Summary
The vacuolar proton pyrophosphatase (H+-PPase) of Toxoplasma gondii (TgVP1), a membrane proton pump, localizes to acidocalcisomes and a novel lysosome-like compartment termed plant-like vacuole (PLV) or vacuolar compartment (VAC). We report the characterization of a T. gondii null mutant for the TgVP1 gene. Propagation of these mutants decreased significantly because of deficient attachment and invasion of host cells, which correlated with deficient microneme secretion. Processing of cathepsin L (CPL) in these mutants was deficient only when the parasites were incubated in the presence of low concentrations of the vacuolar H+-ATPase (V-H+-ATPase) inhibitor bafilomycin A1, suggesting that either TgVP1 or the T. gondii V-H+-ATPase (TgVATPase) are sufficient to support CPL processing. The lack of TgVP1 did not affect processing of micronemal proteins, indicating that it does not contribute to proMIC maturations. The TgVP1 null mutants were more sensitive to extracellular conditions and were less virulent in mice. We demonstrate that T. gondii tachyzoites possess regulatory volume decrease capability during hypo-osmotic stress and this ability is impaired in TgVP1 null mutants implicating TgVP1 in osmoregulation. We hypothesize that osmoregulation is needed for host cell invasion and that TgVP1 plays a role during the normal lytic cycle of T. gondii.
Introduction
Toxoplasma gondii is a protist parasite that has emerged as a major opportunistic pathogen in people with deficient immune systems. Infection with T. gondii is usually asymptomatic and results in the formation of dormant bradyzoites that infect the brain and other tissues for life. The developing fetus and the immunosuppressed patients are at substantial risk of severe disease. The tachyzoite is the rapidly growing asexual form that is seen in acutely infected animals. Tachyzoites enter host cells by a process of active invasion, they replicate inside the cell and, after a certain number of replications exit to infect neighboring cells. While searching for a host cell to invade, the parasite is exposed to an environment with an ionic composition quite different from the host intracellular composition. This sharp change from intracellular to extracellular milieu must pose a challenge to the fitness of the parasite, which needs to glide, extrude its conoid and secrete proteins from apical secretory organelles (micronemes and rhoptries) during invasion to form the moving junction and the parasitophorous vacuole for its next intracellular life.
Membrane pyrophosphatases couple pyrophosphate (PPi) hydrolysis to the active transport of sodium or protons against an electrochemical gradient. Proton pyrophosphatases (H+-PPases) are present in prokaryotes (Baltscheffsky et al., 1966, Serrano et al., 2007) and some eukaryotes including plants (Drozdowicz & Rea, 2001, Maeshima, 2000) and protists (Scott et al., 1998, Rodrigues et al., 2000, Marchesini et al., 2000, Drozdowicz et al., 2003, McIntosh et al., 2001), but are absent in mammalian cells.
H+-PPases are also called vacuolar PPases because they are preferentially localized in vacuolar compartments, like the plant vacuole (Maeshima, 2000), and acidocalcisomes of bacteria (Seufferheld et al., 2003, Seufferheld et al., 2004) and protists (Docampo & Moreno, 2011). In contrast to the multisubunit vacuolar H+-ATPases (V-H+-ATPases), H+-PPases form homodimers (Maeshima, 2000) and are divided in K+-dependent and K+-independent subfamilies (Belogurov & Lahti, 2002). A single amino acid near the cytoplasm-membrane interface, which is an Ala in the K+-dependent and Lys in the K+-independent enzymes (Belogurov & Lahti, 2002), is responsible for this dependence. All membrane H+-PPases require Mg2+ for activity (Baykov et al., 1993) and the three dimensional structure of a H+-PPase (Lin et al., 2012) has recently been reported. In prokaryotes, H+-PPases have roles in generating an ion-motive force for ATP synthesis and transport of metabolites (Baltscheffsky et al., 1999, Lopez-Marques et al., 2004, Garcia-Contreras et al., 2004), while in eukaryotes they acidify intracellular organelles (Maeshima, 2000, Docampo & Moreno, 2011). Overexpression of H+-PPase in plants confers resistance to water/nutrient deprivation, cold, and salinity (Park et al., 2005, Lv et al., 2008, Zhang et al., 2011), while loss of H+-PPase leads to root and shoot growth defects (Li et al., 2005), which are related to its role in auxin transport and organ development. Downregulation of expression of the H+-PPase of Trypanosoma brucei affects acidification of acidocalcisomes and growth of procyclic and bloodstream stages in vitro (Lemercier et al., 2002).
We recently discovered a novel multi-vesicular post-Golgi organelle in T. gondii tachyzoites termed the plant-like vacuole (PLV, the abbreviation used hereafter) (Miranda et al., 2010) or vacuolar compartment (VAC) (Parussini et al., 2010). This organelle can be labeled with antibodies against proteins with similarity to vacuolar plant pumps and channels, such as a K+-sensitive H+-PPase (TgVP1), and a tonoplast-like aquaporin (TgAQP1). Our results showed that the PLV becomes mostly evident during the extracellular stage of the parasite. Our hypothesis is that the TgVP1 present in the PLV is important for parasite survival under extracellular stress and preparation for invasion of host cells. We report the phenotypic analysis of TgVP1 null mutants with the aim of understanding the role of this prominent organelle in extracellular T. gondii tachyzoites.
Results
Targeting of TgVP1 and Complementation
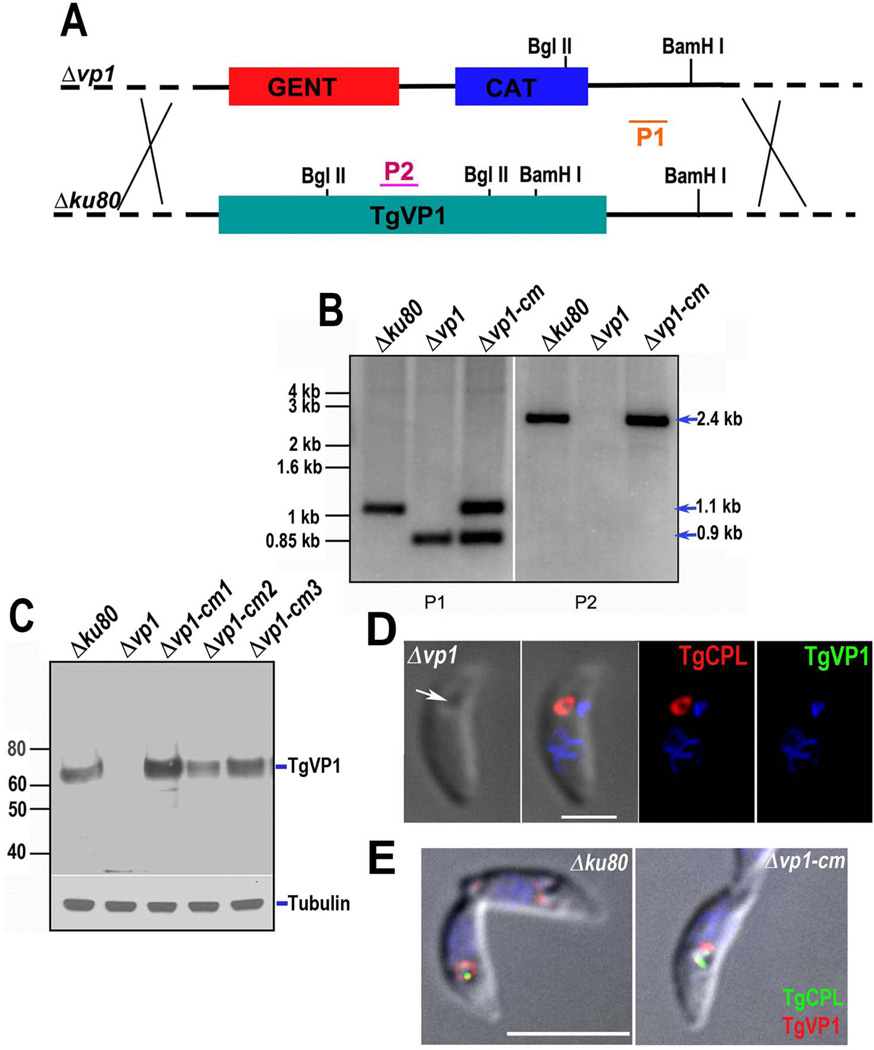
To define the role of TgVP1 in the life cycle of T. gondii, we generated knockout mutants (Δvp1) by targeting the native TgVP1 locus in the Δku80 strain, which favors homologous recombination (Fox et al., 2009, Huynh & Carruthers, 2009). We used a cosmid-based recombineering approach (Brooks et al., 2010), as described under Experimental Procedures. Successful gene deletion (Fig. 1A) was confirmed by Southern blot analysis (Fig. 1B, Δvp1). Western blot analysis using affinity-purified antibody against TgVP1, and antibody against tubulin as loading control, showed lack of expression of the TgVP1 gene (Fig. 1C, Δvp1). The absence of expression of TgVP1 was also verified by immunofluorescence analysis (IFA) of Δvp1 cells with the same anti-TgVP1 antibody (Fig. 1D). IFA with an affinity-purified antibody against T. gondii cathepsin L (CPL) shows labeling of the PLV (Fig. 1D, white arrow) in Δvp1 (Fig. 1D, red), and Δku80 (Fig 1E, green) parasites. Restoration of gene expression was accomplished by transforming Δvp1 with a plasmid that contains the entire coding sequence of the TgVP1 gene. Re-expression of TgVP1 in the complemented clones (Δvp1-cm1, Δvp1-cm2 and Δvp1-cm3) appeared to be at appropriate levels, especially with Δvp1-cm1 (Fig. 1C), which was used for further experiments and termed Δvp1-cm hereafter. IFA staining of tachyzoites confirmed re-expression of TgVP1 in the PLV of clone Δvp1-cm (Fig. 1E, red).
Fig. 1.
Targeted disruption and complementation of the TgVP1 gene. (A) Schematic representation of homologous recombination event in the T. gondii genome for cosmid-based gene disruption. The modified cosmid was transfected into T. gondii tachyzoites (Δku80) following standard procedures and one clone (Δvp1) was selected for further analysis. P1 and P2 indicate probes used for the Southern blot analysis in B. (B) Southern blot analysis of genomic DNA isolated from tachyzoites of Δku80, Δvp1 and Δvp1-cm clones and digested with BamHI and BglII. Probe P1 hybridizes to the 3’ non-coding region present in both loci. P2 hybridizes to a TgVP1 intron only present in the Δku80 and Δvp1-cm clones. (C) Western blot analysis of total tachyzoite lysates using a Guinea pig anti-TgVP1 antibody (1:500). Mouse anti-α-tubulin (1:1,000) was used as loading control. (D) IFA analysis of the Δvp1 clone using mouse anti-CPL (1:1,000) and Guinea pig anti-TgVP1 (1:100). Secondary antibodies were goat-anti-guinea pig (1:1,000) and goat-anti-mouse (1:1,000). The white arrow shows the PLV localization. The PLV is labeled with anti-CPL (red) but shows no signal when using anti-TgVP1 (green) antibody. Scale bar = 2 µm. (E) IFA analysis of the Δku80 and Δvp1-cm strains with mouse anti-CPL (1:200) and rabbit anti-TgVP1 (1:4,000). The PLV is labeled with anti-CPL (green) and anti-TgVP1 (red). Scale bar: 5 µM
TgVP1 is Required for Efficient Attachment and Invasion of Tachyzoites
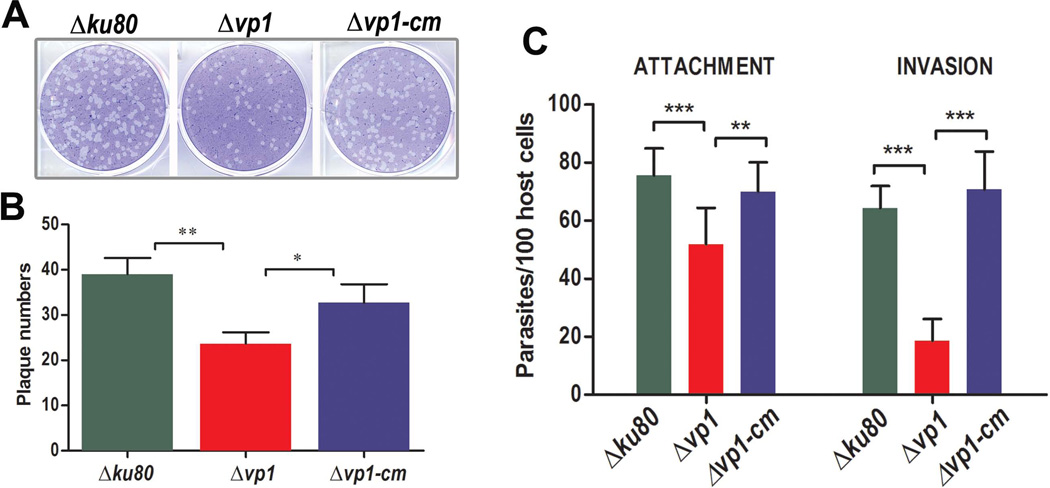
We compared growth of the parental (Δku80), mutant (Δvp1), and complemented (Δvp1-cm) clones in parallel plaque assays (Fig. 2A). Plaque sizes appear similar between the three strains although a lower number of plaques were consistently observed with the Δvp1 mutants. For a more accurate readout we switched to a plaquing efficiency protocol as described (Francia et al., 2011), in which there is only a 30-min contact interval between parasite and host. Under these conditions, clear differences were observed in the number of plaques formed between Δvp1 mutants and the parental cell line (Fig. 2B). The complementation of the gene in Δvp1-cm restored their normal capacity to form plaques (Fig. 2B). These results suggested that once they invade mutant parasites are able to replicate normally and that the reduction in number of plaques could be due to reduced invasion. To test the ability of these clones to invade host cells, we used a red/green invasion assay where external, attached parasites were stained red while internal, penetrated parasites were stained green (Kafsack et al., 2004). Ablation of TgVP1 caused a 33 ± 4.2% reduction in attachment and a 70 ± 7.4% reduction in invasion, while the complemented clone had values similar to the parent clone (Fig. 2C, n = 3). Interestingly no significant difference in gliding motility based on trail deposition was found between Δvp1 parasites and the parental strain Δku80 and Δvp1-cm (Fig. S1).
Fig. 2.
TgVP1 is required for attachment, and invasion of tachyzoites. (A) Plaque assays comparing growth of tachyzoites of the mutant (Δvp1), parental (Δku80) and complemented (Δvp1-cm) clones. Each well was infected with 200 parasites and plaques were stained 9 days later. (B) Plaquing efficiency protocol described in more detail in Experimental Procedures. Data compiled from three independent plaquing efficiency experiments. (C) Red-green assay for quantification of attachment and invasion of T. gondii to hTERT cells. Data were compiled from three independent experiments, each one by triplicate, counting 8 fields/clone. Fields were randomly selected following the same pattern for all samples. Error bars in (B) and (C) are SD. Data were analyzed by GraphPad Prism 5. To compare two groups of data we used One-Way ANOVA test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Δvp1 Tachyzoites are Defective in Microneme Secretion
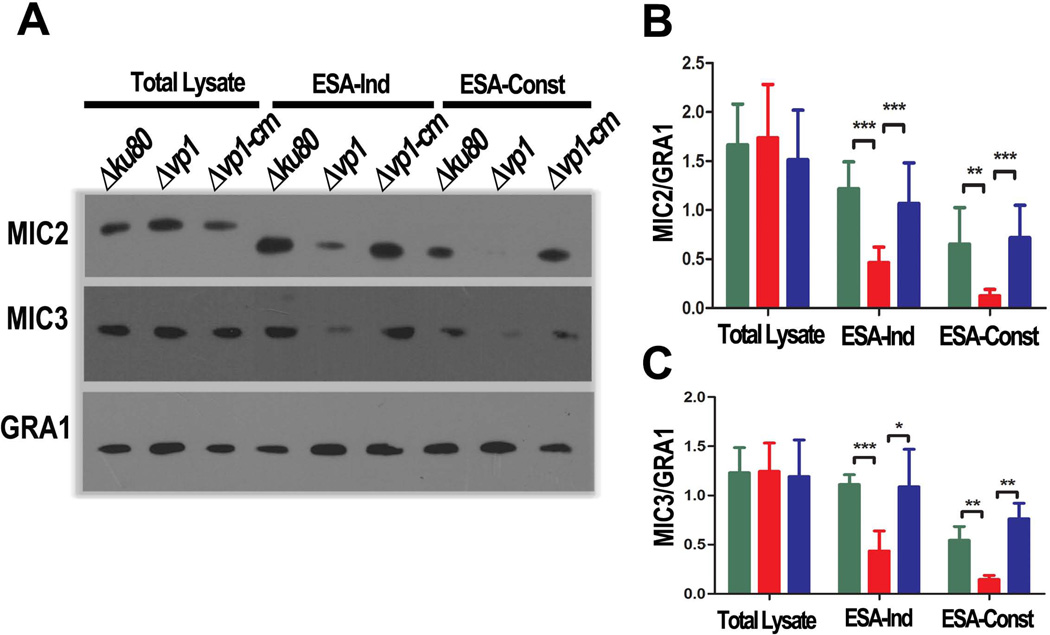
Parasite attachment and invasion is associated with the release of adhesins from micronemes (Carruthers et al., 1999). Hence, we examined the role of TgVP1 in release of the micronemal proteins MIC2 and MIC3 (Fig. 3). We examined the release of these proteins into the excreted/secreted antigen (ESA) fraction collected from supernatants of extracellular parasites without stimulation (ESA-Const.) or after induction of secretion by ethanol (ESA-Ind.) Δvp1 parasites were not able to secrete micronemes constitutively (ESA-Const.) and they responded poorly to stimulation by ethanol (ESA-Ind.) (Fig. 3A). However, the amount of total microneme proteins in the Δvp1 parasite lysate was similar to the parental and complemented strains. Figs. 3B and C show the quantification of three independent experiments using the constitutively secreted GRA1 protein as control.
Fig. 3.
Δvp1 parasites are defective in microneme secretion. (A) Tachyzoites were incubated at 37°C for 30 min with 1 % ethanol (ESA-ind) or in the presence of IM alone (ESA-Const). Aliquots of the supernatants were analyzed by western blot with an anti-MIC2 antibody (upper panel) or anti-MIC3 antibody (middle panel). The membranes were stripped and reprobed with antibodies against GRA1 for secretion control (lower panel). (B) Quantification of MIC2 secretion by Image J (ratio of MIC2/GRA1). Data were compiled from three independent experiments. Error bars are SD. (C) Quantification of MIC3 secretion. Experiments were standardized to secretion control (GRA1) and quantified using Image J (NIH). Data are pooled from three independent experiments. To compare two groups of data we used Two-Way ANOVA test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Δvp1 Tachyzoites Have a Maturation Defect for CPL but not Microneme Proteins in the Presence of Bafilomycin A1
We reported previously that the parasite cathepsin L (CPL) occupies a compartment that also labels with TgVP1 in extracellular parasites, and that this protease mediates the proteolytic maturation of two proproteins (proM2AP and proMIC3) targeted to micronemes (Parussini et al., 2010). Since this proteolytic maturation is pH-dependent (requires acidic conditions), we next asked whether Δvp1 tachyzoites would show differences in maturation of proM2AP, proMIC3, and proMIC5 as compared with those of the parental and complemented clones. Fig. S2 shows that there were no significant differences in the maturation of these proteins under normal conditions.
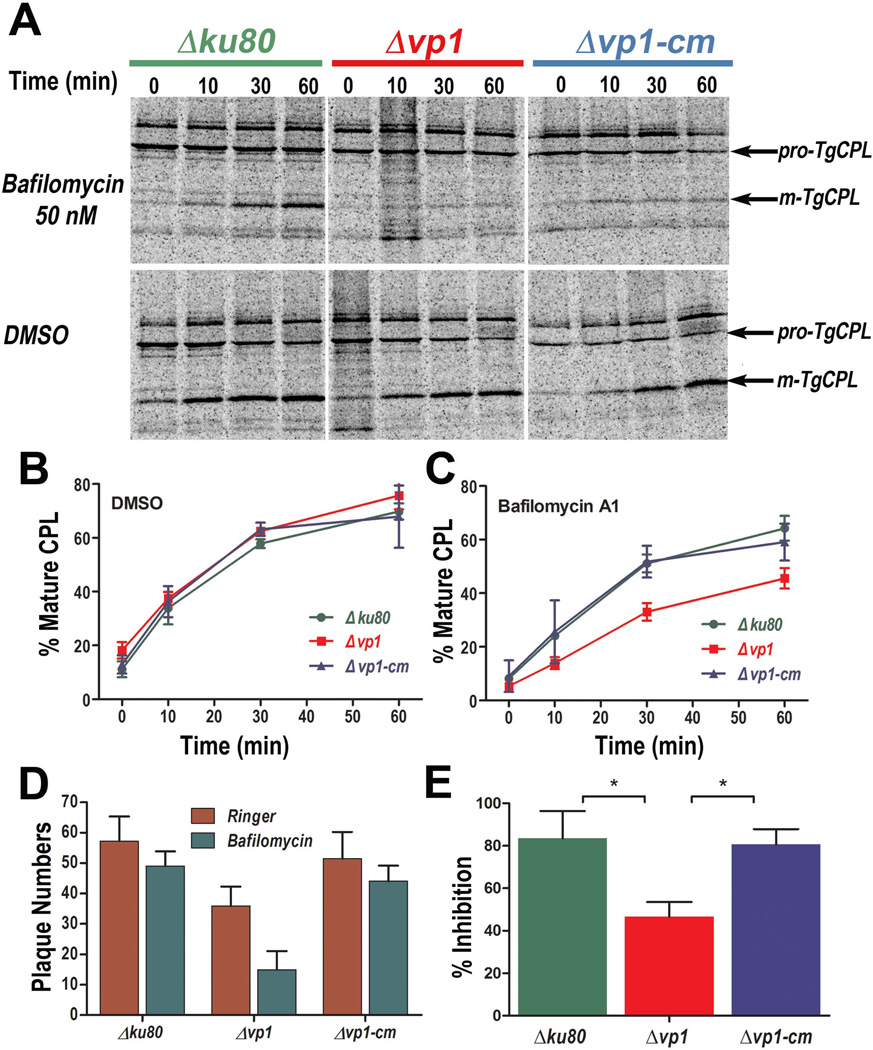
The PLV has two proton pumps, a multisubunit vacuolar H+-ATPase (TgVATPase), and TgVP1 (Miranda et al., 2010). We reasoned that these two proton pumps could have overlapping functions. To test this, we monitored the maturation of M2AP in the absence or presence of bafilomycin A1, which is a specific inhibitor of the V-H+-ATPase when used at very low concentrations (Bowman et al., 1988). Figs. S3A and S3B show that bafilomycin A1 had a concentration-dependent inhibitory effect on maturation of proM2AP that was independent of TgVP1 expression. This suggests that TgVATPase, but not TgVP1, contributes to the acidification of the compartment(s) where proM2AP undergoes maturation. We next investigated the maturation of proCPL (Fig. 4). In the absence of bafilomycin A1, proCPL maturation was normal regardless of TgVP1 status (DMSO, Figs. 4A and 4B) indicating that TgVP1 expression is not required for proCPL maturation when TgVATPase is functional. However, bafilomycin A1 inhibition of TgVATPase activity caused a delay in proCPL maturation exclusively in Δvp1 tachyzoites (Figs. 4C). These findings imply that the proton pumping activities of either TgVATPase or TgVP1 is sufficient for maturation of CPL, indicating a redundancy of function. We also wanted to investigate if the compensatory effect of the TgVATPase would be evident in the lytic cycle of the parasite and for this we used a plaquing efficiency protocol to test whether pre-incubation with bafilomycin A1 affected tachyzoites of the Δvp1 clone to a greater extent. Whereas the low concentration of bafilomycin A1 barely affected plaque formation by the parental or complemented strain, Δvp1 tachyzoites were substantially more sensitive to bafilomycin (Figs. 4D and 4E). Collectively, these findings indicate independent and overlapping functions for TgVATPase and TgVP1.
Fig. 4.
Δvp1 tachyzoites have a TgCPL maturation defect in the presence of bafilomycin A1. (A) Tachyzoites were pre-incubated for 15 min with 50 nM bafilomycin A1 or DMSO as control. Metabolically pulse-labeled tachyzoites from Δku80, Δvp1 and Δvp1-cm clones were either kept on ice (0 min) or chased with medium containing unlabelled Met/Cys for the indicated times (10, 30 or 60 min). CPL proteins were immunoprecipitated and analyzed by SDS-PAGE and autoradiography. Arrows indicate positions of the immature (pro-TgCPL) and mature (m-TgCPL) forms of CPL. Tachyzoites of the Δvp1 clone show impaired maturation of pro-TgCPL after blocking TgVATPase with bafilomycin A1. (B, C) Quantification of TgCPL maturation in tachyzoites from Δku80, Δvp1 and Δvp1-cm clones for each chase time point in the absence (B, DMSO) or presence (C) of 50 nM bafilomycin A1. Values are mean ± SD. Data shown are the combined results of three independent experiments. (D) Plaque numbers after pre-incubation of tachyzoites from Δku80, Δvp1 and Δvp1-cm clones in Ringer buffer contain 50 nM bafilomycin A1. The effect of bafilomycin A1 was more severe in tachyzoites from the Δvp1 clone. (E) Percentage of plaques as compared with controls without bafilomycin A1 considered as 100%, after incubation of tachyzoites of the different clones in Ringer buffer. Tachyzoites from all clones produced ~20% less plaques after incubation with bafilomycin A1 but tachyzoites from the Δvp1 clone produced ~55% less plaques than tachyzoites from Δku80 and Δvp1-cm clones. Data are the combined results of three independent experiments. Data were analyzed by GraphPad Prism 5. To compare two groups of data we used One-Way ANOVA test. *, p <0.05; **, p <0.01; ***, p <0.001.
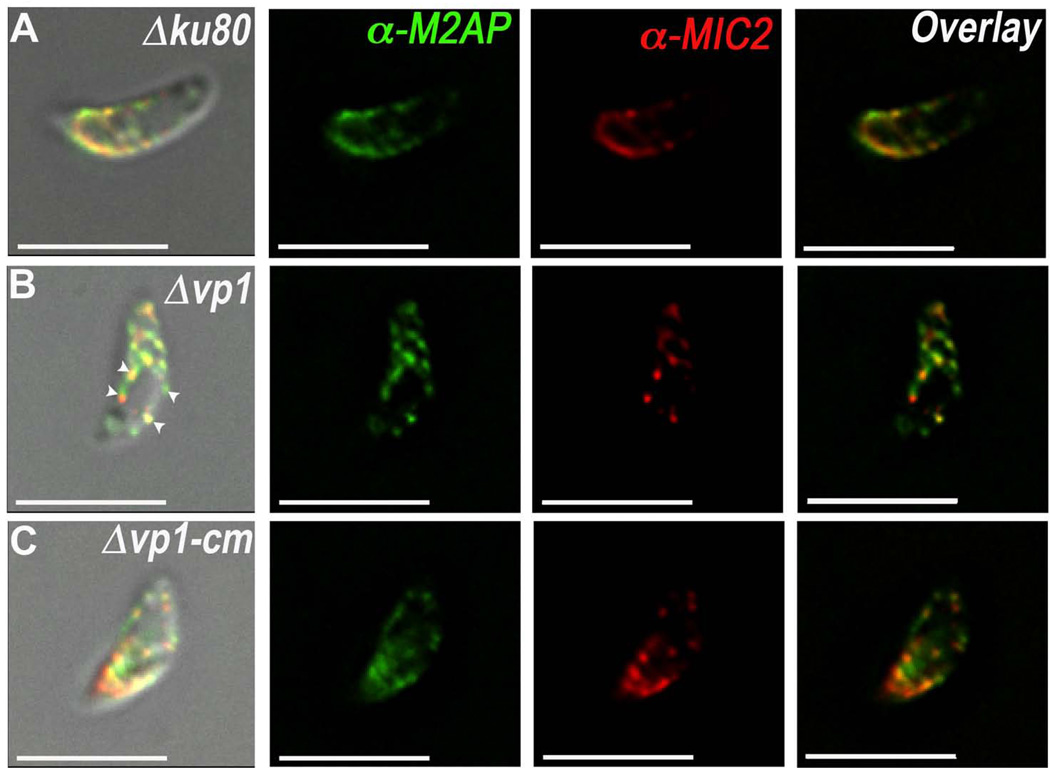
Δvp1 Tachyzoites show a different pattern of microneme organization
Considering that the Δvp1 cells have a microneme secretion defect but their maturation appeared to be normal, we considered the possibility of a defect in the trafficking or biogenesis of micronemes. Fig. 5 shows an IFA analysis with anti-MIC2 and anti-M2AP of Δvp1 mutants compared with parental (Δku80) and complemented (Δvp1-cm) strains. We observed that MIC2 and M2AP localize to vesicles and they appear to concentrate around a large vacuole (Fig. 5B, arrowheads). The difference was quite evident when comparing with the pronounced apical localization of micronemes in the Δku80 and Δvp1-cm strains. Approximately 27% of the Δvp1 parasites showed labeling of MIC2 around a large vacuole. This was markedly different to Δku80, which showed this pattern in only 7% of the cells.
Fig. 5.
Extracellular Δvp1 show an abnormal distribution of MIC2 and M2AP labeling. (A) IFA analysis of Δku80 parasites with microneme markers MIC2 (1:500) and M2AP (1:500). Normal apical distribution for both proteins is observed. 318 parasites were counted from three difference experiments. (B) IFA analysis of Δvp1 parasites showing a large vacuole surrounded by MIC2 and M2AP labeling (arrowheads). 26.7% of Δvp1 parasites show this striking phenotype compared to 7.3% of the Δku80 cells (p = 0.02). 299 Δvp1 parasites were counted from three different preparations. (C) IFA analysis of Δvp1-cm parasites for MIC2 and M2AP. 263 parasites counted. Δku80 and Δvp1, p = 0.0001; Δku80 and Δvp1-cm, p = 0.005; Δvp1 and Δvp1-cm, p = 0.006.
Ca2+ and pH homeostasis
Ca2+ homeostasis was studied in these mutants by examining store content with thapsigargin (endoplasmic reticulum store), nigericin (acidic stores), ionomycin (all neutral stores) and glycyl-phenylalanine 2-naphthylamide (GPN) (lysosomal stores) (Haller et al., 1996, Christensen et al., 2002, Lloyd-Evans et al., 2008). We did not find a significant difference in the amount of Ca2+ released by these ionophores/inhibitors. We found that the cytosolic Ca2+ levels of Δvp1 parasites are elevated compared to the parental and complemented strains (Table S1). We attribute this difference to the cells being stressed. That acidic pools show a similar amount of Ca2+ could be because of the compensatory function of the V-H+-ATPase.
We also measured intracellular pH of mutant parasites and found no significant difference with pHi of control strains (data not shown).
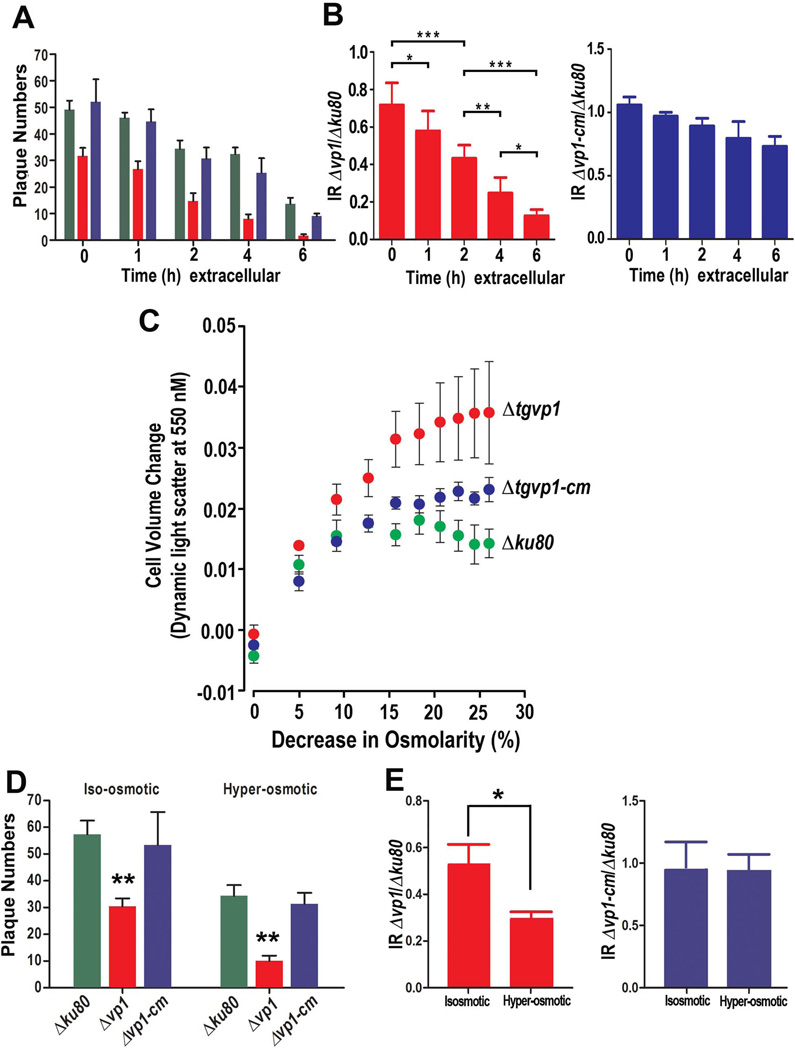
Extracellular Δvp1 Tachyzoites are More Sensitive to Environmental Stress
We reported previously (Miranda et al., 2010) that extracellular tachyzoites overexpressing TgVP1 were more resistant to salt stress than tachyzoites from the parent strain, suggesting this pump plays a role in the response of the parasite to environmental stress. We therefore investigated whether extracellular Δvp1 tachyzoites were more sensitive to environmental stress. We used a plaquing efficiency protocol and counted the number of plaques formed after four days of growth of mutant, parental and complemented parasites pre-incubated in an extracellular buffer for 1–6 h. We observed that the number of plaques was significantly lower for the Δvp1 parasites after being exposed to extracellular ionic conditions for extended lengths of time (Fig. 6A). This difference is more evident when calculating the ratio of invasion (IR) between Δvp1 and Δku80 (Fig. 6B, red columns), which decreases significantly with the time that parasites are left in extracellular buffer. The same IR between Δvp1-cm and Δku80 shows very little change (Fig. 6B, blue columns).
Fig. 6.
Extracellular Δvp1 parasites are more sensitive to environmental stress. (A) Plaque numbers after pre-incubation of parasites in Ringer buffer for different lengths of time (0, 1, 2, 4 or 6 h). (B) Ratio (Invasion Ratio, IR) between the number of plaques formed by Δvp1 and Δku80 after pre-incubation in an extracellular buffer for 0, 1, 2, 4 and 6 h (red columns). Blue columns show the same ratio calculation for Δvp1-cm and Δku80. All strains show decreased number of plaques after long extracellular incubation but the decrease in the number of plaques produced by tachyzoites from Δvp1 clone was more dramatic than that produced by tachyzoites of Δku80 and Δvp1-cm clones. (C) Effect of hyposmotic conditions on volume changes of tachyzoites from different clones as determined by light scattering. Data are the combined results of three independent experiments. (D) Plaque numbers after pre-incubation of parasites under isosmotic or hyperosmotic conditions. (E) Ratio (IR) of the number of plaques formed by Δvp1 and Δku80 submitted to hyperosmotic stress compared to the same ratio for parasites incubated for the same length of time in an isosmotic buffer (red columns). The blue columns show the same calculation between the number of plaques formed by the Δvp1-cm and Δku80 tachyzoites. Tachyzoites from Δvp1 clone were less resistant to this treatment than tachyzoites from Δku80 and Δvp1-cm clones and this is evident in the lower ratio obtained under hyposmotic conditions. Data from (A,B) and (D,E) were analyzed by GraphPad Prism 5. To compare two groups of data we used One-Way ANOVA test. *, p <0.05; **, p <0.01; ***, p <0.001.
The H+-PPase protects plant cells from both salt and osmotic stress (Park et al., 2005, Lv et al., 2008). It is also known that all cells need to regulate their volume continuously (Lang, 2007) so we tested whether tachyzoites of the Δvp1 clone would be more sensitive to osmotic stress. We developed a light-scattering protocol to measure volume recovery, the results of which are highlighted in Fig. 6C. This assay was able to demonstrate that changes in osmotic concentration, independent of ion concentration (i.e. absence of salt stress), resulted in regulatory volume changes in the parasites. Tachyzoites were challenged to increasing hypo-osmotic conditions in 5% intervals while continuously observing their regulatory volume capacity. Tachyzoites were observed to regulate their volume but only within a very narrow range of osmolarity. Parental cell lines initially swelled under these conditions and then partially recovered their volume, as measured by light scattering. Under similar stress conditions, Δvp1 tachyzoites swelled continuously without recovering. Complemented control tachyzoites had an intermediate response in which swelling was observed but not to the degree seen in the Δvp1 tachyzoites (Fig. 6C).
We also investigated the response of tachyzoites to hyperosmotic stress. It has been shown that mutant parasites deficient in the vacuolar type Na+/H+ exchanger (TgNHE3), which localizes to the PLV, are more sensitive to hyperosmotic stress and toxic levels of sodium (Francia et al., 2011). In addition, it is known that some organs that are infected by T. gondii (liver, spleen, lymphoid tissues) have higher osmolarity than serum (330 vs 300 mOsm) (Go et al., 2004) and therefore the ability to tolerate changes in osmolarity would be important for successful infection. To test this response we exposed parasites to a hyper-osmotic buffer for 30 min and then allowed them to form plaques using a plaquing efficiency protocol. Pre-incubation in hyperosmotic conditions (~700 mOsm) for 30 min affected all cell lines (parental, Δvp1 and complemented) but the effect was more pronounced on the Δvp1 parasites (Figs. 6D). The invasion ratio of Δvp1/Δku80 compared with the same ratio of Δvp1-cm/Δku80 highlights this difference (Fig. 6E, compare red and blue columns). In summary, these results indicate that TgVP1 is a critical regulatory element for both the maintenance of salt balance and osmolyte/volume homeostasis.
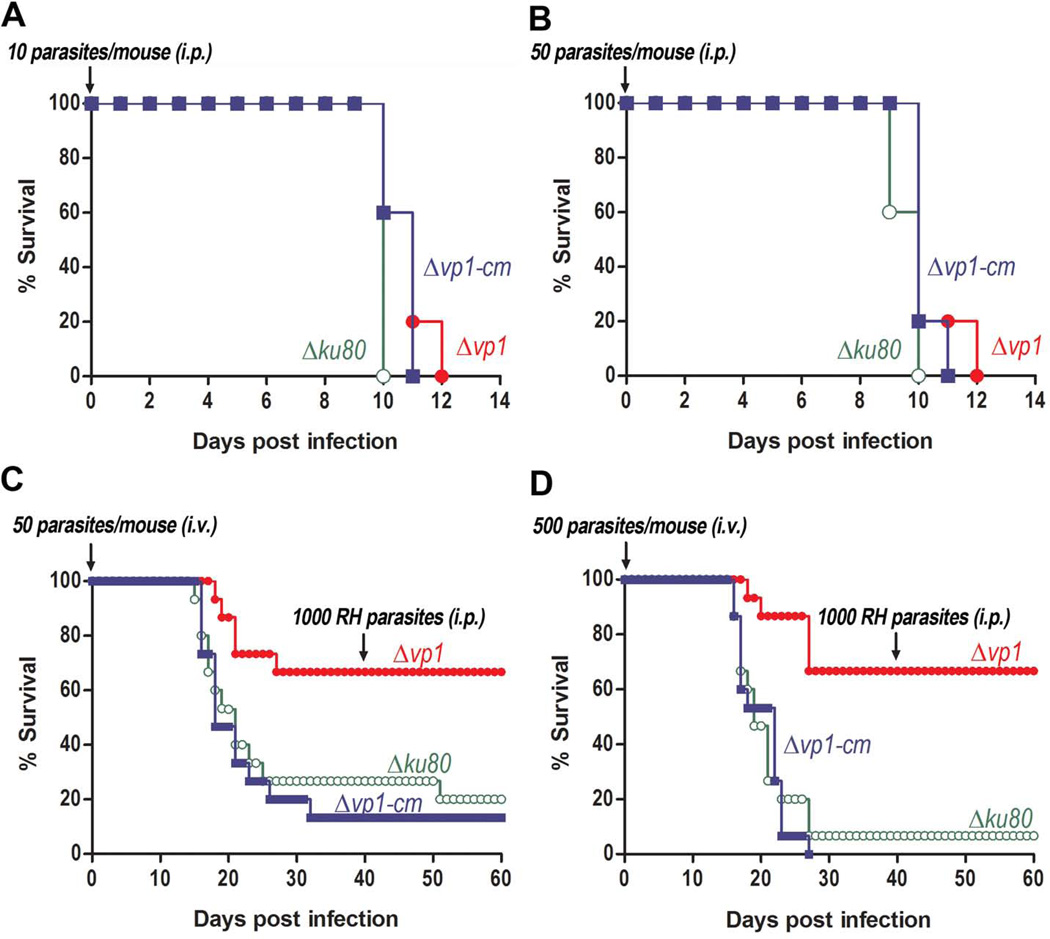
Knockout of TgVP1 Reduces the Virulence of Tachyzoites
As our results showed that Δvp1 tachyzoites were more sensitive to environmental stress we tested their ability to infect mice. We infected mice intraperitoneally (i.p.) with 10 (Fig. 7A) or 50 (Fig. 7B) tachyzoites of the Δvp1, parent, and complemented clones. We observed a 1–2 days difference in survival time between mice infected with Δvp1 vs those infected with parent or complemented clones. This modest difference is probably due to the hyper-virulent nature of the Toxoplasma RH strain masking subtle differences in virulence. Taking into account that dissemination through the circulation is important during natural infection and that this transit exposes tachyzoites to stressful conditions we decided to test the intravenous route (i.v.) to infect mice. We found that 66.7% of mice infected i.v. with 50 or 500 tachyzoites of the Δvp1 clones (Figs. 7C and 7D) survived past 40 days post-infection (p.i.) while only 20% or 6.7% of mice infected with 50 or 500 (respectively) of the Δku80 parasites survived. Mice infected with 50 or 500 Δvp1-cm parasites had a similar survival rate to the parent-infected ones (13.3% and 0%) (Figs. 7C and 7D). Serum from all surviving mice was analyzed 40 days p.i. for the presence of anti-Toxoplasma antibodies and all were seropositive, indicating a successful infection (data not shown). Mice that had been infected with Δvp1 parasites, survived an i.p. inoculation with 1,000 tachyzoites of the RH strain indicating that these mice were protected against a virulent infection. In addition, we monitored weight of living mice (Fig. S4) and we observed that all of them lost weight around 22–24 days p.i., which indicates that they were infected and became ill around that time. All mice recovered a comparable growth rate to the uninfected controls and there were no obvious signs of illness 8–10 days after challenge. In summary, our data indicates that the virulence of the Δvp1 tachyzoites is attenuated compared to the parent and complemented strains.
Fig. 7.
Knockout of TgVP1 reduces the virulence of tachyzoites in mice. Ten (A) or 50 (B) tachyzoites were injected intraperitoneally (i.p.) into groups of five female Swiss Webster mice. Fifty (C) or five hundred (D) tachyzoites were injected intravenously (i.v.) into groups of five female Swiss Webster mice. All surviving mice were tested 40 days p.i. by western blot by probing mouse serum against RH lysate (not shown). All mice that survived infection were seropositive. Challenge was done with 1,000 tachyzoites of the RH strain. Data are pooled from three independent experiments (at least 15 mice per group). Statistical analysis was done following the Log-rank/Mantel-Cox method. For i.v. infection with 50 parasites (C) p = 0.0102; for infection with 500 parasites (D) p = 0.0003. For i.p. infection with 10 parasites (A) p = 0.4221; i.p. infection with 50 parasites p = 0.5118.
Discussion
We present here the phenotypic analysis of a straight knockout of the T. gondii H+-PPase gene. Previous studies have reported downregulation of H+-PPase by RNAi or isolation of loss-of-function mutants in Arabidopsis (Li et al., 2005). Studies of these mutants revealed that the H+-PPase is relevant for development of root, shoot, and flowers, and for fertility (Li et al., 2005). Agricultural plants overexpressing H+-PPase display salt- and drought-tolerant phenotypes (Gaxiola et al., 2001).
H+-PPases are widespread in all domains of life and they are able to use the energy stored in PPi to pump H+ across membranes. The main advantage of these pumps is that they are able to function under stress or with low oxygen levels because they do not need ATP (Maeshima, 2000).
We obtained a clonal line of T. gondii tachyzoites, which lack the TgVP1 gene. These parasites show defects in proper attachment, invasion, microneme secretion, processing of CPL, osmoregulation, and in vivo infection. A redundant function of the H+-ATPase protects these mutant parasites, which are still viable in vitro.
Defects in attachment and invasion correlated with the deficient secretion of micronemal proteins by extracellular parasites under physiological conditions (constitutive secretion) or after ethanol induction (induced secretion), as these are adhesins required for these processes (Carruthers et al., 1999). However, the deficient secretion of micronemal proteins is not due to a deficit in their processing, as suggested by the experiments with proM2AP, proMIC3 and proMIC5. Rather, we found that the localization of MIC2 and M2AP in mutant parasites is altered and a higher percentage of mutant parasites showed a vesicular distribution preferentially around a large vacuole. Our interpretation is that these parasites are defective in the formation of microneme organelles or their trafficking to the apical end of the parasites. Accordingly, these defective micronemes are sub-optimally positioned for secretion, which is predicted to compromise the release of microneme contents, compromising parasite invasion.
Processing of CPL proceeded at a normal rate in the Δvp1 tachyzoites compared to tachyzoites of the parental and complemented clones but showed a significant delay when a V-H+-ATPase inhibitor (bafilomycin A1) was present, suggesting that CPL maturation occurs in a compartment where both TgVP1 and TgVATPase are present. Although CPL has been proposed to function as a maturase for M2AP (Parussini et al., 2010), processing of M2AP proprotein in the presence of bafilomycin A1 was not affected in the Δvp1 tachyzoites. This could be because CPL maturation is not completely blocked by bafilomycin A1 in Δvp1 tachyzoite or because CPL is not the only maturase for M2AP and other maturases might be less dependent upon TgVP1 acidification in the absence of V-H+-ATPase activity. Another explanation could be that the pulse-chase experiments only detect nascent CPL and an existing pool of mature CPL exists in the parasites prior to bafilomycin A1 treatment and this pool is presumably available to catalyze M2AP maturation even when nascent CPL maturation is impaired.
Micronemes are discharged in response to the elevation of parasite intracellular Ca2+ caused naturally by exposure to extracellular conditions or artificially by conditions that elevate intracellular Ca2+ (Carruthers et al., 1999). Interestingly, mutant tachzyoites lacking TgA1, the acidocalcisome Ca2+-ATPase, also show deficient microneme secretion in the presence of ionophores, as compared to control tachyzoites, as well as defects in attachment and invasion (Luo et al., 2001, Luo et al., 2005). We have shown previously that the PLV as well as acidocalcisomes contains Ca2+ (Rohloff et al., 2011) so it is possible that these acidic calcium stores (Patel & Docampo, 2010) are involved in the intracellular Ca2+ increase needed for microneme secretion. Our hypothesis is that the PLV plays a major role in regulating cytoplasmic Ca2+ fluctuations and the acidic environment of the compartment would contribute to its Ca2+ uptake ability. Both TgVP1 and TgVATPase could contribute to this activity. We did not find differences in the amount of Ca2+ released by either nigericin (acidic compartment) or GPN (lysosomal compartment) indicating that the free stored Ca2+ in the PLV has not changed in the Δvp1 mutants. However, the cytosolic Ca2+ levels are elevated in the Δvp1 mutants, which could be the result of a leaky PLV. We also did not find that the endoplasmic reticulum Ca2+ content (tested with thapsigargin) was changed. Another possible explanation of a higher cytosolic Ca2+ would be that these mutant cells are more sensitive to the stress they have to be exposed to during loading with Fura-2 AM leading to higher cytosolic Ca2+. Higher cytosolic Ca2+ has also been observed in other mutants (TgA1 and TgNHE3) associated with a microneme secretion defect (Luo et al., 2005, Francia et al., 2011).
The ability to control cell volume is critical for cell function (Hoffmann et al., 2009). Most cells have the capacity to counteract volume perturbations by the process of Regulatory Volume Decrease (RVD) or Regulatory Volume Increase (RVI) (Hoffmann et al., 2009). When cell volume is disturbed, different signaling events could be triggered by swelling as well as shrinkage, which could play a role in the cell volume regulatory response (Pedersen et al., 2001). The plant vacuole plays in important role in osmoregulation for the plant cell (Barkla & Pantoja, 1996). The organelle acts as a water reservoir for the cytosol and the highly osmosensitive chloroplast. The tonoplast (plant vacuole membrane) is highly permeable to water due to the presence of water channels (Barkla & Pantoja, 1996).
For entering a host cell, T. gondii relies on its own motility and on the ability to establish a structure called the moving junction (Besteiro et al., 2011). During the process of entry, the parasite squeezes itself through the moving junction, a process that results in deformations of its cell shape. Our hypothesis is that these changes in the parasite cell shape are accompanied by large relative changes in cell volume. These changes could be accomplished by the interaction of several solute transporters and exchangers that are linked to the proton gradient established by TgVP1 and TgVATPase. The connectivity of these exchange mechanisms with the proton gradient has been established in the PLV (Miranda et al., 2010, Francia et al., 2011). The phenotypic defects of the Δvp1 mutants would be the result of a defective H+-gradient leading to defective capacity to accumulate osmolytes resulting in a defective cell volume response, which would affect invasion and other stress responses. In this regard, we found that Δvp1 parasites are more sensitive to extracellular ionic concentrations, hyper-osmotic or hypo-osmotic stress. This would be especially evident when the parasite is outside, surrounded by a variable osmotic environment and/or for performing a function requiring cell volume changes like invasion. We found that the action of the V-H+-ATPase partially rescues the defects due to lack of TgVP1. The results from this study further implicate the involvement of TgVP1 as both a mechanism for sodium regulation (Miranda et al., 2010) as well as a participant in osmotic/volume regulatory mechanisms. We found a significantly stronger defect in invasion in the Δvp1 mutants when allowed to invade after a pretreatment with the specific V-H+-ATPase inhibitor, bafilomycin A1. Very low concentrations of the inhibitor were used so it would not affect the normal invasion of parental parasites.
We also tested the levels of PPi because it was previously shown in Arabidopsis that the H+-PPase functions in hydrolysis of PPi and mutants for the enzyme accumulate the substrate during early seedling (Ferjani et al., 2012). We have shown previously that PPi has a regulatory role on glycolysis and an increase in its concentrations could explain some of the observed phenotypes. We did not detect a significant difference in PPi levels in the Δvp1 mutants (data not shown), which are probably controlled by the soluble pyrophosphatase (Pace et al., 2011).
In summary, defects in microneme secretion, attachment, invasion, processing of some proteins and osmotic fragility made Δvp1 tachyzoites less able to grow in vitro and in vivo indicating that TgVP1 fulfills an important role in the T. gondii lytic cycle. The parasite is exposed to dramatic ionic changes upon egress while it actively needs to invade host cells to continue with its lytic cycle. We think that the PLV plays a central role during the extracellular phase of the parasite not only in resisting environmental stress but also as it prepares itself for invading the next host cell. This would be consistent with what is known about the plant vacuole, which is home to a complex set of functions (storage, sorting, stress, etc.). Likewise, it seems feasible that the PLV is involved in a complex set of functions, especially significant during the extracellular phase of T. gondii, and analyzing mutants like Δvp1 will contribute to the elucidation of its role in the lytic cycle of the parasite. Our studies demonstrate that T. gondii tachyzoites can undergo a regulatory volume decrease after hypo-osmotic stress underscoring the role of osmoregulation during invasion.
Experimental Procedures
Parasite cultures and generation of mutant strains
Toxoplasma gondii tachyzoites were grown in hTERT human fibroblasts as described before (Moreno & Zhong, 1996). The parental strain used to generate knockouts were Δku80 (Fox et al., 2009), which was transfected with a modified cosmid generated by recombineering as previously described (Brooks et al., 2010). Cosmid PSBM180 was used and the modification cassette was amplified from plasmid pH3CG, including genes for gentamycin (bacterial selection), and chloramphenicol (T. gondii selection), flanked by 50 bp of TgVP1 sequence. The forward primer was 5'actcgtcgtcttcatctcgtggacacaacagaacgctttcgacttccatcCCTCGACTACGGCTTCCATTGGCAAC-3', and the reverse primer was 5'-atgaacgtacacaaacagataccaaaaaaaattgtacgcgttcaccctgtATACGACTCACTATAGGGCGAATTGG-3'. The PCR product was introduced by electroporation and cells selected with kanamycin and gentamycin. The modified cosmid was used to disrupt the native loci in Δku80 tachyzoites by double homologous recombination and replacement of the entire coding region, and stable clones were derived by chloramphenicol (20 µM) selection. For complementation analysis, the cosmid PSBM180 (which includes the TgVP1 genomic locus plus a selectable marker; DHFR-TS) was transfected into ΔTgvp1 tachyzoites and the parasites were selected with 1 µM pyrimethamine. Although we do not know the fate of the construct (episomal or integrated), the findings in Fig. 1 indicate that the construct did not target to the TgVP1 locus by double crossover homologous recombination since the P1 probe shows that the knockout construct remains at the locus. Although it would have been ideal to target the TgVP1 cosmid to a specific neutral locus, this was not possible due to an inability to insert a specific targeting sequence into the cosmid. For Southern blot analysis total genomic DNA from Δku80, Δvp1 and Δvp1-cm (1.3 µg/lane) were digested with BamHI and BglII, resolved on 0.7% agarose and transferred to nylon membranes. Blots were hybridized with radiolabeled DNA fragments (P1, 413 bp and P2, 700 bp) of TgVP1 prepared by randomly primed synthesis with Klenow DNA polymerase and [α-32P]-dCTP. Probe P1 (TgVP1 intron) hybridizes to native TgVP1 but not to the ectopic minigene copy; probe P2 (3’ noncoding region present in both loci) hybridizes to both. The primers used were, for probe P1: forward, 5'-GCTAGACCACAACGGAAGCAAAC-3' and reverse, 5'-CGAGCAACGGTAACACTCCTGTA-3', and for probe P2: forward 5'-TCGTCGTGTTCTCCTCTATCT-3', and reverse: 5'-GGACCACCTCAATCCAGTCG-3'. Autoradiographs were stripped and reprobed.
Growth assays
Two different protocols were used for plaque assays. For experiments testing growth, plaques of Δku80, Δvp1 and Δvp1-cm parasites (200 parasites/well) were allowed to form in monolayers of hTERT cells for 8–9 days, fixed and stained with crystal violet. Plaquing efficiency was measured infecting hTERT fibroblasts with 1,000 parasites per well and allowing contact with host cells for 30 min. At this point, wells were washed with PBS, fresh media added and parasites allowed to grow for 4 days, fixed and stained with crystal violet. For fitness testing parasites were pre-incubated in Ringer buffer (155 mM NaCl, 3 mM KCl, 1 mM MgCl2, 3 mM NaH2PO4-H2O, 10 mM HEPES, pH 7.2, 10 mM glucose) for the times indicated in the figure. For osmotic stress experiments, parasites were pre-incubated at 37°C for 30 min in either hyperosmotic (64 mM NaCl, 4 mM KCl; 1.8mM CaCl2, 0.53 mM MgCl2, 5.5 mM Glucose, 10 mM Hepes, pH 7.4, 700 mM mannitol) or isosmotic (~300 mOsm) Ringer buffer, and used for infection as for the fitness experiments.
Red/Green invasion assay was performed as described (Kafsack et al., 2004) with some modifications. Briefly, subconfluent hTERT monolayers grown in 12-well plates were challenged with 1×107 tachyzoites, resuspended in Endo buffer (Endo & Yagita, 1990) (44.7 mM K2SO4, 10 mM MgSO4, 106 mM sucrose, 5 mM glucose, 20 mM Tris–H2SO4, 3.5 mg/ml BSA, pH 8.2) and allowed to settle for 20 min at 37°C. Next the buffer was replaced with invasion media (DMEM + 10 mM Hepes, pH 7.4, and 3% FBS) and incubated for 2 min at 37°C. Subsequently, wells were washed with PBS and fixed with 2.5% formaldehyde. Blocking was with 10% FBS for 30 min at room temperature and fixed cells were first incubated with rabbit anti-SAG1 polyclonal antibody (1:1,000), and next with the anti-SAG1 monoclonal antibody (1:500) following a previous protocol (Kafsack et al., 2004). Counting was performed using an Olympus fluorescence microscope and data was compiled from three independent experiments, each one by triplicate, counting eight fields/clone, selected at random, at 400x total magnification. Statistical analysis was performed using the Student’s t-test. Differences were considered significant if P-values were <0.05.
Immunofluorescence and Western blot analysis
Indirect immunofluorescence assays (IFA) were performed on freshly collected tachyzoites washed with buffer A with glucose (BAG, 116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 50 mM HEPES, pH 7.2, and 5.5 mM glucose) and fixed with 4% formaldehyde for 1 h, permeabilized with 0.3% Triton X-100 for 20 min, and blocked with 3% bovine serum albumin (Miranda et al., 2010). Immunofluorescence was performed as previously described (Miranda et al., 2010) and primary and secondary antibodies concentrations are indicated in the figure legends. Fluorescence images were collected with an Olympus IX-71 inverted fluorescence microscope with a Photometrix CoolSnapHQ CCD camera driven by DeltaVision software (Applied Precision, Seattle, WA). Collected images were deconvolved using Softworx deconvolution software (Applied Precision, Seattle, WA). For all images, 15 cycles of enhanced ratio deconvolution were used.
Western blot analysis was performed as previously described (Miranda et al., 2010). We used the anti-TgVP1 polyclonal antibody at a dilution of 1:500 and secondary goat anti-Guinea pig antibody conjugated with HRP at 1:5,000. Mouse anti-α-tubulin at a dilution of 1:1,000 was used as a loading control.
Gliding Motility
Glass chamber slides were coated overnight at 4°C with 50% fetal bovine serum in PBS (pH7.4) and slides washed with PBS (Wetzel et al., 2004). Freshly lysed parasites, washed and suspended in HHE (Hank’s Balanced Salt, 10mM HEPES, 1mM EGTA) were allowed to glide on FBS coated slides, at 37°C for 15 min. Fixation was with 4% paraformaldehyde and staining was performed with Rabbit anti-SAG1 antibody 1:1,000 directly conjugated to Alexa 488 fluorochrome 1:1,000. A trail was considered circular if the diameter was 11 µm or less; trails that were larger in diameter or straight were counted as non-circular. Approximately 100 trails were enumerated per strain in each experiment. Although Δvp1 show a small decrease in circular trails when compared to Δku80 and Δvp1-cm, we did not find a significant difference among the three strains. Values are mean ± SD. Data are the combined results of three independent experiments and analyzed by GraphPad Prism 5.
Microneme Secretion
The secretion of micronemes by tachyzoites of the Δku80, Δvp1, and Δvp1-cm1 clones were measured as described (Carruthers et al., 1999). Tachyzoites were harvested and washed twice in invasion medium (IM), and resuspended in IM at 1.6 × 108 cells per ml. Excretion/secretion antigen (ESA) fraction was obtained by collecting the supernatant of parasites incubated for 30 min in a 37°C water bath (ESA-Const.). The stimulated secretion of micronemes was obtained by incubating parasites for 2 min in IM containing 1 % ethanol. Secretion was stopped by placing the tubes on ice. Cells were removed by centrifugation at 1,000 g for 10 min at 4°C. Proteins in the supernatants were separated by SDS-PAGE, and used for western blot analysis. Mouse anti-MIC2 serum was used at a dilution of 1:8,000, and secondary Goat anti-mouse HRP-conjugated antibody. Rabbit anti-MIC3 was used at 1:500. Mouse anti-GRA1. Quantification was done using ImageJ (NIH). Data are pooled from three independent experiments.
Metabolic labeling and immunoprecipitation
Immunoprecipitation was performed as described before (Parussini et al., 2010). Freshly lysed tachyzoites were harvested and re-suspended in Met/Cys-free DMEM containing 10 mM HEPES, pH 7.0, and 2 mM L-Gln. Parasites were pre-incubated for 15 min, at room temperature with solvent control (DMSO) or bafilomycin A1, then pulsed-labeled with 300 µCi [35S] Met/Cys (Perkin Elmer) for 10 min, at 37°C, chased in unlabeled medium with 5 mM methionine, 5 mM cysteine and bafilomycin A1 for the indicated times. Parasites were washed with medium, re-suspended in 0.8 ml RIPA buffer (50 mM Tris, pH 7.5, 100 mM NaCl, 5 mM EDTA, pH 8.0, 1% Triton X-100, 0.5% sodium deoxycholate, 0.2% SDS, 10 mg/ml RNase A, 20 µg/ml DNase I, and protease inhibitors), incubated 30 min at 0°C, and insoluble material was removed by centrifugation (13,000 r.p.m., 10 min, at 4°C). Antibodies to CPL, MIC3, MIC5, or M2AP were incubated with 400 µl of extract for 1 h, at 4°C, followed by addition of 100 µl 10 % (v/v) slurry of protein G-sepharose beads, and 1 h incubation at 4°C, with gentle rocking. Immune complexes were washed four times with 1 ml RIPA buffer before boiling in SDS-PAGE sample buffer containing 2% β-mercaptoethanol, separated by SDS-PAGE, incubated in fluorographic enhancer (Amplify, Amersham), dried in cellophane, and exposed to Storage Phosphor Screen.
Intracellular Ca2+ and pH measurements
Parasites were loaded with Fura 2-AM as described (Moreno & Zhong, 1996). After harvesting and purifying the parasites they were washed twice at 500xg for 10 min at in BAG. Cells were resuspended to a final density of 1 × l09 cells/ml in loading buffer, which consisted of BAG plus 1.5% sucrose and 5 µM Fura 2-AM. The suspensions were incubated for 25 min in a 26°C water bath with mild agitation. Subsequently, the cells were washed twice and resuspended to a final density of 1 × 109 cells/ml in the same buffer. A 50 µl aliquot of the cell suspension was diluted into 2.5 ml of Ringer buffer (2 × l07 cells/ml final density) in a cuvette placed in a Hitachi F-4500 spectrofluorometer. Excitation was at 340 and 380 nm and emission at 510 nm. The Fura 2 response was calibrated from the ratio of 340/380 nm fluorescence values after subtraction of the background fluorescence of the cells at 340 and 380 nm as previously described (Grynkiewicz et al., 1985). For pH measurements parasites were loaded following a similar procedure but using BCECF-AM (2',7'-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein, Acetoxymethyl Ester) as intracellular pH indicator. Calibrations were done following published protocols (Moreno et al., 1998).
Cell volume regulation during hypo-osmotic stress
Analysis of cell volume change was based on previous studies in which changes in cell volume were determined by the degree of light scattering at 550 nM (Rohloff et al., 2003). These approaches have worked very well for other unicellular parasites such as trypanosomes (Rohloff et al., 2003). The reagents employed in these experiments were designed to ensure that ionic conditions (for example Na+ and K+ concentrations) were held constant during hypo-osmotic treatment. Hypo-osmotic conditions were therefore manipulated through variations in the amount of mannitol in the buffers (de Jesus et al., 2010). Cells were harvested and resuspended in isosmotic buffer (64 mM NaCl, 4.0 mM KCl, 0.5 mM MgCl2, 1.8 mM CaCl2, 5.0 mM Hepes-Na buffer, pH 7.3, 5.5 mM glucose, and 150 mM mannitol; final osmolarity of 282 mOsm). Cells (2 ml initial volume at a final concentration of 3 × 107 cells/ml) were transferred to a 4 ml cuvette and light scattering at 550 nm continuously monitored during addition of hypo-osmotic buffer (64 mM NaCl, 4.0 mM KCl, 0.5 mM MgCl2, 1.8 mM CaCl2, 5.0 mM HEPES-Na buffer, pH 7.3, 5.5 mM glucose, final osmolarity 132 mOsm). Over the course of 20 min the initial isosmotic conditions were diluted with hypo-osmotic buffer at a rate of 200 µl addition every 2 min (initial osmotic dilution rate at 5%). Light scattering was monitored continuously during this time using a SpectraMax M2c plate reader. Hypo-osmotic dilution experiments resulted in a stepwise decrease in absorption at 550 nm every 2 min. This decrease was due to 2 factors: the dilution of cells that occurs by adding buffer and the change in cell volume that results from the addition of hypo-osmotic buffer. To distinguish between the 2 phenomena, controls were done in which an equal volume of iso-osmotic buffer was added to control for the contribution of the dilution effect to the light scattering measurements.
In vivo virulence assay
Groups of five female Swiss Webster mice 8–9 weeks old were infected intraperitoneally (i.p.) or intravenously (i.v.) (Lagal et al., 2010). Serum from all surviving mice was assayed 40 days post infection by western blot by probing it against an RH tachyzoite lysate (not shown). Surviving mice were challenged at this time with 1,000 RH parasites (i.p. inoculation). Mice were monitored daily over a period of 8 weeks after infection. Data were pooled from three independent experiments. Statistical analysis was done following the Log-rank/Mantel-Cox method.
Supplementary Material
Acknowledgments
We thank David Bzik for the Δku80 parasites and Boris Striepen for cosmids, plasmids and advise. Samantha Lie Tjauw provided excellent technical support. This work was supported by NIH grants AI096836 (to SNJM) and AI063263 (to VC). D.A.P. was partially supported by an NIH T32 training grant AI-60546 to the Center for Tropical and Emerging Global Diseases.
The abbreviations used are
- H+-PPase
vacuolar-H+-transporting pyrophosphatase
- TgVP1
T. gondii H+-PPase
- V-H+-ATPase
vacuolar-H+-transporting ATPase, TgVATPase, T. gondii V-H+-ATPase
- CPL
T. gondii cathepsin L
- MIC2
microneme protein 2
- M2AP
MIC2-associated protein
- proM2AP
pro form of M2AP
- hTERT
human telomerase reverse transcriptase immortalized cells
- PLV/VAC
plant-like vacuole/vacuolar compartment
Footnotes
Present address for Douglas Pace: California State University, Long Beach, CA
No conflict of interest to declare from any of the authors.
References
- Baltscheffsky H, Von Stedingk LV, Heldt HW, Klingenberg M. Inorganic pyrophosphate: formation in bacterial photophosphorylation. Science. 1966;153:1120–1122. doi: 10.1126/science.153.3740.1120. [DOI] [PubMed] [Google Scholar]
- Baltscheffsky M, Schultz A, Baltscheffsky H. H+ -PPases: a tightly membrane-bound family. FEBS Lett. 1999;457:527–533. doi: 10.1016/s0014-5793(99)90617-8. [DOI] [PubMed] [Google Scholar]
- Barkla BJ, Pantoja O. Physiology of Ion Transport across the Tonoplast of Higher Plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:159–184. doi: 10.1146/annurev.arplant.47.1.159. [DOI] [PubMed] [Google Scholar]
- Baykov AA, Bakuleva NP, Rea PA. Steady-state kinetics of substrate hydrolysis by vacuolar H(+)-pyrophosphatase. A simple three-state model. Eur J Biochem. 1993;217:755–762. doi: 10.1111/j.1432-1033.1993.tb18303.x. [DOI] [PubMed] [Google Scholar]
- Belogurov GA, Lahti R. A lysine substitute for K+. A460K mutation eliminates K+ dependence in H+-pyrophosphatase of Carboxydothermus hydrogenoformans. J Biol Chem. 2002;277:49651–49654. doi: 10.1074/jbc.M210341200. [DOI] [PubMed] [Google Scholar]
- Besteiro S, Dubremetz JF, Lebrun M. The moving junction of apicomplexan parasites: a key structure for invasion. Cell Microbiol. 2011;13:797–805. doi: 10.1111/j.1462-5822.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CF, Johnsen H, van Dooren GG, Muthalagi M, Lin SS, Bohne W, Fischer K, Striepen B. The toxoplasma apicoplast phosphate translocator links cytosolic and apicoplast metabolism and is essential for parasite survival. Cell Host Microbe. 2010;7:62–73. doi: 10.1016/j.chom.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, Giddings OK, Sibley LD. Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cell Microbiol. 1999;1:225–235. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- de Jesus TC, Tonelli RR, Nardelli SC, da Silva Augusto L, Motta MC, Girard-Dias W, Miranda K, Ulrich P, Jimenez V, Barquilla A, Navarro M, Docampo R, Schenkman S. Target of rapamycin (TOR)-like 1 kinase is involved in the control of polyphosphate levels and acidocalcisome maintenance in Trypanosoma brucei. J Biol Chem. 2010;285:24131–24140. doi: 10.1074/jbc.M110.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Moreno SN. Acidocalcisomes. Cell Calcium. 2011;50:113–119. doi: 10.1016/j.ceca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdowicz YM, Rea PA. Vacuolar H(+) pyrophosphatases: from the evolutionary backwaters into the mainstream. Trends Plant Sci. 2001;6:206–211. doi: 10.1016/s1360-1385(01)01923-9. [DOI] [PubMed] [Google Scholar]
- Drozdowicz YM, Shaw M, Nishi M, Striepen B, Liwinski HA, Roos DS, Rea PA. Isolation and characterization of TgVP1, a type I vacuolar H+-translocating pyrophosphatase from Toxoplasma gondii. The dynamics of its subcellular localization and the cellular effects of a diphosphonate inhibitor. J Biol Chem. 2003;278:1075–1085. doi: 10.1074/jbc.M209436200. [DOI] [PubMed] [Google Scholar]
- Endo T, Yagita K. Effect of extracellular ions on motility and cell entry in Toxoplasma gondii. J Protozool. 1990;37:133–138. doi: 10.1111/j.1550-7408.1990.tb05883.x. [DOI] [PubMed] [Google Scholar]
- Ferjani A, Segami S, Horiguchi G, Sakata A, Maeshima M, Tsukaya H. Regulation of pyrophosphate levels by H+-PPase is central for proper resumption of early plant development. Plant Signal Behav. 2012;7:38–42. doi: 10.4161/psb.7.1.18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BA, Ristuccia JG, Gigley JP, Bzik DJ. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot Cell. 2009;8:520–529. doi: 10.1128/EC.00357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia ME, Wicher S, Pace DA, Sullivan J, Moreno SN, Arrizabalaga G. A Toxoplasma gondii protein with homology to intracellular type Na(+)/H(+) exchangers is important for osmoregulation and invasion. Exp Cell Res. 2011;317:1382–1396. doi: 10.1016/j.yexcr.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Contreras R, Celis H, Romero I. Importance of Rhodospirillum rubrum H(+)-pyrophosphatase under low-energy conditions. J Bacteriol. 2004;186:6651–6655. doi: 10.1128/JB.186.19.6651-6655.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci U S A. 2001;98:11444–11449. doi: 10.1073/pnas.191389398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc. Natl. Acad. Sci. USA. 2004;101:10673–10678. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Haller T, Dietl P, Deetjen P, Volkl H. The lysosomal compartment as intracellular calcium store in MDCK cells: a possible involvement in InsP3-mediated Ca2+ release. Cell Calcium. 1996;19:157–165. doi: 10.1016/s0143-4160(96)90084-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- Huynh MH, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009;8:530–539. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack BF, Beckers C, Carruthers VB. Synchronous invasion of host cells by Toxoplasma gondii. Mol Biochem Parasitol. 2004;136:309–311. doi: 10.1016/j.molbiopara.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Lagal V, Binder EM, Huynh MH, Kafsack BF, Harris PK, Diez R, Chen D, Cole RN, Carruthers VB, Kim K. Toxoplasma gondii protease TgSUB1 is required for cell surface processing of micronemal adhesive complexes and efficient adhesion of tachyzoites. Cell Microbiol. 2010;12:1792–1808. doi: 10.1111/j.1462-5822.2010.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F. Mechanisms and significance of cell volum regulation. J Am Coll Nutr. 2007;26:613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- Lemercier G, Dutoya S, Luo S, Ruiz FA, Rodrigues CO, Baltz T, Docampo R, Bakalara N. A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J Biol Chem. 2002;277:37369–37376. doi: 10.1074/jbc.M204744200. [DOI] [PubMed] [Google Scholar]
- Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, Titapiwantakun B, Undurraga S, Khodakovskaya M, Richards EL, Krizek B, Murphy AS, Gilroy S, Gaxiola R. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- Lin SM, Tsai JY, Hsiao CD, Huang YT, Chiu CL, Liu MH, Tung JY, Liu TH, Pan RL, Sun YJ. Crystal structure of a membrane-embedded H+-translocating pyrophosphatase. Nature. 2012;484:399–403. doi: 10.1038/nature10963. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- Lopez-Marques RL, Perez-Castineira JR, Losada M, Serrano A. Differential regulation of soluble and membrane-bound inorganic pyrophosphatases in the photosynthetic bacterium Rhodospirillum rubrum provides insights into pyrophosphate-based stress bioenergetics. J Bacteriol. 2004;186:5418–5426. doi: 10.1128/JB.186.16.5418-5426.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Ruiz FA, Moreno SN. The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol. 2005;55:1034–1045. doi: 10.1111/j.1365-2958.2004.04464.x. [DOI] [PubMed] [Google Scholar]
- Luo S, Vieira M, Graves J, Zhong L, Moreno SN. A plasma membrane-type Ca(2+)-ATPase co-localizes with a vacuolar H(+)-pyrophosphatase to acidocalcisomes of Toxoplasma gondii. Embo J. 2001;20:55–64. doi: 10.1093/emboj/20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S, Zhang K, Gao Q, Lian L, Song Y, Zhang J. Overexpression of an H+-PPase gene from Thellungiella halophila in cotton enhances salt tolerance and improves growth and photosynthetic performance. Plant Cell Physiol. 2008;49:1150–1164. doi: 10.1093/pcp/pcn090. [DOI] [PubMed] [Google Scholar]
- Maeshima M. Vacuolar H(+)-pyrophosphatase. Biochim Biophys Acta. 2000;1465:37–51. doi: 10.1016/s0005-2736(00)00130-9. [DOI] [PubMed] [Google Scholar]
- Marchesini N, Luo S, Rodrigues CO, Moreno SN, Docampo R. Acidocalcisomes and a vacuolar H+-pyrophosphatase in malaria parasites. Biochem J. 2000;1(347 Pt):243–253. [PMC free article] [PubMed] [Google Scholar]
- McIntosh MT, Drozdowicz YM, Laroiya K, Rea PA, Vaidya AB. Two classes of plant-like vacuolar-type H(+)-pyrophosphatases in malaria parasites. Mol Biochem Parasitol. 2001;114:183–195. doi: 10.1016/s0166-6851(01)00251-1. [DOI] [PubMed] [Google Scholar]
- Miranda K, Pace DA, Cintron R, Rodrigues JC, Fang J, Smith A, Rohloff P, Coelho E, Haas Fde, Souza Wde, Coppens I, Sibley LD, Moreno SN. Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol Microbiol. 2010;76:1358–1375. doi: 10.1111/j.1365-2958.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno SN, Zhong L. Acidocalcisomes in Toxoplasma gondii tachyzoites. Biochem J. 1996;313(Pt 2):655–659. doi: 10.1042/bj3130655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno SN, Zhong L, Lu HG, Souza WD, Benchimol M. Vacuolar-type H+-ATPase regulates cytoplasmic pH in Toxoplasma gondii tachyzoites. Biochem J. 1998;330(Pt 2):853–860. doi: 10.1042/bj3300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace DA, Fang J, Cintron R, Docampo MD, Moreno SN. Overexpression of a cytosolic pyrophosphatase (TgPPase) reveals a regulatory role of PP(i) in glycolysis for Toxoplasma gondii. Biochem J. 2011;440:229–240. doi: 10.1042/BJ20110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA. Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci U S A. 2005;102:18830–18835. doi: 10.1073/pnas.0509512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parussini F, Coppens I, Shah PP, Diamond SL, Carruthers VB. Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii. Mol Microbiol. 2010;76:1340–1357. doi: 10.1111/j.1365-2958.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Hoffmann EK, Mills JW. The cytoskeleton and cell volume regulation. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:385–399. doi: 10.1016/s1095-6433(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Rodrigues CO, Scott DA, Bailey BN, Souza WDe, Benchimol M, Moreno B, Urbina JA, Oldfield E, Moreno SN. Vacuolar proton pyrophosphatase activity and pyrophosphate (PPi) in Toxoplasma gondii as possible chemotherapeutic targets. Biochem J. 2000;3(349 Pt):737–745. doi: 10.1042/bj3490737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohloff P, Miranda K, Rodrigues JC, Fang J, Galizzi M, Plattner H, Hentschel J, Moreno SN. Calcium uptake and proton transport by acidocalcisomes of Toxoplasma gondii. PLoS One. 2011;6:e18390. doi: 10.1371/journal.pone.0018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohloff P, Rodrigues CO, Docampo R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol Biochem Parasitol. 2003;126:219–230. doi: 10.1016/s0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- Scott DA, de Souza W, Benchimol M, Zhong L, Lu HG, Moreno SN, Docampo R. Presence of a plant-like proton-pumping pyrophosphatase in acidocalcisomes of Trypanosoma cruzi. J Biol Chem. 1998;273:22151–22158. doi: 10.1074/jbc.273.34.22151. [DOI] [PubMed] [Google Scholar]
- Serrano A, Perez-Castineira JR, Baltscheffsky M, Baltscheffsky H. H+-PPases: yesterday, today and tomorrow. IUBMB Life. 2007;59:76–83. doi: 10.1080/15216540701258132. [DOI] [PubMed] [Google Scholar]
- Seufferheld M, Lea CR, Vieira M, Oldfield E, Docampo R. The H(+)-pyrophosphatase of Rhodospirillum rubrum is predominantly located in polyphosphate-rich acidocalcisomes. J Biol Chem. 2004;279:51193–51202. doi: 10.1074/jbc.M406099200. [DOI] [PubMed] [Google Scholar]
- Seufferheld M, Vieira MC, Ruiz FA, Rodrigues CO, Moreno SN, Docampo R. Identification of organelles in bacteria similar to acidocalcisomes of unicellular eukaryotes. J Biol Chem. 2003;278:29971–29978. doi: 10.1074/jbc.M304548200. [DOI] [PubMed] [Google Scholar]
- Wetzel DM, Chen LA, Ruiz FA, Moreno SN, Sibley LD. Calcium-mediated protein secretion potentiates motility in Toxoplasma gondii. J Cell Sci. 2004;117:5739–5748. doi: 10.1242/jcs.01495. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li J, Wang X, Chen J. OVP1, a vacuolar H+-translocating inorganic pyrophosphatase (V-PPase), overexpression improved rice cold tolerance. Plant Physiol Biochem. 2011;49:33–38. doi: 10.1016/j.plaphy.2010.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.