Article Synopsis
As the modern era of combination antiretroviral therapy has increased life expectancy for HIV infected individuals, Type II Diabetes Mellitus (DM) and disorders of glucose metabolism have emerged as an important issue in the care of this population. Multiple mechanisms, both specific and nonspecific to HIV, underlie a significant prevalence. While best practice diagnostic testing remains unclear, the risks associated with diabetes in the setting of HIV are well characterized, ranging from organ-specific damage to socioeconomic decline. Population specific treatment data is limited, and thus current guidelines serve as a basis for ongoing management.
Keywords: Diabetes, HIV, disorders of glucose metabolism, cART, cardiovascular disease
The landscape of HIV care has changed dramatically in the last 10 years. While the advent of combination antiretroviral therapy (cART) has saved an estimated 14 million life-years in low- and middle-income countries, it has also altered the natural history of HIV infection. Numerous studies have demonstrated that, as HIV patients gain control of the disease (as measured by viral load below the limit of detection and rising CD4 count), there is a parallel rise in chronic medical illness. (1). A recent study from Kim et. al.(2) observed that 2/3 of a treated HIV-infected cohort had the presence of multimorbidity, or the clustering of two or more chronic medical illnesses. These diseases, including cardiovascular disease and diabetes, are not benign, and collectively contribute to the decreased life expectancy of HIV-infected patients on treatment.
Diabetes mellitus (DM) and disorders of glucose metabolism are increasingly common in the US and impact morbidity and cardiovascular disease in all populations, including persons living with HIV. In 2011 the American Diabetic Association estimated 25.8 million, or 8.5% of the United States population, carry a diagnosis of type 2 DM. Pre-diabetes, defined by a fasting glucose of 100–126 mg/dl or a hemoglobin A1c level of 5.7–6.4%, affects an additional 79 million Americans. While lifestyle associated risk factors are thought to be the main progenitor of this epidemic, HIV-infected patients with disorders of glucose metabolism have established a divergent and multifactorial pathogenesis compared to their non-infected counterparts. In addition to traditional risk factors such as obesity, both direct and indirect effects of cART medication, HIV-associated conditions such as Hepatitis C and opiate drug use, and the HIV virus itself have been proposed as mechanisms of hyperglycemia and diabetes in this context (see Figure 1).
Figure 1.
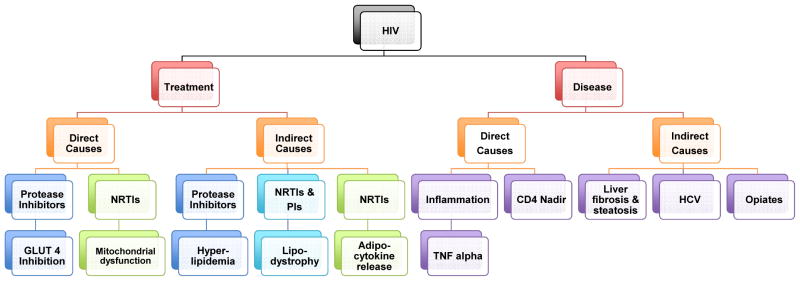
Schema for the multifactorial HIV-specific mechanisms responsible for insulin resistance and diabetes in persons with HIV infection
DM is a systemic disease, and carries risk for HIV-infected patients beyond cardiovascular disease. Disorders of glucose metabolism in this population have been associated with increased prevalence and worsened outcomes in a diverse array of conditions ranging from neurocognitive changes to renal impairment and albuminuria. Hence, the diagnosis and treatment of this condition represents a potential wide net of impact on general health.
Diabetes is an emerging issue in the HIV-infected population that is important to characterize as the clinical course of HIV changes. This review will seek to discuss (1) the epidemiology of DM in HIV; (2) the proposed mechanisms of glucose intolerance; (3) challenges in diagnosis; (4) associated health risks; and (5) current treatment strategies.
Epidemiology
As the use of cART spread in the late 1990s, there was early recognition that disorders of glucose metabolism were linked to cART. Early studies evaluated this in the context of specific medications such as protease inhibitors, and emerging conditions such as lipodystrophy.
Calza et. al.(3) evaluated the prevalence of DM and metabolic syndrome in an observational cohort of 775 HIV-infected patients on cART seen in an Italian clinic between July-September 2009. Diabetes was diagnosed through one time fasting blood glucose >126 mg/dl, random glucose >200 mg/dl, or history of hypoglycemic medications. The prevalence of DM was 4.5%, impaired fasting glucose was 9.4%, hyperinsulinemia was 11.9%, and metabolic syndrome was 9.1%. There were no significant differences found in type of cART, presence of liver disease, cholesterol levels, or blood pressure measurements between diabetic and non-diabetic patients.
Similarly, Galli et. al.(4) conducted a cross sectional investigation of 4,249 HIV-infected clinic patients and 9,148 healthy controls Diabetes was diagnosed using fasting blood glucose >126 mg/dl, reported history of DM, or prescription of hypoglycemic medication. The prevalence of DM was 4% compared to 2.5% within the non HIV-infected population.
In 1999 a large, multinational cohort was established to prospectively evaluate potential adverse effects of cART. The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) cohort of 33,389 HIV-infected patients found the prevalence of diabetes, defined by fasting glucose > 126 mg/dl on two separate readings, physician record or antidiabetic therapy, was 2.85% at entry to study.(5)
Taken together, a conservative estimate from largely European cohort data estimates the prevalence of diabetes among HIV-infected patients at 3–4%. It should be noted that these study populations were overwhelmingly male, relatively young, with limited ethnic and racial diversity. Previous data from the Multicenter AIDS Cohort Study (MACS), which evaluated 533 HIV-infected men and contemporary uninfected controls from multiple US cities between 1999–2003, found an overall prevalence of DM of 14% in HIV vs. 5% in the non-infected control group.(6) The higher prevalence may be related to the definition of diabetes, which included fasting glucose >126 mg/dl as well as self-report, and that the body mass index (BMI) tended to be higher than other cohorts. Numerous demographic and social factors which contribute to DM prevalence should be taken into account, but in the studies that included matched uninfected subjects as a comparator group, the prevalence of diabetes was increased among those with HIV.
Of note, Capeau and colleagues (7) determined the incidence of DM in a cohort of 1046 HIV-infected patients prospectively followed between 1999 and 2009 as they started cART. While the overall incidence was 14.1 cases per 1000 person-years (py), there was a trend for the incidence of DM to decline over time. For example, in the period between 1999–2000, the incidence was 23.2/1000 py, whereas it was as low as 2.7 cases/1000 py in the period of 2005–2006. Elements of cART, as well as traditional risk factors such as age, BMI, and waist-to-hip ratio were associated with incident DM, but the decline in incidence over time may reflect changes in cART practices and the development of less toxic cART alternatives.
In the US, diabetes is known to disproportionately affect those of African-American, Hispanic, and Native American descent. Within the HIV-infected diabetic population, data on the influence of race and ethnicity are limited. The aforementioned studies of prevalence consist largely of white men, limiting the power to evaluate association with minority race. Adeyemi et. al.(8) utilized the Women’s Interagency HIV Study data, a prospective study of predominantly African American and Hispanic HIV-infected and matched HIV-uninfected women from multiple US cities, to evaluate factors related to insulin resistance. In this report of non-diabetic women (754 HIV-infected and 328 uninfected), there was a higher rate of insulin resistance as measured by HOMA-IR among Hispanic women, but no difference in insulin resistance by HIV status. Hispanic race remained a significant associated risk factor for HOMA-IR ≥ 2.6 after adjusting for age, BMI, hepatitis C infection and protease inhibitor use. Further studies are necessary to further outline the impact of race and ethnicity on the prevalence of diabetes in the HIV-infected population.
Mechanisms
Despite the contribution of several mechanisms of pathogenesis unique to HIV, traditional risk factors play a significant role in the development of diabetes in the HIV-infected population. While studies have demonstrated a lower BMI in HIV-infected patients with diabetes compared to non-infected matched cohorts,(9) the association between DM and obesity persists in HIV. For example, Galli et. al.(4) found the prevalence of DM in those with normal BMI was 3.2% in HIV patients and 1.1% in HIV uninfected. However, in the overweight and obese categories, DM prevalence rose to 3.9% and 12.7% among HIV-infected patients compared with 3.1% and 7.8% respectively in HIV-uninfected patients. Similarly, the D:A:D cohort demonstrated an increase in relative rate of new-onset DM with increasing weight category.(10) Obesity in HIV will be discussed as a separate topic for this journal, but the association between obesity and diabetes, as in non-infected patients, is strong.
Similarly, genetics appear to contribute in the development of diabetes in HIV. Recent genomic studies have established a number of common single nucleotide polymorphisms (SNPs) associated with diabetes in the general population. Rotger et. al.(11) evaluated 22 SNPs associated with the development of DM in 94 patients with diabetes in the Swiss HIV Cohort and in 550 HIV-infected non-diabetic controls. Four common SNPs were found to influence DM risk, and this association became stronger as the number of risk alleles increased. Further, the relative contribution of genetic variation and other risk factors to the development of diabetes was assessed; SNPs accounted for 14% of DM risk variability, whereas cART exposure accounted for 3% and age 19% of the variability in DM. This emerging area of research highlights the importance of non-HIV risk factors in the pathogenesis of DM in HIV.
Various studies investigated ART naïve populations in order to assess any inherent connection between the HIV virus and development of glucose disorders. Unfortunately, the available data do not suggest a clear answer. Brown et. al.(12) evaluated cumulative exposure to cART and markers of insulin resistance in 533 HIV positive men of the MACS cohort, and found that ART naïve patients demonstrated a lower QUICKI score (−0.04; 95% CI −0.07–0.01) as well as higher odds of fasting hyperinsulinemia (1.08 OR; 95% CI 1.02–1.13). However, this relationship was not born out in subsequent studies. Galli et. al.(4) found HIV-infected patients who were ART naïve had a lower prevalence of diabetes (0.8%) compared to HIV-infected patients on therapy (4%), or uninfected comparison cohort (2.5%).
One proposed pathway for the influence of HIV on diabetes and glucose is through generalized inflammation, with upregulation of chemokines involved in insulin regulation. For example, Brown et. al.(13) found that higher markers of TNF-α activation after 48 weeks of cART was associated with increased odds of subsequent diabetes after adjustment for age, BMI, CD4 count, and type of cART. However, conflicting data provide no definitive conclusion to this hypothesis. More studies have focused on the relative contribution of cART to the incidence of diabetes in HIV.
In the D:A:D cohort, DeWit et. al.(5) found that cumulative exposure to cART carried an adjusted relative risk of 1.11 per year (95% CI: 1.07–1.15, p=0.0001). Exposure to the specific nucleoside reverse transcriptase inhibitor (NRTI) stavudine was associated with an increased risk of diabetes (RR 1.19 per year exposure; 95% CI: 1.15–1.25, p=0.0001), as were didanosine and zidovudine, but to a lesser extent. In another large prospective trial, Capeau et. al.(7) found stavudine use was associated with a significant increase in hazard ratio for DM, peaking in the second year of use (HR 2.81 95% CI: 1.4–5.64, p=0.004). The protease inhibitor indinavir was also associated with increased hazard ratio of 2.55 (95% CI: 1.36–4.79) although this declined in the subsequent years of use. Utilizing the MACS Cohort, Brown et. al.(12) also found stavudine exposure was associated with significantly lower QUICKI value and increased risk of hyperinsulinemia; lamivudine and indinavir exposure demonstrated similar results. The WIHS (14) study found that NRTI exposure was associated with a higher median HOMA compared with no NRTI in a cohort of HIV-infected women and uninfected controls. Each additional year of NRTI use was associated with a 2% increase in median HOMA. Median HOMA increased 6% per year of stavudine exposure. No significant relationship between markers of insulin resistance and exposure to lamivudine, zidovudine, abacavir, tenofovir were identified, nor did they find any significant relationship with PIs or NNRTIs as classes of antiretroviral therapy.
Overall, data support an increased prevalence of diabetes among HIV-infected patients treated with stavudine, with mixed data regarding indinavir and other agents. Observed discrepancies may be attributed to changes in cART prescribing practices over time, with more recent studies reporting less use of a first generation PIs and thymidine analogues. For initiation of HIV therapy, current treatment guidelines do not include the described agents implicated in diabetes and insulin resistance. However, observations regarding risk associated with agents such as stavudine remain applicable, as it continues to be utilized in resource limited settings globally. Future studies will be needed to reevaluate the influence of contemporary antiretroviral treatment practices on diabetes and glucose metabolism in the modern era of antiretroviral therapy.
Side effects of cART such as lipodystrophy and hyperlipidemia play an important role in the association between diabetes and HIV, and will be addressed directly in other sections of this review. Other conditions known to occur with increased frequency in HIV-infected patients have also been found to influence the development of disordered glucose metabolism. For example, opioid use is common in HIV-infected patients, and has been linked to aberrant glucose metabolism in animal studies. In an investigation of women with or at risk of HIV by Howard and colleagues, opiate use was associated with increased prevalence of DM amongst past opioid users (18% vs. 9%) as well as current opioid users (15% vs. 10%), independent of multiple factors including Hepatitis C infection.(15) Opiate use in the cohort was also associated with an increased incidence of diabetes during the period of 2000–2006.
This study also found a higher prevalence of diabetes among hepatitis C co-infected patients (16% vs. 10% among those without hepatitis C), a finding which has been identified in other studies of both HIV-infected and uninfected populations. This is thought to be mediated by hepatic steatosis and liver fibrosis, with resultant insulin resistance. In a cross sectional study among 432 HIV-monoinfected patients, DallaPiazza et. al.(16) identified liver fibrosis estimated by APRI (AST to platelet ratio, where score of >1.5 is predictive of significant liver fibrosis) in association with diabetes. While the overall prevalence of DM was 8%, this increased to 17% in patients with APRI > 1.5. While the design and sample size cannot establish causality, diabetes was the most significant risk factor associated with fibrosis. The authors suggest that liver fibrosis, independent of viral hepatitis, may be prevalent in HIV monoinfected patients, with important implications in the risk of diabetes.
Taken together, the above data demonstrate the wide array of pathogenesis in the development of insulin resistance and diabetes in HIV. Though multifactorial, the disease is related to known demographic risk factors, in particular age, obesity, and genetic variants, but specific medications such as stavudine, and associated conditions such as lipodystrophy, opiate use, and liver fibrosis are also contributory for many patients.
Diagnostics
The definition of DM in the aforementioned studies demonstrates the variability of diagnostic criteria available today, and may underlie the differences in prevalence and incidence among various cohorts. The current American Diabetes Association guidelines underscore that either fasting blood glucose, hemoglobin A1c, oral glucose tolerance test (OGTT), or random glucose with classic symptoms of hyperglycemia can be performed to diagnose diabetes (17). For the first three strategies, outside of unequivocal hyperglycemia, repeat testing should occur for confirmation. Interval testing is recommended to occur every three years. The only group of distinction remains pregnant women, for whom OGTT is the diagnostic test of choice. Studies have evaluated the performance of diagnostic strategies in the setting of HIV, but optimal diabetes screening guidelines have not been established specifically for HIV-infected patients.
One study determined the proportion of incident DM cases among 377 HIV-infected or at-risk adults using fasting and OGTT 120 minute glucose levels tested a year and half apart.(18) Of those diagnosed with DM during the study, 44% were detected only by a fasting plasma glucose, 31% were detected only by the 120-min plasma glucose level on OGTT, and 25% were detected by both tests, with no difference between HIV and non-infected patients. A similar pattern of detection was found for diagnosis of prediabetes. The authors conclude that given 1/3 of diabetes cases were detected only by use of OGTT, performing fasting glucose alone may miss a significant number of individuals with diabetes.
Recently, Tien et. al.(19) also explored the effect of definition on case detection of DM, using the WIHS cohort. The researchers used three definitions of diabetes: one (I) being the measures (FBG >126 mg/dl, reported DM diagnosis or medications for hyperglycemia) utilized by most of the large cohort studies, two (II) including a second confirmatory fasting blood glucose, and three (III) including a HgbA1c of >6.5%. The results demonstrated the highest case detection for definition I, with an incidence rate of 2.44 per 100 person-years in the HIV infected population, and 1.89 per 100 person-years in the uninfected comparison, a difference of 1.2 fold. While the greatest prevalence in both infected and non-infected patients was seen with single fasting glucose >126 mg/dl, the addition of confirmatory testing or an HgbA1c level increased the accuracy of diagnosis and only slightly attenuated the association between DM and HIV.
In a retrospective chart review of 395 HIV-infected, primarily black and Hispanic men, Eckhardt et. al.(20) compared elevated fasting blood glucose with HgbA1c. Using fasting blood glucose as the gold standard, investigators found that the sensitivity of HgbA1c was poor at 40.9%, but specificity high at 97.5%; peak sensitivity and specificity was found at a HgbA1c cutoff of 5.8%. This investigation and others have noted that elevations in MCV in patients with HIV tend to underestimate glycemia when using HgbA1c.(9) Despite these observations, HgbA1c can be used in conjunction with FBG for clinical decision making.
Overall, there are no specific recommendations for diabetes surveillance or testing in the context of HIV infection. For practical purposes, most investigations conclude that fasting blood glucose as well as HgbA1c are the diagnostic tests of choice, and that there is not enough evidence in the HIV population to recommend for or against either strategy. The recently updated Infectious Disease Society of America (IDSA) primary care guidelines recommend fasting glucose and/or HgbA1c, and suggest consideration of a 5.8% HgbA1c threshold cutoff (21). The IDSA guidelines also recommend testing every 6–12 months in HIV-infected patients, which does deviate from the ADA recommendations for the general population to screen every three years. Currently, there is insufficient evidence to recommend an ideal time interval, especially as the only study which addressed this used the oral glucose tolerance test. However, given the prevalence of diabetes in HIV, this additional guidance can be employed in conjunction with clinical evaluation.
Effects
Diabetes is a systemic illness with well-established, targeted end organ effects. In the setting of HIV, strong data supports the association between diabetes and the risk of cardiovascular disease. In a study utilizing the D:A:D cohort, Worm et. al.(22) investigated the predictive value of preexisting DM and heart disease for subsequent cardiac events in the context of HIV infection. Of the 33,347 patients enrolled, the prevalence of diabetes was 2.9%, and the prevalence for heart disease was 1.1%. There were 698 incident cardiac events (incidence of 4.4 per 1000 person years). These events were divided into four risk categories: previous heart disease alone, diabetes alone, both prior CHD (coronary heart disease) and DM, and neither diagnosis. While previous cardiac disease had a stronger relative risk (RR 9.04, 95% CI: 7.1–11.49) than diabetes (RR 3.03, 95% CI: 2.34–3.93) for incident cardiac events, their combined effect was substantial (RR 11.66 with 95% CI: 7.42–18.3). In this cohort of mainly 30–40 year old adults, the average length of diabetes diagnosis was only five years, which may be insufficient time for resultant vascular damage to occur. In a subanalysis, the risk of heart disease increased with longer duration of diabetes, highlighting the potential for a stronger relationship with extended clinical follow up. Additionally, the study noted the high prevalence of cardiac risk factors such as tobacco use, which is often over-represented among HIV-infected populations. A study by Calvo-Sanchez et. al.(23) further illustrates these findings. This retrospective chart review utilized two parallel case-controls to evaluate the rate of acute coronary syndrome (ACS) in HIV, with matched populations of HIV-infected patients without ACS, HIV-uninfected patients with ACS, and healthy volunteers. While the population attributable risk (PAR) of diabetes was 6.57 (95% CI: −8.87–16.96), smoking held the largest fraction, with a PAR 54.35 (95% CI: 29.33–70.51), which was significantly larger than that observed in the HIV-uninfected population.
The effects of diabetes in cardiovascular health potentially extend beyond incidence of cardiac events. In a study by Lorgis et. al.(24), data from a French database of 277,303 patients with acute myocardial infarction (MI) was separated into parallel cohorts of HIV-infected and uninfected patients. After one year of follow-up, multiple post-MI complications were followed including hospitalizations, heart failure, and recurrent MI. A diagnosis of diabetes was associated with an increased risk of heart failure, with an odds ratio of 5.34 (95% CI: 2.39–11.9) within this period. Similarly, in the HIV-HEART study, Reinsch et. al.(25) investigated the prevalence of diastolic dysfunction, a potential early marker of coronary artery disease, in a cross-sectional cohort of 698 HIV-infected patients. Patients with diabetes were found to be four times as likely to have diastolic dysfunction, despite a prevalence of only 2% in the study population.
While cardiac disease is an important contributor to mortality in persons living with HIV, liver disease also disproportionately affects this population. Much of this is related to co-infection with Hepatitis C and B. However, significant liver abnormalities have also been found in HIV-monoinfected patients, and diabetes may play a role in modulation of this. As noted previously, DallaPiazza et. al.(16) showed that HIV-infected patients with a diagnosis of diabetes were three fold times more likely to have an APRI score indicative of liver fibrosis compared to those without diabetes. While data were not collected on potential confounders such as cART and other hepatotoxic medications, this study sheds light on important interplay between diabetes and liver disease in HIV.
Renal disease is also a significant risk in the HIV-infected population, and the presence of diabetes appears to have an additive affect. To assess the interaction between diabetes and development of chronic kidney disease (CKD), Medapalli et. al.(26) measured progression to GFR < 45 mL/min/1.73m2 using data from the large Veterans Aging Cohort Study. The presence of both HIV and DM was associated with a hazard ratio of 4.47 (95% CI: 3.87–5.17) for development of CKD. In a subgroup of 7328 with HIV infection, excluding those with acute or chronic renal injury, the incidence of progression to CKD was 9.7%, and diabetes was associated with a hazard ratio of 1.78 (95% CI 1.49–2.12), representing a stronger association with development of CKD than use of cART, HIV RNA, or CD4 count. The additive effect of DM and HIV is also seen in earlier measures of renal disease, such as albuminuria. In a cross sectional study of HIV-infected patients with and without diabetes, and matched uninfected controls with diabetes, Kim et. al.(27) identified a high prevalence of albuminuria in HIV-infected diabetics (34% HIV/DM vs. 13% HIV vs. 16% DM). The association with HIV and DM remained significant when a multivariate model controlled for multiple potential confounders including age, race, blood pressure, gender, BMI, and ACE/ARB use. These studies demonstrate that the untoward effects of DM in the context of HIV infection may contribute to renal dysfunction and chronic kidney disease.
One of the most common complications of uncontrolled DM in the general population is peripheral neuropathy. In the HIV population, this relationship is confounded by the independent risk of HIV- and cART-mediated neuropathy. In an AIDS Clinical Trials Group study of neuropathy,(28) 2141 patients who initiated cART from 2000 to 2007 were followed to determine the prevalence and risk factors for peripheral neuropathy in HIV. Throughout the evolution of treatment, both before, during and after discontinuation of neurotoxic cART, diabetes remained positively associated with symptomatic and asymptomatic neuropathy. These investigators subsequently showed that use of glucose lowering therapies was protective against neuropathy and its progression in HIV-infected patients.(29) The impact of diabetes and insulin resistance on the nervous system may also apply to the brain and cognition. To examine the relationship between HIV neurocognitive disorder and metabolic variables, McCutchan et. al.(30) completed a cross-sectional substudy of 130 HIV-infected patients. In the population aged > 55 years, those with neurocognitive impairment (defined as a global deficiency score of > 0.5) were more likely to have a concomitant diagnosis of DM (52.4% vs. 29.9%, p= 0.05)
The above studies support the idea that diabetes is a significant risk factor for organ system dysfunction the context of HIV. Yet the implications of diabetes in the HIV infected population may also extend to psychosocial factors. A study by Dray-Spira et. al.(31), evaluated the risk of work cessation in a cohort of persons living with chronic HIV infection. They found that one third of patients stopped working within five years of their HIV diagnosis. Looking at multiple risk factors, diabetes was associated with a hazard ratio of 5.6 (95% CI 1.5–18.5, p<0.005), while HIV disease severity and HIV discrimination had no significant relationship with work cessation. The authors concluded that comorbid conditions such as DM constitute a major barrier to retained employment in French HIV-infected patients.
Taken together, these studies demonstrate the widespread impact of diabetes as a risk factor for multiple pathways of morbidity and mortality in the HIV infected population.
Treatment
Currently, there are no randomized control trials of diabetes treatment specific to patients with HIV infection. Multiple studies have analyzed the use of thiazolidinedione (TZD) medication in the setting of lipodystrophy, with improvements seen in measures of insulin resistance. However, given the declining prevalence of lipodystrophy along with proposed increase in cardiac risk with use of TZDs, application beyond this clinical setting is not currently recommended. Similarly, switching classes or specific combinations of antiretroviral therapy in the absence of stavudine or indinavir induced hyperglycemia has not been shown to improve measures of insulin resistance.
Despite the lack of clinical data regarding various treatment options, several studies have looked at the efficacy of overall diabetes treatment and management in the HIV-infected population. Satlin et. al.(32) completed a retrospective cross sectional study of 142 HIV-infected adults with type 2 DM. They found that one third of patients had inadequate glycemic control, as defined by HgbA1c > 7.5 for more than six months over the year. While most uncontrolled patients were on insulin (60% vs. 20%, p < 0.001), this may simply reflect that insulin initiation is in part triggered by measures of A1c. The overall frequency that ADA clinical goals were achieved was poor, with approximately 30% who met HDL and TG goals, and 40% who met blood pressure goals. A study set in a Malawi diabetes clinic discovered similar findings. Cohen et. al.(33) analyzed 620 patients, separating outcomes between HIV infected and non-infected patients. HIV diabetes patients had similar poor control to their non-infected counterparts, with an average HbA1c of 9.0% vs. 9.5% respectively. In short, HIV-infected patients appear to have a similar profile of disease control compared to their non-infected counterparts. However, it should be noted that this is a poor level of control, and given the additive effects of diabetes in the setting of HIV, carries an additional layer of risk.
As such, the current ADA guidelines serve as the basis for ongoing management of DM in the HIV-infected population. Further studies are needed to assess the question of optimal therapeutics.
Conclusions
Successful screening, diagnosis, treatment, and management of diabetes has emerged as an important component of HIV care. HIV cohort studies estimate the prevalence of diabetes at 3–4% amongst HIV-infected individuals. Studies on the impact of ethnicity and race on this prevalence are limited.
As in the general population, traditional risk factors such as age, BMI, and genetics play a significant role in development of diabetes in persons living with HIV. However, multiple other mechanisms contribute to the pathogenesis of DM in HIV-infected patients. Antiretroviral agents, as well as drug effects such as hyperlipidemia and lipodystrophy, have been associated with increased incidence of diabetes. HIV is a risk factor for other conditions such as opiate use and liver fibrosis and steatosis; in these groups diabetes is found in increased prevalence.
The diagnosis of diabetes in HIV can be made using both fasting blood glucose as well as HgbA1c, as current studies do not favor a specific diagnostic strategy. Recognition and diagnosis is necessary, however, given the far ranging health effects associated with diabetes in this population. Increased rates of cardiovascular disease and cardiac complications have been found in HIV-infected patients with diabetes. Diabetes in HIV has also been associated with worsened liver disease, measures of chronic kidney disease, and increased rates of unemployment.
There are currently no studies comparing diabetes treatment strategies in the setting of HIV, and as such, current ADA guidelines serve as the guiding tool for treatment. Future research should address this knowledge gap, and address specific subpopulations related to HIV, such as those with advanced liver disease. In addition, the recently updated HIV treatment guidelines demonstrate the rapidity of turnover in cART recommendations. As HIV treatment practices evolve, recurrent studies of diabetes prevalence are necessary to recalibrate the scope of this issue.
Acknowledgments
Support: This review was prepared with support from the Intramural Research Program of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Footnotes
Disclosures: The authors have no potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Colleen Hadigan, Email: hadiganc@niaid.nih.gov, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, NIH, 10 Center Drive, Bethesda, MD 20892, Telephone (301) 594-5754, Fax (301) 402-4097.
Sarah Kattakuzhy, Email: sarah.kattakuzhy@nih.gov, Laboratory of Immunoregulation, Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, Maryland 21702, Telephone (301) 594-7807, Fax (301) 402-1137.
References
- 1.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–9. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 2.Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, Ritchie C, et al. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr. 2012;61(5):600–5. doi: 10.1097/QAI.0b013e31827303d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calza L, Masetti G, Piergentili B, Trapani F, Cascavilla A, Manfredi R, et al. Prevalence of diabetes mellitus, hyperinsulinaemia and metabolic syndrome among 755 adult patients with HIV-1 infection. Int J STD AIDS. 2011;22(1):43–5. doi: 10.1258/ijsa.2010.010256. [DOI] [PubMed] [Google Scholar]
- 4.Galli L, Salpietro S, Pellicciotta G, Galliani A, Piatti P, Hasson H, et al. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur J Epidemiol. 2012;27(8):657–65. doi: 10.1007/s10654-012-9707-5. [DOI] [PubMed] [Google Scholar]
- 5.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31(6):1224–9. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 7.Capeau J, Bouteloup V, Katlama C, Bastard JP, Guiyedi V, Salmon-Ceron D, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26(3):303–14. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 8.Adeyemi OM, Livak B, Orsi J, Glesby MJ, Villacres MC, Weber KM, et al. Vitamin D and insulin resistance in non-diabetic women’s interagency HIV study participants. AIDS Patient Care STDS. 2013;27(6):320–5. doi: 10.1089/apc.2012.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim PS, Woods C, Georgoff P, Crum D, Rosenberg A, Smith M, et al. A1C underestimates glycemia in HIV infection. Diabetes Care. 2009;32(9):1591–3. doi: 10.2337/dc09-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petoumenos K, Worm SW, Fontas E, Weber R, De Wit S, Bruyand M, et al. Predicting the short-term risk of diabetes in HIV-positive patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. J Int AIDS Soc. 2012;15(2):17426. doi: 10.7448/IAS.15.2.17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rotger M, Gsponer T, Martinez R, Taffe P, Elzi L, Vernazza P, et al. Impact of single nucleotide polymorphisms and of clinical risk factors on new-onset diabetes mellitus in HIV-infected individuals. Clin Infect Dis. 2010;51(9):1090–8. doi: 10.1086/656630. [DOI] [PubMed] [Google Scholar]
- 12.Brown TT, Li X, Cole SR, Kingsley LA, Palella FJ, Riddler SA, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19(13):1375–83. doi: 10.1097/01.aids.0000181011.62385.91. [DOI] [PubMed] [Google Scholar]
- 13.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33(10):2244–9. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tien PC, Schneider MF, Cole SR, Levine AM, Cohen M, DeHovitz J, et al. Antiretroviral therapy exposure and insulin resistance in the Women’s Interagency HIV study. J Acquir Immune Defic Syndr. 2008;49(4):369–76. doi: 10.1097/qai.0b013e318189a780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard AA, Hoover DR, Anastos K, Wu X, Shi Q, Strickler HD, et al. The effects of opiate use and hepatitis C virus infection on risk of diabetes mellitus in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2010;54(2):152–9. doi: 10.1097/QAI.0b013e3181d0c911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V., 3rd Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 (Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 18.Polsky S, Floris-Moore M, Schoenbaum EE, Klein RS, Arnsten JH, Howard AA. Incident hyperglycaemia among older adults with or at-risk for HIV infection. Antivir Ther. 2011;16(2):181–8. doi: 10.3851/IMP1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tien PC, Schneider MF, Cox C, Karim R, Cohen M, Sharma A, et al. Association of HIV infection with incident diabetes mellitus: impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr. 2012;61(3):334–40. doi: 10.1097/QAI.0b013e31826bfc32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckhardt BJ, Holzman RS, Kwan CK, Baghdadi J, Aberg JA. Glycated Hemoglobin A(1c) as screening for diabetes mellitus in HIV-infected individuals. AIDS Patient Care STDS. 2012;26(4):197–201. doi: 10.1089/apc.2011.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 22.Worm SW, De Wit S, Weber R, Sabin CA, Reiss P, El-Sadr W, et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study) Circulation. 2009;119(6):805–11. doi: 10.1161/CIRCULATIONAHA.108.790857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo-Sanchez M, Perello R, Perez I, Mateo MG, Junyent M, Laguno M, et al. Differences between HIV-infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case-control studies. HIV Med. 2013;14(1):40–8. doi: 10.1111/j.1468-1293.2012.01057.x. [DOI] [PubMed] [Google Scholar]
- 24.Lorgis L, Cottenet J, Molins G, Benzenine E, Zeller M, Aube H, et al. Outcomes after acute myocardial infarction in HIV-infected patients: analysis of data from a French nationwide hospital medical information database. Circulation. 2013;127(17):1767–74. doi: 10.1161/CIRCULATIONAHA.113.001874. [DOI] [PubMed] [Google Scholar]
- 25.Reinsch N, Neuhaus K, Esser S, Potthoff A, Hower M, Brockmeyer NH, et al. Prevalence of cardiac diastolic dysfunction in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials. 2010;11(3):156–62. doi: 10.1310/hct1103-156. [DOI] [PubMed] [Google Scholar]
- 26.Medapalli RK, Parikh CR, Gordon K, Brown ST, Butt AA, Gibert CL, et al. Comorbid diabetes and the risk of progressive chronic kidney disease in HIV-infected adults: data from the Veterans Aging Cohort Study. J Acquir Immune Defic Syndr. 2012;60(4):393–9. doi: 10.1097/QAI.0b013e31825b70d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim PS, Woods C, Dutcher L, Georgoff P, Rosenberg A, Mican JA, et al. Increased prevalence of albuminuria in HIV-infected adults with diabetes. PLoS One. 2011;6(9):e24610. doi: 10.1371/journal.pone.0024610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, et al. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25(7):919–28. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans SR, Lee AJ, Ellis RJ, Chen H, Wu K, Bosch RJ, et al. HIV peripheral neuropathy progression: protection with glucose-lowering drugs? J Neurovirol. 2012;18(5):428–33. doi: 10.1007/s13365-012-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78(7):485–92. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dray-Spira R, Legeai C, Le Den M, Boue F, Lascoux-Combe C, Simon A, et al. Burden of HIV disease and comorbidities on the chances of maintaining employment in the era of sustained combined antiretoviral therapies use. AIDS. 2012;26(2):207–15. doi: 10.1097/QAD.0b013e32834dcf61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satlin MJ, Hoover DR, Glesby MJ. Glycemic control in HIV-infected patients with diabetes mellitus and rates of meeting American Diabetes Association management guidelines. AIDS Patient Care STDS. 2011;25(1):5–12. doi: 10.1089/apc.2010.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen DB, Allain TJ, Glover S, Chimbayo D, Dzamalala H, Hofland HW, et al. A survey of the management, control, and complications of diabetes mellitus in patients attending a diabetes clinic in Blantyre, Malawi, an area of high HIV prevalence. Am J Trop Med Hyg. 2010;83(3):575–81. doi: 10.4269/ajtmh.2010.10-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]


