Abstract
OBJECTIVE(S)
Clinical research characterizing the mechanisms responsible for sex-based outcome differences post-injury remain conflicting. We sought characterize an X-chromosome linked IRAK-1 polymorphism as an alternative mechanism responsible for sex differences post-injury. IRAK-1 is key intermediate in the Toll Like Receptor (TLR) pathway thought to drive inflammation post-injury.
METHODS
A prospective cohort study was performed over a 24-month period. Blunt injured patients requiring ICU admission were enrolled while patients with isolated brain and spinal cord injuries were excluded. Outcomes of interest included Multiple Organ Failure (MOF, Marshall MODscore > 5) and mortality. Logistic regression was utilized to determine the independent risk of poor outcome associated with the IRAK-1 variant after controlling for important differences.
RESULTS
In an enrolled cohort of 321 patients, the IRAK-1 variant was common (12.5%). Patients with and without the variant were similar in age, injury severity and 24hr blood transfusion. After controlling for important confounders, the IRAK1 variant was independently associated with over a 8-fold (OR 8.4, p=0.005, 95% CI 1.9–37.1) and 11-fold (OR 11.8, p=0.037, 95% CI 1.1–121) greater risk of MOF and mortality, respectively. These differences were most prominent in males, while females heterozygous for the variant demonstrated worse outcome in a dose-dependent fashion.
CONCLUSIONS
The IRAK1 polymorphism is a strong independent predictor of MOF and mortality post-injury and represents a common variant with prognostic potential. These data demonstrate the importance of TLR signaling post-injury and supports that a genetic mechanism may drive sex outcome differences post-injury.
Introduction
Although significant advances in the care of the injured patient have occurred over the last decade, those who survive their initial injury continue to be plagued with the development of coagulopathy, multiple organ failure, nosocomial infection and their attributable morbid effects.1-6 A persistent finding has been that males and females respond differently following traumatic injury with significant protection afforded to the female sex.7, 8 Controversy exists regarding the clinical explanation and underlying mechanisms responsible for this female protective effect.7, 9,10
A large body of laboratory evidence suggests a sex-hormone based mechanism (estrogen being protective) is responsible for these post-injury differences.8, 11-14 In prior work, our group has shown that the protective effect afforded to females following severe injury is independent of age and the hormonal status of the female, suggesting other mechanisms may be involved clinically.7, 15 Males and females also are different genetically, primarily due to the method of inheritance of, and the genes which reside on, the X-chromosome. Secondary to the known mosaic expression of the X-chromosome, females would be less affected by unfavorable X-linked genetic variants.16 Importantly, increasing evidence also has demonstrated that the Toll-like receptor (TLR) signaling cascade plays an essential role in the early activation of the innate immune response following traumatic injury.17-24 The IL-1 receptor-associated kinase (IRAK-1) is a protein constituent member of the TLR signaling cascade which resides on the X-chromosome and has been demonstrated to have two haplotypes. The IRAK-1 variant haplotype has been demonstrated to be relatively common and associated with worse outcome in septic patients, thought to be secondary to an excessive innate immune response brought about by upregulated NF-κB signaling.25-27 No evidence currently exists regarding the significance of this TLR pathway variant which resides on the X chromosome on pertinent outcomes following traumatic injury. We sought to characterize the IRAK-1 variant as an alternative mechanism responsible for sex based outcome differences post-injury. We hypothesized that the IRAK-1 variant would be common and independently associated with poor clinical outcome following traumatic injury.
Methods
A prospective observational cohort study was performed over a 24-month time period (2011– 2012) with the overarching goal to further characterize the mechanisms responsible for sex (male vs. female) based outcome differences following traumatic injury. Inclusion criteria for the study included blunt injured patients greater than 17 years of age requiring ICU admission. Patients > 90 years of age, with isolated traumatic brain injury, pre-existing immune-suppression, or those with an anticipated survival of < 24 hours were excluded from enrollment. Blood samples were obtained within 6 hours of injury for serial cytokine measurements, coagulation assessment (INR, TEG analysis) and DNA isolation and haplotype discrimination. Clinical outcomes assessed included the development of multiple organ failure (MOF), nosocomial infection (NI), and mortality.
The IRAK-1 variant haplotype was determined by genotyping the single nucleotide polymorphism on the X chromosome where a T→ C substitution [rs1059703] at position 1,595 in exon12 results in a non-synonymous mutation (532, L→ S). Probe and primer combinations were designed for genotyping this polymorphism and polymerase chain reaction (PCR) was performed using an Applied Biosystems 7300 Real-Time PCR system using methods previously described.25, 27 Allelic discrimination was verified by direct DNA sequencing of a small subgroup of patients of each haplotype (males and females: wild-type, variant, and heterozygous, respectively) to assure the PCR based assay was sufficiently accurate.
Multiple organ failure was evaluated using the well-validated Marshall Multiple Organ Dysfunction Score (MODScore).5, 28, 29 A MODScore > 5 beyond 48 hours from injury and ICU admission was classified as multiple organ failure (MOF). Primary infectious outcomes of interest include ventilator associated pneumonia, blood stream infection (excluding those associated with an intra-abdominal abscess), and urinary tract infections.30 These were selected in attempts to use those infectious outcomes which can be used as a marker for the degree of relative immune suppression. The development of these nosocomial infections was based upon positive culture evidence.
Blood samples were serially obtained at 6 hours, 24 hours and 72 hours of injury and serum was separated and frozen at −80°C until assayed for cytokine analysis. Cytokine expression including; IL-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-15, IFN-α, IFN-γ were measured from patients serum using a Luminex™ 100 IS System and commercially available human specific beadsets. Thromboelastography (TEG) was performed within the first 6 hours of injury and at 24 hours using on a TEG® 5000 Thromboelastograph® Hemostasis Analyzer and standard TEG parameters were recorded including r value, k time, α-angle, maximal amplitude (MA), G value, and fibrinolysis at 30min (LY30) as previously described.31-35
First, patients with and without the IRAK-1 polymorphism underwent unadjusted comparison of demographics, injury characteristics, resuscitation and transfusion requirements and clinical outcomes. Multivariable logistic regression analysis was then utilized to determine the independent risks of our clinical outcomes associated with the IRAK-1 variant. Covariates adjusted for in the regression model included age, sex (male vs. female), race, body mass index (BMI) injury severity score (ISS), presenting systolic blood pressure (SBP), presenting Glasgow Coma Score (GCS), intubation status, presenting coagulopathy (INR>1.5), 24 hour crystalloid and blood component transfusion requirements. Due to the X chromosome location of the polymorphism, we then characterized the risk of our clinical outcomes across whether the IRAK-1 variant existed in a homozygous manner (male-1 variant allele, female -2 variant alleles) or heterozygous manner (females -1 variant allele) to determine if a dose response relationship existed. Finally, we characterized serial cytokine expression and TEG parameters for patients with and without the IRAK-1 polymorphism.
All data were summarized as mean ± SD, median [IQR, inter-quartile range], or percentage (%). Student-t or Mann-Whitney statistical tests were used to compare continuous variables, while χ2 or Fischer's Exact test was used for categorical variables. A p-value of ≤ 0.05 was considered statistically significant. The institutional review board at the University of Pittsburgh approved this study.
Results
Over the study time period, 321 patients met inclusion and exclusion criteria and constituted the study cohort. The overall study cohort had a mean age of 50±16 years, was 70% male, and had a median ISS of 16 [10,21]. The cohort had an average ICU length of stay of 5.3±6 days and an overall incidence of MOF, NI and mortality for the cohort was 8.1%, 27.0% and 4.4%, respectively. The prevalence of the IRAK-1 polymorphism across males and females in the study cohort was 21.5% when heterozygous females (n=29) were also included. For the purposes of the principal analyses, only homozygous patients (males- 1 variant allele, females-2 variant alleles) were considered to have the IRAK-1 variant (12.5%). In the IRAK-1 variant group, 5 patients were female and were homozygous for the variant allele.
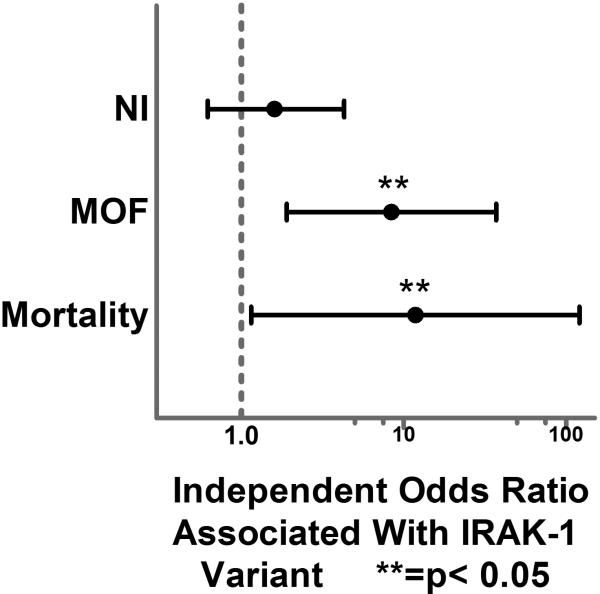
When IRAK-1 variant patients were compared to those with the normal haplotype, patients were similar in demographics, presenting vital and GCS, overall injury severity and 24-hour resuscitation and transfusion requirements. (Table 1.). IRAK-1 patients were more commonly male and had a significantly higher rate of MOF and mortality in unadjusted comparison. Our logistic regression model was an excellent predictor of mortality with an area under the curve (AUC) of 0.94 via Receiver Operating Characteristic (ROC) curve analysis. The model was also a strong predictor of MOF and adequate predictor of NI with AUC of 0.90 and 0.70, respectively. After controlling for all important confounders, the IRAK-1 variant was not a significant independent risk factor for the development of NI (OR 1.6, p=0.315, 95%CI 0.62– 4.3). When both MOF and mortality were analyzed, the IRAK-1 variant was significantly associated with over an 8-fold greater independent odds of MOF (OR 8.4, p=0.005, 95% CI 1.9– 37.1) and over an 11-fold greater independent odds of mortality (OR 11.8, p=0.037, 95% CI 1.1– 121) (Fig. 1.).
Table 1.
Unadjusted comparison of IRAK-1 variant and normal haplotype demographics, injury characteristics and outcomes.
| IRAK-1 Variant (n=40) | Normal Haplotype (n=281) | p-value | |
|---|---|---|---|
| Age (years) | 47±22 | 50±19 | 0.388 |
| Sex (%Male) | 87.5% | 67.3% | 0.009 |
| Race (%Caucasian) | |||
| Caucasian | 72.5% | 81.5% | |
| African American | 7.5% | 2.5% | 0.176 |
| Other/Unknown | 20% | 16.0% | |
| ED SBP (mmHg) | 130±25 | 129±28 | 0.817 |
| ED GCS | 15 [14,15] | 15 [14,15] | 0.645 |
| Injury Severity Score (ISS) | 17 [13,20] | 16 [10,21] | 0.442 |
| Intubation Status (%yes) | 16.7% | 11.2% | 0.573 |
| Body Mass Index (BMI) | 26.9±4 | 29.2±7 | 0.078 |
| ICU Days | 6.3±7 | 5.2±6 | 0.289 |
| Length Of Stay | 12±10 | 11±9 | 0.337 |
| 24 hour Crystalloid (cc) | 3770±2900 | 3290±2160 | 0.249 |
| 24 hour Blood Transfusion (cc) | 447±820 | 437±1010 | 0.956 |
| 24 hour Plasma Tranfusion (cc) | 179±653 | 218±790 | 0.786 |
| 24 hour Platelet Transfusion (cc) | 114±326 | 70±227 | 0.328 |
| Nosocomial Infection | 33.3% | 26.1% | 0.381 |
| Pneumonia | 21.2% | 18.3% | 0.683 |
| MOF% | 18.2% | 5.4% | 0.006 |
| Mortality % | 12.5% | 3.2% | 0.007 |
ED SBP – emergency department systolic blood pressure; GCS- glascow coma score; ICU- intensive care unit, MOF-multiple organ failure
Figure 1.
Forest plot depicting the independent Odds Ratio for the development of nosocomial infection (NI), multiple organ failure (MOF), and mortality associated with the IRAK-1 variant.
To characterize significance of homozygous or heterozygous status of the IRAK-1 variant we first looked at the incidence of MOF and mortality across the haplotype designation (Table 2.). This unadjusted comparison revealed a dose response relationship with heterozygous females having an intermediate incidence of MOF and mortality relative to the normal haplotype and homozygous IRAK-1 variant. When the haplotype (CT and CC relative to the normal haplotype TT) of the IRAK-1 variant was analyzed concurrently in the regression model, as compared to the odds of poor outcome associated with the normal haplotype, both the heterozygous haplotype and homozygous IRAK-1 variant were significant independent risk factors for MOF (p-value 0.012 and 0.003, respectively). Only the homozygous IRAK-1 variant (CC) remained a significant independent risk factor for mortality when both variant haplotypes were included in the model.
Table 2.
Unadjusted rates of multiple organ failure (MOF) and mortality across haplotype of IRAK-1 gene.
| Normal Haplotype (TT, n=281) | Variant Haplotype Heterozygous (CT, n=29) | Variant Haplotype Homozygous (CC, n=40) | p-value | |
|---|---|---|---|---|
| MOF | 4.7% | 11.1% | 18.2% | 0.012 |
| Mortality | 2.4% | 10.3% | 12.5% | 0.004 |
When serial cytokine measurements were characterized, early IL-6 and IL-10 levels were significantly correlated in a positive direction with the propensity to develop MOF and mortality, however, there was no significant relationship with serial cytokine expression and the IRAK-1 variant or IRAK-1 haplotype (TT,CT,CC).
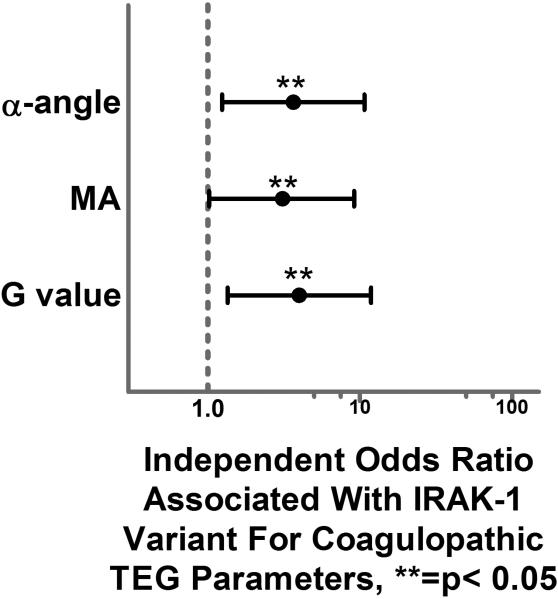
When coagulopathy was further characterized, we first excluded those patients who presented on oral anticoagulation or antiplatelet medications (n = 91). We then looked the continuous TEG parameter variables and the extreme quartile (> 75th percentile or < 25th percentile) associated with coagulopathy for each TEG parameter (r value, k time, α-angle, MA, G value and LY30). When these were compared across TEG measurements drawn in the first 6 hours from injury, there were no significant differences found across those with and without the IRAK-1 variant. When TEG measurements that were performed at 24 hours out from injury were analyzed, there were significantly higher k time, α-angle, MA and G values (p=0.029, p=0.021, p=0.45, p=0.043, respectively) in those patients with the IRAK-1 variant. As this represents a potential evolving coagulopathy over the initial 24 hours post injury we verified these significant differences in a regression model which also controlled for differences in demographics, injury severity and 24 hour resuscitation and transfusion requirements (age, sex, ISS, 24 hour blood, plasma, platelet and crystalloid). After controlling for important differences across the groups, the IRAK-1 variant remained significantly associated in 3 out of the 4 TEG parameters with over a 3-fold greater independent risk of coagulopathic tendency (α-angle- p=0.018, MA-p=0.047, and G value- p=0.012, Fig. 2.).
Figure 2.
Forest plot depicting the independent odds ratio for the development of coagulopathic TEG parameters at 24 hours from injury associated with the IRAK-1 variant.
Discussion
As few effective interventions exist which alter the morbidity and mortality that inherently follows traumatic injury, investigation into novel mechanisms which result in a protective effect may provide a route to reduce these sequelae post-injury. The ultimate elucidation of the mechanisms responsible for sex based outcome differences will provide insight and understanding of novel therapeutic targets which have significant potential to improve outcomes in both males and females post-injury.
Toll-like receptors (TLRs) are an evolutionarily conserved family of protein receptors which are central to NF-κB cellular signaling and the initiation of the innate immune response to infection.36-38 Accumulating evidence suggests that TLRs also recognize endogenous ligands that arise from cellular damage that are unrelated to infection.21-24 Compelling evidence has revealed that the TLR receptor, specifically TLR4, is required and plays a critical role in the early activation and up regulation of the innate immune response, the resultant systemic inflammatory response, and the secondary organ dysfunction which is known to complicate and follow traumatic injury.17-20 Concurrently, it is known that females would potentially be less affected by an unfavorable X-linked genetic polymorphism due to the mosaic expression pattern of the X-chromosome, which has been shown in other disease processes to be protective for females.16, 39-41
The results of the current prospective analysis suggest the an IRAK-1 polymorphism, which is a TLR signaling pathway constituent that also resides on the X-chromosome that is known to result in increased NF-κB cellular signalling, is strongly associated with the development of MOF and mortality in a prospectively enrolled cohort of injured patients that required ICU admission. Further confirmation of the significance of these findings is demonstrated by prevalence of the polymorphism in a single center injured population and the dose response relationship determined by the haplotype (heterozygous or homozygous expression) of the IRAK-1 variant. The current results verify that the IRAK-1 polymorphism represents a common variant with prognostic potential and demonstrates the importance of TLR signaling post-injury and further supports that a genetic mechanism may in part drive sex based outcome differences post-injury.
These results compliment prior studies demonstrating a detrimental association of the IRAK-1 variant in patients with sepsis.25, 26 Arcaroli and colleagues studied the same polymorphism to identify the IRAK-1 variant haplotype and characterized its association with clinical outcomes in a septic population (n=155). The IRAK-1 variant was relatively common in this septic population (prevalence = 21.3%) and was associated with increased nuclear translocation of NF-κB (synonymous with NF-κB activation), more severe organ dysfunction, and independently associated with a higher risk of mortality, in this cohort of patients. Similarly, evidence exists that racial disparities exist in the strength of sex base outcome differences which correlates with the known prevalence of the IRAK-1 variant across different racial groups.42
Importantly, the current results are not simply an extension of sex based outcomes which have been previously demonstrated following traumatic injury in multiple studies. 7, 15, 43-51 In the current 321 patient cohort, there were no significant differences in the clinical outcomes (NI, MOF or mortality) across male and female sex nor was sex a significant covariate in any of the regression models. Prior studies demonstrating sex based outcome differences post-injury have utilized larger retrospective and prospective injured populations and have demonstrated a significantly lower magnitude of risks of poor outcome across males and females with few able to characterize the risk of multiple organ failure and attributable complications. 7, 43-51 The strength of the current findings, demonstrated in a relatively small cohort of patients, provides insight into the magnitude of effect the IRAK-1 variant may have on clinical outcomes post-injury. Despite the strength of these clinical outcome findings, the underlying mechanism responsible remains less clearly characterized.
It is somewhat surprising that no differences in serial cytokine expression between patients with and without the IRAK-1 variant were found. It is known that IL-6 levels are a strong predictor of the development of MOF52, 53 and despite the strong association between the IRAK-1 variant and MOF, no differences were found for IL-6 or other cytokine expression. Importantly, the full spectrum of circulating mediators that might contribute to the immune response driven by TLR and IRAK-1 were not able to be measured for the analysis. The most current paradigm holds that increased innate immune activity leads to proinflammatory mediators and subsequent organ dysfunction and attributable morbidity and mortality.54 The lack of any significant measurable differences in the proteomic (cytokine) response may call into question this paradigm in this particular circumstance. It may be that either early proteomic or innate immune response differences exist but were not able to be appropriately measured by standard cytokine analysis which was undertaken, or that the clinical outcome differences found follow an alternative paradigm or model in this specific situation.
We attempted to characterize the early and evolving coagulopathy for the cohort as it has been shown to be a significant risk factor for poor outcome and we have previously demonstrated significant differences in the risk of coagulopathy across male and female sex post-injury.55-60 Although no TEG parameter differences were demonstrated early (6hrs) post injury, a strong relationship with evolving coagulopathy over the first 24 hours was demonstrated. It may be that patients at high risk for MOF have a tendency toward coagulopathy or these early differences in coagulation may be in part driving the risk of MOF. Mechanistic possibilities include that the TLR signaling cascade by way of the IRAK-1 polymorphism in some way drives this evolving coagulopathy. It has been previously demonstrated that hypoperfusion and activated protein C are principal drivers of trauma induced coagulopathy. 61, 62 Importantly, hemorrhagic shock and traumatic injury are principal drivers of TLR activation.63, 64 It may be that the principal drivers of these occurrences overlap. Importantly, there were no significant differences in the initial 24 hour transfusion or resuscitation requirements across patients with and without the IRAK-1 variant. The TEG parameters which were found to be significantly abnormal after adjustment were the α-angle, MA and G value. The α-angle characterizes the rate of thrombin generation, conversion of fibrinogen to fibrin and the interactions among fibrinogen, fibrin and platelets. Both the MA and G value TEG parameters characterize the overall clot strength with contributors to clot strength including platelet and fibrinogen function. The current results verify there is an association with the IRAK-1 variant in this cohort with evolving coagulopathy based upon serial TEG measurements. However, the current analysis is unable to provide causal information regarding these developments and the interaction of MOF, coagulopathy and the early innate immune response post-injury.
The current analysis does have several limitations that deserve discussion. First, this study was performed at a single, level I trauma center and may not be generalizable or pertinent to other centers with differing admission demographics, injury characteristics or management practices. Although the data collected for the prospective cohort analysis was extensive, potential unknown or unmeasured confounding variables may be responsible for the associations described and the conclusions formulated. The study group represents a smaller cohort than previous sex studies but is substantially larger than some of the prior sepsis studies for which the IRAK-1 variant has been characterized clinically.15, 25 There was a lower than expected incidence of the selected pertinent outcomes of the study including MOF and mortality which can have an exaggeratory effect on the odds ratios presented in certain circumstances. Importantly, it has been previously demonstrated that a large portion of the most critically injured patients suffer mortality relatively early, commonly within the first 48 hours.65 Due to the requirement of informed consent the most critically ill patients had a lower consent rate significantly reducing the incidence of mortality for the study cohort. Although the within 6 hour early cytokine expression measurements which were performed represents a relatively early time point compared to most other studies, this may still represent a delayed measurement for cytokine expression which drives the development of MOF and mortality. Finally, our current understanding of the early coagulopathy which complicates trauma is just beginning to expand due to the complex nature of the process. The most appropriate analysis of TEG parameters remains controversial with variability in the methods of comparison across studies.31-35 We utilized the extreme quartile, either > 75% or < 25% depending on the specific parameter for logistic regression modeling. This possibly may result in an underestimation or overestimation of coagulopathic tendency for specific patients.
In conclusion, the IRAK-1 polymorphism is a strong independent predictor of MOF and mortality post-injury and represents a common variant with prognostic potential. These data demonstrate the importance of TLR signaling post-injury and supports that an X-chromosome linked genetic mechanism may drive sex based outcome differences post-injury.
Acknowledgments
Funding: This work was funded by NIH NIGMS K23GM093032 and Award # NTI-NTI-TRA-09-030 from the National Trauma Institute and sponsored by the Department of the Army, # W81XWH-10-1-0924. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense
Footnotes
This paper was presented as an oral presentation at the annual meeting of the American Surgical Association in Boston, MA, April 10-April 12, 2014.
Author Contributions: J.L.S. and S.Z. designed the study, performed the literature search, data collection and analysis. All authors contributed to data interpretation, manuscript preparation, and critical revision of the manuscript.
References
- 1.Manship L, McMillin RD, Brown JJ. The influence of sepsis and multisystem and organ failure on mortality in the surgical intensive care unit. Am Surg. 1984;50:94–101. [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Nathens AB, Marshall JC. Sepsis, SIRS, and MODS: what's in a name? World J Surg. 1996;20:386–391. doi: 10.1007/s002689900061. [DOI] [PubMed] [Google Scholar]
- 5.Carrico CJ, Meakins JL, Marshall JC, et al. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 6.Roumen RM, Redl H, Schlag G, et al. Inflammatory mediators in relation to the development of multiple organ failure in patients after severe blunt trauma. Crit Care Med. 1995;23:474–80. doi: 10.1097/00003246-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Sperry JL, Minei JP. Gender dimorphism following injury: making the connection from bench to bedside. J Leukoc Biol. 2008;83:499–506. doi: 10.1189/jlb.0607360. [DOI] [PubMed] [Google Scholar]
- 8.Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006;6:127–135. doi: 10.2174/187153006777442422. [DOI] [PubMed] [Google Scholar]
- 9.Dossett LA, Swenson BR, Evans HL, et al. Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surg Infect (Larchmt) 2008;9:41–48. doi: 10.1089/sur.2007.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dossett LA, Swenson BR, Heffernan D, et al. High levels of endogenous estrogens are associated with death in the critically injured adult. J Trauma. 2008;64:580–585. doi: 10.1097/TA.0b013e31816543dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angele MK, Schwacha MG, Ayala A, et al. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama Y, Schwacha MG, Samy TS, et al. Gender dimorphism in immune responses following trauma and hemorrhage. Immunol Res. 2002;26:63–76. doi: 10.1385/ir:26:1-3:063. [DOI] [PubMed] [Google Scholar]
- 13.Choudhry MA, Schwacha MG, Hubbard WJ, et al. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24(Suppl 1):101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 14.Yang S, Hu S, Chen J, et al. Mechanism of hepatoprotection in proestrus female rats following trauma-hemorrhage: heme oxygenase-1-derived normalization of hepatic inflammatory responses. J Leukoc Biol. 2009;85:1015–1026. doi: 10.1189/jlb.0508288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperry JL, Nathens AB, Frankel HL, et al. Characterization of the gender dimorphism after injury and hemorrhagic shock: are hormonal differences responsible? Crit Care Med. 2008;36:1838–1845. doi: 10.1097/CCM.0b013e3181760c14. [DOI] [PubMed] [Google Scholar]
- 16.Migeon BR. The role of X inactivation and cellular mosaicism in women's health and sex-specific diseases. JAMA. 2006;295:1428–1433. doi: 10.1001/jama.295.12.1428. [DOI] [PubMed] [Google Scholar]
- 17.Kaczorowski DJ, Mollen KP, Edmonds R, et al. Early events in the recognition of danger signals after tissue injury. J Leukoc Biol. 2008;83:546–552. doi: 10.1189/jlb.0607374. [DOI] [PubMed] [Google Scholar]
- 18.Levy RM, Prince JM, Yang R, et al. Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4. Am J Physiol Regul Integr Comp Physiol. 2006;291:R970–976. doi: 10.1152/ajpregu.00793.2005. [DOI] [PubMed] [Google Scholar]
- 19.Prince JM, Levy RM, Yang R, et al. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 2006;202:407–417. doi: 10.1016/j.jamcollsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Mollen KP, Anand RJ, Tsung A, et al. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 21.Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172:20–24. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Carpio DF, Zheng Y, et al. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128–7135. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi K, Burkart V, Flohe S, et al. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 24.Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 25.Arcaroli J, Silva E, Maloney JP, et al. Variant IRAK-1 haplotype is associated with increased nuclear factor-kappaB activation and worse outcomes in sepsis. Am J Respir Crit Care Med. 2006;173:1335–1341. doi: 10.1164/rccm.200603-341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toubiana J, Courtine E, Pene F, et al. IRAK1 functional genetic variant affects severity of septic shock. Crit Care Med. 2010;38:2287–2294. doi: 10.1097/CCM.0b013e3181f9f9c7. [DOI] [PubMed] [Google Scholar]
- 27.Liu G, Park YJ, Abraham E. Interleukin-1 receptor-associated kinase (IRAK) -1-mediated NF-kappaB activation requires cytosolic and nuclear activity. FASEB J. 2008;22:2285–2296. doi: 10.1096/fj.07-101816. [DOI] [PubMed] [Google Scholar]
- 28.Marshall JC. Organ dysfunction as an outcome measure in clinical trials. Eur J Surg Suppl. 1999:62–67. doi: 10.1080/11024159950188583. [DOI] [PubMed] [Google Scholar]
- 29.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core--standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60:1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 31.Holcomb JB, Minei KM, Scerbo ML, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256:476–486. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 32.Cotton BA, Faz G, Hatch QM, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71:407–414. doi: 10.1097/TA.0b013e31821e1bf0. discussion 414-417. [DOI] [PubMed] [Google Scholar]
- 33.Kashuk JL, Moore EE, Wohlauer M, et al. Initial experiences with point-of-care rapid thrombelastography for management of life-threatening postinjury coagulopathy. Transfusion. 2012;52:23–33. doi: 10.1111/j.1537-2995.2011.03264.x. [DOI] [PubMed] [Google Scholar]
- 34.Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251:604–614. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 35.Kashuk JL, Moore EE, Le T, et al. Noncitrated whole blood is optimal for evaluation of postinjury coagulopathy with point-of-care rapid thrombelastography. J Surg Res. 2009;156:133–138. doi: 10.1016/j.jss.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Armant MA, Fenton MJ. Toll-like receptors: a family of pattern-recognition receptors in mammals. Genome Biol. 2002:3. doi: 10.1186/gb-2002-3-8-reviews3011. REVIEWS3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 38.Kimbrell DA, Beutler B. The evolution and genetics of innate immunity. Nat Rev Genet. 2001;2:256–267. doi: 10.1038/35066006. [DOI] [PubMed] [Google Scholar]
- 39.Migeon BR. X-chromosome inactivation: molecular mechanisms and genetic consequences. Trends Genet. 1994;10:230–235. doi: 10.1016/0168-9525(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 40.Migeon BR. X chromosome inactivation: theme and variations. Cytogenet Genome Res. 2002;99:8–16. doi: 10.1159/000071568. [DOI] [PubMed] [Google Scholar]
- 41.Migeon BR. X inactivation, female mosaicism, and sex differences in renal diseases. J Am Soc Nephrol. 2008;19:2052–2059. doi: 10.1681/ASN.2008020198. [DOI] [PubMed] [Google Scholar]
- 42.Sperry JL, Vodovotz Y, Ferrell RE, et al. Racial disparities and sex-based outcomes differences after severe injury. J Am Coll Surg. 2012;214:973–980. doi: 10.1016/j.jamcollsurg.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wohltmann CD, Franklin GA, Boaz PW, et al. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181:297–300. doi: 10.1016/s0002-9610(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 44.Bowles BJ, Roth B, Demetriades D. Sexual dimorphism in trauma? A retrospective evaluation of outcome. Injury. 2003;34:27–31. doi: 10.1016/s0020-1383(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 45.Croce MA, Fabian TC, Malhotra AK, et al. Does gender difference influence outcome? J Trauma. 2002;53:889–894. doi: 10.1097/00005373-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Oberholzer A, Keel M, Zellweger R, et al. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 47.Rappold JF, Coimbra R, Hoyt DB, et al. Female gender does not protect blunt trauma patients from complications and mortality. J Trauma. 2002;53:436–441. doi: 10.1097/00005373-200209000-00007. discussion 441. [DOI] [PubMed] [Google Scholar]
- 48.Coimbra R, Hoyt DB, Potenza BM, et al. Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! J Trauma. 2003;54:689–700. doi: 10.1097/01.TA.0000058314.31655.5F. [DOI] [PubMed] [Google Scholar]
- 49.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–8. doi: 10.1001/archsurg.134.9.935. discussion 938-940. [DOI] [PubMed] [Google Scholar]
- 50.George RL, McGwin G, Jr., Metzger J, et al. The association between gender and mortality among trauma patients as modified by age. J Trauma. 2003;54:464–471. doi: 10.1097/01.TA.0000051939.95039.E6. [DOI] [PubMed] [Google Scholar]
- 51.George RL, McGwin G, Jr., Windham ST, et al. Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003;19:28–32. doi: 10.1097/00024382-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Sperry JL, Friese RS, Frankel HL, et al. Male gender is associated with excessive IL-6 expression following severe injury. J Trauma. 2008;64:572–578. doi: 10.1097/TA.0b013e3181650fdf. discussion 578-579. [DOI] [PubMed] [Google Scholar]
- 53.Cuschieri J, Bulger E, Schaeffer V, et al. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock. 2010;34:346–351. doi: 10.1097/SHK.0b013e3181d8e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown JB, Cohen MJ, Minei JP, et al. Characterization of acute coagulopathy and sexual dimorphism after injury: females and coagulopathy just do not mix. J Trauma Acute Care Surg. 2012;73:1395–1400. doi: 10.1097/TA.0b013e31825b9f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacLeod J, Lynn M, McKenney MG, et al. Predictors of mortality in trauma patients. Am Surg. 2004;70:805–810. [PubMed] [Google Scholar]
- 57.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 58.Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 59.Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–1463. doi: 10.1097/TA.0b013e318174e8bc. discussion 1463-1465. [DOI] [PubMed] [Google Scholar]
- 61.Cohen MJ, Call M, Nelson M, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255:379–385. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mollen KP, Levy RM, Prince JM, et al. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J Leukoc Biol. 2008;83:80–88. doi: 10.1189/jlb.0407201. [DOI] [PubMed] [Google Scholar]
- 64.Fan J, Li Y, Levy RM, Fan JJ, et al. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 65.Gunst M, Ghaemmaghami V, Gruszecki A, et al. Changing epidemiology of trauma deaths leads to a bimodal distribution. Proc (Bayl Univ Med Cent) 2010;23:349–354. doi: 10.1080/08998280.2010.11928649. [DOI] [PMC free article] [PubMed] [Google Scholar]