Abstract
Background
Operant responding paradigms quantify a subject’s motivation for reward, but such studies employing ingested alcohol cannot assure the same incremental increase in brain exposure to alcohol across subjects because of substantial variability in absorption kinetics. We developed a human progressive ratio (PR) paradigm using the computer-assisted self-infusion of ethanol (CASE) system that overcomes such variability, and conducted a pilot study to assess its utility for detecting an interaction of subjects’ GABRA2 or GABRG1 genotype and pre-treatment with 1mg of lorazepam (LZ) vs. placebo on their willingness to work for alcohol rewards.
Methods
20 healthy, non-dependent drinkers, aged 21–27 were balanced on rs279871 and rs2350439 single nucleotide polymorphisms in the GABRA2 and GABRG1 genes respectively. Subjects worked for alcohol, with water as an alternative reinforcer, using a progressive schedule of a task that required constant attention and adapted to both fatigue and drug effects. Testing began 1-hour after pre-treatment with 1 mg LZ or placebo in a crossover design.
Results
The CASE system performed well and the constant attention task was perceived as work by all subjects. GABRA2 homozygosity did not significantly predict either breakpoint or cumulative work, whereas a significant GABRG1 genotype by LZ pre-treatment interaction for cumulative work was detected (p=0.04). Breakpoint revealed a weak trend towards pre-treatment drug effects (p= 0.11), and a somewhat stronger interaction of LZ pre-treatment with GABRG1 genotype (p=0.06). GABRG1 status revealed a more complex relationship with respect to motivation for alcohol with and without LZ pre-treatment; AG and GG individuals worked more for alcohol under both pre-treatment conditions while AA individuals worked more for the alternative reinforcer.
Conclusion
The CASE PR paradigm shows promise as a laboratory method for use in drug-development and phenotyping studies.
Keywords: operant behavior, alcohol, progressive ratio, GABRG1, GABRA2, physiologically-based pharmacokinetic model
Introduction
Laboratory alcohol self-administration is a method used to examine human consumption behavior in a controlled setting using one of two basic designs. In a typical free-access (FA) design, subjects may consume as many alcohol containing beverages as they prefer in a specified interval. Progressive-ratio (PR) paradigms, used extensively in animal studies, require an increasing response or ‘work’ requirement while subjects self-administer alcohol, and are designed to quantify the point at which motivation for achieving the reward is no longer worth the effort. The objective of this pilot study was to explore the application of a PR paradigm in humans using intravenous (IV) alcohol self-administration to explore the effects of genotype, pre-treatment with a relevant drug, lorazepam (LZ), on the subject’s willingness to work for alcohol.
Alcohol self-administration paradigms have two general areas of potential utility: phenotyping and pharmaceutical development; see (Zimmermann et al., 2011) for a review. In phenotyping experiments, some difference that accounts for separate clusters of behavioral or subjective measures is sought and then examined for etiology or association with risk (de Wit and McCracken, 1990; Petrakis et al., 2002). Drug efficacy paradigms examine the effects of a pharmaceutical upon the consumption behavior (George et al., 2010; McKee et al., 2009; Sarid-Segal et al., 2009), and/or subjective response to alcohol in specific populations of interest (Davidson et al., 1999; McKee et al., 2008; O'Malley et al., 2002; Setiawan et al., 2011). In this context, a successful pharmaceutical intervention could be defined through direct or indirect mechanisms, such as a decrease in alcohol self-administration or modulation of a subjective response. Most human alcohol self-administration experiments to date have employed FA paradigms.
Recently, a few studies have employed PR paradigms to examine various modulators of alcohol self-administration in humans. For example, Spiga et al. (1997) investigated the relationship between the response requirement and administered alcohol dosage. Perkins et al. (2000) showed that males self-administered more alcohol than females after cigarette smoking. Barrett et al. (2006) found that nicotine increased alcohol self-administration in non-dependent male smokers and then investigated dopaminergic modulation on motivation (Barrett et al., 2008). Setiawan et al. (2011) found gender related subjective differences, but not alcohol self-administration differences, after naltrexone administration in subjects with the A1188G polymorphism in the μ opioid receptor gene. These studies have all relied upon oral alcohol administration and were limited by several concerns: standardizing the dose and time course of alcohol exposure, safety, choice of vehicle beverage, and selection of an alternative reinforcer (AR).
We have developed a human laboratory PR paradigm using the Computer Assisted Self-infusion of Ethanol (CASE) system and have begun a program of research to demonstrate its utility for both phenotyping and drug-development applications. The CASE system was developed to minimize variation in the incremental time course of breath alcohol concentration (BrAC) across subjects in experiments involving alcohol self-administration (Zimmermann et al., 2009; Zimmermann et al., 2008). CASE utilizes a physiologically-based pharmacokinetic (PBPK) model of the distribution and elimination of alcohol, with parameters tailored to the individual subject (Plawecki et al., 2007; Plawecki et al., 2008). This approach avoids the 3-fold range of peak BrAC encountered when ingestion of alcohol is employed (Ramchandani et al., 2009). The combination of an IV route of administration and real-time, PBPK model-based calculations permit CASE to achieve an identical incremental time course of BrAC (thus brain exposure to alcohol) in every subject for each alcohol reward.
In order to test the utility of the CASE PR paradigm, we devised a pharmaceutical development-type experiment where subjects who were homozygous for a GABRA2 SNP worked for alcohol or water after pre-treatment with a GABAergic drug. Our pilot study goals were to validate performance of the “work” task we developed, to document alcohol as the primary reinforcer when the alternative was water, and to examine the extent to which pharmaceutical intervention altered measures of that reinforcement.
Extensive evidence has demonstrated an association of alcohol dependence with several single nucleotide polymorphisms (SNPs) in GABRA2, the gene encoding for the α2 subunit of the gamma-aminobutyric acid (GABA) receptor (Bierut et al., 2010; Covault et al., 2004; Edenberg et al., 2004; Fehr et al., 2006; Lappalainen et al., 2005). However, the direction of association is not uniform (Covault et al., 2008; Drgon et al., 2006; Matthews et al., 2007; Onori et al., 2010) and the underlying mechanism is unclear. The A allele at rs279871 (along with many other alleles in a large block of linkage disequilibrium) has been reported to be over-transmitted to individuals with alcohol dependence (Edenberg et al., 2004) and is associated with a fast EEG (Lydall et al., 2011) while other studies have shown a higher frequency of the G allele in alcohol-dependent individuals (Fehr et al., 2006; Haughey et al., 2008). Studies have also shown that individuals with the AA genotype at this SNP report feeling significantly less high and less intoxicated than AG individuals (Kareken et al., 2010). Furthermore, rs279871 is in high linkage disequilibrium with other GABRA2 SNPs, including rs279858, which has also been shown to be associated with alcohol dependence and again with opposite alleles (Covault et al., 2004; Edenberg et al., 2004; Enoch et al., 2006; Fehr et al., 2006; Haughey et al., 2008; Lappalainen et al., 2005). Studies have shown that individuals with 1 or 2 copies of the A allele at this SNP showed greater subjective response to both oral and intravenous alcohol than those with the G allele which was associated with alcohol dependence (Pierucci-Lagha et al., 2005; Roh et al., 2010). The lack of a clear underlying mechanism has led investigators to consider other possibilities as the source of these associations, including the contribution of neighboring variations and genes.
GABRG1, the gene encoding the γ1 subunit of the GABA receptor, has been the target of such investigations. SNPs in this gene have been shown to be in moderate linkage disequilibrium with GABRA2 (Covault et al., 2008; Enoch et al., 2009; Ittiwut et al., 2008) and associated with alcohol dependence (Covault et al., 2008; Enoch et al., 2009). Variation in GABRG1 has also been associated with level of response to alcohol, drinking behavior, and alcohol problems in a sample selected for drinkers with an ongoing hazardous consumption pattern (Ray and Hutchison, 2009).
While the associations of individual genetic contributions to alcohol dependence have been replicated, including GABRA2 and GABRG1, the effects appear to be modest. Further, they do not explain the strength of the familial aggregation observed in alcohol dependence and the strong heritability found for many alcoholism-related endophenotypes; other genes, and gene×gene interactions undoubtedly make additional contributions.
We chose LZ, a benzodiazepine, as a proxy for drugs used as a treatment for alcohol dependence in this pilot study of the utility of the CASE PR paradigm because the sample population had been characterized by GABA receptor genotype. Alcohol and benzodiazepines are both GABAergic drugs (Harris et al., 1998) that influence the perception of anxiety, the liability for amnestic episodes, the potential for developing tolerance and cross-tolerance to each drug, and the potential for physiologic addiction with similar withdrawal syndromes (Kumar et al., 2009). GABA is an inhibitory neurotransmitter which reduces neuronal activity and LZ is an FDA-approved drug that enhances GABA activity. Alcohol also influences activity of the GABA receptor. In theory, then, pre-treatment with LZ could influence alcohol self-administration in all subjects. On the other hand, we postulated that GABA receptor polymorphisms may differentially influence operant alcohol self-administration when subjects were pre-treated with LZ. This paper reviews the methodology employed and the results obtained in the pilot study.
Materials and Methods
Subjects
Twenty subjects were identified from those who completed a parent study of GABRA2 or GABRG1 polymorphisms upon initial and adaptive responses to a prescribed 3-hr steady-state exposure to a BrAC of 60mg%. All subjects had been genotyped for their GABRA2 SNP rs279871 status, and ten each were selected for this pilot based upon their AA or GG homozygous status atat that SNP. Subjects had also been genotyped at the rs2350439 GABRG1 SNP.
Other inclusion criteria were: Caucasian ancestry, between 21–28 years of age and good health as determined by medical history, physical exam, and laboratory tests. Exclusion criteria were: Current or prior history of any serious disease, alcohol or drug dependence, current or prior history of alcohol-induced flushing reactions, current history of Axis-I psychiatric illness excepting alcohol abuse, consumption of less than 10 standard (12 gm ethanol) drinks per week in the preceding month according to a 90-day Time-Line Follow-Back assessment (TLFB) (Sobell et al., 1996; Sobell and Sobell, 1992), a positive result on urine drug screen at the time of testing, use of medications known to interact with alcohol within 2 weeks of the study, the intention to become pregnant or a positive urinary pregnancy test in women at the start of each study session.
In the parent study, the subjects had responded to local advertisement, been interviewed by phone twice, signed an informed consent approved by the Indiana University School of Medicine IRB, and undertook an in-depth face-to-face interview. Recruitment for the pilot study was performed by inviting eligible subjects, and signing of a separate informed consent. Each subject then undertook a brief face-to-face interview regarding any alcohol problems since the earlier interview, provided another TLFB assessment of recent drinking history, and provided samples for retesting of urine and blood.
Experimental Design
The pilot study employed a within-subject, 2-session, double-blind, randomized-order, crossover design with pre-treatment with either LZ or placebo, and operant self-administration of intravenous alcohol on both days, with infused half-normal saline (water) available as an alternative reinforcer (AR).
Subjects were requested to avoid consumption of alcohol after 4 pm on the day before testing. They were admitted to the outpatient section of the Indiana Clinical Research Center (ICRC) of the Indiana Clinical and Translational Sciences Institute at 10 AM, asked to provide a BrAC sample documenting no alcohol, and then fitted with an indwelling 20 ga. intravenous catheter in a vein of the ante-cubital fossa of the non-dominant arm. A standardized lunch was provided at 11:30 AM, and at 12:00 PM, one hour before the beginning of alcohol self-administration, the subject ingested 1 mg of LZ or placebo. The second session was conducted using identical procedures with no less than 3 nor more than 7 days separating the sessions.
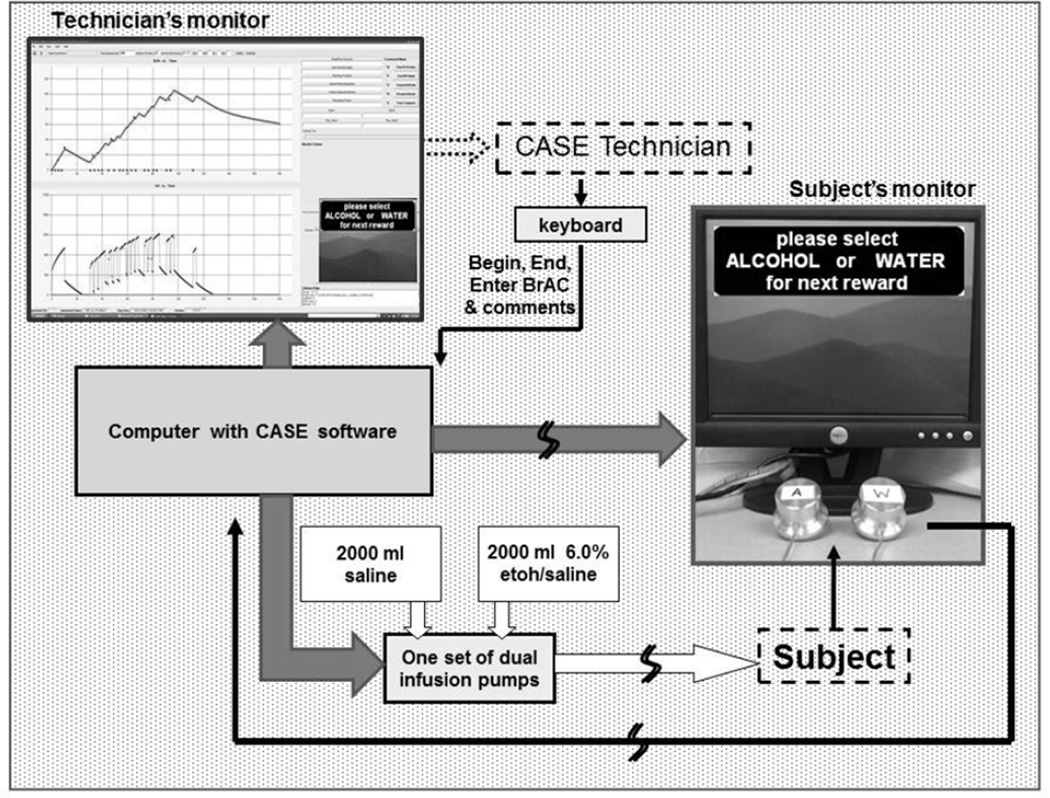
The experimental session employed the CASE apparatus, Figure 1, developed by the Indiana Alcohol Research Center (IARC). The apparatus enabled subjects to choose if/when to work for an alcohol reward or for the AR.
Figure 1.
CASE comprises an IARC software application that manages the real-time interaction between a subject, a PBPK model, MatLab Simulink, a technician, infusion pumps and two computer monitors. The physiologically-based pharmacokinetic (PBPK) model describes alcohol distribution and elimination with parameters tailored to that subject; Simulink solves the model’s differential equations once every 3 sec to accurately predict the future time course of the subject’s brain exposure for the remainder of the 2 hour experiment. When an alcohol reward is earned, the infusion rate is recalculated to yield an increment in breath alcohol concentration (BrAC, mg%) that increases steadily over 2.5 min by a prescribed amount and then decreases at a constant rate until another alcohol reward is delivered. CASE includes a preset BrAC safety limit and will not infuse alcohol if that infusion would yield a BrAC exceeding the safety limit (120 mg% in this experiment, although the work schedule precluded maximum BrACs above 75 mg%). Through infusion, CASE dissociates expectation based on taste and smell from the pharmacological effects of alcohol.
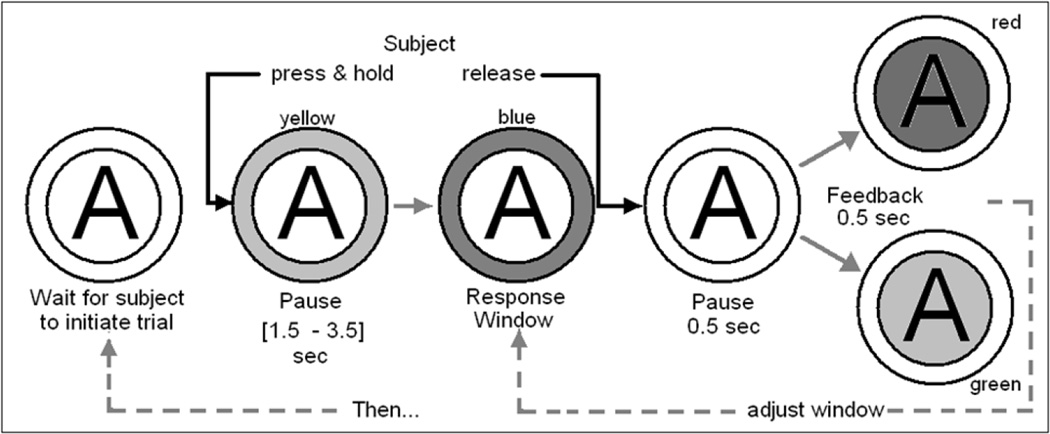
The progressive work paradigm task was designed such that the “work” would require the constant attention of the subject to perform it successfully despite any effects of LZ, alcohol and fatigue. A work-set comprised successful completion of a predetermined number of trials of the Constant Attention Task (CAT), Figure 2. The progression of successful CAT trials required per work-set, Table 1, was identical for alcohol and AR, but progress was accounted separately.
Figure 2.
CAT video monitor display flowchart; only one symbol appears on the subject’s video monitor at any instant. The illustration represents the sequence of actions taken by the subject and resultant changes in the symbol displayed on the subject’s CASE screen for a single trial of the CAT. “A” denotes the selection of an alcohol reward is under way (as opposed to a “W” when working for water).
Table 1.
Progression of the number of correct CAT trials required to complete the next work-set. The increasing amounts of time required to complete successive work-sets, combined with a steady decline in BrAC following the delivery of alcohol rewards, yielded an inevitable decline in the maximum BrAC achievable in the second half of the voluntary self-administration interval.
| Work- set # |
Correct CAT trials per work set |
Cumulative correct CAT trials |
Minutes per work-set at Max. rate |
|---|---|---|---|
| 1 | 1 | 1 | 0.15 |
| 2 | 2 | 3 | 0.30 |
| 3 | 3 | 6 | 0.45 |
| 4 | 5 | 11 | 0.75 |
| 5 | 8 | 19 | 1.20 |
| 6 | 12 | 31 | 1.80 |
| 7 | 18 | 49 | 2.70 |
| 8 | 27 | 76 | 4.05 |
| 9 | 40 | 116 | 6.00 |
| 10 | 59 | 175 | 8.85 |
| 11 | 87 | 262 | 13.05 |
| 12 | 129 | 391 | 19.35 |
| 13 | 190 | 581 | 28.50 |
| 14 | 280 | 861 | 42.00 |
A CAT trial began at the sole discretion of the subject, and was initiated by pressing and holding down a 2-in diameter button with a 0.25 in travel requiring 0.003 ft-pounds of effort (Griffin Technologies PowerMate®). The press-and-hold initiated a change in the button symbol; the outer ring turned from white to yellow signifying the beginning of an unpredictable interval (uniformly distributed in 2.0–3.5 sec). While the outer ring was yellow, the subject prepared to release the button as soon as, but not before the outer ring changed from yellow to blue. To count as a successful trial, the subject had to release the button within a response window before the outer ring changed from blue to white. One second after the response or end of the response window, whichever occurred first, the inner ring color changed for 0.5 sec: either from white to green to white, signifying success and progress towards earning the next reward, or from white to red to white, signifying failure of the trial. If successful, the response window for the next trial was shortened by 25 msec; if failed, the response window was lengthened by 25 msec. Thus, the subject’s success rate remained near 50% despite any eventual psychomotor effects caused by alcohol, LZ, or fatigue. Unlike the conventional work task of simple button presses, the CAT cannot be performed successfully if the subject is attending to anything else.
Each CASE session began with a 30 minute alcohol priming interval. Subjects were prompted to select the alcohol reward 4 times consecutively with a progression of 2, 4, 6 and 8 correct CAT trials required per set. After completion of each work-set, the subject was notified to wait for completion of reward delivery before another work-set was prompted. As soon as a work-set was completed, CASE delivered the infusion pump rate profile that, starting at the current value of BrAC, raised it at +2 mg% per min for 2.5 min, then decreased it at −0.8 mg% per min until the next alcohol delivery started. Prompting choices for alcohol yielded a mandated BrAC prime of approximately 20 mg% at 11 min., and a BrAC of approximately 5mg% when the 2-hour interval of voluntary self-administration began 19 min later.
During the voluntary interval, subjects initiated a work-set by pre-selecting the next reward; pressing 1 of 2 available buttons, labeled “A” for alcohol or “W” for water, respectively. Subjects could take as much time as they desired to complete the work-set, including the options of pausing or ceasing altogether, but were required to finish it and receive the respective reward before another work-set could be initiated. As soon as a work-set conducted for the AR was finished, a 2.5 min infusion of half-normal saline at 36 ml per min began. During work-sets, subjects could take bathroom breaks ad-lib (while infusions continued), but interaction with the CASE technician was limited to obtaining BrAC samples approximately every 20 min. Subjects were not allowed other distractions including TV, phone, reading, etc. and were required to remain in the testing room for the duration of the experiment. They were also required to remain on the ICRC until 6 pm or until their BrAC dropped below 20 mg%, whichever occurred later, to maximize safety and in order to discourage curtailing work for an early release. Subjects were paid $75 cash immediately before release from the ICRC after the first CASE session and $150 before release from the second session.
In real time, CASE used actual BrAC measurements obtained during the experiment to refine the infusion calculations and to estimate the continuous time course of BrAC that minimized the error between the measured and modeled data. The time-stamped CAT trial initiation, response time, response window duration and trial outcome were recorded for each trial performed, and the delivery times of the rewards were also automatically noted.
The outcome measures used for analyses were breakpoint (the total CAT trials in the last completed work set) and cumulative work (the total of all CAT trials) for each reward.
Statistical Analyses
Hardy-Weinberg equilibrium and minor allele frequency of rs279871 in GABRA2 and rs2350439 in GABRG1 were estimated using the full sample of the parent study using Haploview (Barrett et al., 2005).
Differences in demographic data between gender and genotype were examined using Fisher’s exact tests for association. A t-test was used to test for differences by session in average and rate of change of CAT performance and GABRA2 genotype for the quantitative variables: age, height, weight, number of drinks in the past 30 days, number of drinking days in the past 30 days, and number of drinks per drinking day in the past 30 days. An analysis of variance (ANOVA) model was employed to test for differences of these variables and the GABRG1 genotype. A paired t-test was used to detect overall differences across sessions.
A repeated measures ANOVA model was employed to test the within-subject effect of type of reward selected (alcohol vs. water) and the effect of the pre-treatment drug (LZ vs. placebo). The between-subject effects of GABRA2 genotype (AA vs. GG) or GABRG1 (AA vs. AG vs. GG) were included as independent variables. Hotelling’s T2 test was used to test for an overall effect of genotype. The order of the pre-treatment drug administered in the two sessions (LZ first vs. placebo first) was included in the original model, but was not significant for breakpoint or cumulative work (both p>0.19) and was subsequently excluded from all analyses. Due to the small sample size, the only three-way interactions examined were with reward type×pre-treatment×genotype. Each genotype was tested separately, due to the moderate linkage disequilibrium between them. Due to the small sample size and lack of heterozygotes for the GABRA2 SNP, haplotypes were not estimated for analysis.
Results
The rs279871 GABRA2 SNP was in Hardy-Weinberg equilibrium (p=0.89) as was rs2350439 (p=0.34), based on the 107 genotyped individuals in the parent study. The observed heterozygosities were 0.475 and 0.533, and minor allele frequencies = 0.41 (G) and 0.46 (A) for rs279871 and rs2350439 respectively. There was moderate linkage disequilibrium between the two SNPs: D’ = 0.70, and r2 = 0.28.
The demographic characteristics of the genotype groups are summarized in Table 2. The GABRA2 genotypes of the 20 individuals who completed the CASE PR pilot study were equally balanced, with 10 AA and 10 GG. The number of individuals in each GABRG1 genotype group was slightly smaller, with 5 AA, 7 AG and 8 GG individuals. Gender was moderately confounded with GABRA2 genotype (Table 2). Gender was initially included in the GABRG1 models, but was not significant (all p>0.20). Therefore gender was excluded from all models in order to maximize power in analyses of the sample size. There were no differences in any of the quantitative demographic variables between GABRA2 genotype (all p>0.59) or GABRG1 genotype (all p>0.17,), with the exception of a trend toward association of GABRG1 genotype and height (p=0.08). In addition, there were no differences in recent alcohol consumption (GABRA2 genotype p>0.38, GABRG1 genotype p>0.60).
Table 2.
Demographic information by genotype. Average values with standard deviations displayed except for Genotype information. Recent Drinking History as determined from a 30 day TLFB and with DD indicating Drinking Days.
| GABRA2 Genotype | Gender (M:F) | Age (Years) | Height (cm) | Weight (kg) | DD (Past 30) | DD/Week | Drinks/DD | |
| AA | 7:3 | 24.3 (2.1) | 171.6 (6.7) | 75.6 (15.1) | 14.8 (5.3) | 3.5 (1.2) | 4.2 (2.5) | |
| GG | 2:8 | 23.8 (2.0) | 171.1 (4.6) | 74.02 (8.3) | 12.5 (6.1) | 2.9 (1.4) | 5 (2.5) | |
| p-value | 0.07 | 0.59 | 0.84 | 0.78 | 0.38 | 0.38 | 0.46 | |
| Gender (M:F) | Age (Years) | Height (cm) | Weight (kg) | DD (Past 30) | DD/Week | Drinks/DD | ||
| GABRG1 Genotype | AA | 4:1 | 24.4 (1.7) | 175.3 (6.8) | 75.0 (17.2) | 16 (3.5) | 3.7 (0.8) | 4.5 (3.0) |
| AG | 3:4 | 24.4 (2.3) | 168 (3.7) | 74.7 (13.5) | 12.9 (6.2) | 3.0 (1.4) | 4.4 (2.9) | |
| GG | 2:6 | 23.5 (2.1) | 171.7 (5.0) | 74.8 (7.7) | 12.9 (6.6) | 3.0 (1.5) | 4.9 (1.9) | |
| p-value | 0.17 | 0.63 | 0.08 | 0.99 | 0.60 | 0.60 | 0.92 | |
To verify adaptive performance of the CAT task, the average accuracy rate and rate of change per 60 preceding trials, a window corresponding to one-half the average breakpoint for either reward, was determined for each session. Average CAT accuracy was not significantly different between LZ and placebo, with values of 45.9% and 45.6% (S.D. of 2.4 and 2.6), respectively (p=0.71). Regression analysis confirmed that the rates of change of CAT accuracy as a function of attempted trial by pre-treatment drug were negligible and not significantly different (−0.0037 and −0.0013 % per Trial for LZ and placebo; respectively; p=0.46).
Overall, alcohol was more rewarding than the AR under both pre-treatment conditions. Under placebo, the average surfeit of effort performed for alcohol, compared to the alternative, was 80 trials for breakpoint and 233 trials for cumulative work. Under LZ, the average additional effort for breakpoint was 65 trials; for cumulative work, 249 trials. A comparison of the cumulative work differences, connoting greater motivation for alcohol between pre-treatment conditions (233 versus 249), trended towards significance (p=0.08).
For GABRA2, repeated measures ANOVA revealed no significant results for cumulative work. Specifically, there were no repeated measures effects of: pre-treatment (Hotelling-Lawley Trace [HLT] F(1,17)=0.80, p=0.38), interaction of pre-treatment with genotype or reward chosen, interaction of reward genotype; 3-way interaction of pre-treatment×reward×genotype (all HLT p>0.18). Hotelling’s test revealed no overall repeated measures effects of genotype (p=0.67). Similar negative results were obtained for breakpoint with no repeated measures effect of pre-treatment (HLT F(1,17)=1.25, p=0.28). There was no overall effect of genotype (HLT F(4,14)=0.61, p=0.66).The same effects and interactions were tested among GABRG1 genotypes, resulting in a very weak trend for a pre-treatment drug effect on breakpoint (HLT F(1,16)=2.87, p=0.11) and a stronger trend for an interaction of pre-treatment drug×genotype. (HLT F(2,16)=3.30, p=0.06).
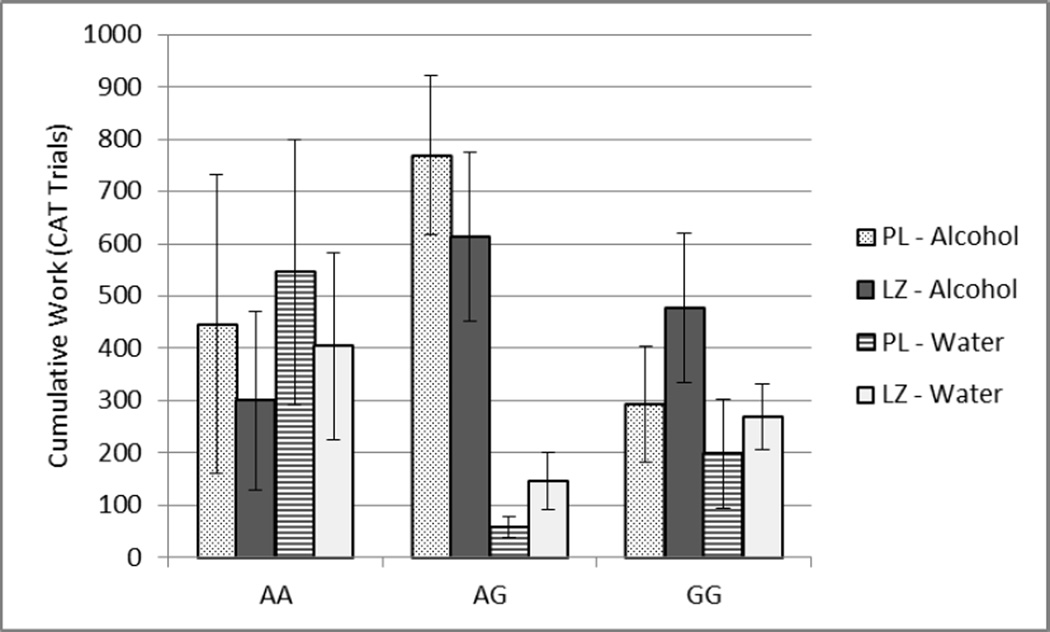
Repeated measures ANOVA for influences on cumulative work, using the same models as stated above, revealed no main effect of pre-treatment with LZ (HLT F(1,16)=2.47, p=0.14), nor genotype (HLT F(8,16)=1.54, p=0.22) but did reveal a significant interaction between pre-treatment drug and genotype (HLT F(2,16)=3.88; p=0.04). GABRG1 AG and GG individuals worked more for alcohol under both pre-treatments while AA individuals worked more for the AR, Figure 3. Compared to placebo, LZ pre-treatment was associated with a decrease in AA and AG work for alcohol versus an increase in work for both rewards in those with the GG genotype. All other interactions were non-significant (all HLT p>0.17).
Figure 3.
Cumulative Work +/− SEM for alcohol and water by pre-treatment condition and GABRG1 genotype, with PL and LZ indicating placebo and lorazepam pretreatment respectively.
Discussion
This pilot study demonstrates the potential utility of the CASE PR paradigm for human operant alcohol self-administration studies and for detecting the effects of pharmaceuticals in laboratory studies. This experiment examined the effect of pre-treatment with a GABAergic agent (LZ) in a sample population comprised of individuals with known GABA receptor genotype. As proof of principle, a benzodiazepine was selected as the “treatment.” Since both alcohol and LZ affect the GABA neurotransmitter system, the design attempted to detect the production of a likely pharmacogenetic interaction signal. Important experimental design considerations included the nature of the work performed, the AR employed, and the use of a priming dose of ethanol.
The CASE methodology worked well from an operational perspective; all subjects expressed understanding of the task, no subject dropped out for any reason, the CAT performed well, and the database will be useful for the development of properly-powered future studies. Face validity of the PR paradigm is supported by the outcome that, in both treatment conditions, subjects found that alcohol was more motivating than the AR. Examination of the interaction between pre-treatment with a GABAergic drug and GABA receptor genotype showed that GABRA2 status did not yield significant influences in this small sample, but that GABRG1 status did. The pharmacogenetic interaction on cumulative work is a new finding to our knowledge, and attempts to replicate and expand this finding appear to be warranted.
Although the small size of this pilot study yielded overall estimated effect sizes of 0.15–0.22, and thus precludes any definitive conclusions, individual subgroup trends proved of interest. Comparing AA and GG GABRG1 subgroups, AA individuals were relatively less motivated for alcohol, and GABAergic pre-treatment appeared to decrease their motivation. GG subjects appeared less motivated to work for either reward, but motivation for alcohol was increased by LZ pre-treatment. AG individuals were dramatically more motivated for alcohol than either AA or GG, and were relatively insensitive to GABAergic modulation. These results would appear to support further investigation in properly powered sample populations.
While alcohol is - by definition - a rewarding substance in the recruited population, the lab conditions (solitary performance, hospital setting, rewards lacking odor or taste, unusual time of day for alcohol exposure, etc.) all served to reduce the rewarding properties of alcohol, making self-administration more sensitive to the availability of alternate activities.
The nature of the work (defined as an activity one would not choose to perform except for its potential to earn a reward or avoid a punishment) also posed a dilemma. In other projects, we have found that subjects with nothing else to do will persist in performing a task such as button-pushing, for little or no reward other than to relieve boredom. On the other hand, we were concerned that the opportunity to engage in other activities might undermine subject’s attention to the experimental task. Thus, we chose to forbid any competing activities, such as reading, watching TV, interaction with the technicians, or use of all personal devices during the session. Development of the CAT succeeded as a self-adjusting work task unaffected by LZ pre-treatment, alcohol intoxication, or fatigue, but of sufficient difficulty to induce the cessation of work despite the lack of environmental stimuli.
Selection of any AR remains an important consideration in the design of a PR paradigm. Since CASE dissociates the odor, texture and taste associated with oral alcohol administration, an appetitive control was not required. As humans regularly include “alternative” beverages during periods of alcohol consumption, IV delivery of half-normal saline was chosen as our AR. Besides water, we had considered money or small consumables (e.g. peanuts, candy or crackers). The use of saline in this pilot study allowed for consistency of the delivered reward properties: both were IV and could neither be saved nor converted to another form at a later time period.
A more common AR in human experiments is money. Compared to an alternative beverage, money is an equally or more naturalistic alternative, since people work for money which is then traded for access to alcohol. Money was not utilized as an AR, despite its potential superiority, because it is subject to delay discounting, a complexity beyond the scope of the resources available for this pilot study.
Other than the pre-treatment drug, experimental conditions were maintained across sessions, including a mandated alcohol priming interval before voluntary self-administration began. Thus, this pilot study cannot address the potential effects of an alcohol prime superimposed upon LZ pre-treatment. In social drinkers, alcohol priming has largely, but inconsistently, been shown to increase consumption behavior (de Wit and Chutuape, 1993; Duka et al., 1998; Kirk and de Wit, 2000), and to increase subjective craving (Chutuape et al., 1994; Kirk and de Wit, 2000; Rose and Duka, 2006; Schoenmakers and Wiers, 2010). Moreover, the response to alcohol priming is potentially modulated by some pharmacologic interventions, at least in treatment-seeking alcohol-dependent individuals (Hammarberg et al., 2009). Finally, as expected given their related pharmacology (Harris et al., 1998), regular use of benzodiazepines are hypothesized to increase subsequent alcohol usage (Malcolm, 2003), perhaps due to cross-tolerance.
Our results should be interpreted relative to several beneficial and limiting methodological considerations. While effective, this PR paradigm is artificial in nature and necessarily performed in a laboratory setting. During debriefing, some subjects speculated that they might make different choices in a social setting, but we believe the within-subjects design mitigated this concern. Their statements also raise the possibility of confounding through demand characteristics. i.e. trying to guess what response the experimenter wanted. Although not documented, it is also possible that some subjects may have inhibited repeated selection of alcohol over saline in a laboratory experiment, particularly if any concern about personal drinking style motivated participation. Fortunately, if this effect was present, it would have likely reduced the magnitude of the signals of interest and made their detection more difficult. In an extreme case, such an effect could invert the relationship between alcohol and saline, but no inversion was observed in this sample population. Further, while, an expectancy effect of the result of pretreatment may have been produced, we believe a double-blind approach minimized its impact. Further, each subject was provided an identical reward in terms of brain exposure to alcohol, dissociated from other conditioned cues associated with the consumption of alcohol. However, alcohol is not consumed IV in the community and the incremental BrAC reward did not follow a “standard” alcohol oral dose trajectory, peaking more quickly than an ingested quantlet of alcohol would achieve. The experimental design, including recruitment by GABRA2 homozygosity and pre-treatment with a GABAergic drug, maximized the potential for surveying useful applications of the PR paradigm. In this sample, however, the lack of GABRA2 effect could be secondary to gender effects, although there is no a priori reason that gender should influence motivation for alcohol under the effect of GABAergic manipulation.
Until now, studies using CASE have employed the FA paradigm. The CASE PR paradigm demonstrates potential for application to pharmaceutical development when the motivation to seek alcohol is the target of intervention. CASE also incorporates technology to permit integration with imaging devices for eventual identification of the areas of the brain associated with work and observed preferences, but these were not employed in this pilot study.
Acknowledgements
We appreciate the efforts of Jim Hays, Kurt White and Tet Lu who provided the recruiting and data-collection expertise required for the execution of this pilot study, and of Bonnie Klank and the Indiana University Hospital Investigational Drug Services staff in the maintenance of drug blinding. The authors also wish to express gratitude to Dr. Conrad Wong for his guidance and intellectual curiosity. This pilot study was funded by the Indiana Alcohol Research Center grant, P60 AA07611-23/4 and was performed using the resources of the Indiana CTSI, grant UL RR025761. Research time for the first author was made available by the Indiana University Department of Psychiatry Section of Child and Adolescent Psychiatry and the Psychopharmacology Fellowship jointly sponsored by Indiana University and Eli Lilly and Company.
References
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Pihl RO, Benkelfat C, Brunelle C, Young SN, Leyton M. The role of dopamine in alcohol self-administration in humans: individual differences. Eur Neuropsychopharmacol. 2008;18:439–447. doi: 10.1016/j.euroneuro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug and Alcohol Dependence. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, Fisher S, Fox L, Howells W, Bertelsen S, Hinrichs AL, Almasy L, Breslau N, Culverhouse RC, Dick DM, Edenberg HJ, Foroud T, Grucza RA, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Krueger RF, Kuperman S, Lynskey M, Mann K, Neuman RJ, Nothen MM, Nurnberger JI, Jr, Porjesz B, Ridinger M, Saccone NL, Saccone SF, Schuckit MA, Tischfield JA, Wang JC, Rietschel M, Goate AM, Rice JP. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutuape MaD, Mitchell SH, De Wit H. Ethanol Preloads Increase Ethanol Preference Under Concurrent Random-Ration Schedules in Social Drinkers. Experimental and Clinical Psychology. 1994;2:310–318. [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Jensen K, Anton R, Kranzler HR. Markers in the 5'-region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology. 2008;33:837–848. doi: 10.1038/sj.npp.1301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res. 1999;23:195–203. [PubMed] [Google Scholar]
- De Wit H, Chutuape MA. Increased ethanol choice in social drinkers following ethanol preload. Behav Pharmacol. 1993;4:29–36. [PubMed] [Google Scholar]
- De Wit H, Mccracken SG. Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcohol Clin Exp Res. 1990;14:63–70. doi: 10.1111/j.1530-0277.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Drgon T, D'addario C, Uhl GR. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:854–860. doi: 10.1002/ajmg.b.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Tasker R, Stephens DN. Alcohol choice and outcome expectancies in social drinkers. Behav Pharmacol. 1998;9:643–653. doi: 10.1097/00008877-199811000-00019. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O'connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Hodgkinson CA, Yuan Q, Albaugh B, Virkkunen M, Goldman D. GABRG1 and GABRA2 as independent predictors for alcoholism in two populations. Neuropsychopharmacology. 2009;34:1245–1254. doi: 10.1038/npp.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- George DT, Herion DW, Jones CL, Phillips MJ, Hersh J, Hill D, Heilig M, Ramchandani VA, Geyer C, Spero DE, Singley ED, O'malley SS, Bishai R, Rawlings RR, Kunos G. Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in nontreatment-seeking heavy alcohol drinkers. Psychopharmacology (Berl) 2010;208:37–44. doi: 10.1007/s00213-009-1704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg A, Jayaram-Lindstrom N, Beck O, Franck J, Reid MS. The effects of acamprosate on alcohol-cue reactivity and alcohol priming in dependent patients: a randomized controlled trial. Psychopharmacology (Berl) 2009;205:53–62. doi: 10.1007/s00213-009-1515-6. [DOI] [PubMed] [Google Scholar]
- Harris RA, Mihic SJ, Valenzuela CF. Alcohol and benzodiazepines: recent mechanistic studies. Drug Alcohol Depend. 1998;51:155–164. doi: 10.1016/s0376-8716(98)00073-8. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Ray LA, Finan P, Villanueva R, Niculescu M, Hutchison KE. Human gamma-aminobutyric acid A receptor alpha2 gene moderates the acute effects of alcohol and brain mRNA expression. Genes Brain Behav. 2008;7:447–454. doi: 10.1111/j.1601-183X.2007.00369.x. [DOI] [PubMed] [Google Scholar]
- Ittiwut C, Listman J, Mutirangura A, Malison R, Covault J, Kranzler HR, Sughondhabirom A, Thavichachart N, Gelernter J. Interpopulation linkage disequilibrium patterns of GABRA2 and GABRG1 genes at the GABA cluster locus on human chromosome 4. Genomics. 2008;91:61–69. doi: 10.1016/j.ygeno.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Liang T, Wetherill L, Dzemidzic M, Bragulat V, Cox C, Talavage T, O'connor SJ, Foroud T. A polymorphism in GABRA2 is associated with the medial frontal response to alcohol cues in an fMRI study. Alcohol Clin Exp Res. 2010;34:2169–2178. doi: 10.1111/j.1530-0277.2010.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JM, De Wit H. Individual differences in the priming effect of ethanol in social drinkers. J Stud Alcohol. 2000;61:64–71. doi: 10.15288/jsa.2000.61.64. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, Gelernter J. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Lydall GJ, Saini J, Ruparelia K, Montagnese S, Mcquillin A, Guerrini I, Rao H, Reynolds G, Ball D, Smith I, Thomson AD, Morgan MY, Gurling HM. Genetic association study of GABRA2 single nucleotide polymorphisms and electroencephalography in alcohol dependence. Neurosci Lett. 2011;500:162–166. doi: 10.1016/j.neulet.2011.05.240. [DOI] [PubMed] [Google Scholar]
- Malcolm RJ. GABA systems, benzodiazepines, and substance dependence. J Clin Psychiatry. 2003;64(Suppl 3):36–40. [PubMed] [Google Scholar]
- Matthews AG, Hoffman EK, Zezza N, Stiffler S, Hill SY. The role of the GABRA2 polymorphism in multiplex alcohol dependence families with minimal comorbidity: within-family association and linkage analyses. J Stud Alcohol Drugs. 2007;68:625–633. doi: 10.15288/jsad.2007.68.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee SA, Harrison EL, O'malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee SA, O'malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology (Berl) 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Onori N, Turchi C, Solito G, Gesuita R, Buscemi L, Tagliabracci A. GABRA2 and alcohol use disorders: no evidence of an association in an Italian case-control study. Alcohol Clin Exp Res. 2010;34:659–668. doi: 10.1111/j.1530-0277.2009.01135.x. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Buonopane A, O'malley S, Cermik O, Trevisan L, Boutros NN, Limoncelli D, Krystal JH. The effect of tryptophan depletion on alcohol self-administration in non-treatment-seeking alcoholic individuals. Alcohol Clin Exp Res. 2002;26:969–975. doi: 10.1097/01.ALC.0000021338.38350.95. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Decarlo R, Ramchandani VA, O'connor S. Improved Transformation of Morphometric Measurements for a Priori Parameter Estimation in a Physiologically-Based Pharmacokinetic Model of Ethanol. Biomed Signal Process Control. 2007;2:97–110. doi: 10.1016/j.bspc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Han JJ, Doerschuk PC, Ramchandani VA, O'connor SJ. Physiologically based pharmacokinetic (PBPK) models for ethanol. IEEE Trans Biomed Eng. 2008;55:2691–2700. doi: 10.1109/TBME.2008.919132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li T-K, O'connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcoholism, clinical and experimental research. 2009;33:938–944. doi: 10.1111/j.1530-0277.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Associations among GABRG1, level of response to alcohol, and drinking behaviors. Alcohol Clin Exp Res. 2009;33:1382–1390. doi: 10.1111/j.1530-0277.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh S, Matsushita S, Hara S, Maesato H, Matsui T, Suzuki G, Miyakawa T, Ramchandani VA, Li TK, Higuchi S. Role of GABRA2 in Moderating Subjective Responses to Alcohol. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Duka T. Effects of dose and time on the ability of alcohol to prime social drinkers. Behav Pharmacol. 2006;17:61–70. doi: 10.1097/01.fbp.0000189814.61802.92. [DOI] [PubMed] [Google Scholar]
- Sarid-Segal O, Knapp CM, Burch W, Richardson MA, Bahtia S, Dequattro K, Afshar M, Richambault C, Sickels L, Devine E, Ciraulo DA. The anticonvulsant zonisamide reduces ethanol self-administration by risky drinkers. Am J Drug Alcohol Abuse. 2009;35:316–319. doi: 10.1080/00952990903060150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers TM, Wiers RW. Craving and attentional bias respond differently to alcohol priming: a field study in the pub. Eur Addict Res. 2010;16:9–16. doi: 10.1159/000253859. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SM, Gianoulakis C, Palmour RM, Benkelfat C, Leyton M. The effect of naltrexone on alcohol's stimulant properties and self-administration behavior in social drinkers: influence of gender and genotype. Alcohol Clin Exp Res. 2011;35:1134–1141. doi: 10.1111/j.1530-0277.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. In: Timeline Follow-back: A technique for assessing self-reported ethanol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods. Allen J, Litten RZ, editors. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, O'connor S. Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self-infusion of ethanol (CASE) Psychopharmacology (Berl) 2009;202:689–697. doi: 10.1007/s00213-008-1349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O'connor S. Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): a new method to study alcohol self-administration in humans. Alcohol Clin Exp Res. 2008;32:1321–1328. doi: 10.1111/j.1530-0277.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O'connor S, Ramchandani VA. Modeling Alcohol Self-Administration in the Human Laboratory. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_149. Epub ahead of print. [DOI] [PubMed] [Google Scholar]