Abstract
Objectives
The purpose of this study was to compare the results of acute type A aortic dissection (ATAAD) repair before and after implementation of a multidisciplinary thoracic aortic surgery program (TASP) at our institution, with dedicated high-volume thoracic aortic surgeons, a multidisciplinary approach to thoracic aortic disease management, and a standardized protocol for ATAAD repair.
Background
Outcomes of ATAAD repair may be improved when operations are performed at specialized high-volume thoracic aortic surgical centers.
Methods
Between 1999 and 2011, 128 patients underwent ATAAD repair at our institution. Records of patients who underwent ATAAD repair 6 years before (n = 56) and 6 years after (n = 72) implementation of the TASP were retrospectively compared. Expected operative mortality rates were calculated using the International Registry of Acute Aortic Dissection pre-operative prediction model.
Results
Baseline risk profiles and expected operative mortality rates were comparable between patients who underwent surgery before and after implementation of the TASP. Operative mortality before TASP implementation was 33.9% and was statistically equivalent to the expected operative mortality rate of 26.0% (observed-to-expected mortality ratio 1.30; p = 0.54). Operative mortality after TASP implementation fell to 2.8% and was statistically improved compared with the expected operative mortality rate of 18.2% (observed-to-expected mortality ratio 0.15; p = 0.005). Differences in survival persisted over long-term follow-up, with 5-year survival rates of 85% observed for TASP patients compared with 55% for pre-TASP patients (p = 0.002).
Conclusions
ATAAD repair can be performed with results approximating those of elective proximal aortic surgery when operations are performed by a high-volume multidisciplinary thoracic aortic surgery team. Efforts to standardize or centralize care of patients undergoing ATAAD are warranted.
Keywords: aortic dissection, aortic surgery, outcomes
Despite advances in diagnosis and surgical techniques, operative mortality rates for acute type A aortic dissection (ATAAD) repair continue to exceed 20% in the United States and abroad (1-4). However, recent studies have suggested that outcomes of thoracic aortic surgery may be improved when operations are performed at high-volume surgical centers with focused expertise in thoracic aortic surgery (1,3,5,6). Beginning in July 2005, a thoracic aortic surgery program (TASP) was introduced at our institution based on the hypothesis that performance of operations by a multidisciplinary aortic surgery team led by dedicated high-volume thoracic aortic surgeons would lead to improved outcomes. In this report, we compared the results of ATAAD repair before and after implementation of the TASP at our institution.
Methods
Patient population and data collection
This study was approved by the institutional review board of Duke University, and the need for individual patient consent was waived. We retrospectively reviewed the records of all patients at our institution diagnosed with spontaneously occurring ATAAD (less than 2 weeks from symptom onset) 6 years before (June 30, 1999 to June 30, 2005) and 6 years after (July 1, 2005 to July 1, 2011) implementation of the TASP. Patient records were identified from the prospectively maintained Duke Thoracic Aortic Surgery Database (2005 to present) (7-9) as well as from a query of the Duke Enterprise Data Unified Content Explorer for all patients with a discharge diagnosis code (International Classification of Diseases-Ninth Revision-Clinical Modification) of 441.0 (dissection of aorta) or 441.01 (dissection of aorta, thoracic). Patients with iatrogenic ATAAD secondary to cardiac surgery or thoracic endovascular aortic repair were excluded from the analysis because risk estimation variables related to hospital presentation could not be ascertained in cases of iatrogenic ATAAD identified intraoperatively. Comorbid conditions and post-operative complications were defined using Society of Thoracic Surgeons definitions (http://www.sts.org). Long-term follow-up and survival were assessed from the medical record and the Social Security Death Index. Individual surgeon ATAAD procedural volume was calculated by dividing the number of ATAAD repairs performed by the number of academic years in which they performed at least 1 ATAAD operation.
Thoracic aortic surgery program
The multidisciplinary TASP at Duke University Medical Center includes cardiothoracic surgery, vascular surgery, cardiac anesthesia, cardiovascular medicine, cardiac critical care, radiology, neurology, pathology, medical genetics, blood bank, nursing, and perfusion specialists. Elective and nonelective thoracic aortic operations are performed primarily by 2 principal surgeons (G.C.H. and J.G.G.) who received advanced fellowship training in thoracic aortic surgery. During the first 6 years after program implementation, 520 open proximal thoracic aortic operations (ascending, root, and/or arch replacement) were performed, for an average institutional volume of 87 proximal aortic repairs per year. Before implementation of the TASP, ATAAD repairs were performed by the general cardiac surgeon on call.
Patient selection and operative technique
Our current approach to ATAAD repair represents an adaptation of the techniques championed by Bavaria et al. in 2001 (10) and described previously by our group in prior publications (7,8). Unstable patients (ongoing pain, hemodynamic compromise, tamponade, or malperfusion) and those who presented within 48 h of symptom onset were taken emergently to the operating room for immediate surgical repair. Stable, asymptomatic patients who arrived more than 48 h after initial symptom onset underwent urgent operation after completion of a pre-operative evaluation (5,11,12). Patients with established visceral malperfusion, especially if static malperfusion or severe limb ischemia with loss of motor function, underwent a “complication-specific approach” in an attempt to first alleviate the malperfusion syndrome (13,14). For patients with debilitating pre-operative stroke, operation was delayed by 1 to 2 weeks in coordination with the neurology service if the patient was hemodynamically stable without rupture or tamponade. Patients with uncomplicated acute type A intramural hematoma with an ascending aortic diameter less than 5 cm were managed medically (15). Operation was deferred in patients with multisystem organ failure or prohibitive comorbidities or in patients undergoing cardiopulmonary resuscitation with chest compressions.
All operations were performed by median sternotomy with intraoperative transesophageal echocardiographic and invasive hemodynamic monitoring. Methylprednisolone (1 g intravenously) was administered for pharmacological neuroprotection to all patients pre-operatively. The standard operation involved aortic valve resuspension with ascending aorta and hemi-arch replacement (7,10). Aortic root replacement was performed selectively for aortic root aneurysm, intrinsic aortic valve pathology not amenable to repair, or extensive destruction of the intima of the aortic root by the dissection process. Total arch replacement was similarly performed for aneurysm or extensive destruction of the transverse arch. For hemi-arch or total arch repair, the open distal anastomosis was completed under a period of deep hypothermic circulatory arrest with the duration of cooling guided by electroencephalographic monitoring, when available (16). In the unmonitored cases, patients were cooled for a minimum of 50 min or until the nasopharyngeal temperature was below 18°C (17). Adjunctive antegrade cerebral perfusion via the right axillary artery was the preferred cerebral protection strategy in circulatory arrest cases, with retrograde cerebral perfusion being used in cases in which the right axillary artery was not suitable for cannulation, as previously described (7). Transfusion practices were performed as previously described, with intraoperative low-dose recombinant activated factor VII (rFVIIa) administered to patients with severe coagulopathy refractory to routine transfusions (7-9).
Statistical analysis
Categorical variables were represented as number and percent, and continuous variables were represented by mean, SD, and range. Predicted surgical mortality was calculated using the International Registry of Acute Aortic Dissection (IRAD) pre-operative prediction model (18). Estimates of long-term survival were calculated for all patients using the Kaplan-Meier method and compared between eras using the log-rank test. The Fisher exact test was used for all other statistical comparisons, including comparisons of observed and expected mortality rates. Calculations were performed using STATA 11.1 (StataCorp, College Station, Texas).
Results
Patient presentation
Between July 1999 and July 2011, 150 patients at our institution were diagnosed with ATAAD, of whom 128 (85%) underwent operative repair. Of these, 56 underwent repair before and 72 underwent repair after implementation of the TASP. Baseline characteristics of the operative cohort are shown in Table 1. No statistically significant differences in demographic or comorbid characteristics were noted between the pre-TASP and TASP groups.
Table 1.
Baseline Characteristics
| Variable | Pre-TASP (n = 56) | TASP (n = 72) | p Value |
|---|---|---|---|
| Age, yrs | 58 ± 15 (20–83) | 54 ± 14 (20–87) | 0.13 |
|
| |||
| Male | 39 (70) | 52 (72) | 0.75 |
|
| |||
| White race | 31 (55) | 42 (58) | 0.74 |
|
| |||
| Body mass index, kg/m2 | 28 ± 6 (16-48) | 29 ± 6 (17-52) | 0.35 |
|
| |||
| Hypertension | 46 (82) | 57 (79) | 0.67 |
|
| |||
| Hyperlipidemia | 16 (29) | 26 (36) | 0.37 |
|
| |||
| Active or recent tobacco use | 23 (41) | 38 (53) | 0.19 |
|
| |||
| Diabetes | 5 (9) | 2 (3) | 0.13 |
|
| |||
| Coronary artery disease | 8 (14) | 14 (19) | 0.44 |
|
| |||
| History of prior stroke | 4 (7) | 7 (10) | 0.61 |
|
| |||
| Chronic obstructive pulmonary disease | 4 (7) | 6 (8) | 0.80 |
|
| |||
| Baseline creatinine >1.5 mg/dl | 7 (13) | 17 (24) | 0.11 |
|
| |||
| Peripheral vascular disease | 2 (4) | 4 (6) | 0.60 |
|
| |||
| Connective tissue disease | 7 (13) | 7 (10) | 0.62 |
|
| |||
| Prior aortic surgery | 7 (13) | 3 (4) | 0.08 |
|
| |||
| Dissection type | 0.56 | ||
| DeBakey type 1 | 40 (71) | 48 (67) | |
| DeBakey type 2 | 16 (29) | 24 (33) | |
|
| |||
| Transfer from outside institution | 40 (71) | 55 (76) | 0.52 |
Values are mean ± SD (range) or n (%).
TASP = thoracic aortic surgery program.
Expected operative mortality
The IRAD pre-operative prediction model was used to calculate expected operative mortality rates for the operative and nonoperative patient cohorts before and after implementation of the TASP (Table 2). Expected operative mortality was similar between eras for operative patients as well as for nonoperative patients who declined surgery, died prior to surgery, or in whom operation was deferred.
Table 2.
International Registry of Acute Aortic Dissection Pre-operative Prediction Model Variables
| Variable | Pre-TASP | TASP | p Value |
|---|---|---|---|
|
| |||
| Operative patients | n = 56 | n = 72 | |
| Age ≥70 yrs | 13 (23) | 10 (14) | 0.17 |
| History of aortic valve replacement | 3 (5) | 1 (1) | 0.20 |
| Presenting hypotension, shock, or tamponade | 9 (16) | 6 (8) | 0.18 |
| Migrating chest pain | 49 (88) | 64 (89) | 0.81 |
| Pre-operative cardiac tamponade | 4 (7) | 5 (7) | 0.97 |
| Any pulse deficit | 15 (27) | 21 (30) | 0.77 |
| ECG infarct, new Q waves, ST-segment elevation, or ischemia | 5 (9) | 1 (1) | 0.05 |
| Expected operative mortality | 26% ± 29% | 18% ± 18% | 0.36 |
| (4%–100%) | (4%–84%) | ||
|
| |||
| Nonoperative patients | n = 10 | n = 12 | |
|
| |||
| Age ≥70 years | 9 (90) | 2 (17) | 0.001 |
| History of aortic valve replacement | 1 (10) | 0 | 0.26 |
| Presenting hypotension, shock, or tamponade | 3 (30) | 6 (50) | 0.34 |
| Migrating chest pain | 8 (80) | 6 (50) | 0.15 |
| Pre-operative cardiac tamponade | 3 (30) | 4 (33) | 0.87 |
| Any pulse deficit | 0 | 7 (58) | 0.003 |
| ECG infarct, new Q waves, ST-segment elevation, or ischemia | 0 | 1 (8) | 0.35 |
| Expected operative mortality | 46% ± 40% | 42% ± 37% | 0.53 |
| (8%–100%) | (4%–98%) | ||
Values are n (%) or mean ± SD (range).
ECG = electrocardiogram; other abbreviation as in Table 1.
Operative details
Operative parameters are shown in Table 3. Fewer TASP patients underwent emergent surgery and fewer underwent surgery during the night. Several important differences in operative technique were further observed between eras, with a higher proportion of arch replacements, aortic valve resuspensions, and nonmechanical aortic root replacements performed in the TASP era. Concomitant procedures were less common in the TASP era primarily due to a decreased proportion of patients undergoing concomitant coronary artery bypass grafting, and mean cardiopulmonary bypass (CPB) time was reduced by 20%. A higher proportion of cases used circulatory arrest in the TASP era, and the circulatory arrest cases employed lower cooling temperatures, shorter circulatory arrest times, and increased use of selective cerebral perfusion when compared with the pre-TASP era. Use of rFVIIa was restricted to the TASP era and was administered intraoperatively in 27 (38%) and post-operatively in 3 (4%) TASP patients.
Table 3.
Operative Parameters
| Variable | Pre-TASP (n = 56) | TASP (n = 72) | p Value |
|---|---|---|---|
| Case status | 0.04 | ||
| Urgent | 6 (11) | 18 (25) | |
| Emergent | 50 (89) | 54 (75) | |
|
| |||
| Operation within 24 h of symptoms | 39 (70) | 49 (68) | 0.85 |
|
| |||
| Operation beginning at night (5 pm–6 am) | 27 (48) | 21 (29) | 0.03 |
|
| |||
| Redo sternotomy | 7 (13) | 8 (11) | 0.81 |
|
| |||
| Ascending aorta replacement | 55 (98) | 72 (100) | 0.26 |
|
| |||
| Arch replacement | 14 (25) | 70 (97) | <0.0001 |
|
| |||
| Arch type | 0.52 | ||
| Hemi-arch | 14 (25) | 68 (94) | |
| Total arch | 0 | 2 (3) | |
|
| |||
| Aortic valve resuspension | 22 (39) | 55 (76) | <0.0001 |
|
| |||
| Aortic valve replacement | 12 (21) | 0 | <0.0001 |
|
| |||
| Aortic valve repair | 0 | 4 (6) | 0.07 |
|
| |||
| Aortic root replacement | 16 (29) | 17 (24) | 0.52 |
|
| |||
| Root type | 0.001 | ||
| Valve sparing (David-V) | 0 | 6 (8) | |
| Mechanical composite | 15 (27) | 4 (6) | |
| Stentless bioprosthetic | 0 | 4 (6) | |
| Pericardial composite | 1 (2) | 3 (4) | |
|
| |||
| Concomitant procedure | 21 (38) | 12 (17) | 0.008 |
| Coronary artery bypass grafting | 16 (29) | 8 (11) | |
| Mitral valve replacement | 1 (2) | 0 | |
| Mitral valve repair | 1 (2) | 0 | |
| Maze procedure | 1 (2) | 0 | |
| Innominate bypass | 2 (4) | 0 | |
| Aortic arch debranching | 0 | 1 (1) | |
| Descending thoracic aortic endograft | 0 | 1 (1) | |
| Atrial septal defect closure | 0 | 1 (1) | |
| Pulmonary artery embolectomy | 0 | 1 (1) | |
| Femoral bypass | 1 (2) | 1 (1) | |
| Extracorporeal membrane oxygenation | 1 (2) | 0 | |
|
| |||
| Aortic cross-clamp time, min | 128 ± 50 (47–301) | 122 ± 37 (48–250) | 0.62 |
|
| |||
| Cardiopulmonary bypass time, min | 270 ± 94 (107–665) | 214 ± 48 (78–377) | <0.0001 |
|
| |||
| Use of circulatory arrest | 38 (68) | 70 (97) | <0.0001 |
|
| |||
| Nadir NP temperature, °C | 16.3 ± 1.4 (12.0–19.0) | 14.6 ± 1.9 (11.0–19.0) | <0.0001 |
|
| |||
| Circulatory arrest time, min | 33 ± 12 (12–53) | 25 ± 8 (13–61) | 0.002 |
|
| |||
| Selective cerebral perfusion | 26 (68) | 70 (100) | <0.0001 |
| Antegrade cerebral perfusion | 5 (13) | 67 (96) | |
| Retrograde cerebral perfusion | 21 (55) | 3 (4) | |
|
| |||
| Recombinant activated factor VII use | 0 | 30 (42) | <0.0001 |
Values are n (%) or mean ± SD (range).
NP = nasopharyngeal; other abbreviation as in Table 1.
Outcomes
The 30-day/in-hospital adverse events are listed in Table 4. Significant reductions in the rates of reoperation for bleeding, delayed sternal closure, and operative mortality were observed between the TASP and pre-TASP eras. Operative mortality before TASP implementation was 33.9% and was statistically equivalent to the IRAD-predicted mortality rate of 26.0% (observed-to-expected mortality ratio 1.30; p = 0.54) (Fig. 1). Five deaths occurred intraoperatively, and the causes of death were hemorrhage in 5, withdrawal of care due to severe neurological injury in 5, cardiac arrest in 3, multisystem organ failure in 3, failure to wean from CPB in 2, and ruptured descending aorta in 1. Operative mortality after TASP implementation fell to 2.8% and was statistically improved compared with the IRAD-predicted mortality rate of 18.2% (observed-toexpected mortality ratio 0.15; p = 0.005) (Fig. 1). No patients died intraoperatively, and the causes of death included cardiac arrest in 1 and acute myocardial infarction in 1. Differences in survival persisted over long-term follow-up, with 5-year survival rates of 85% observed for TASP patients (mean follow-up 52 ± 23 months) compared with 55% for pre-TASP patients (mean follow-up 104 ± 38 months) (Fig. 2).
Table 4.
30-Day/In-Hospital Adverse Events
| Complication | Pre-TASP (n = 56) | TASP (n = 72) | p Value |
|---|---|---|---|
| Reoperation for bleeding | 11 (19.6) | 3 (4.2) | 0.005 |
| Delayed sternal closure | 6 (10.7) | 1 (1.4) | 0.02 |
| Deep sternal wound infection | 2 (3.6) | 2 (2.8) | 0.80 |
| Acute renal failure (Cr >2.0 mg/dl and >2 × baseline) | 15 (26.8) | 12 (16.7) | 0.16 |
| New-onset dialysis | 4 (7.1) | 4 (5.6) | 0.71 |
| Prolonged ventilation (> 24 h) | 16 (28.6) | 15 (20.8) | 0.31 |
| Tracheostomy | 5 (8.9) | 6 (8.3) | 0.91 |
| Myocardial infarction | 1 (1.8) | 1 (1.4) | 0.86 |
| Stroke (neurologic deficit lasting >72 h) | 7 (12.5) | 4 (5.6) | 0.16 |
| Post-operative length of stay, days | 10 ± 12 (0–55) | 12 ± 12 (4–69) | 0.05 |
| 30-day/in-hospital death | 19 (33.9) | 2 (2.8) | <0.0001 |
Values are mean ± SD (range) or n (%).
Cr = creatinine; other abbreviation as in Table 1.
Figure 1. Observed and Expected Operative Mortality Rates for Acute Type A Aortic Dissection Repair.
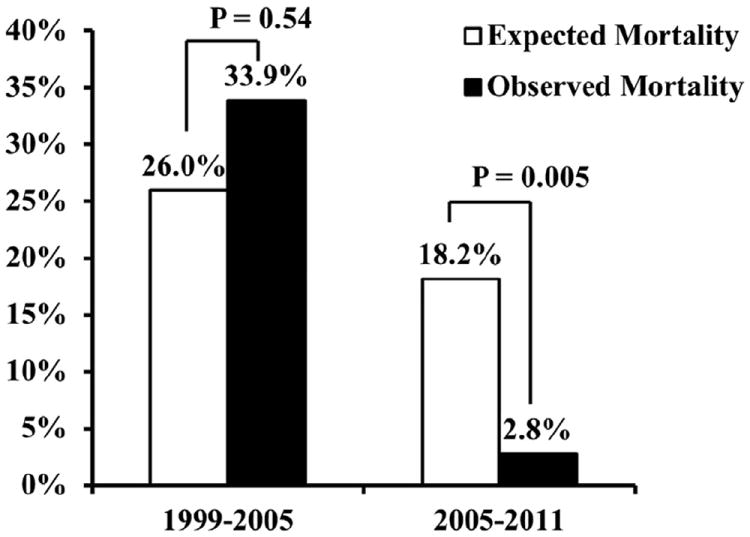
Figure 2. Kaplan-Meier Estimates of Survival Stratified by Era.
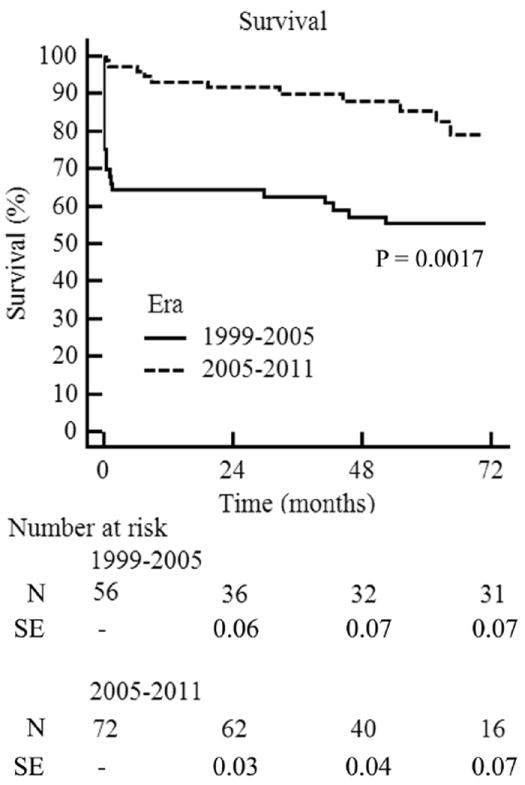
N = number at risk; SE = standard error.
Surgeon-specific procedural volume and outcomes
During the 6-year period prior to TASP implementation, an average of 9 ATAAD repairs were performed per year at the institution. These operations were performed by 11 different surgeons with average annual procedural volumes of 2.0 ± 1.3 ATAAD repairs/year (range 1 to 4 ATAAD repairs/ year). Surgeon-specific mortality rates ranged from 20% to 67% for the 8 surgeons who performed more than 1 ATAAD operation during the study period (Fig. 3). During the first 6 years after TASP implementation, an average of 12 ATAAD repairs were performed per year at the institution. Seventy (97%) of these cases were performed by the 2 principal TASP surgeons, of whom surgeon 12 performed an average of 9.7 ATAAD repairs/year with a surgeon-specific mortality rate of 1.7% and surgeon 13 performed an average of 4.0 ATAAD repairs/year with a surgeon-specific mortality rate of 8.3% (Fig. 3).
Figure 3. Distribution of Surgeons Performing Acute Type A Aortic Dissection Repairs Over Time.
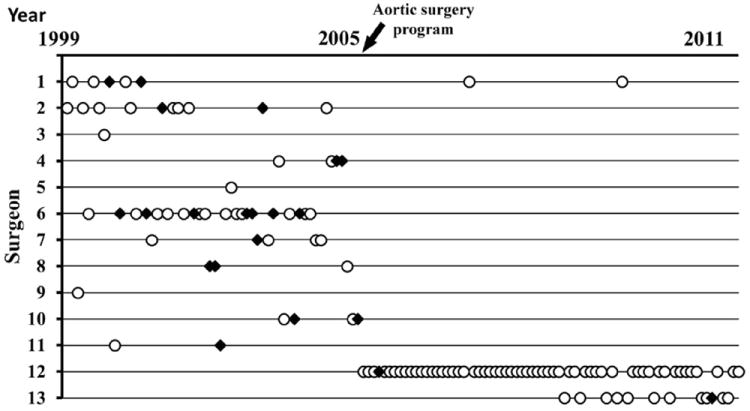
Between 1999 and 2011, 128 acute type A aortic dissection repairs were performed by 13 different surgeons. Open circles = survivors and black diamonds = deaths.
Discussion
In this single-center experience, we demonstrated a significant reduction in operative mortality for ATAAD repair following the implementation of a TASP with dedicated high-volume thoracic aortic surgeons, a multidisciplinary approach to thoracic aortic disease management, and a standardized protocol for ATAAD repair. The contemporary operative mortality rate of 2.8% appears improved compared with cumulative operative mortality rates reported by national and international registries (1-3,19) but similar to other experienced aortic centers where operative mortality rates below 10% have been achieved (10,20-22).
Although patient characteristics and expected operative mortality rates were statistically equivalent between eras, there were several important differences in patient risk profiles that likely influenced outcomes. Specifically, TASP patients were noted to be younger and had decreased rates of hypotension, shock, tamponade, and myocardial infarction at presentation compared with pre-TASP patients. Correspondingly, the expected operative mortality for TASP patients was numerically lower than for pre-TASP patients (18.2% vs. 26.0%). As a result, the observed-to-expected mortality improved only 9-fold after TASP introduction (0.15 vs. 1.30), whereas raw mortality rates improved by 12-fold (2.8% vs. 33.9%), suggesting that the reduction in operative mortality was partially due to operation on lowerrisk patients. However, deferral of surgery in high-risk patients did not appear to be the underlying source of the reduced operative risk, given that the rate of nonoperation remained unchanged at 15% and the predicted mortality of the nonoperative patients was nearly identical between eras. In addition, the rates of medical management in our series were similar to those reported in the IRAD database (9.9% to 28%), suggesting that our selection criteria were not overly selective (19,23). However, these data highlight that patient selection and differences in baseline risk are powerful drivers of outcomes that must be objectively assessed when comparing outcomes between centers and studies.
One of the most important advances following implementation of the TASP was the modernization and standardization of operative technique and cerebral protection strategies. Prior to the TASP, marked variability in operative technique and circulation management was observed. During this period, the rate of stroke was 12.5% and 5 deaths (8.9%) were the consequence of unrecoverable neurological injury. Essential additions to our modern operative strategy included right axillary cannulation, root replacement for standardized indications, routine hemi-arch reconstruction, use of circulatory arrest with deep hypothermia, electroencephalographic monitoring when available, and antegrade cerebral perfusion as the preferred cerebral protection adjunct. With these measures, the rate of stroke was 5.6% and no deaths resulted from neurological injury.
The second major area of improvement following implementation of the TASP was reduced morbidity and mortality from bleeding complications. Prior to TASP implementation, nearly a quarter of patients underwent reoperation for bleeding and/or delayed sternal closure and 5 patients (8.9%) succumbed to refractory hemorrhage. In the modern cohort, rates of reoperation for bleeding and delayed sternal closure were significantly reduced and no patients died of hemorrhage. These improvements are likely attributable to the advent of intraoperative low-dose rFVIIa protocols as well as standardization and optimization of transfusion maneuvers with deep hypothermia (8,9).
The timing and expediency of surgery were also noted to change significantly between eras—the proportion of cases initiated at night decreased from 48% to 29% and the number of emergent cases decreased from 89% to 75% during the TASP era. It is our current practice to defer surgery until daylight hours in stable patients who present more than 48 h after symptom onset, given the low rate of rupture or acute decompensation in these patients (5). There are several theoretical benefits of such an approach, including performance of a thorough pre-operative evaluation as well as embarking upon these challenging operations with the most qualified ancillary team members and without the undue effects of sleep deprivation on all participants. Despite these benefits, we continue to operate emergently on patients who present within 48 h of symptom onset, even if asymptomatic and stable, given the increased risk of death within this time frame as well as medicolegal considerations (5,12).
Lastly, our data provide additional support to the growing body of literature suggesting that outcomes of proximal aortic operations are improved when performed by high-volume centers and high-volume surgeons. Hospital procedure volume has been strongly associated with post-operative mortality for a number of complex cardiovascular procedures, including both elective and emergent proximal aortic operations (1,3,6). Although our institutional ATAAD repair case volumes between 9 and 12 per year may appear modest, a review of the Nationwide Inpatient Sample (NIS) by Knipp et al (1) defined high-volume centers as those that performed more than 2.5 cases per year, and only 15 NIS-participating centers in the entire country averaged more than 10 ATAAD repairs per year. However, in an updated NIS study of aortic dissection repair, the lowest operative mortality rate of 16.4% was achieved in centers that performed more than 13 aortic dissection repairs per year (although both type A and type B repairs were included in the analysis) (3). Given that our program achieved a substantially lower mortality rate without significantly exceeding this volume threshold, we suspect the total number of all proximal aortic cases performed yearly is likely a better predictor of outcomes than the number of isolated ATAAD repairs performed. No prior volume-outcomes study of ATAAD repair has explored this hypothesis.
Interestingly, the improvement in outcomes observed before and after TASP implementation occurred without a marked increase in institutional ATAAD case volume, suggesting that the more important component of the volume-outcomes relationship may be surgeon volume (3). Prior to the TASP, surgeon coverage for aortic emergencies was provided by the full complement of cardiothoracic surgeons, many of whom were without expertise or recent experience in thoracic aortic surgery and performed only 1 or 2 ATAAD repairs per year. Following implementation of the TASP, ATAAD repairs were restricted to 2 high-volume aortic surgeons; as a result, surgeon case volumes increased substantially for each of the 2 principal surgeons. Further, the same 2 surgeons also performed all of the elective proximal aortic operations, dramatically increasing each surgeon’s yearly proximal aortic case numbers. We suspect this supersubspecialization in proximal aortic surgery may be the major contributor to our improved results by leading to fewer technical errors, shorter CPB and circulatory arrest times, more complex root and arch operations, and avoidance of intraoperative deaths. Creation of similar programs with designated aortic surgeons at other tertiary centers may therefore represent an important way to improve outcomes for this disease process.
The current study and others further suggest that outcomes of ATAAD repair could be improved through centralization of care, possibly through the credentialing and designation of regional referral centers for aortic emergencies and establishment of protocols for rapid patient transport, given that few centers are likely to achieve the center and surgeon volume thresholds associated with the best outcomes. Although emergent operations are generally considered less amenable to triage and transfer, most patients with ATAAD are able to be stabilized and survive transfer, as evidenced by the approximately 75% of patients in our series and others who were transferred from other institutions (5). In addition, a framework for such an enterprise that could serve as a template for other regions was recently described by Harris et al. (24) who developed a laudable community outreach program at more than 30 regional hospitals to expedite transfer of aortic dissection patients to an aortic referral center in Minneapolis, Minnesota. This initiative resulted in significant reductions in time to diagnosis, transfer, and surgery for patients with ATAAD (24).
Study limitations
Several limitations of this study should be acknowledged. TASP patients were compared with historical controls from the era immediately preceding program implementation. Therefore, unmeasured confounders related to patient selection and disease severity at presentation may influence the comparison of results between eras. A selection bias may further exist with regard to patients who were not accepted for transfer or died during transfer. The study is prone to type II error and is underpowered to detect small differences in outcomes between groups. Finally, results may not be generalizable to other institutions due to unique differences in patient populations, practitioners, and locoregional factors.
Conclusions
This study demonstrated that ATAAD repair can be performed with results approximating those of elective proximal aortic surgery when operations are performed by a high-volume multidisciplinary thoracic aortic surgery team. These data suggest that efforts to standardize or centralize care of ATAAD patients are warranted.
Acknowledgments
Dr. Andersen was supported by a Thoracic Surgery Foundation for Research and Education Research Fellowship. Dr. Williams was supported by National Institutes of Health grant U01- HL088953.
Abbreviations and Acronyms
- ATAAD
acute type A aortic dissection repair
- CPB
cardiopulmonary bypass
- IRAD
International Registry of Acute Aortic Dissection
- NIS
Nationwide Inpatient Sample
- rFVIIa
recombinant activated factor VII
- TASP
thoracic aortic surgery program
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Knipp BS, Deeb GM, Prager RL, Williams CY, Upchurch GR, Jr, Patel HJ. A contemporary analysis of outcomes for operative repair of type A aortic dissection in the United States. Surgery. 2007;142:524–8. doi: 10.1016/j.surg.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Williams JB, Peterson ED, Zhao Y, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol. 2012;60:1156–62. doi: 10.1016/j.jacc.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chikwe J, Cavallaro P, Itagaki S, Seigerman M, Diluozzo G, Adams DH. National outcomes in acute aortic dissection: influence of surgeon and institutional volume on operative mortality. Ann Thorac Surg. 2013;95:1563–9. doi: 10.1016/j.athoracsur.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD) Eur J Vasc Endovasc Surg. 2009;37:149–59. doi: 10.1016/j.ejvs.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011;58:2455–74. doi: 10.1016/j.jacc.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 6.Hughes GC, Zhao Y, Rankin JS, et al. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg. 2013;145:166–70. doi: 10.1016/j.jtcvs.2011.10.094. [DOI] [PubMed] [Google Scholar]
- 7.Lima B, Williams JB, Bhattacharya SD, et al. Results of proximal arch replacement using deep hypothermia for circulatory arrest: is moderate hypothermia really justifiable? Am Surg. 2011;77:1438–44. [PMC free article] [PubMed] [Google Scholar]
- 8.Williams JB, Phillips-Bute B, Bhattacharya SD, et al. Predictors of massive transfusion with thoracic aortic procedures involving deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011;141:1283–8. doi: 10.1016/j.jtcvs.2010.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen ND, Bhattacharya SD, Williams JB, et al. Intraoperative use of low-dose recombinant activated factor VII during thoracic aortic operations. Ann Thorac Surg. 2012;93:1921–8. doi: 10.1016/j.athoracsur.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bavaria JE, Pochettino A, Brinster DR, et al. New paradigms and improved results for the surgical treatment of acute type A dissection. Ann Surg. 2001;234:336–42. doi: 10.1097/00000658-200109000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies RR, Coe MP, Mandapati D, et al. Thoracic Surgery Directors Association Award. What is the optimal management of late-presenting survivors of acute type A aortic dissection? Ann Thorac Surg. 2007;83:1593–601. doi: 10.1016/j.athoracsur.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Elefteriades JA, Barrett PW, Kopf GS. Litigation in nontraumatic aortic diseases—a tempest in the malpractice maelstrom. Cardiology. 2008;109:263–72. doi: 10.1159/000107790. [DOI] [PubMed] [Google Scholar]
- 13.Parsa CJ, McCann RL, Hughes GC. Novel approach to the treatment of distal malperfusion secondary to ascending aortic dissection. J Card Surg. 2010;25:220–2. doi: 10.1111/j.1540-8191.2009.00991.x. [DOI] [PubMed] [Google Scholar]
- 14.Patel HJ, Williams DM, Dasika NL, Suzuki Y, Deeb GM. Operative delay for peripheral malperfusion syndrome in acute type A aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg. 2008;135:1288–95. doi: 10.1016/j.jtcvs.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Kitai T, Kaji S, Yamamuro A, et al. Clinical outcomes of medical therapy and timely operation in initially diagnosed type A aortic intramural hematoma: a 20-year experience. Circulation. 2009;120:S292–8. doi: 10.1161/CIRCULATIONAHA.108.843615. [DOI] [PubMed] [Google Scholar]
- 16.James ML, Andersen ND, Swaminathan M, et al. Predictors of electrocerebral inactivity with deep hypothermia. J Thorac Cardiovasc Surg. 2013 Apr 11; doi: 10.1016/j.jtcvs.2013.03.022. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest: I. effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. 2001;71:14–21. doi: 10.1016/s0003-4975(00)01592-7. [DOI] [PubMed] [Google Scholar]
- 18.Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55–61. doi: 10.1016/j.athoracsur.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 20.Girardi LN, Krieger KH, Lee LY, Mack CA, Tortolani AJ, Isom OW. Management strategies for type A dissection complicated by peripheral vascular malperfusion. Ann Thorac Surg. 2004;77:1309–14. doi: 10.1016/j.athoracsur.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Qi R, Zhu J, Liu Y, Chang Q, Zheng J. Repair of acute type A dissection: our experiences and results. Ann Thorac Surg. 2011;91:1147–52. doi: 10.1016/j.athoracsur.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Westaby S, Saito S, Katsumata T. Acute type A dissection: conservative methods provide consistently low mortality. Ann Thorac Surg. 2002;73:707–13. doi: 10.1016/s0003-4975(01)03449-x. [DOI] [PubMed] [Google Scholar]
- 23.Tsai TT, Evangelista A, Nienaber CA, et al. Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2006;114:I350–6. doi: 10.1161/CIRCULATIONAHA.105.000497. [DOI] [PubMed] [Google Scholar]
- 24.Harris KM, Strauss CE, Duval S, et al. Multidisciplinary standardized care for acute aortic dissection: design and initial outcomes of a regional care model. Circ Cardiovasc Qual Outcomes. 2010;3:424–30. doi: 10.1161/CIRCOUTCOMES.109.920140. [DOI] [PubMed] [Google Scholar]


