant support from Merck, Pfizer, Bristol-Myers-Squibb and Dr. Eron is a consultant to GSK/ViiV, Gilead, BMS, and Tibotec. Dr. Floris-Moore has received research funding from Janssen Services, LLC.
Appendix
We conducted a sensitivity analysis to examine the impact of a potential unmeasured clinical factor on our observed results using the methodology proposed by Schneeweiss.22 Figure 1 displays the magnitude of the association between a potential unmeasured clinical confounder and exposure (cART regimen) (OREC) as well as the association between the same unmeasured confounder and outcome (MI) (ORCD) needed nullify the observed relationship. The prevalence of the unmeasured clinical factor was set at 50% and the prevalence of exposure was set at 25%.
Figure 1.
Strength of an unmeasured clinical factor needed to eliminate observed relationship between initiation of cART containing abacavir vs. cART containing tenofovir.
Footnotes
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole responsibility of the author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006 Sep;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008 Apr 26;371(9622):1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strategies for Management of Anti-retroviral Therapy/INSIGHT; DAD Study Groups. Use of Nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;(22):F17–F24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durand M, Sheehy O, Baril JG, et al. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr. 2011 Jul 1;57(3):245–253. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 5.Obel N, Farkas DK, Kronborg G, et al. Abacavir and risk of myocardial infarction in HIV-infected patients on highly active antiretroviral therapy: a population-based nationwide cohort study. HIV medicine. 2010 Feb;11(2):130–136. doi: 10.1111/j.1468-1293.2009.00751.x. [DOI] [PubMed] [Google Scholar]
- 6.Lang S, Mary-Krause M, Cotte L, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Archives of internal medicine. 2010 Jul 26;170(14):1228–1238. doi: 10.1001/archinternmed.2010.197. [DOI] [PubMed] [Google Scholar]
- 7.Choi AI, Vittinghoff E, Deeks SG, et al. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011 Jun 19;25(10):1289–1298. doi: 10.1097/QAD.0b013e328347fa16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedimo RJ, Westfall AO, Drechsler H, et al. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Jul 1;53(1):84–91. doi: 10.1093/cid/cir269. [DOI] [PubMed] [Google Scholar]
- 9.Cruciani M, Zanichelli V, Serpelloni G, et al. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS. 2011 Oct 23;25(16):1993–2004. doi: 10.1097/QAD.0b013e328349c6ee. [DOI] [PubMed] [Google Scholar]
- 10.Brothers CH, Hernandez JE, Cutrell AG, et al. Risk of Myocardial Infarction and Abacavir Therapy: No Increased Risk Across 52 GlaxoSmithKline-Sponsored Clinical Trials in Adult Subjects. J Acquir Immune Defic Syndr. 2009 Mar 11; doi: 10.1097/QAI.0b013e31819ff0e6. [DOI] [PubMed] [Google Scholar]
- 11.Ding X, Andraca-Carrera E, Cooper C, et al. No association of myocardial infarction with abacavir use: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr. 2012;61:441–447. doi: 10.1097/QAI.0b013e31826f993c. [DOI] [PubMed] [Google Scholar]
- 12.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. The New England journal of medicine. 2007 Apr 26;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 13.Lundgren JD, Reiss P, Worm SW, et al. Risk of myocardial infarction and exposure to specific antiretrovirals from the PI, NNRTI, and NRTI drug classes: The D-A-D study. Conference on Retroviruses and Opportunistic Infections (CROI); Montreal. 2009. [Google Scholar]
- 14.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American journal of epidemiology. 2003 Nov 1;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 15.Hernan MA. With great data comes great responsibility: publishing comparative effectiveness research in epidemiology. Epidemiology. 2011 May;22(3):290–291. doi: 10.1097/EDE.0b013e3182114039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carolina Cost and Quality Initiative. [Accessed December 1, 2008];2008 Accessed at: http://www.shepscenter.unc.edu/ccqi/index.htmal.
- 17.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA : the journal of the American Medical Association. 2012 Jul 25;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 18.Brouwer ES, Napravnik S, Eron JJ, et al. Validation of Medicaid claims-based diagnosis of myocardial infarction using an HIV clinical cohort. Medical care. 2013 doi: 10.1097/MLR.0b013e318287d6fd. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin Dc, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14:680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 21.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 22.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiology and drug safety. 2006 May;15(5):291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 23.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003 Aug 1;33(4):506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 24.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009 Sep 10;23(14):1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AC, Rizk N, O'Riordan MA, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009 Oct 1;49(7):1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross AC, Armentrout R, O'Riordan MA, et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J Acquir Immune Defic Syndr. 2008 Dec 15;49(5):499–506. doi: 10.1097/QAI.0b013e318189a794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Larranaga GF, Wingeyer SD, Puga LM, et al. Relationship between hepatitis C virus (HCV) and insulin resistance, endothelial perturbation, and platelet activation in HIV-HCV-coinfected patients under highly active antiretroviral treatment. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2006 Feb;25(2):98–103. doi: 10.1007/s10096-006-0090-6. [DOI] [PubMed] [Google Scholar]
- 28.de Larranaga GF, Bocassi AR, Puga LM, et al. Endothelial markers and HIV infection in the era of highly active antiretroviral treatment. Thrombosis research. 2003 May 1;110(2-3):93–98. doi: 10.1016/s0049-3848(03)00291-3. [DOI] [PubMed] [Google Scholar]
- 29.Martin A, Amin J, Cooper DA, et al. Abacavir does not affect circulating levels of inflammatory or coagulopathic biomarkers in suppressed HIV: a randomized clinical trial. AIDS. 2010 Nov 13;24(17):2657–2663. doi: 10.1097/QAD.0b013e32833f147f. [DOI] [PubMed] [Google Scholar]
- 30.Hsue PY, Hunt PW, Wu Y, et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. Aids. 2009 Sep 24;23(15):2021–2027. doi: 10.1097/QAD.0b013e32832e7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palella FJ, Jr, Gange SJ, Benning L, et al. Inflammatory biomarkers and abacavir use in the Women's Interagency HIV Study and the Multicenter AIDS Cohort Study. Aids. 2010 Jul 17;24(11):1657–1665. doi: 10.1097/QAD.0b013e3283389dfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baum PD, Sullam PM, Stoddart CA, et al. Abacavir increases platelet reactivity via competitive inhibition of soluble guanylyl cyclase. AIDS. 2011 Nov 28;25(18):2243–2248. doi: 10.1097/QAD.0b013e32834d3cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satchell CS, O'Halloran JA, Cotter AG, et al. Increased Platelet Reactivity in HIV-1-Infected Patients Receiving Abacavir-Containing Antiretroviral Therapy. Journal of Infectious Diseases. 2011 Oct 15;204(8):1202–1210. doi: 10.1093/infdis/jir509. [DOI] [PubMed] [Google Scholar]
- 34.Kowalska JD, Kirk O, Mocroft A, et al. Implementing the number needed to harm in clinical practice: risk of myocardial infarction in HIV-1-infected patients treated with abacavir. HIV Medicine. 2010;11:200–208. doi: 10.1111/j.1468-1293.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 35.Anderson KM, Odell PM, Wilson PW, et al. Cardiovascular disease risk profiles. American heart journal. 1991 Jan;121(1 Pt 2):293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 36.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. The New England journal of medicine. 2001 Jul 12;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 37.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial - The role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008 Sep 16;118(12):1294–1303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 38.Menezes P, Miller WC, Wohl DA, et al. Does HAART Efficacy Translate to Effectiveness? Evidence for a Trial Effect. PloS one. 2011 Jul 13;6(7) doi: 10.1371/journal.pone.0021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stürmer T, Jonsson Funk M, Poole C, et al. Nonexperimental comparative effectiveness research using linked healthcare databases. Epidemiology. 2011 May;22(3):298–301. doi: 10.1097/EDE.0b013e318212640c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stürmer T, Schneeweiss S, Avorn J, Glynn RJ. Adjusting effect estimates for unmeasured confounding with validation data using propensity score calibration. American journal of epidemiology. 2005 Aug 1;162(3):279–289. doi: 10.1093/aje/kwi192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glynn RJ, Knight EL, Levin R, Avorn J. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology. 2001 Nov;12(6):682–689. doi: 10.1097/00001648-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Brookhart MA, Sturmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Medical care. 2010 Jun;48(6 Suppl):S114–120. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz B. U.S. busts $108 million black market in Medicaid drugs. [Accessed May 7, 2013];Reuters. 2012 [Google Scholar]