Abstract
Negative-sense (NS) RNA viruses deliver into cells a mega-dalton RNA-protein complex competent for transcription. Within this complex, the RNA is protected in a nucleocapsid protein (NP) sheath which the viral polymerase negotiates during RNA synthesis. The NP-RNA templates come as nonsegemented (NNS) or segmented (SNS), necessitating distinct strategies for transcription by their polymerases. Atomic-level understanding of the NP-RNA of both NNS and SNS RNA viruses show that the RNA must be transiently dissociated from NP during RNA synthesis. Here we summarize and compare the polymerases of NNS and SNS RNA viruses, and the current structural data on the polymerases. Those comparisons inform us on the evolution of related RNA synthesis machines which use two distinct mechanisms for mRNA cap formation.
Negative-strand (NS) RNA viruses encompass some of the most significant human and agricultural pathogens extant [1,2]. The viruses can be divided into two main groups based on their genomic RNA: non-segmented NS (NNS) RNA viruses and segmented NS (SNS) RNA viruses. The NNS RNA viruses comprise four families, the Rhabdoviridae (vesicular stomatitis virus (VSV) and rabies virus), Paramyxoviridae (measles and respiratory syncytial viruses (RSV)), Filoviridae (Ebola and Marburg viruses) and the Bornaviridae (Borna disease virus). The SNS RNA viruses comprise three families, the Arenaviridae (lymphocytic choriomeningitis virus and Machupo virus (MACV)), Bunyaviridae (Rift Valley fever virus) and Orthomyxoviridae (influenza A virus).
The NS RNA viruses share a common replicative machinery comprising a protein–RNA complex in which the viral genomic RNA is found within a capsid protein sheath to form the nucleocapsid (NP) protein–RNA complex. Those NP-RNA templates are copied by the virally encoded RNA dependent RNA polymerase (RdRP) in two synthetic modes: mRNA transcription and genome replication. For the NNS RNA viruses that single genome contains a tandem array of 5–10 genes that are sequentially copied by the viral polymerase, whereas the SNS RNA virus polymerases copy each template into a single mRNA (for review see: [3]). The enzymatic activities necessary for copying of the NP-RNA templates include an RdRP, as well as the enzymes required for mRNA cap formation that are only utilized during mRNA transcription. All of the necessary enzymatic activities reside within a 250 kDa large (L) polymerase protein, except for orthomyxoviruses where 3 separate proteins assemble into a complex of similar size (for review see: [4,5]).
The mechanism of mRNA cap formation, and consequently the enzymatic activities involved differ between the NNS and SNS RNA viruses. The NNS RNA viruses synthesize their mRNA cap-structures. They employ an L encoded polyribonucleotidyltransferase (PRNTase) to transfer nascent RNA onto a GDP acceptor to form a GpppN cap structure, through a covalent L–pRNA intermediate [6,7]. The cap structure is then subsequently modified by an unusual dual specificity methyltransferase that adds both 2′-O and guanine-N-7 modifications to form the 7mGpppNpmNp cap structure [7–9]. By contrast, SNS RNA viruses cannibalize host cell mRNA cap structures to serve as primers of transcription, and employ a cap-dependent endonuclease activity to do so [10–14]. The unique mechanisms of the “cap-snatching” reaction employed by SNS RNA viruses, and the PRNTase employed by NNS RNA viruses hold promise as potential targets for development of antiviral drugs.
The various enzymatic activities required for RNA synthesis have been mapped within the corresponding polymerases. The smaller polymerase fragments of influenza virus facilitated a greater biochemical and structural understanding of this tripartite complex than for NS RNA virus L proteins [15–17]. This in part reflects the large size (~250–450 kDa) of L, and the presence of flexible domains or connecting “hinge” regions that likely separate independent enzymatic activities [18]. Despite those challenges, a combination of sequence analysis, expression and purification of polymerases, in vitro biochemistry and low and high-resolution structural studies have provided a map of the different enzymatic activities on the polymerases (Figure 1) [19–23].
Figure 1. Structural architecture and organization of NS RNA viral polymerases.
Conserved architecture and domain organization found in nonsegmented (top, purple) and segmented (bottom, orange) polymerases. The linear amino acid sequence of L and the tripartite influenza virus polymerase contain highly conserved regions dedicated to RNA synthesis (blue boxes). L and the influenza virus polymerase also contain blocks of conservation dedicated to 5′ cap formation (maroon boxes), including an endonuclease domain for cap-snatching (domain I or PA, segmented NS RNA viruses) or PRNTase / MTase domains for de novo cap synthesis (domains V and VI, nonsegmented NS RNA viruses). The regions containing cap formation enzymatic activities are also required for RNA synthesis, and the exact function of domains II and IV in the L protein of segmented NS RNA viruses remains unknown. EM of purified L from Machupo virus and vesicular stomatitis virus reveals a shared structural architecture conserved within NS RNA viral L proteins. L consists of a central ring-like RNA polymerase domain (blue highlight in cartoon) and a large appendage dedicated to 5′ cap formation (maroon highlight in cartoon) attached with a flexible linkage. As described in the text, comparison with EM analysis and 3D reconstruction of the influenza virus polymerase complex provides further insight into the architecture of L[19,22,27]. (Fig. adapted from ref. 4)
The overall architecture of the NNS and SNS polymerases were investigated by single particle electron microscopy. In each case, the start point for these studies was functional enzyme that retains RNA synthesis activity. The first such structures came from studies of the heterotrimeric influenza A virus complex [19,22,23], comprising PA, PB1 and PB2 which assemble into a complex that shares features that were subsequently observed in analyses of the L proteins of VSV, a cap synthesizing NNS RNA virus, and MACV a cap snatching SNS RNA virus [20,21]. The structures of VSV and MACV L reveal a shared ring-like domain that contains the RdRP and this domain is decorated with structurally distinct appendages to which the capping machinery was mapped (Figure 1). For VSV, the PRNTase activity maps to the globular domain adjacent to the ring-like domain, and the very C-terminus of the protein provides the two remaining globules to which the MTase activity maps [21]. Rather than a set of globules appended to the ring-like domain, MACV contains an “arm-like” appendage that likely contains the cap-dependent endonuclease [20]. This EM characterization also demonstrates that the capping activities adopt different orientations in relation to the ring-like domain between different classes of images. Such an observation is consistent with the presence of the flexible linkers or hinge domains present within the L proteins.
The ring-like domain is also reminiscent of the RdRPs of the double-stranded RNA viruses reovirus and rotavirus. The dsRNA virus polymerases are similar in size to the portion of L required to assemble the ring (approximately 130 kDa). The atomic structures of the reovirus and rotavirus polymerases reveal that the RdRP domain is bracketed between an N-terminal bridging domain and a C-terminal bracelet domain that serve to fully enclose the active site [24,25]. This cage like structure provides a secondary RNA exit tunnel that facilitates the release of the mRNA. Like the L proteins, the RdRPs of the dsRNA viruses engage in two tasks: mRNA synthesis and genome replication. Similar four-tunnel architecture may also be required by NS RNA viruses to facilitate the separation of the template and replication RNAs and permit the simultaneous re-encapsidation of the template strand, and encapsidation of the nascent strand.
The influenza A virus polymerase structures are at considerably higher resolution and permit the docking of the atomic structures of the individual fragments of the influenza proteins (Figure 1) [19,26,27]. The overall dimensions and the presence of a central “ring-like” structure are strikingly similar to those of the VSV and MACV L proteins. The large flexible appendages observed for VSV and MACV L are absent in the influenza structure, although the polymerase adopts a more open conformation in the absence of the NP-RNA template. Intriguingly, those structural rearrangements appear to involve the amino-terminus of PB2 underscoring the flexibility of capping domains in relation to the RdRP. Such flexibility may be essential for discriminating mRNA synthesis from genome replication in which the capping activities are not required and indeed may interfere with encapsidation of the nascent RNA.
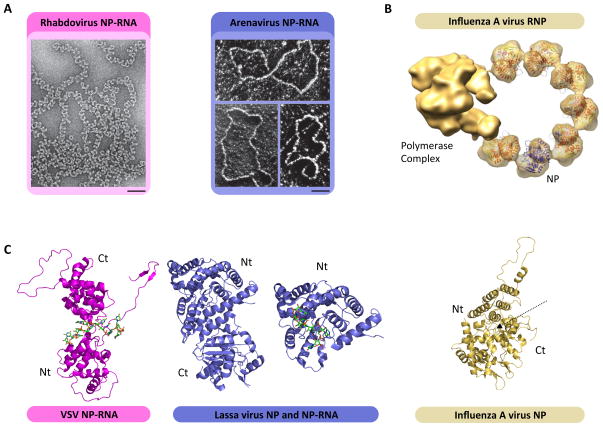
X-ray crystal structures of recombinant NP and model NP-RNA complexes reveal the structural basis of RNA coordination (Figure 2) [26–36]. 3D EM reconstructions have allowed the construction of pseudoatomic models of encapsidated NS RNA genomes [19,29,35]. NP from NNS RNA viruses adopts a conserved bi-lobed architecture that forms a positive groove between amino- and carboxyl-terminal globules that coordinates and shields the genomic RNA. Crystallographic structures of NP-RNA complexes from VSV, rabies virus and RSV revealed NP–NP interactions that tightly lock monomers together resulting in the continuous rigid encapsidated structure characteristic of NNS RNA viral NP-RNA templates [28,30,35]. In contrast, NP structures from SNS RNA viruses instead reveal varied structural architecture and less coordinated NP–NP contact consistent with a loosely organized template. The structures also explain why the NP-RNA of the NNS RNA viruses is more resistant to RNase digestion than those of the SNS RNA viruses (for review see: [37]).
Figure 2. Architecture of the NP-RNA complex.
(A) EM images of rhabdovirus (NNS RNA viruses, magenta) and arenavirus (SNS RNA viruses, blue) NP-RNA genomic template isolated from purified virions. (Scale bar: 50 nm) [37,52]. (B) Pseudoatomic model of influenza A virus RNP. The tripartite polymerase complex is bound to a truncated NP-RNA template. Atomic structures of the NP are docked inside the EM density. Adapted from (ref). (C) Crystal structure of VSV (magenta), Lassa virus (blue) and influenza A virus NP. The N-terminal (Nt) and C-terminal (Ct) domains are indicated. VSV NP is shown in complex with a RNA oligomer that binds NP in between the N and C-teminal domain of the protein. The full Lassa NP is represented devoid of RNA (left), while the N-terminal domain is shown in complex with a RNA oligomer (right). The influenza A NP is represented free of RNA, the putative RNA binding site is indicated by a dashed arrow.
The polymerases of NNS and SNS RNA viruses negotiate their corresponding NP-RNA templates by different mechanisms. SNS RNA virus polymerases directly access NP-RNA and produce a single transcript or replication product from each segment. By contrast L proteins of NNS RNA viruses cannot directly engage the NP-RNA but require an accessory factor the phosphoprotein (P) to bridge interactions between L and the NP-RNA. In addition to the cap-synthesis vs cap-snatching properties of the two classes of polymerase a further fundamental distinction for L proteins of NNS RNA viruses is that they sequentially copy their NP-RNA into a series of 5–10 monocistronic mRNAs through a start-stop mode of transcription. Those distinctions may be linked to a requirement for P to dissociate the NP-RNA complex, and to keep L associated with the template during the termination and re-initiation events that occur at gene junctions.
Recent structural studies on VSV have also revealed other functions for the P protein. VSV P forms non-globular homodimers and consists of three domains separated by two intrinsically disordered linkers [38–41]. The P N-terminal domain which binds nascent RNA-free N (N0), the central domain (PCD) that constitutes a dimerization interface, and the C-terminal domain (PCTD) which binds the N-RNA (Figure 3A, B and C). The long disordered linkers confer to P a large flexibility in between the different domains important for the functioning of the replicative machinery. The EM studies reveal that P affects the overall structural architecture of L [21] such that the appendage containing the capping activities appears to be reordered and now folds back on the ring-like domain in a general conformation resembling a “6” (Figure 3D, E). That rearrangement in the domain containing the capping activities of L therefore strengthens an involvement of the overall architecture of L in the coordination between polymerization and capping of the newly synthesized mRNA. The ability of P to induce the conformational alterations in L is fully recapitulated by a region of P that extends from residue 41–106 [42].
Figure 3. Molecular architecture of VSV P and L.
(A) Schematic organization of VSV P. P consists of three domains connected by two intrinsically disordered regions (Black line). The N-terminal (Nt) and C-terminal (Ct) are indicated. The P N-terminal domain (PNTD, red) contains the RNA-free N (N0) binding region, the central domain (PCD, orange) consists of an oligomerization region and the C-terminal domain (PCTD, green) is involved in the N-RNA binding. (B) Crystallographic structure of VSV PNTD (top), a dimer of PCD (middle) and PCTD (bottom). (C) Structure of PNTD in complex with N0 (magenta) (top) and PCTD in complex with the C-teminal domain of N (magenta) (bottom). (D) EM image (left) and model (right) of L organization. L is arranged with a core ring structure harboring the RdRP domain and a flexible appendage containing the activities necessary for cap formation (capping + methylation). The arrows depict putative flexible linkers. (E) EM image (right) and model (left) of the L-P complex. Upon binding to P or P41-106, the appendage of L undergoes a structural rearrangement. When L binds full length P, the L-P complex is isolated in two forms a monomer and a dimer in which the L pairs are likely bridged by interaction with an oligomer of P (top). The arrow represents the variable orientation of L proteins in the dimers. When L binds P41-106 the L-P41-106 complex exits only as a monomer (bottom).
The structural insights obtained from NS RNA virus templates indicate that the RNA must be displaced from NP to facilitate its copying by polymerase. Such a displacement must involve relatively few molecules of NP at any given time, because for the NNS RNA viruses the template remains resistant to nuclease digestion even during transcription. Those observations catalyzed the development of an improved system to study polymerase biology in vitro in which polymerase alone could use naked RNA as template. In agreement with a model of N dissociation, it has been demonstrated via a simple in vitro system that VSV L, in the absence of NP and P, initiates RNA synthesis on a naked RNA corresponding to the first 19 nucleotides of the genomic RNA (Figure 4) [43]. Thus, it provides the first evidence that the minimal RNA synthesis components of NNS RNA viruses are L and RNA, supporting the model that the RNA is dissociated from NP during polymerization. Similarly, parallel experiments conducted with MACV L demonstrate that this in vitro assay is broadly applicable to the study of NS RNA virus polymerases [44].
Figure 4. VSV L in vitro RNA synthesis assay.
(A) De novo RNA synthesis by VSV L, in the absence and presence of P, on a synthetic template corresponding to the first 19 nt of the leader (Le19). Activity assays were set using 0.2 μM of Le19, 0.2 μM of protein and [α32P]-GTP as the radio-labeled nucleotide. Reactions were quenched by the addition EDTA/formamide and analyzed on a 20% polyacrylamide/7 M urea gel. De novo initiation product sizes are indicated on the left. (B) RNA synthesis by VSV L on a naked 50 (Le50) nucleotides long RNAs, and the encapsidated RNA (N-RNA) templates. (C) RNA synthesis by MACV L on a naked RNA (left), or a naked mutated RNA (right).
This new in vitro assay is a powerful system to study RNA synthesis initiation by NS RNA virus L proteins and is revealing fundamental new insights into the biology of the RNA synthesis machinery. For the NNS RNA viruses, work with VSV L demonstrates in addition to the structural roles of P in interacting with N and L [21,38], P is a bona fide processivity factor for L and an enhancer of initiation (Figure 4A) [43]. The difficulties of obtaining NP-RNA template completely devoid of P, have interfered with a precise analysis of the influence of P on L activity. Results from this new in vitro assay convincingly demonstrate that P, independent of its role in engagement of the template-associated NP protein, has direct functional effects on the enzymatic properties of L. The mechanism by which P affects L catalytic activity remains unclear, however EM analysis shows that L undergoes conformational changes on P binding that may facilitate the increased processivity. While P binding increases L processivity, this L–P complex is not fully processive on a naked RNA template. Full processivity requires the template associated NP (Figure 4B), perhaps reflecting a need to keep the template relatively unstructured to not impede the progress of L [43]. The tripartite influenza virus polymerase is also fully processive only in presence of NP demonstrating that all NS RNA viruses polymerases require the template associated NP for full processivity [45]. This simple in vitro assay has consequently uncovered previously unappreciated roles for the replication proteins of NS RNA viruses.
RdRPs can initiate RNA synthesis by two distinct mechanisms: primer-dependent and primer-independent or de novo initiation. It is clear that both mechanisms operate for the NS RNA viruses. The polymerases of NNS RNA viruses were widely thought to initiate RNA synthesis by a de novo mechanism, and work with naked RNA templates provides the first direct evidence for such a mechanism. De novo initiation requires a higher Km for the first nucleotide (NTPi) incorporated during synthesis which engages in specific interactions within the active site (for a review see: [46,47]). Intriguingly, VSV L require high concentrations of the first two nucleotides incorporated during the synthesis suggesting that both nucleotides might bind specifically the active site to generate a stable initiation complex [43]. Engineered templates show that the first two positions at the 3′end of the template are essential for correct initiation by VSV L, with the third position playing an enhancing role. These results suggest that the major requirement of the initiation complex is a double base pair between the first two nucleotides incorporated during the initiation and the first two nucleotides of the RNA template. Although VSV L initiates RNA synthesis in a template-dependent manner, work with RSV indicates that its L uses a non-template initiation mechanism for incorporation the first two nucleotides of the antigenome [48–50]. This raises the possibility that the initiating nucleotides are differently selected by the two polymerases.
Based on the initiation site on the template two mechanisms of de novo initiation are known to be used by RdRPs: a 3′ terminal initiation and internal initiation. Although VSV L can initiate RNA synthesis by both mechanisms, internal initiation is dramatically less efficient. This suggests that the active site of the RdRP of VSV L is sufficiently flexible to allow the 3′end of the template to overshoot its position and permit a correct initiation. The adaptation of the naked RNA template assay to the study of RSV uncovers efficient initiation at two positions, +1 and +3, at the 3′ end of the genome [50,51], lending further support to a distinction in the mechanism of initiation.
The above summarizes advances in our structural understanding of the RNA synthesis machinery of NS RNA viruses that provide an invaluable new framework for further functional analysis. There remain, however, significant gaps in our understanding of both structure and function of these dynamic machines. There remains no complete atomic level structure of any NS RNA polymerases. Such structures should further our understanding of how NP-RNA complexes are transiently dissociated during RNA synthesis as well as how the capping and polymerization activities are coordinated during transcription. The flexible nature of the positioning of the capping and RdRP activities raise the possibility of a significant structural rearrangement of the machinery during replication. As the resolution of structures improves some of these secrets may be revealed. The continued application of newly described in vitro assays to study multiple NS RNA viruses demonstrates the broad utility of such approaches. Those assays are already uncovering the mechanisms by which initiation and elongation proceed as well as informing on the mechanism by which polymerase inhibitors function. As some NNS RNA virus polymerases depend upon cofactors in addition to the P protein, notably the M2 protein of RSV, and the VP30 protein of Ebola virus the application of this in vitro approach should permit an unequivocal analysis of the function of such proteins.
Acknowledgments
The authors gratefully acknowledge their productive collaboration with Thomas Walz and Andreas Schenk on the electron microscopic analysis of the MACV and VSV L proteins, and Robin Ross and the support of the New England Regional Center for Excellence in Biodefense and Emerging Infectious Disease for assistance with protein production. The authors are also grateful to Juan Ortin for insightful discussions on the influenza polymerase. This work was supported in part by grants from the National Institutes of Health AI059371 and AI057159. SPJW is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10:S110–121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- 2.Pappu HR, Jones RA, Jain RK. Global status of tospovirus epidemics in diverse cropping systems: successes achieved and challenges ahead. Virus Res. 2009;141:219–236. doi: 10.1016/j.virusres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Whelan SP, Barr JN, Wertz GW. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr Top Microbiol Immunol. 2004;283:61–119. doi: 10.1007/978-3-662-06099-5_3. [DOI] [PubMed] [Google Scholar]
- 4.Kranzusch PJ, Whelan SP. Architecture and regulation of negative-strand viral enzymatic machinery. RNA Biol. 2012;9:941–948. doi: 10.4161/rna.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morin B, Whelan SP. La protéine L des Mononegavirales. Virologie. 2012;16:258–268. doi: 10.1684/vir.2012.0457. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Rahmeh A, Morelli M, Whelan SP. A conserved motif in region v of the large polymerase proteins of nonsegmented negative-sense RNA viruses that is essential for mRNA capping. J Virol. 2008;82:775–784. doi: 10.1128/JVI.02107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogino T, Banerjee AK. Unconventional mechanism of mRNA capping by the RNA-dependent RNA polymerase of vesicular stomatitis virus. Mol Cell. 2007;25 :85–97. doi: 10.1016/j.molcel.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Wang JT, Whelan SP. A unique strategy for mRNA cap methylation used by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2006;103:8493–8498. doi: 10.1073/pnas.0509821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahmeh AA, Li J, Kranzusch PJ, Whelan SP. Ribose 2′-O methylation of the vesicular stomatitis virus mRNA cap precedes and facilitates subsequent guanine-N-7 methylation by the large polymerase protein. J Virol. 2009;83 :11043–11050. doi: 10.1128/JVI.01426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias A, Bouvier D, Crepin T, McCarthy AA, Hart DJ, Baudin F, Cusack S, Ruigrok RW. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature. 2009;458:914–918. doi: 10.1038/nature07745. [DOI] [PubMed] [Google Scholar]
- 11.Guilligay D, Tarendeau F, Resa-Infante P, Coloma R, Crepin T, Sehr P, Lewis J, Ruigrok RW, Ortin J, Hart DJ, et al. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat Struct Mol Biol. 2008;15:500–506. doi: 10.1038/nsmb.1421. [DOI] [PubMed] [Google Scholar]
- *12.Morin B, Coutard B, Lelke M, Ferron F, Kerber R, Jamal S, Frangeul A, Baronti C, Charrel R, de Lamballerie X, et al. The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription. PLoS Pathog. 2010;6:e1001038. doi: 10.1371/journal.ppat.1001038. First crystallographic structure of an arenavirus L protein domain revealing the N-terminal end of L as the endonuclease domain involved in cap snacthing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Reguera J, Weber F, Cusack S. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Pathog. 2010;6:e1001101. doi: 10.1371/journal.ppat.1001101. First crystallographic structure of a bunyavirus L protein domain revealing the N terminal end of L as the endonuclease domain involved in cap snacthing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan P, Bartlam M, Lou Z, Chen S, Zhou J, He X, Lv Z, Ge R, Li X, Deng T, et al. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature. 2009;458:909–913. doi: 10.1038/nature07720. [DOI] [PubMed] [Google Scholar]
- 15.Boivin S, Cusack S, Ruigrok RW, Hart DJ. Influenza A virus polymerase: structural insights into replication and host adaptation mechanisms. J Biol Chem. 2010;285:28411–28417. doi: 10.1074/jbc.R110.117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das K, Aramini JM, Ma LC, Krug RM, Arnold E. Structures of influenza A proteins and insights into antiviral drug targets. Nat Struct Mol Biol. 2010;17:530–538. doi: 10.1038/nsmb.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resa-Infante P, Jorba N, Coloma R, Ortin J. The influenza virus RNA synthesis machine: advances in its structure and function. RNA Biol. 2011;8:207–215. doi: 10.4161/rna.8.2.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poch O, Blumberg BM, Bougueleret L, Tordo N. Sequence comparison of five polymerases (L proteins) of unsegmented negative-strand RNA viruses: theoretical assignment of functional domains. J Gen Virol. 1990;71 (Pt 5):1153–1162. doi: 10.1099/0022-1317-71-5-1153. [DOI] [PubMed] [Google Scholar]
- 19.Coloma R, Valpuesta JM, Arranz R, Carrascosa JL, Ortin J, Martin-Benito J. The structure of a biologically active influenza virus ribonucleoprotein complex. PLoS Pathog. 2009;5:e1000491. doi: 10.1371/journal.ppat.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Kranzusch PJ, Schenk AD, Rahmeh AA, Radoshitzky SR, Bavari S, Walz T, Whelan SP. Assembly of a functional Machupo virus polymerase complex. Proc Natl Acad Sci U S A. 2010;107:20069–20074. doi: 10.1073/pnas.1007152107. First EM images of a SNS RNA virus L protein showing that L consists of a core ring-like domain decorated with appendages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Rahmeh AA, Schenk AD, Danek EI, Kranzusch PJ, Liang B, Walz T, Whelan SP. Molecular architecture of the vesicular stomatitis virus RNA polymerase. Proc Natl Acad Sci U S A. 2010;107:20075–20080. doi: 10.1073/pnas.1013559107. First EM images of a NNS RNA virus L protein showing that L consists of a core ring-like domain decorated with appendages that are required for mRNA cap formation, comparison with the previous reference demonstrates that the appendages to the ring domain are likely the cap-dependent endonuclease in the SNS RNA viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resa-Infante P, Recuero-Checa MA, Zamarreno N, Llorca O, Ortin J. Structural and functional characterization of an influenza virus RNA polymerase-genomic RNA complex. J Virol. 2010;84:10477–10487. doi: 10.1128/JVI.01115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torreira E, Schoehn G, Fernandez Y, Jorba N, Ruigrok RW, Cusack S, Ortin J, Llorca O. Three-dimensional model for the isolated recombinant influenza virus polymerase heterotrimer. Nucleic Acids Res. 2007;35:3774–3783. doi: 10.1093/nar/gkm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butcher SJ, Grimes JM, Makeyev EV, Bamford DH, Stuart DI. A mechanism for initiating RNA-dependent RNA polymerization. Nature. 2001;410:235–240. doi: 10.1038/35065653. [DOI] [PubMed] [Google Scholar]
- 25.Tao Y, Farsetta DL, Nibert ML, Harrison SC. RNA synthesis in a cage--structural studies of reovirus polymerase lambda3. Cell. 2002;111:733–745. doi: 10.1016/s0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- **26.Arranz R, Coloma R, Chichon FJ, Conesa JJ, Carrascosa JL, Valpuesta JM, Ortin J, Martin-Benito J. The structure of native influenza virion ribonucleoproteins. Science. 2012;338:1634–1637. doi: 10.1126/science.1228172. Cryo-EM images of the NP-RNA complex of influenza virus showing a detailed double-helical conformation. [DOI] [PubMed] [Google Scholar]
- **27.Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA. Organization of the influenza virus replication machinery. Science. 2012;338:1631–1634. doi: 10.1126/science.1227270. Cryo-EM images of the influenza virus tripartite polymerase bound to the NP-RNA showing how the polymerase interacts on the encapsidated template. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albertini AA, Wernimont AK, Muziol T, Ravelli RB, Clapier CR, Schoehn G, Weissenhorn W, Ruigrok RW. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science. 2006;313:360–363. doi: 10.1126/science.1125280. [DOI] [PubMed] [Google Scholar]
- 29.Ferron F, Li Z, Danek EI, Luo D, Wong Y, Coutard B, Lantez V, Charrel R, Canard B, Walz T, et al. The hexamer structure of Rift Valley fever virus nucleoprotein suggests a mechanism for its assembly into ribonucleoprotein complexes. PLoS Pathog. 2011;7:e1002030. doi: 10.1371/journal.ppat.1002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green TJ, Zhang X, Wertz GW, Luo M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science. 2006;313:357–360. doi: 10.1126/science.1126953. [DOI] [PubMed] [Google Scholar]
- 31.Hastie KM, Kimberlin CR, Zandonatti MA, MacRae IJ, Saphire EO. Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3′ to 5′ exonuclease activity essential for immune suppression. Proc Natl Acad Sci U S A. 2011;108:2396–2401. doi: 10.1073/pnas.1016404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hastie KM, Liu T, Li S, King LB, Ngo N, Zandonatti MA, Woods VL, Jr, de la Torre JC, Saphire EO. Crystal structure of the Lassa virus nucleoprotein-RNA complex reveals a gating mechanism for RNA binding. Proc Natl Acad Sci U S A. 2011;108:19365–19370. doi: 10.1073/pnas.1108515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi X, Lan S, Wang W, Schelde LM, Dong H, Wallat GD, Ly H, Liang Y, Dong C. Cap binding and immune evasion revealed by Lassa nucleoprotein structure. Nature. 2010;468:779–783. doi: 10.1038/nature09605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudolph MG, Kraus I, Dickmanns A, Eickmann M, Garten W, Ficner R. Crystal structure of the borna disease virus nucleoprotein. Structure. 2003;11:1219–1226. doi: 10.1016/j.str.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Tawar RG, Duquerroy S, Vonrhein C, Varela PF, Damier-Piolle L, Castagne N, MacLellan K, Bedouelle H, Bricogne G, Bhella D, et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science. 2009;326:1279–1283. doi: 10.1126/science.1177634. [DOI] [PubMed] [Google Scholar]
- 36.Ye Q, Krug RM, Tao YJ. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature. 2006;444:1078–1082. doi: 10.1038/nature05379. [DOI] [PubMed] [Google Scholar]
- 37.Ruigrok RW, Crepin T, Kolakofsky D. Nucleoproteins and nucleocapsids of negative-strand RNA viruses. Curr Opin Microbiol. 2011;14:504–510. doi: 10.1016/j.mib.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Green TJ, Luo M. Structure of the vesicular stomatitis virus nucleocapsid in complex with the nucleocapsid-binding domain of the small polymerase cofactor, P. Proc Natl Acad Sci U S A. 2009;106:11713–11718. doi: 10.1073/pnas.0903228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanov I, Crepin T, Jamin M, Ruigrok RW. Structure of the dimerization domain of the rabies virus phosphoprotein. J Virol. 2010;84:3707–3710. doi: 10.1128/JVI.02557-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leyrat C, Jensen MR, Ribeiro EA, Jr, Gerard FC, Ruigrok RW, Blackledge M, Jamin M. The N(0)-binding region of the vesicular stomatitis virus phosphoprotein is globally disordered but contains transient alpha-helices. Protein Sci. 2011;20:542–556. doi: 10.1002/pro.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leyrat C, Schneider R, Ribeiro EA, Jr, Yabukarski F, Yao M, Gerard FC, Jensen MR, Ruigrok RW, Blackledge M, Jamin M. Ensemble structure of the modular and flexible full-length vesicular stomatitis virus phosphoprotein. J Mol Biol. 2012;423:182–197. doi: 10.1016/j.jmb.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Rahmeh AA, Morin B, Schenk AD, Liang B, Heinrich BS, Brusic V, Walz T, Whelan SP. Critical phosphoprotein elements that regulate polymerase architecture and function in vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2012;109:14628–14633. doi: 10.1073/pnas.1209147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **43.Morin B, Rahmeh AA, Whelan SP. Mechanism of RNA synthesis initiation by the vesicular stomatitis virus polymerase. EMBO J. 2012;31:1320–1329. doi: 10.1038/emboj.2011.483. Development of a new in vitro assay to study NNS RNA virus polymerases, demonstrates that the polymerase copies naked RNA and defining functions for the template associated NP and the phosphoprotein in regulating polymerase processivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kranzusch PJ, Whelan SP. Arenavirus Z protein controls viral RNA synthesis by locking a polymerase-promoter complex. Proc Natl Acad Sci U S A. 2011;108:19743–19748. doi: 10.1073/pnas.1112742108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turrell L, Lyall JW, Tiley LS, Fodor E, Vreede FT. The role and assembly mechanism of nucleoprotein in influenza A virus ribonucleoprotein complexes. Nat Commun. 2013;4:1591. doi: 10.1038/ncomms2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kao CC, Singh P, Ecker DJ. De novo initiation of viral RNA-dependent RNA synthesis. Virology. 2001;287:251–260. doi: 10.1006/viro.2001.1039. [DOI] [PubMed] [Google Scholar]
- 47.van Dijk AA, Makeyev EV, Bamford DH. Initiation of viral RNA-dependent RNA polymerization. J Gen Virol. 2004;85:1077–1093. doi: 10.1099/vir.0.19731-0. [DOI] [PubMed] [Google Scholar]
- 48.Noton SL, Cowton VM, Zack CR, McGivern DR, Fearns R. Evidence that the polymerase of respiratory syncytial virus initiates RNA replication in a nontemplated fashion. Proc Natl Acad Sci U S A. 2010;107:10226–10231. doi: 10.1073/pnas.0913065107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noton SL, Fearns R. The first two nucleotides of the respiratory syncytial virus antigenome RNA replication product can be selected independently of the promoter terminus. RNA. 2011;17:1895–1906. doi: 10.1261/rna.2813411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tremaglio CZ, Noton SL, Deflube LR, Fearns R. Respiratory syncytial virus polymerase can initiate transcription from position 3 of the leader promoter. J Virol. 2013;87:3196–3207. doi: 10.1128/JVI.02862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noton SL, Deflube LR, Tremaglio CZ, Fearns R. The respiratory syncytial virus polymerase has multiple RNA synthesis activities at the promoter. PLoS Pathog. 2012;8:e1002980. doi: 10.1371/journal.ppat.1002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young PR, Howard CR. Fine structure analysis of Pichinde virus nucleocapsids. J Gen Virol. 1983;64 (Pt 4):833–842. doi: 10.1099/0022-1317-64-4-833. [DOI] [PubMed] [Google Scholar]