Abstract
The primary aims of this work were to: 1) establish a calibrator surrogate matrix for quantification of amyloid-β (Aβ)42 in human cerebrospinal fluid (CSF) and preparation of quality control samples for LC-MS-MS methodology, 2) validate analytical performance of the assay, and 3) evaluate its diagnostic utility and compare it with the AlzBio3 immunoassay. The analytical methodology was based on a 2D-UPLC-MS-MS platform. Sample pretreatment used 5 M guanidine hydrochloride and extraction on μElution SPE columns as previously described. A column cleaning procedure involved gradual removal of aqueous solvents by acetonitrile assured consistent long-term chromatography performance. Receiver-operator characteristic (ROC) curve and correlation analyses evaluated the diagnostic utility of UPLC-MS-MS compared to AlzBio3 immunoassay for detection of Alzheimer’s disease (AD). The surrogate matrix, artificial CSF containing 4 mg/mL of BSA, provides linear and reproducible calibration comparable to human pooled CSF as calibration matrix. Appropriate cleaning of the trapping and analytical columns provided every-day, trouble-free runs. Analyses of CSF Aβ42 showed that UPLC-MS-MS distinguished neuropathologically-diagnosed AD subjects from healthy controls with at least equivalent diagnostic utility to AlzBio3. Comparison of ROC curves for these two assays showed no statistically significant difference (p = 0.2229). Linear regression analysis of Aβ42 concentrations measured by this mass spectrometry-based method compared to the AlzBio3 immunoassay showed significantly higher but highly correlated results. In conclusion, the newly established surrogate matrix for 2D-UPLC-MS-MS measurement of Aβ42 provides selective, reproducible, and accurate results. The documented analytical performance and diagnostic performance for AD versus controls supports consideration as a candidate reference method.
Keywords: Alzheimer’s disease, amyloid-β42, cerebrospinal fluid, mass spectrometry
INTRODUCTION
Alzheimer’s disease (AD), the most common form of dementia, is characterized by loss of mental ability that interferes with normal activities of daily living. It was estimated that in 2012 in the United States (US) there were 5.2 million individuals, age 65 or older, with AD and that number will reach nearly 16 million by 2050, with 7 million aged 85 years or older [1]. AD is the sixth-leading cause of death in the US and the only disease among the top 10 that currently cannot be prevented, cured, or even slowed. The current costs of dementia care in the US estimated by the Rand Corporation are equal to or exceeds those of neoplastic and cardiovascular diseases. Costs will increase in the coming years due to the inexorably rising prevalence of AD [2]. The pathological hallmarks of AD are the deposition of amyloid-β (Aβ) in the extracellular space of the brain (plaques) and intraneuronal tangles formed by abnormal tau proteins. These pathologic changes start occurring two or more decades before the earliest clinical signs of the disease [3] and are reflected by lowered concentrations of Aβ42 and increased tau protein concentrations in cerebrospinal fluid (CSF) [4–7]. Proposed clinical research diagnostic guidelines have described addition of assays for CSF Aβ42, and tau proteins in AD research settings to: (a) enhance the pathophysiological specificity of the clinical diagnosis of AD, (b) increase the level of certainty that AD pathology is the cause of cognitive decline in patients with a clinical diagnosis of mild cognitive impairment (MCI), and (c) help define the preclinical stage of AD neuropathology [8–10].
Immunoassays are the most widely used methods for quantification of tau and Aβ biomarkers in CSF [11–13]. Several limitations of the current Aβ-immunoassays include differences in epitope recognition by differing antibodies, matrix effects [14], and lack of a CSF-based standard reference material with Aβ42 concentrations based on qualified mass spectrometry reference methodology. Recently two papers described a new, alternative non-immunological method for accurate quantitation of Aβ42 in human CSF based on liquid chromatography with tandem mass spectrometric detection [15, 16]. A choice of matrix for calibrators and quality control sample preparation was one of the most important challenges for this assay. Lame and colleagues [15] used artificial CSF (aCSF) containing 5% rat plasma to act as a carrier and decrease nonspecific binding. However diagnostic utility of this method was not assessed/reported. Pannee and co-workers [16] prepared calibrators in human CSF using stable isotope labeled Aβ42 as a standard and the endogenous Aβ42 as an internal standard, but for clinical samples, isotope labeled Aβ42 served as an internal standard. Using freshly obtained human CSF for calibrator preparation would be ideal, but we lack access to pools of freshly prepared CSF so for us it is impractical.
The goal of the current study was to establish and assess reproducible, practical surrogate matrix for calibrators and quality control sample preparation; validate the analytical performance of this method based on the surrogate matrix using three different lots of standard material; and assess the diagnostic utility of the method and compare it with the well-established AlzBio3 immunoassay kit (Innogenetics/Fujirebio, Ghent, Belgium).
MATERIAL AND METHODS
Chemicals and reagents
Human synthetic peptide Aβ42 and internal standard (uniformly labeled, Nitrogen-15) Aβ42 both with trifluoracetate as counter ion, were purchased from r-Peptide. Certificate of Analysis from rPeptide contained information about Aβ42 purity (>97% for Aβ42 and > 95% for 15N-Aβ42), amino acid sequence and molecular weight assessment by Maldi TOF mass spectrometry.
Accuracy of the weight of this material is based on amino acid analysis of the standards used for HPLC analyses of each lot of material (Jane Wightman, personal communication). Discarded human CSF samples were obtained from the clinical laboratory at the Hospital of the University of Pennsylvania (Philadelphia, PA, USA). Purchases of aCSF perfusion fluid (Na 150 mM, K 3.0 mM, Ca 1.4 mM, Mg 0.8 mM, P 1.0 mM, and Cl 155 mM) and normal rat plasma were from Harvard Apparatus and GeneTex, respectively. Guanidine hydrochloride (GuCl) and trifluoroethanol (TFE) were purchased from Sigma-Aldrich. All other chemical reagents were purchased from Fisher Scientific.
Sample preparation
Standard and internal standard Aβ42 peptide delivered in lyophilized form were dissolved in DMSO at the concentration of 50 μg/mL. This stock solution was subsequently diluted with DMSO to obtain two independent sets of two working solutions (500 and 50 ng/mL) (separately prepared working solutions for calibrator and QC samples preparation) and one working solution for internal standard (500 ng/mL). Working solutions were aliquoted and stored at −80°C.
Spiking solutions for each calibrator and quality control (QC) sample were prepared by diluting working solutions of Aβ42 standards with diluent (acetonitrile/water/concentrated NH4OH [50/49/1]). They were aliquoted and stored at −80°C. Three different lots of Aβ42 standard material were used for method validation. Spiking solution of internal standard (2 ng/mL of CSF) was prepared fresh on the day of analysis, in aCSF with rat plasma (5%). Calibrators and three QC samples (250, 400, and 800 pg/mL) were prepared on the day of analysis in surrogate matrix by spiking 0.95 mL of the matrix with 0.05 mL of each Aβ42 spiking solution. Two surrogate matrices were tested: aCSF with 5% rat plasma and aCSF with BSA (bovine serum albumin [lot number: 111328], Cohn fraction V, heat-shock treated, DNase, RNase and protease free) (4 mg/mL), referred to as BSA/aCSF throughout this manuscript.
Calibrators, QC samples, and frozen samples of human CSF were equilibrated at room temperature for 30 min before analysis. The Aβ42 calibrator range was 50 to 3,000 pg/mL. A 0.25 mL aliquot of each calibrator, QC sample, and human CSF was diluted with a mixture of 5 M GuCl, and internal standard (0.25 mL of 5 M GuCl and 0.005 mL of spiking solution of internal standard), shaken at room temperature 45 min at 1800 rpm (Multi-Tube Vortexer VX-2500, VWR) then diluted further with 0.25 mL of aqueous 4% H3PO4.
Following pre-treatment, samples were loaded on Oasis MCX μelution 96-well plates as previously described [15]. Briefly, the plate was first equilibrated with 0.2 mL of methanol followed by 0.2 mL of aqueous H3PO4. Then 0.6 mL of the sample was loaded onto the SPE microcolumns, washed with 0.2 mL of H3PO4 followed by 0.2 mL of 10% acetonitrile in water. Aβ42 peptide was eluted into a collection plate with 2 × 0.025 mL of acetonitrile/water/concentrated ammonia solution (75: 15: 10). Each eluate was diluted with 0.05 mL of deionized water. The content of the wells was mixed on a horizontal shaker (Vortex Genie-2; Fisher Scientific) for 10 s and placed in the autosampler.
2D-UPLC-MS-MS system characteristics
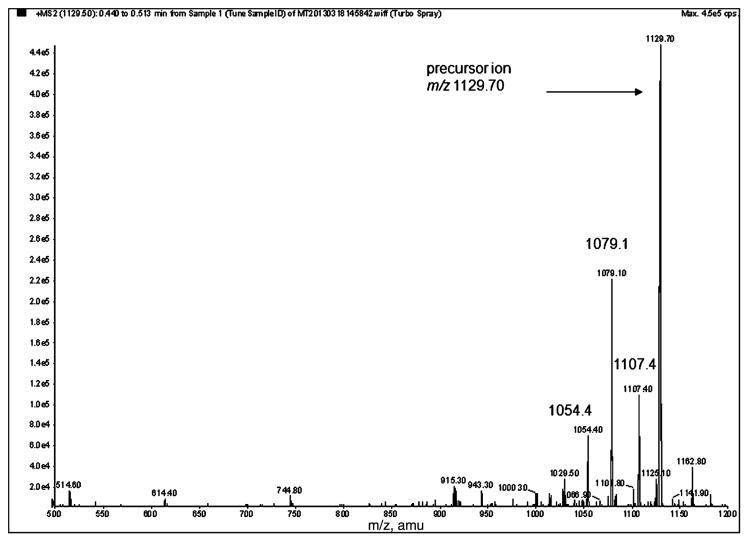
Analysis of Aβ42 peptide was carried out on an API 5000 triple quadrupole mass spectrometer (ABSciex) with an electrospray probe in a TurboV ion source, interfaced with an ACQUITY Ultra Performance LC system (Waters) including sample manager, two pumps, and a column oven with two columns: trapping (xBridge BEH C18, 2.1 × 30 mm, 3.5 μm; Waters) and analytical (ACQUITY UPLC BEH-300 C18, 2.1 × 150 mm, 1.7 μm: Waters) both maintained at 60°C. MassLynx v. 4.1 software (Waters) was used for UPLC system control and Analyst 1.4.2 software (ABSciex) was used for mass spectrometer control and data processing. Mass spectrometer operation was in the positive ion mode and MRM scan type. The first and third quadrupoles were set to isolate the 4+ charged precursor and fragment ion pairs: m/z 1129.5 → 1079.1 (Aβ42) and m/z 1142.5 → 1091.5 (15N-Aβ42). For quantification one ion transition was used, the highest intensity fragments were selected for our method. These ion pairs were consistent with the reported data [15, 16] (Fig. 1).
Fig. 1.
Product ions spectrum of positively charged (+4) precursor ion of Aβ42 (5 μg/mL) in acetonitrile:water (50: 50 with 0.1% NH4OH). Peptide was infused into the mass spectrometer with a syringe pump at 10 μL/min while the mixture of acetonitrile and water (50: 50) was delivered by LC pump (0.2 mL/min). The most abundant products of precursor ion m/z 1129.7 were: m/z 1079.1, 1107.4, and 1054.4.
Fifty microliters of sample was injected into the trap column. Following a 1-min desalting period with trapping solvent (water/acetonitrile [98/2] with 0.1% ammonia) at a flow rate of 0.6 mL/min directed to waste, the valve was switched and analytes were transferred to the analytical column in the reverse direction. Aβ42 and its internal standard were eluted under linear gradient conditions of A-0.1% ammonia in water and B-acetonitrile/MeOH/TFE (75/25/5, v/v/v) from 10%B to 45%B over 7.3 min at 0.2 mL/min. As Aβ42 was eluted from the analytical column, the trap column was regenerated with acetonitrile, methanol, isopropanol, and water mixture (60/20/10/5). Total run time was 12 min, including 2 min of post-run equilibration of the analytical column under the initial gradient conditions to prepare for the next injection. At the end of each analytical run the entire system was cleaned using a solvent [NH4OH (0.1%)/ACN; 90/10)] that was gradually replaced by organic solvent, up to 100% ACN, over 2 h.
Subjects of the clinical study
A set of pre-mortem CSF samples from 41 autopsy-confirmed AD cases (mean age 70.2 [range 44.2–86.2] years old) and 41 age-matched cognitively normal living elderly subjects (NC) (mean age 69.4 [range 51.3–88.2] years old) from the University of Pennsylvania Alzheimer’s Disease Center Core (ADCC) were analyzed by the assay. Cases and control subjects were clinically evaluated as described [17–19]. All of the CSF samples were collected using standardized methodology [17] and stored at −80°C. Demographic characteristics of these 82 subjects are summarized in Supplementary Table 1. The neuropathological diagnosis, performed according to previously described procedures [20], confirmed that all 41 cases had high probability AD [21]. All cases had a V/VI Braak stage [22] and CERAD score of C [23] for all subjects except one patient with a CERAD B score. In addition, six cases had a coincident neuropathological diagnosis of dementia with Lewy bodies, two subjects had coincident progressive supranuclear palsy and one subject had coincident frontotemporal lobar degeneration with TDP-43 deposits. Neuropathological images and details of the neuropathological examination are described in the supplementary material (Supplementary Figure 1 and Supplementary Table 2) as reported previously [20]. Written informed consent was obtained for participation in these studies, which was approved by the University of Pennsylvania IRB.
Statistical analysis
On completion of analyses of AD and NC subject CSF samples, statistical analyses were performed to determine diagnostic performance of CSF Aβ42 for distinguishing between AD and NC subjects. Since there was Aβ42 concentration data for these samples based on prior measurements using a qualified AlzBio3 immunoassay [24], we also compared these two methods for their diagnostic performance. Statistical analysis, including receiver-operator characteristic (ROC) curve analyses, was done using R 2.15.2 [25] and statistical comparison between the AlzBio3 immunoassay and 2D-UPLC-MS-MS assay ROC AUCs was done according to DeLong [26]. For linear regression and t-test analysis we used Graph-PadPrism 5 software.
RESULTS
Selection of calibrator matrix
Prevention of Aβ42 from precipitating out of solution and provision of reproducible and accurate calibration required development of a matrix for the peptide calibrators that achieved these goals. Use of human CSF as the matrix would be preferable, but not practical. Thus, we considered alternatives to human CSF including: 1) rat plasma mixed into aCSF (5% v/v) that mimics the electrolyte composition of human CSF [15] and 2) BSA/aCSF. However, in our system Aβ42 standards in aCSF with rat plasma had lower peak intensities compared with standards prepared in BSA/aCSF or human CSF. Therefore, all standards were prepared in BSA/aCSF. They were stable in this matrix at least 4 h at room temperature (Supplementary Table 3). This surrogate matrix did not affect the ionization process; no ion suppression or enhancement was observed, as tested by the post-column infusion method [27].
Validation of the surrogate-based method
Dynamic range, linearity, and lower limit of quantification
The lower limit of quantification (LLOQ) was defined as the lowest concentration of Aβ peptides that could be measured with a reproducibility of ≤20% and an accuracy of 80–120% [28]. The lower limit of quantification for Aβ42 was 50 pg/mL (signal to noise 4.55 ± 0.3) and mean LLOQ accuracy was 99.7% (n = 18; CV = 10.8%) (Table 1). Calibration curve ranged from 50 to 3000 pg/mL and r2 values were >0.996 (n = 10) for three different lots of Aβ42 standard material. Retention time average (n = 50 samples) for Aβ42 was 7.74 ± 0.05 min over a 5 day period.
Table 1.
Between day precision and accuracy for calibrators and three different batches of QC samples. Data were collected from a replication experiment in which each sample was run in duplicate on a different day (n = 5–9). Concentrations of Aβ42 for QC samples lot#242 and 142 were obtained from the calibration curve prepared from standard material lot# 942
| Calibrator (pg/mL) lot#942
|
Mean accuracy (%) | Mean SD | Mean CV (%) | n | |
|---|---|---|---|---|---|
| Nominal | Reads | ||||
| 50 | 49.9 | 99.7 | 10.7 | 10.8 | 18 |
| 100 | 105.1 | 105.1 | 7.9 | 7.4 | 18 |
| 200 | 200.9 | 100.4 | 6.7 | 6.6 | 18 |
| 350 | 345.3 | 98.6 | 5.5 | 5.6 | 18 |
| 500 | 482.5 | 96.5 | 4.7 | 4.8 | 18 |
| 750 | 738.2 | 98.4 | 7.2 | 7.3 | 18 |
| 1000 | 1003.5 | 100.4 | 6.2 | 6.1 | 18 |
| 3000 | 3019.5 | 100.6 | 2.7 | 2.6 | 18 |
| QC samples lot#942 | |||||
| 250 | 255.6 | 102.2 | 24.9 | 9.7 | 14 |
| 400 | 404.7 | 101.2 | 33.3 | 8.2 | 14 |
| 800 | 793.6 | 99.2 | 50.7 | 6.4 | 14 |
| QC samples lot#242 | |||||
| 250 | 238.2 | 95.8 | 22.0 | 9.2 | 10 |
| 400 | 366.8 | 91.7 | 19.1 | 5.2 | 10 |
| 800 | 782.1 | 97.8 | 79.3 | 10.1 | 10 |
| QC samples lot#142 | |||||
| 250 | 249.0 | 99.6 | 24.2 | 9.7 | 10 |
| 400 | 402.4 | 100.6 | 29.8 | 7.4 | 10 |
| 800 | 770.4 | 96.3 | 62.7 | 8.1 | 10 |
Precision, analytical, and absolute recovery for standards and QC samples
Within-day precision and accuracy were assessed for two QC samples prepared from one lot of standard material. These samples were tested 10 times on one day. All data collected from a replication experiment in which each sample was run in duplicate on a different day are reported as between-day precision and accuracy in Table 1. Within-day precision was 6.9% and 7.3% with accuracy 91.6% and 107.3% for two QC samples (250 and 800 pg/mL). For all calibrators and three QC samples, between-day accuracy was within 10.0% of expected values, ranging from 91.7 to 105.1%. Mean between-day precision for calibrators ranged from 2.6% to 10.8% and for quality control samples, from 5.2% to 10.1%. No significant difference in precision or accuracy was found between three different lots of Aβ42 standard material (Table 1), no carry over effect was observed, and no peak was detected in the blank sample run immediately after the highest standard.
To measure absolute recovery of Aβ42 peptide from SPE micro-columns, paired sets of samples were prepared. The first set contained samples, spiked after extraction on SPE micro-columns; the second set included aliquots of the same samples spiked before extraction. The average absolute recovery was 70 ± 4.6% (n = 6).
Precision of human CSF measurements and analytical recovery of Aβ42 peptide standard spiked into CSF pools
Pools of human CSF were prepared by mixing residual CSF samples from the Hospital of the University of Pennsylvania clinical laboratory. Between-day measurement precision for Aβ42 in ten different CSF pools ranged from 2.2% to 11.1% (n = 4–15) and within-day mean precision for two different CSF pools was 5.2% (n = 8).
Analytical recovery of Aβ42 peptide from CSF was assessed by overspiking five different CSF pools with Aβ42 (200, 500, 750, and 1000 pg/mL). For each pair of results (basal and spiked value) average bias, the difference between the expected and obtained results expressed as percentage, was −0.8% (ranging from −18.8 to 8.9%) (Table 2).
Table 2.
Analytical recovery of Aβ42 from five different pools of human CSF
| pg/mL Basal value (n = 4) | Pool 1 708 (CV = 6.6%) |
Pool 4 688 (CV = 4.4%) |
Pool 5 864 (CV = 2.2%) |
Pool 16 286 (CV = 10.5%) |
Pool 32 362 (CV = 9.3%) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiked-in Amount | 200 | 500 | 750 | 1000 | 200 | 500 | 750 | 1000 | 200 | 500 | 750 | 1000 | 200 | 500 | 750 | 1000 | 200 | 500 | 750 | 1000 |
| Spiked Value | 989 | 1260 | 1455 | 1760 | 875 | 1095 | 1340 | 1830 | 1037 | 1425 | 1610 | 1730 | 496 | 844 | 969 | 1295 | 543 | 863 | 1115 | 1360 |
| Theoretical | 908 | 1208 | 1458 | 1708 | 888 | 1188 | 1438 | 1688 | 1064 | 1364 | 1614 | 1864 | 486 | 786 | 1039 | 1286 | 562 | 1062 | 1112 | 1362 |
| Bias (%) | 8.9 | 4.3 | −0.2 | 3.0 | −1.5 | −7.8 | −6.8 | 8.4 | −2.5 | 4.5 | −0.3 | −7.2 | 2.0 | 7.4 | −6.5 | 0.7 | −3.5 | −18.8 | 0.3 | −0.2 |
Assay selectivity
Assay selectivity was assessed by measurement of Aβ42 concentration in two kinds of samples in BSA/aCSF: one set contained Aβ42 alone and the other besides Aβ42 (1,000 pg/mL) contained three different concentrations of Aβ40 (750, 3,000, and 10,000 pg/mL) and Aβ38 (750, 3,000, and 7,500 pg/mL). The difference in Aβ42 concentration for these two kinds of samples was below 10% and random, from −7.9 to 9.1% providing substantiation for the selectivity of our assay. Furthermore the total ion chromatogram showed very good separation of the three Aβ peptide peaks for artificial and human CSF (Supplementary Figures 2 and 3).
Assessment of the surrogate matrix
The method of standard additions was used to compare performance of calibrators in CSF pools with the performance of the BSA/aCSF surrogate matrix. Nine CSF pools with previously measured concentrations of Aβ42 were spiked with five different concentrations of Aβ42 (0, 200, 500, 750, and 1000 pg/mL) to prepare calibration curves and calculate Aβ42 concentration in each pool. Average bias for results from calibration curves in BSA/aCSF versus those in human CSF was −3.3% (ranging from −12.1 to +8.4%) (Table 3). Figure 2 shows the correlation between these two sets of Aβ42 results (r2 = 0.984; correlation equation, y = 1.07X – 11.3). These results provide substantiation for the equivalence of BSA/aCSF compared to human CSF as the calibration matrix.
Table 3.
Concentration of Aβ42 obtained for 9 pools of human CSF from calibration curve prepared in BSA/aCSF and in human CSF (method of standards addition)
| Aβ42 | Pool 1 | Pool 2A | Pool 4 | Pool 5 | Pool 8 | Pool 9 | Pool 16 | Pool 32 | Pool 10 |
|---|---|---|---|---|---|---|---|---|---|
| Conc. from curve in art CSF with BSA | 708 | 392 | 688 | 864 | 1006 | 676 | 286 | 362 | 526 |
| Conc. from curve in human CSF | 695 | 360 | 691 | 857 | 884 | 670 | 310 | 358 | 467 |
| Difference (%) | −1.8 | −8.2 | +0.4 | −0.8 | −12.1 | −0.9 | +8.4 | −1.1 | −11.2 |
Fig. 2.

Correlation of Aβ42 results measured in 9 CSF pools using calibration curves prepared in surrogate human CSF matrix [aCSF with BSA (4 mg/mL)] versus measured concentrations using human CSF. For the latter, each of the 9 CSF pools served as calibrator matrix (method of standard additions).
Analysis of Aβ42 in clinical samples: Diagnostic utility assessment
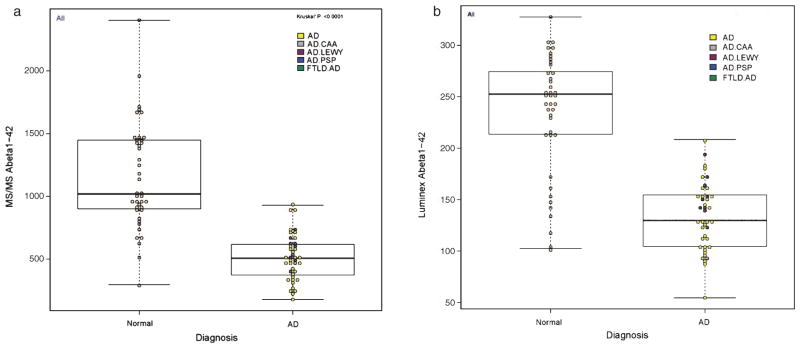
To qualify this method for use in clinical investigations, we measured the concentration of Aβ42 in 82 human CSF samples. The mean Aβ42 concentration for the healthy controls was 1152 ± 421.2 pg/mL (median 1015 pg/mL) and was 2.25 times greater than the AD group mean value (511 ± 185.2 pg/mL; median 504 pg/mL) (Fig. 3A). ROC AUC analyses of the two groups (Fig. 4) provided a cut point concentration achieved at optimal test efficiency, and assessments of the diagnostic sensitivity and specificity, efficiency, and positive and negative predictive values for this test. The ROC AUC value was 0.938; values for sensitivity, specificity, negative and positive predictive values, and the diagnostic efficiency were, respectively: 92.7%, 85.4%, 92.1%, 86.4%, and 89%. Thus, in CSF Aβ42 has sensitivity of 92.7% and negative predictive value of 92.1% (the probability that AD is not present when the Aβ42 concentration is greater than the cutoff value of 789 pg/mL comparing AD with healthy controls). Notably, the diagnostic specificity value of 85.4% is in part due to the presence of amyloid plaques in some healthy controls, estimated to occur in 30% or more of healthy controls >60 years old [7, 9, 13, 24]. Diagnostic test efficiency (the percentage of all results for the AD versus healthy controls that are classified correctly) was 89%.
Fig. 3.
Distribution of Aβ42 results in the group of 41 autopsy proven Alzheimer’s disease subjects and 41 age matched control group; A) 2D-UPLC-MS-MS, B) AlzBio3 Luminex.
Fig. 4.

Comparison of ROC curves for 2D-UPLC-MS-MS and AlzBio3 Luminex. The ROC AUC value for 2D-UPLC-MS-MS was 0.938, and for AlzBio3 Luminex immunoassay the AUC value was 0.900.
The group of 82 subjects was also analyzed in our laboratory for Aβ42 concentration using the multiplex xMAP Luminex (AlzBio3) immunoassay platform as a part of a previous study (Fig. 3B) [24]. The ROC AUC value for the immunoassay was 0.90 and diagnostic sensitivity, specificity, positive and negative predictive values, and test efficiency values were: 100%, 78%, 82%, 100%, and 89%, respectively (Fig. 4). Comparison of these two correlated ROC curves, using DeLong’s test [26], showed no statistically significant difference (p = 0.2229). Even though the results of Aβ42 concentration obtained by UPLC-MS-MS assay were on average 4.5 times higher compared to results by AlzBio3 immunoassay, we found statistically significant linear correlation between these two methods with r2 = 0.67 (p < 0.0001) (Fig. 5).
Fig. 5.

Correlation of Aβ42 results in human CSF obtained by AlzBio3 immunoassay versus UPLC-MS-MS assay.
DISCUSSION
Quantification of Aβ peptides in biological fluids has relied mainly on immunoassays, primarily ELISA and Luminex-based test platforms [13, 29]. These immunoassays are precision, not accuracy-based, although they have provided foundational data regarding clinical utilities for CSF tau and Aβ measurements to distinguish between AD and healthy controls and many studies have shown their diagnostic value for AD in patients with a diagnosis of probable AD or even MCI [13]. However, the biggest concerns with immunoassays are the large differences in biomarker concentrations obtained in the same CSF sample using different immunoassays [30, 31]. Thus, the urgent need for a highly specific, antibody-independent, reproducible methodology for quantification of Aβ peptides in biological fluids to serve as a reference method [32].
Here we described a LC-MS-MS method with a newly established and assessed surrogate matrix for calibrators and QC samples preparation, designed for accurate, reliable, and efficient quantification of Aβ42 in human CSF. We also reported the ruggedness defined as the method capacity to remain unaffected by the small variations expected to occur such as pipet volume delivery variance and clinical utility of this assay compared with the AlzBio3 immunoassay in AD patients with an autopsy-based diagnosis, and age-matched living controls.
The choice of surrogate matrix for calibrators and quality control samples was critical to successful validation of this method. We found that BSA (4 mg/mL)/aCSF calibrator matrix provides a linear and reproducible calibration curve, with an LLOQ of 50 pg/mL, comparable to that afforded by human pooled CSF.
The approach to Aβ peptide quantification included: 1) use of a surrogate CSF matrix for calibrators and QC samples, 2) denaturation of aggregated forms of Aβ42 in CSF using high concentration GuCl, and 3) use of mixed-mode ion exchange sample cleanup step in a 96 well plate format. Several key advantages of this method over others used for quantification of amyloid peptides include: 1) matrix effects-resistant Aβ42 quantitation, 2) discrimination of post-translationally modified forms of this peptide, 3) antibody-independent sample preparation, 4) potential for low inter-laboratory variability, and 5) the ability to simultaneously quantify diverse Aβ species. Thus, we confirmed that measurement of Aβ42 in CSF using surrogate matrix-based LC-MS/MS technology under denaturing conditions and SPE sample cleanup provides a solid basis for development of reference methodology to measure Aβ42 concentration accurately in CSF samples.
The reasons why measurement of Aβ42 in CSF is challenging include its presence at low concentration (pg/mL range), its poor aqueous solubility and non-specific binding to other peptides/proteins and/or to the walls of tubes and pipette tips, and tendency to aggregate. The high concentration GuCl used for sample preparation is presumed to denature Aβ peptides from various aggregated and oligomeric and polymeric forms to monomeric peptide [33, 34]. Following this step, aliquots of GuCl-treated samples were applied to pre-conditioned SPE cartridge columns to remove endogenous contaminant species as previously described [15, 16]. The Oasis MCX sorbent is a novel, mixed-mode polymeric sorbent that has been optimized to achieve higher selectivity and sensitivity for extracting basic compounds with cation-exchange groups without requiring evaporation or reconstitution that can cause loss of amyloid peptide.
The method described here uses automated column-switching together with on-line sample concentration/cleanup. Optimization of trapping and analytical column mobile phases was needed for this highly selective assay with a short run time of 12 min. We used 0.1% NH4OH as mobile phase A, similar to Oe et al. [35] since 0.1% ammonia affords better signal-to-noise ratio for Aβ42 peaks compared to higher concentrations such as 0.3% NH4OH. 2D chromatography provided an additional on-line cleaning step improving sample purity. The trap column was cleaned after each injection, using a mixture of organic solvents, during the time when analytes were eluted from the analytical column to the mass spectrometer. It was critical to remove all impurities from the columns after each analytical run. A cleaning procedure, developed by us, involved an aqueous solvent (NH4OH (0.1%)/ACN; 90/10) that is gradually removed and exchanged by organic solvent, up to 100% of ACN, over 2 h. Such treatment of the analytical and trap columns allows for more than 1000 injections of human and BSA/aCSF samples without loss of performance or increase of back pressure.
Analysis of Aβ42 in pre-mortem human CSF samples was a final method validation step. The results show that our methodology distinguished AD subjects, diagnosed at autopsy, including nine subjects with various co-pathologies, from healthy controls with at least equivalent diagnostic utility to that of a qualified immunoassay method for CSF Aβ42. Biologic markers of AD should have a sensitivity >80% for detecting AD and specificity >80% for discriminating other forms of dementia [36]. Our method with 92.7% sensitivity and 85.4% specificity exceeds these requirements. These results and the described analytical performance provide strong support for use of this method for both precision and accuracy-based measurement of Aβ42 in CSF. The possibility that matrix interference-free measurement of Aβ42 in CSF can be achieved across multiple laboratories using the sample preparation approach described here based on previous publications is the basis of a collaborative effort in the Global Biomarker Standardization Consortium of the Alzheimer’s Association [32]. One important outcome of this collaborative effort is the formation of a CSF biomarker working group sponsored by the International Federation of Clinical Chemistry and the Institute for Reference Materials and Measurements to develop a standard reference material that is CSF-based and participating laboratories will measure Aβ42 using their tandem mass spectrometry methodology for assigning accuracy-based concentrations to this material [32]. It is expected that this “standard reference material” would be made available for immunoassay calibrator standardization once all of the required steps for its preparation and Aβ42 concentration value assignments are completed.
We took advantage of mass spectrometric detection performance for Aβ42 and added two additional Aβ peptides, Aβ40 and Aβ38, to quantify them together with Aβ42 with excellent separation of the Aβ peptides (Supplementary Figures 2 and 3) and precision and accuracy performance similar to that described here for Aβ42 in the singleplex method was achieved. This provides direct support for the accuracy of this multiplex methodology reported by others [15, 16].
This study serves as an important step to advance research on mass spectrometric detection of Aβ peptides. Given its effective performance, this method may be used for diagnostic and prognostic assessments in AD research. Since a number of different types of anti-Aβ antibodies with varying specificity are used in AD research and in clinical trials, this methodology may serve as a potential basis for a consensus reference method leading to assay standardization for these measurements of Aβ42 in biological fluids. Furthermore the multiplex method will permit investigation of Aβ peptides in research studies of production and clearance of peptides derived from the processing of the amyloid-β protein precursor.
Supplementary Material
Acknowledgments
This research was supported by the ADNI U01 NIA grant AG024904 and NIA grants AG10124 and NS053488. John Q. Trojanowski is the William Maul Measey-Truman G. Schnabel, Jr., Professor of Geriatric Medicine and Gerontology. We are grateful for helpful discussions and suggestions of our colleagues in the Global Biomarker Standardization Consortium of the Alzheimer’s Association, and from members of the IFCC Scientific Division.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=2133).
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-132489.
References
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 4.Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ, Coart E, Morris JC, Holtzman DM. Comparison of analytical platforms for cerebrospinal fluid measures of beta-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol. 2011;68:1137–1144. doi: 10.1001/archneurol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, Andreasen N, Hofmann-Kiefer K, DeBernardis J, Kerkman D, McCulloch C, Kohnken R, Padberg F, Pirttila T, Schapiro MB, Rapoport SI, Moller HJ, Davies P, Hampel H. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002;59:1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- 6.Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, Sjoegren M, DeBernardis J, Kerkman D, Ishiguro K, Ohno H, Vanmechelen E, Vanderstichele H, McCulloch C, Moller HJ, Davies P, Blennow K. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: A comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol. 2010;45:30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampel H, Shen Y, Walsh DM, Aisen P, Shaw LM, Zetterberg H, Trojanowski JQ, Blennow K. Biological markers of amyloid beta-related mechanisms in Alzheimer’s disease. Exp Neurol. 2010;223:334–346. doi: 10.1016/j.expneurol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang JH, Korecka M, Toledo JB, Trojanowski JQ, Shaw LM. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-β(1-42) and τ proteins as Alzheimer disease biomarkers. Clin Chem. 2013;59:903–916. doi: 10.1373/clinchem.2013.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattsson N, Andreasson U, Persson S, Arai H, Batish SD, Bernardini S, Bocchio-Chiavetto L, Blankenstein MA, Carrillo MC, Chalbot S, Coart E, Chiasserini D, Cutler N, Dahlfors G, Duller S, Fagan AM, Forlenza O, Frisoni GB, Galasko D, Galimberti D, Hampel H, Handberg A, Heneka MT, Herskovits AZ, Herukka SK, Holtzman DM, Humpel C, Hyman BT, Iqbal K, Jucker M, Kaeser SA, Kaiser E, Kapaki E, Kidd D, Klivenyi P, Knudsen CS, Kummer MP, Lui J, Lladó A, Lewczuk P, Li QX, Martins R, Masters C, McAuliffe J, Mercken M, Moghekar A, Molinuevo JL, Montine TJ, Nowatzke W, O’Brien R, Otto M, Paraskevas GP, Parnetti L, Petersen RC, Prvulovic D, de Reus HP, Rissman RA, Scarpini E, Stefani A, Soininen H, Schröder J, Shaw LM, Skinningsrud A, Skrogstad B, Spreer A, Talib L, Teunissen C, Trojanowski JQ, Tumani H, Umek RM, Van Broeck B, Vanderstichele H, Vecsei L, Verbeek MM, Windisch M, Zhang J, Zetterberg H, Blennow K. The Alzheimer’s association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;4:386–395. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lame ME, Chambers EE, Blatnik M. Quantitation of amyloid beta peptides Abeta(1-38), Abeta(1-40), and Abeta(1-42) in human cerebrospinal fluid by ultra-performance liquid chromatography-tandem mass spectrometry. Anal Biochem. 2011;419:133–139. doi: 10.1016/j.ab.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Pannee J, Portelius E, Oppermann M, Atkins A, Hornshaw M, Zegers I, Höjrup P, Minthon L, Hansson O, Zetterberg H, Blennow K, Gobom J. A selected reaction monitoring (SRM)-based method for absolute quantification of Aβ38, Aβ40 and Aβ42 in CSF of Alzheimer’s disease patients and healthy control. J Alzheimers Dis. 2013;33:1021–1032. doi: 10.3233/JAD-2012-121471. [DOI] [PubMed] [Google Scholar]
- 17.Clark CM, Davatzikos C, Borthakur A, Newberg A, Leight S, Lee VM, Trojanowski JQ. Biomarkers for early detection of Alzheimer pathology. Neurosignals. 2008;16:11–18. doi: 10.1159/000109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark CM1, Pratico D, Shaw LM, Leight S, Xie SX, Gu A, Lee VM, Trojanowski JQ. Commentary on “Optimal design of clinical trials for drug designed to slow the course of Alzheimer’s disease”. Biochemical biomarkers of latelife dementia. Alzheimers Dement. 2006;4:287–293. doi: 10.1016/j.jalz.2006.05.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, Xie SX, Lee VM, Shaw LM, Trojanowski JQ. CSF biomarkers cutoffs: The importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124:23–35. doi: 10.1007/s00401-012-0983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toledo JB, Van Derlin VM, Lee EB, Suh E, Baek Y, Robinson J, Xie SX, McBride J, Wood EM, Schuck T, Irwin D, Gross RG, Hurtig H, McCluskey L, Elman L, Karlawish J, Schellenberg G, Chen-Plotkin A, Wolk D, Grossman M, Arnold SE, Shaw LM, Lee VM, Trojanowski JQ. A platform for discover: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.06.003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 24.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ Alzheimer’s Disease Neuroimaging, Initiative . Cerebrospinal fluid biomarker signature in Alzheimer’s Disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RCoreTeam. A language and environment for statistical computing. 2012 http://www.R-project.org.
- 26.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 27.Annesley T. Ion suppression in mass spectrometry. Clin Chem. 2003;49:1041–1044. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. 2001 www.fda.gov/downloads/.../ucm070107.pdf.
- 29.Skovronsky DM, Wang J, Lee VM, Doms RW. Quantifying Abeta(1-40) and Abeta (1-42) Using Sandwich-ELISA. Methods Mol Med. 2000;32:79–89. doi: 10.1385/1-59259-195-7:79. [DOI] [PubMed] [Google Scholar]
- 30.Irwin DJ, McMillan CT, Toledo JB, Arnold SE, Shaw LM, Wang LS, Van Deerlin V, Lee VM, Trojanowski JQ, Grossman M. Comparison of CSF levels of tau and Abeta 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms. Arch Neurol. 2012;69:1018–1025. doi: 10.1001/archneurol.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang LS, Leung YY, Chang SK, Leight S, Knapik-Czajka M, Baek Y, Shaw LM, Lee VM, Trojanowski JQ, Clark CM. Comparison of xMAP and ELISA assay for detecting CSF biomarkers of Alzheimer’s disease. J Alzheimers Dis. 2012;31:439–445. doi: 10.3233/JAD-2012-120082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrillo MC, Blennow K, Soares H, Lewczuk P, Mattsson N, Oberoi P, Umek R, Vandijck M, Salamone S, Bittner T, Shaw LM, Stephenson D, Bain L, Zetterberg H. Global standardization measurement of cerebral spinal fluid for Alzheimer’s disease: An update from the Alzheimer’s Association Global Biomarkers Consortium. Alzheimers Dement. 2013;9:137–140. doi: 10.1016/j.jalz.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Monera OD, Kay CM, Hodges RS. Protein denaturation with guanidine hydrochloride or urea provides a different estimate of stability depending on the contributions of electrostatic interactions. Protein Sci. 1994;3:1984–1991. doi: 10.1002/pro.5560031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer I, Wingfield PT. Preparation and extraction of insoluble (inclusion-body) proteins from Escherichia coli. Curr Protoc Protein Sci. 2012;Chapter 6(Unit 6.3) doi: 10.1002/0471140864.ps0603s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oe T, Ackermann BL, Inoue K, Berna MJ, Garner CO, Gelfanova V, Dean RA, Siemers ER, Holtzman DM, Farlow MR, Blair IA. Quantitative analysis of amyloid beta peptides in cerebrospinal fluid of Alzheimer’s disease patients by immunoaffinity purification and stable isotope dilution liquid chromatography/negative electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3723–3735. doi: 10.1002/rcm.2787. [DOI] [PubMed] [Google Scholar]
- 36.Frank RA, Galasko D, Hampel H, Hardy J, de Leon MJ, Mehta PD, Rogers J, Siemers E, Trojanowski JQ. Biological markers for therapeutic trials in Alzheimer’s disease. Proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer’s disease. Neurobiol Aging. 2003;24:521–536. doi: 10.1016/s0197-4580(03)00002-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.