Abstract
Urinary bladder dysfunction presents a major problem in the clinical management of patients suffering from pathological conditions and neurological injuries or disorders. Currently, the etiology underlying altered visceral sensations from the urinary bladder that accompany the chronic pain syndrome, bladder pain syndrome (BPS)/interstitial cystitis (IC), is not known. Bladder irritation and inflammation are histopathological features that may underlie BPS/IC that can change the properties of lower urinary tract sensory pathways (e.g., peripheral and central sensitization, neurochemical plasticity) and contribute to exaggerated responses of peripheral bladder sensory pathways. Among the potential mediators of peripheral nociceptor sensitization and urinary bladder dysfunction are neuroactive compounds (e.g., purinergic and neuropeptide and receptor pathways), sensory transducers (e.g., transient receptor potential channels) and target-derived growth factors (e.g., nerve growth factor). We review studies related to the organization of the afferent limb of the micturition reflex and discuss neuroplasticity in an animal model of urinary bladder inflammation to increase the understanding of functional bladder disorders and to identify potential novel targets for development of therapeutic interventions. Given the heterogeneity of BPS/IC and the lack of consistent treatment benefits, it is unlikely that a single treatment directed at a single target in micturition reflex pathways will have a mass benefit. Thus, the identification of multiple targets is a prudent approach, and use of cocktail treatments directed at multiple targets should be considered.
Keywords: urothelium, dorsal root ganglia, adenosine triphosphate, neuropeptides, transient receptor potential channels, neural growth factor
the storage and elimination of urine is a central and peripheral nervous system reflex involving coordinated activity between the urinary bladder and urethra. These tissues are regulated by neural circuits in the brain and spinal cord that determine appropriate smooth and striated muscle function in the lower urinary tract (LUT) (53). Disruption to the neural circuits underlying storage and elimination may often be observed with neurological injuries [e.g., spinal cord injury (SCI), stroke] or disorders (e.g., multiple sclerosis, Parkinson's disease) and may contribute to functional disorders of the urinary bladder, including overactive bladder (OAB) and bladder pain syndrome (BPS)/interstitial cystitis (IC) (5, 35, 52). We have hypothesized that BPS/IC, as well as OAB, may involve alterations to the afferent limb of the micturition reflex, including bladder afferent neurons in the dorsal root ganglia (DRG) and urothelial cells in the bladder wall mucosa. The following sections will review studies related to bladder sensory neuroplasticity following injury, disease, and/or inflammation in an attempt to advance functional bladder disorder understanding and to identify potential targets for therapeutic intervention.
Afferent and Spinal Pathways to the Urogenital Tract
The micturition reflex is organized as an on-off system, switching between two modes of action in the urinary bladder. During the storage phase, somatic and sympathetic excitatory inputs to the urethral sphincters and sympathetic inhibitory inputs to the bladder wall are tonically active, whereas parasympathetic pathways are inactive (34, 79). In contrast, during reflexive or voluntary micturition, parasympathetic input to the bladder wall is excited, and somatosympathetic input to the bladder wall and urethral sphincters is inhibited (100). Unlike local spinal reflexes that underlie most of the storage phase, these LUT micturition reflex mechanisms are predominantly influenced by supraspinal modulation in the pontine micturition center (59).
The switch from the storage to the elimination phase is elicited by slowly adapting mechanoreceptors in the urinary bladder wall (77). As hydrostatic pressure increases, bladder afferent (thinly myelinated Aδ) fibers amplify their signal transduction along the hypogastric and pelvic nerves (53). Bladder afferent nerves that terminate peripherally throughout the tunica mucosa and tunica muscularis propria may also incorporate unmyelinated C-fibers, which respond to nociceptive stimulation by chemicals [e.g., capsaicin (Cap), menthol], extreme intravesical pressure, and inflammation (4, 62, 97). Although typically quiescent during bladder filling, C-fiber activation may contribute to the development of LUT symptoms and pathological conditions of the urinary bladder (32, 159).
Bladder afferent fibers in the pelvic nerve travel centrally via dorsal roots and project into Lissauer's tract, where collateral branches extend ventromedially and ventrolaterally along the superficial layers of the dorsal horn (4, 34, 41). The ventromedial branches follow the medial edge of the dorsal horn into the dorsal commissure and are largely projections from the pudendal nerve and urogenital structures (4, 34, 41). The ventrolateral branches, on the other hand, project to the lateral edge of dorsal horn (laminae I) into the sacral parasympathetic nucleus (SPN) and are termed the lateral collateral pathway (4, 34, 41). Ultimately, the medial and lateral collateral pathways extend to the dorsal commissure and SPN (laminae V–VII), respectively, which contain cell bodies of preganglionic parasympathetic neurons that project to the periphery (34, 36, 41, 95). Primary bladder afferent fibers not only synapse on preganglionic parasympathetic neurons, but also synapse on interneurons in the lumbosacral dorsal commissure, superficial dorsal horn, and SPN (34, 53). These interneurons are important in the maintenance of micturition reflex function and project locally in the spinal cord or to supraspinal cortical integration centers (34, 53).
Urothelial Signaling
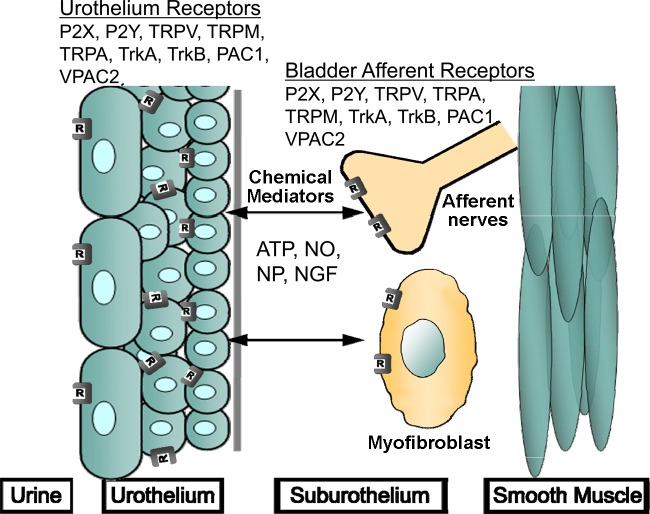
The transitional epithelium lining the urinary bladder mucosa, termed the urothelium, has been demonstrated to detect mechanical, chemical, and thermal stimuli (13). In response to these stimuli, urothelial cells secrete factors like urinary proteins (e.g., urokinase) and signaling molecules (e.g., ATP, ACh, and nitric oxide) through the apical and/or serosal surfaces, suggesting the urothelium may have a role in urinary bladder sensory transduction (13, 15). Additionally, urothelial cells express receptors (e.g., purinergic, cholinergic, and adrenergic) and mechanosensitive channels (e.g., transient receptor potential, TRP) on their surface that are responsive to signals in the extracellular environment (21, 78, 102, 150). As a result of the sensory influence, the urothelium may have in micturition reflex function, any perturbation to urothelial signaling mechanisms and/or the underlying nervous network may contribute to functional disorders of the urinary bladder (13). The following sections will address the distribution, function, and regulation of neuroactive compounds and associated receptors, sensory transducers, and target-derived growth factors, including ATP, neuropeptides, TRP channels, and NGF in micturition reflex pathways under normal and pathological conditions that have been demonstrated to affect LUT function (Fig. 1).
Fig. 1.
Expression of neuroactive chemical/receptor systems and sensory transducers in afferent pathways of the micturition reflex emphasizing urothelium and bladder afferent nerve participation. Receptor activation and channel stimulation on urothelial cells elicits secretion of sensory mediators that may affect adjacent cells and tissues, including bladder afferent nerves in the suburothelial plexus, myofibroblasts, and detrusor smooth muscle. Urothelial cells are also responsive to neurotransmitters released from bladder nerves and other cell types, including inflammatory cells. ATP, adenosine triphosphate; TrkA, receptor tyrosine kinase A; TrkB, receptor tyrosine kinase B; NO, nitric oxide; NGF, nerve growth factor; NP, neuropeptides; PAC1, pituitary adenylate cyclase-activating polypeptide (PACAP) selective receptor; VPAC2, receptor with equal and high affinity for vasoactive intestinal polypeptide and PACAP; TRP, transient receptor potential; V, vanilloid family; M melastatin family; A, ankyrin family; P2, purinergic receptor; R, receptor/channel expression. See text for additional details. Figure modified from Arms and Vizzard (9) [Springer, Handbook of Experimental Pharmacology 202: 395–423, L. Arms and M. A. Vizzard, Fig. 1; with kind permission from Springer Science and Business Media].
ATP in Micturition Reflex Function and Dysfunction
The urothelium responds to changes in hydrostatic pressure by releasing factors, such as ATP, from its mucosal and serosal surfaces (50, 150). ATP released from the serosal surface may then interact with nerve terminals, interstitial cells, and/or basolateral urothelial P2 purinoceptors to transduce sensory information or regulate its release, respectively (150). In functional disorders of the urinary bladder, such as BPS/IC, however, urothelial cells have been demonstrated to increase stretch-evoked ATP release relative to symptom-free controls (125). It has been suggested that the increased ATP release may, in part, underlie the development of lower urinary tract symptoms in micturition reflex dysfunction due to the capacity of intravesical purinergic agonist (ATP or α,β-meATP) instillation to increase the firing rate of urinary bladder afferent nerves and induce bladder overactivity (105, 109, 161). Taken together, these studies have begun to establish the influence of purinergic signaling in urinary bladder sensory transduction and demonstrate its possible role in micturition reflex dysfunction.
P2X and P2Y receptor expression in the LUT.
P2 purinoceptors are classified as ligand-gated ion channels, P2X, or G protein-coupled receptors, P2Y (54). There are currently seven P2X subunits (P2X1–7) that may arrange as heteromeric or homomeric ligand-gated ion channels and eight metabotropic P2Y subunits (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14) that may couple to Gs, Gi, or Gq (1, 106). While the kinetics and tissue distribution of each P2 purinoceptor differ, there is substantial evidence that many of these subunits are expressed throughout the urinary bladder urothelium, lamina propria, and detrusor smooth muscle.
The distribution of P2X and P2Y receptors in the urothelium has been described in multiple species, including rodents, felines, and humans. P2X2 and P2X4–7 receptor immunoreactivity (IR) was detected in the rodent urothelium, whereas, positive IR was detected for P2X1–7 in the feline urothelium (18, 83, 135) (Fig. 1). In the human bladder urothelium, glycosylated P2X2 and P2X3 transcript and protein expression have also been detected (128). There appears to be less diversity in the urothelial distribution of P2Y receptors where P2Y2 and P2Y4 transcript and protein expression has been demonstrated in cultured rat urothelial cells (26). Additionally, in the human urothelial cell line, UROtsa, P2Y1, P2Y2, and P2Y11 transcript expression was detected (116).
The urinary bladder lamina propria is adjacent to the mucosal basement membrane and includes loose connective tissue, vasculature, lymphatics, nerves, and interstitial cells (7). A population of interstitial cells, termed myofibroblasts, in the lamina propria generates intracellular calcium and membrane transients in response to purinergic agonists and express P2X3, P2Y2, P2Y4, and P2Y6 receptors (123, 124). The presence of ATP-dependent transients suggests myofibroblasts may have a role in influencing urinary bladder sensory transduction and warrants further investigation (123, 124).
Afferent (and efferent) nerves terminating in the urothelium, lamina propria, and detrusor smooth muscle have received much of the attention in characterizing P2 purinoceptor distribution. While the transcript and protein expression of all seven P2X subunits (P2X1–7) has been detected in the rodent DRG, there appears to be a differential distribution of P2X2 and P2X3 depending on spinal cord level (24, 113, 155) (Fig. 1). P2X2 mRNA has been detected in both thoracolumbar and lumbosacral urinary bladder afferent neurons, but transcripts at the thoracolumbar level appear to be coexpressed with P2X3 (24). P2X3 transcripts, on the other hand, appear to be restricted to small- and medium-diameter afferent neurons and have a greater frequency of expression in thoracolumbar than lumbosacral neurons (20, 24, 113). Similar to the urothelium, there appears to be less diversity in the distribution of P2Y receptors in DRG neurons. P2Y1, P2Y2, and P2Y4 transcript expression has been detected in rodent DRG neurons with P2Y1 restricted to small-diameter neurons and P2Y4 to medium- and large-diameter neurons (114). P2Y2 and P2Y4, in particular, have been detected in thoracolumbar and lumbosacral bladder afferent neurons (25).
Lastly, P2X and P2Y receptors are expressed on urinary bladder smooth muscle cells. P2X1–6 receptor IR has been demonstrated in rodent urinary bladder smooth muscle cells (44, 83). In contrast, evidence for the expression of P2Y receptors in the detrusor smooth muscle is sparse where P2Y6 has been detected and determined to augment P2X-mediated contractile force (160).
P2X and P2Y receptor expression and function with cystitis.
Of the seven P2X subunits, P2X2 and P2X3 have been suggested to be involved in the sensitization of urinary bladder sensory transduction with cystitis. Specifically, P2X2 and P2X3 protein expression in the human bladder urothelium has been demonstrated to increase in IC (128). Likewise, P2X2 transcript expression was increased in mouse thoracolumbar DRG neurons with cyclophosphamide (CYP)-induced cystitis (24). Inhibition of P2X3 or P2X2/3 receptors with A-317491 following CYP-induced cystitis reduced nonvoiding contractions and residual urine volume and increased intermicturition intervals, suggesting a role for purinergic signaling in bladder hyperreflexia with cystitis (67).
CYP-induced cystitis has also been demonstrated to increase peak urinary bladder afferent nerve activity that was significantly decreased following P2X receptor antagonist (TNP-ATP or PPADS) instillation (161). The significance of P2X3 specifically in bladder afferent nerve sensitization was demonstrated with P2X3-null mice and its attenuation of P2X agonist (ATP or α,β-meATP)-induced afferent nerve excitation (148). Additionally, following CYP-induced cystitis, thoracolumbar rat DRG neurons increased homomeric P2X3 current, whereas, lumbosacral rat DRG neurons increased heteromeric P2X2/3 current (30). While CYP-treated urinary bladder neurons exhibit increased responsiveness to purinergic agonist application, the changes in P2X subtypes do not appear to be conserved across species (24, 30). For instance, homomeric P2X2 currents not seen in rat DRG neurons increased in mouse lumbosacral DRG neurons (24).
Although the role of P2Y receptors in urinary bladder sensory transduction is still maturing, P2Y2 has been implicated in bladder afferent hyperexcitability. Application of UTP, a P2Y2 and P2Y4 agonist, depolarized the resting membrane potential and increased action potential frequency in bladder afferent neurons (25). UTP application also potentiated homomeric P2X2 current in wild-type (WT) but not P2Y2-null mice, suggesting P2Y2 may contribute to P2X-mediated afferent nerve sensitization that was observed with cystitis (25).
Neurochemical Plasticity in the LUT with Bladder Inflammation
Neurochemistry of micturition pathways.
Bladder afferent fibers contain a variety of neuropeptides, including calcitonin-gene-related peptide (CGRP), substance P (SP), neurokinin A, neurokinin B, vasoactive intestinal polypeptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), cholecystokinin, and enkephalins (9, 33, 37, 41, 73, 136, 141). With the exception of CGRP, all of these substances are predominantly expressed in small-diameter (presumably C-fiber) afferents (33, 37, 41, 45, 74, 122, 142, 144–147). The administration of Cap, which acts selectively on small-diameter afferent fibers to deplete neurotransmitter stores and induce neuronal degeneration, reduces the levels of SP, neurokinin A, and CGRP within the pelvic viscera but does not affect VIP or enkephalin expression (31). These findings are consistent with SP, related tachykinins, and CGRP expression in afferent pathways to the pelvic viscera (31). The following sections will focus on the expression, distribution, and functional plasticity of members of the VIP, secretin, the glucagon family of hormones, PACAP, and VIP (Fig. 1). The contributions of other peptides to micturition reflex pathways have recently been described (9).
PACAP, VIP, and Associated Receptor Signaling in Micturition Reflexes
PACAP mechanisms facilitate micturition dysfunction.
Dense PACAP-IR sensory fibers are present in the urinary bladder, including in the suburothelial nerve plexus (19, 58). PACAP receptor expression has also been demonstrated in the detrusor smooth muscle, urothelium, lumbosacral DRG, and spinal cord, suggesting that PACAP signaling participates at multiple levels in the LUT (19, 58) (Fig. 1). Notably, in the detrusor smooth muscle, PACAP has been demonstrated to facilitate contractility by increasing smooth muscle tone and potentiating electrical field stimulation-induced contractions (19).
Following CYP-induced bladder inflammation, PACAP/receptor expression in the DRG and afferent projections to the spinal cord are upregulated (141). The increase in PACAP expression with cystitis may evoke ATP release from the urothelium to enhance bladder sensory transduction (58). Inhibiting the PAC1 receptor with PACAP6–38 following cystitis has been demonstrated to reduce voiding frequency, suggesting PACAP signaling may have a functional role in the inflammation-induced neuroplasticity of the afferent limb of the micturition reflex (19). Differential effects of PACAP have also been demonstrated in peripheral nociception (63, 71, 72). These differences may reflect the diversity of PACAP-mediated second messengers (63), which may result in increased neuropeptide/neurotransmitter production and secretion, as well as facilitation of neuronal depolarization by modulating nonselective cationic channels (e.g., TRP channels) (12).
Disproportionate contribution of PACAP and VIP to altered bladder function.
Urinary bladder function and somatic sensitivity were recently evaluated in PACAP null (PACAP−/−), littermate heterozygote (PACAP+/−), and WT mice (96). PACAP−/− mice exhibit morphological differences in the urinary bladder, including hypertrophy of the lamina propria and detrusor smooth muscle (96). Consistent with an increased bladder mass, PACAP−/− mice exhibit increased bladder capacity, void volume, and intercontraction intervals (96). Furthermore, PACAP−/− mice are less responsive to somatic stimulation and, unlike WT mice, do not exhibit altered urodynamic measurements following intravesical instillation of acetic acid (0.5%) (96). This transgenic mouse model demonstrated that PACAP gene disruption is associated with changes in bladder morphology, bladder function, and reduced somatic and visceral sensitivity (96).
Unlike PACAP, the closely related neuropeptide, VIP, exhibits minimal innervation to the urinary bladder and is predominantly expressed in postganglionic efferent neurons of the pelvic ganglia (22, 49, 118, 152). Minimal innervation of VIP to the urinary bladder is consistent with the lack of functional effects on urinary bladder contractions (66). Application of VIP to detrusor smooth muscle had no effect on spontaneous or carbachol-induced bladder contractions; however, intrathecal or intraarterial administration of VIP-facilitated micturition in the rat (66). The diverse and conflicting roles for VIP demonstrated in the literature (46, 64, 66, 132) may be attributable to differential VIP receptor distribution across species and target tissues. Taken together, it appears that PACAP/receptor signaling is a more prominent regulator of rat bladder physiology compared with VIP/receptor signaling (19, 46, 64, 66, 132).
TRP Channel Expression and Function in the LUT
Studies indicate that several TRP channels, including three members of the vanilloid family (TRPV1, TRPV2, and TRPV4), one member of the melastatin family (TRPM8), and one member of the ankyrin family (TRPA1), are expressed in the urinary bladder and may act as sensory transducers of stretch and/or chemical irritation in the lower urinary tract (6) (Fig. 1). Most of these nonselective, cation channels are also implicated in bladder disorders, such as OAB and BPS/IC (47, 103). Two members of the vanilloid family, in particular, TRPV1 and TRPV4, have received considerable attention in micturition reflex sensory transduction due to their expression on urothelial cells and afferent nerve cells.
TRPV1.
The TRPV1 channel is activated by heat, protons, vanilloids (e.g., Cap), and endovanilloids (e.g., anandamide) and remains the only member of the TRP channel superfamily targeted for therapeutic use in the urinary bladder (6). TRPV1 channel functional expression (11) has been demonstrated in both small-diameter primary afferent neurons (10, 82, 126) and rodent urothelial cells (16). In 2002, Birder et al. (17) indirectly examined the role of TRPV1 in bladder afferent fibers using TRPV1 knockout (KO) mice. Birder et al. (17) demonstrated that TRPV1 KO mice had increased bladder capacity and decreased voiding contractions relative to WT mice, suggesting decreased afferent input to micturition reflex pathways. In support of this interpretation, they demonstrated a decrease in c-Fos-expressing cells in the sacral spinal cord in TRPV1 KO mice compared with WT mice (17).
The direct role of TRPV1 in micturition reflex sensory transduction was examined by Daly et al. (29) using a bladder-pelvic nerve preparation in TRPV1 KO and WT mice. Distension-induced afferent nerve activity was significantly diminished in TRPV1 KO mice relative to WT controls (29). Additionally, administration of a TRPV1 agonist, Cap, increased afferent nerve activity, whereas the TRPV1 antagonist, capsazepine, attenuated distension-induced nerve activity in WT mice (29). Collectively, these studies confirm a role for TRPV1 in the afferent limb of the micturition reflex.
The functional expression of TRPV1 in the urothelium is also relevant to micturition reflex sensory transduction. Data exist in support of the hypothesis that TRPV1 at the level of the urothelium could indirectly activate peripheral bladder afferent terminals. For example, Cap and H+ not only activate urothelial TRPV1 channels, but also induce the secretion of signaling mediators like nitric oxide (16). Furthermore, TRPV1 KO mice exhibit decreased stretch-evoked ATP release from the urothelium, suggesting a role for TRPV1 in purinergic signal transduction (17).
TRPV4.
The TRPV4 channel is a calcium-permeable, stretch-activated, nonselective cation channel that is expressed throughout the renal epithelium, endothelial cells, and urinary bladder epithelium (6, 8). In addition to TRPV4 expression in urothelial cells, the channels are highly expressed in primary sensory neurons, suggesting a role in nociception and mechanosensation and thermosensation (14, 56, 78, 101, 156) (Fig. 1). TRPV4 was originally identified as a channel activated by hypotonic cell swelling (85, 149) but has since been shown to be activated by other physical and chemical stimuli, including mechanical or shear stress, heat (>27°C), arachidonic acid, anandamide, and the synthetic ligands 4α-phorbol 12,13-didecanoate (4α-PDD) and GSK1016790A (GSK) (6, 8).
Similar to the characterization of TRPV1 in urinary bladder function, transgenic mice have been essential to study the role of TRPV4. TRPV4 KO mice have been demonstrated to have decreased voiding frequency and increased frequency of nonvoiding contractions, intermicturition intervals, and total urine output, suggesting diminished afferent input into micturition reflex pathways (48, 56, 129). To further determine the role of TRPV4 in bladder afferent nerve excitability, Aizawa et al. (2) recorded single-unit afferent activity in Aδ-fibers, as well as Cap-sensitive and Cap-insensitive C-fibers. While afferent activity was not altered in Aδ-fibers or Cap-sensitive C-fibers following GSK instillation, Cap-insensitive C-fiber activity significantly increased with the first instillation of GSK (2). These studies suggest TRPV4 may facilitate sensory transduction of the micturition reflex via Cap-insensitive C-fiber afferent nerves (2).
TRPV4 at the level of the urothelium is expressed throughout the basal and intermediate cell layers and has been suggested to be involved in regulating stretch-evoked ATP release (48, 56). Functional TRPV4 channels were demonstrated in urothelial cells when the application of a TRPV4 agonist, 4α-PDD, increased [Ca2+]i, and its influx was attenuated by a nonselective TRP antagonist, ruthenium red (RR) (14). 4α-PDD mediated [Ca2+]i was dependent on both TRPV4 and extracellular calcium, suggesting functional expression of TRPV4 on the urothelium (101). Furthermore, there is a prominent decrease in stretch-evoked ATP release in TRPV4 KO mice and following the depletion of extracellular calcium (56, 101). Taken together, these studies provide evidence for the surface expression of TRPV4 on urothelial cells and suggest a role for TRPV4 in purinergic signaling and bladder sensory transduction.
TRPV channels and nociception.
The literature suggests that the TRPV channel subfamily is involved in the detection of acute noxious thermal, mechanical, and chemical stimuli (134). Hypersensitivity and pain in various pathological conditions are often due to upregulated expression and/or increases in sensitivity of TRPV channels (104). For example, CYP-induced bladder inflammation resulted in mechanical hyperreactivity and hypersensitivity of the hindpaw in WT, but not TRPV1 KO, mice, suggesting TRPV1 is essential for referred mechanosensitivity in peripheral tissues with visceral inflammation (151). Similarly, TRPV1 KO mice show decreased responsiveness to noxious thermal stimuli and fail to develop inflammatory thermal hyperalgesia (104). The TRPV4 channel has also been implicated in nociception and inflammatory pain because of expression on primary afferent nociceptors (23). Similar to TRPV1 KO mice, TRPV4 KO mice fail to develop thermal hyperalgesia and also have decreased sensitivity to tail pressure and deficits in mechanically evoked paw withdrawal responses (14). These studies together suggest TRPV1 and TRPV4 may have roles in the etiology of thermal allodynia and hyperalgesia and may serve as therapeutic targets for pathological pain conditions.
Role of Nerve Growth Factor and Associated Receptors in LUT Plasticity After Inflammation
Neurotrophic factors.
A potential mechanism underlying the morphological (158), electrophysiological (69, 158), and neurochemical (136, 137, 140–142) changes in bladder afferent neurons after cystitis may involve bladder neurotrophic factors (NTFs) and/or neural activity (138). The concept of trophic interactions between nerve cells and their targets is clearly demonstrated during embryonic and postnatal development (81, 87, 108, 133) and more recently in adult tissues (43, 119–121, 130, 131, 138). Studies from our laboratory (138) have demonstrated that chronic CYP-induced cystitis or chronic SCI also alters the expression of nerve growth factor (NGF) and NGF mRNA in the urinary bladder, as well as a variety of other NTFs; however, the role of NGF and associated receptors in LUT pathways, as well as the contribution to LUT reflex plasticity, is the focus of this section.
NGF.
Cytokines and growth factors, including NGF, are upregulated at the site of tissue injury, inflammation, and/or target organ hypertrophy (42, 84, 86, 99, 153). Following noxious peripheral stimulation, for example, levels of neuroactive compounds [e.g., enkephalin (84), dynorphin (115), CGRP (40, 55, 153), SP (55, 84, 115, 136), neuropeptide Y (84), nNOS (139, 142, 143), and PACAP (71, 141)] have been demonstrated to increase in DRG and spinal cord neurons. NGF, in particular, is also released from the target organ for tyrosine kinase receptor (Trk) type 1 (TrkA) binding and retrograde transport in DRG afferent neurons (70) (Fig. 1). The subsequent increase in NGF expression within the DRG neurons may induce increased production of neuropeptides (i.e., SP, CGRP, and PACAP) and alter sensory transduction (40, 55, 153).
NTF receptors.
A large percentage of pelvic visceral afferent neurons express NTF receptors, including Trk for NGF and related substances (98, 111, 112, 154). Following cystitis, NTF receptors exhibit neuroplastic increases in TrkA-IR and TrkB-IR and Trk phosphorylation in bladder afferent neurons (111, 112). Elevated levels of neurotrophins have also been detected in the urine (107) or in the urinary bladder (93) of women with BPS/IC. The activation of neurotrophin/Trk complex in the neuronal cell bodies could activate signal cascade(s) to induce long-term changes in cells, including: 1) neurotransmitter phenotype, 2) synaptic reorganization, 3) increased synaptic efficacy, and 4) target organ dysfunction (94).
Pharmacological manipulation of NGF/receptor signaling in LUT pathways.
NTF overexpression in the urinary bladder may underlie or contribute to the diverse changes in the properties of bladder afferent neurons after CYP-induced cystitis (136, 141). As previously stated, CYP-induced bladder inflammation alters NGF and associated receptor expression in LUT tissues, including the urinary bladder, DRG, and major pelvic ganglion (138). The increased LUT expression of NGF may facilitate bladder activity and afferent cell hyperexcitability (27, 157, 162). Additionally, overexpression of NGF in the bladder smooth muscle has been demonstrated to contribute to bladder hyperinnervation and bladder overactivity (28). The LUT dysfunction mediated by NGF can be reduced by instillation of anti-NGF (39) or a scavenging agent, REN1820 (65), suggesting NGF may underlie, in part, the morphological, functional, and electrophysiological alterations in micturition reflex pathways with cystitis.
Transgenic mouse model with chronic urothelial overexpression of NGF.
Our laboratory has characterized a transgenic mouse model of urothelium-specific NGF overexpression (OE) that represents a novel approach to exploring the role of NGF in urinary bladder inflammation and sensory function (117). Functionally, NGF-OE mice exhibit urinary bladder hyperreflexia with frequent urination and the presence of nonvoiding bladder contractions (NVCs), as well as referred somatic pelvic hypersensitivity (117). NGF-OE mice may represent a useful animal model of BPS/IC because the changes observed in the urinary bladders of these mice are consistent with certain changes observed in this syndrome. Clinically, increased NGF levels have been detected in the bladder urothelium of patients with BPS/IC (93). Elevated NGF levels were also detected in the urine of patients with BPS/IC or OAB symptoms associated with detrusor overactivity (DO), stress urinary incontinence, or bladder outlet obstruction (BOO) (75, 76, 88–92), and patients with DO, who responded to treatment, had reduced urinary NGF levels (90, 92). Our studies with NGF-OE mice are consistent with numerous other studies demonstrating involvement of NGF in altered bladder sensory function (38, 39, 60, 61, 65, 68, 80, 157, 162). Pleiotropic changes, subsequent to NGF overexpression, including changes in the expression of growth factors, neuroactive compounds, and ion channels (e.g., TRP channels) (3, 110) can also directly modulate pain and bladder and visceral sensory function and could contribute to altered urinary bladder function in NGF-OE mice (51, 110, 127, 159) (Fig. 1). Recent studies (57) have demonstrated that TRPV4 transcript and protein expression is significantly increased in the urothelium, suburothelium, and suburothelial nerve plexus of the urinary bladder and in small- and medium-sized lumbosacral (L1, L2, L6-S1) DRG cells from NGF-OE mice compared with littermate WT mice. These studies demonstrate NGF may, in part, regulate TRPV4 expression in LUT tissues to alter urinary bladder sensory transduction. This transgenic mouse model provides us with the opportunity to understand the contribution of NGF overexpression in the urothelium to voiding frequency, nonvoiding contractions, referred somatic sensitivity, and the mechanisms underlying these changes.
Significance of NVCs with Urinary Bladder Dysfunction
Increases in micturition pressure during the filling phase without the release of urine (NVCs) are often associated with OAB and have been observed, as previously noted, in preclinical animal models with urinary bladder dysfunction, including the NGF-OE mouse (117) and following CYP treatment (67) and BOO (75, 76). In addition to the increased appearance of NVCs, these animal models (NGF-OE, CYP, and BOO) also demonstrate increased voiding frequency and decreased void volumes. On the other hand, it has also been reported that urinary bladder dysfunction in TRPV1−/− and TRPV4−/− mice is associated with increased NVCs, reduced voiding frequency, and increased void volumes (17, 48, 56, 129). Increased NVCs and increased voiding frequency have been suggested to represent an increase in bladder afferent activity (57, 75, 76, 117), whereas decreases in bladder afferent activity have been suggested with reduced voiding frequency and increased NVCs (17, 48, 56, 129). Completely different interpretations of NVCs and their relation to bladder afferent activity most likely reflect an incomplete understanding of NVCs, including their origin [neurogenic (afferent, efferent) and/or myogenic], sites of initiation in the urinary bladder, pharmacology, and overall relationship to sensation. A better understanding of NVCs would be aided by additional studies, including the use of an ex vivo bladder-nerve preparation in which simultaneous recordings of bladder pressure and bladder afferent activity during bladder filling could determine the relationship between NVCs and bladder afferent nerve activity under control conditions and following urinary bladder dysfunction.
Perspectives and Significance
Complex neural circuitry underlies the micturition reflex, and perhaps because of this intricacy, micturition reflex function is often compromised as a result of diverse neural injuries, diseases, chronic pain, and inflammatory conditions. We have emphasized the chronic pain condition, BPS/IC, and an animal model of urinary bladder inflammation induced by intraperitoneal injection of CYP in this review to illustrate the neurochemical plasticity that occurs in the sensory components (e.g., bladder afferent cells, the suburothelial plexus of the urinary bladder, and the urothelium) of the micturition reflex. Changes in the expression of numerous chemical mediators, receptors, sensory transducers, as well as target-derived factors are associated with preclinical models of BPS/IC. Given the heterogeneity of BPS/IC, it is unlikely that identification of a single target for pharmacological intervention will have a broad impact. We draw attention to other potential targets (Fig. 1) in the sensory components of the micturition reflex to consider for therapeutic interventions, either alone or in combination, to improve bladder function, including neuroactive compounds/receptor systems, the TRP family of sensory transducers, as well as the target-derived growth factor, NGF.
GRANTS
Research from the Vizzard laboratory described herein was funded by the National Institutes of Health (NIH) grants DK-051369 (M. A. Vizzard), DK-060481 (M. A. Vizzard). Additional support was also provided by grants from the National Center for Research Resources (5 P30 RR 032135) and the National Institute of General Medical Sciences (8 P30 GM 103498) from the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.J.G., L.M., and M.A.V. performed experiments; E.J.G., L.M., and M.A.V. analyzed data; E.J.G., L.M., and M.A.V. interpreted results of experiments; E.J.G. and M.A.V. drafted manuscript; E.J.G., L.M., and M.A.V. edited and revised manuscript; E.J.G., L.M., and M.A.V. approved final version of manuscript; M.A.V. conception and design of research; M.A.V. prepared figures.
ACKNOWLEDGMENTS
The authors acknowledge the technical expertise provided by current laboratory members Dr. Beatrice Girard, Susan Malley, and Abbey Peterson, who contributed to studies from the Vizzard laboratory described.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aizawa N, Wyndaele JJ, Homma Y, Igawa Y. Effects of TRPV4 cation channel activation on the primary bladder afferent activities of the rat. Neurourol Urodyn 31: 148–155, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond) 110: 175–191, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Andersson KE. Bladder activation: afferent mechanisms. Urology 59: 43–50, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Andersson KE. Mechanisms of disease: central nervous system involvement in overactive bladder syndrome. Nat Clin Pract Urol 1: 103–108, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Andersson KE, Gratzke C, Hedlund P. The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int 106: 1114–1127, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Andersson KE, McCloskey KD. Lamina propria: The functional center of the bladder? Neurourol Urodyn 33: 9–16, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Angelico P, Testa R. TRPV4 as a target for bladder overactivity. F1000 Biol Rep 2: 1–6, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arms L, Vizzard MA. Neuropeptides in lower urinary tract function. Hand Exp Pharmacol 202: 395–423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avelino A, Cruz C, Nagy I, Cruz F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience 109: 787–798, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Avelino A, Cruz F. TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications. Naunyn Schmiedebergs Arch Pharmacol 373: 287–299, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Beaudet MM, Parsons RL, Braas KM, May V. Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons. J Neurosci 20: 7353–7361, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birder L, Andersson KE. Urothelial signaling. Physiol Rev 93: 653–680, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birder L, Kullmann FA, Lee H, Barrick S, de Groat W, Kanai A, Caterina M. Activation of urothelial transient receptor potential vanilloid 4 by 4α-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther 323: 227–235, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Birder L, Wyndaele JJ. From urothelial signalling to experiencing a sensation related to the urinary bladder. Acta Physiol (Oxf) 207: 34–39, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 98: 13396–13401, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol 287: F1084–F1091, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol 290: R951–R962, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 12: 256–268, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Bschleipfer T, Schukowski K, Weidner W, Grando SA, Schwantes U, Kummer W, Lips KS. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci 80: 2303–2307, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Chapple CR, Milner P, Moss HE, Burnstock G. Loss of sensory neuropeptides in the obstructed human bladder. Br J Urol 70: 373–381, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Alessandri-Haber N, Levine JD. Marked attenuation of inflammatory mediator-induced C-fiber sensitization for mechanical and hypotonic stimuli in TRPV4−/− mice. Mol Pain 3: 31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Gebhart GF. Differential purinergic signaling in bladder sensory neurons of naive and bladder-inflamed mice. Pain 148: 462–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Molliver DC, Gebhart GF. The P2Y2 receptor sensitizes mouse bladder sensory neurons and facilitates purinergic currents. J Neurosci 30: 2365–2372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopra B, Gever J, Barrick SR, Hanna-Mitchell AT, Beckel JM, Ford AP, Birder LA. Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol 294: F821–F829, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165: 975–979, 2001 [PubMed] [Google Scholar]
- 28.Clemow DB, Steers WD, McCarty R, Tuttle JB. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol Regul Integr Comp Physiol 275: R1279–R1286, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol 583: 663–674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol 99: 49–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Groat WC. Neuropeptides in pelvic afferent pathways. Experientia 43: 801–813, 1987 [DOI] [PubMed] [Google Scholar]
- 32.de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: The Autonomic Nervous System, edited by C. A. M. London : Harwood Academic Publishers, 1993, p. 227–290 [Google Scholar]
- 33.de Groat WC, Kawatani M, Hisamitsu T, Booth AM, Roppolo JR, Thor K, Tuttle P, Nage J. Neural control of micturition: the role of neuropeptides. J Autonom Nerv Syst Suppl: 369–387, 1986 [Google Scholar]
- 34.de Groat WC, Kruse MN. Central processing and morphological plasticity in lumbosacral afferent pathways from the lower urinary tract. In: Basic and Clinical Aspects of Chronic Abdominal Pain Pain Research and Clinical Management, edited by Mayer EA, Raybould HE. Amsterdam: Elsevier Science Publishers, 1993, p. 219–235 [Google Scholar]
- 35.de Groat WC, Kruse MN, Vizzard MA, Cheng CL, Araki I, Yoshimura N. Modification of urinary bladder function after spinal cord injury. In: Advances in Neurology, edited by Seil FJ. New York: Raven Press, 1997, p. 347–364 [PubMed] [Google Scholar]
- 36.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst 3: 135–160, 1981 [DOI] [PubMed] [Google Scholar]
- 37.de Groat WC, Vizzard MA, Araki I, Roppolo J. Spinal interneurons and preganglionic neurons in sacral autonomic reflex pathways. Prog Brain Res 107: 97–111, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain 66: 87–97, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Dmitrieva N, Shelton D, Rice ASC, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78: 449–459, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene- related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo. Neuroscience 49: 693–698, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Donovan MK, Winternitz SR, Wyss JM. An analysis of the sensory innervation of the urinary system of the rat. Brain Res Bull 11: 321–324, 1983 [DOI] [PubMed] [Google Scholar]
- 42.Dray A. Inflammatory mediators of pain. Br J Anaesth 75: 125–131, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol 166: 1111–1118, 2001 [PubMed] [Google Scholar]
- 44.Dutton JL, Hansen MA, Balcar VJ, Barden JA, Bennett MR. Development of P2X receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J Neurocytol 28: 4–16, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Ek A, Alm P, Andersson KE, Persson K. Adrenergic and cholinergic nerves of the human urethra and urinary bladder. Acta Physiol Scand 99: 345–352, 1977 [DOI] [PubMed] [Google Scholar]
- 46.Erol K, Ulak G, Donmez T, Cingi MI, Alpan RS, Ozdemir M. Effects of vasoactive intestinal polypeptide on isolated rat urinary bladder smooth muscle. Urol Int 49: 151–153, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Everaerts W, Gevaert T, Nilius B, De Ridder D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn 27: 264–273, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience 83: 1261–1272, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–a possible sensory mechanism? J Physiol 505: 503–511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP, Cockayne DA. Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol 147 Suppl 2: S132–S143, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fowler CJ. Neurological disorders of micturition and their treatment. Brain 122: 1213–1231, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev 46: 143–156, 1994 [PMC free article] [PubMed] [Google Scholar]
- 55.Gary MB, Hargreaves KM. Enhanced release of immunoreactive CGRP and substance P from spinal dorsal horn slices occurs during carriageenan inflammation. Brain Res 582: 139–142, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453–3462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girard BM, Merrill L, Malley S, Vizzard MA. Increased TRPV4 expression in urinary bladder and lumbosacral dorsal root ganglia in mice with chronic overexpression of NGF in urothelium. J Mol Neurosci 51: 602–614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36: 310–320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffiths DJ. The pontine micturition centres. Scand J Urol Nephrol Suppl 21–26, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193–197, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295: R111–R122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol 166: 4–17, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hernandez M, Barahona MV, Recio P, Benedito S, Martinez AC, Rivera L, Garcia-Sacristan A, Prieto D, Orensanz LM. Neuronal and smooth muscle receptors involved in the PACAP- and VIP-induced relaxations of the pig urinary bladder neck. Br J Pharmacol 149: 100–109, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide-induced cystitis. J Urol 173: 1016–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Igawa Y, Persson K, Andersson KE, Uvelius B, Mattiasson A. Facilitatory effect of vasoactive intestinal polypeptide on spinal and peripheral micturition reflex pathways in conscious rats with and without detrusor instability. J Urol 149: 884–889, 1993 [DOI] [PubMed] [Google Scholar]
- 67.Ito K, Iwami A, Katsura H, Ikeda M. Therapeutic effects of the putative P2X3/P2X2/3 antagonist A-317491 on cyclophosphamide-induced cystitis in rats. Naunyn-Schmiedeberg's Arch Pharmacol 377: 483–490, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth 83: 442–448, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Jennings LJ, Vizzard MA. Cyclophosphamide-induced inflammation of the urinary bladder alters electrical properties of small-diameter afferent neurons from dorsal root ganglia. FASEB J 13: A57, 1999 [Google Scholar]
- 70.Johnson EM, Jr, Taniuchi M, Clark HB, Springer JE, Koh S, Tayrien MW, Loy R. Demonstration of the retrograde transport of nerve growth factor receptor in the peripheral and central nervous system. J Neurosci 7: 923–929, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jongsma H, Danielsen N, Sundler F, Kanje M. Alteration of PACAP distribution and PACAP receptor binding in the rat sensory nervous system following sciatic nerve transection. Brain Res 853: 186–196., 2000 [DOI] [PubMed] [Google Scholar]
- 72.Jongsma H, Pettersson LME, Zhang YZ, Reimer MK, Kanje M, Waldenstrom A, Sundler F, Danielsen N. Markedly reduced chronic nociceptive response in mice lacking the PAC(I) receptor. Neuroreport 12: 2215–2219, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Keast JR. Patterns of co-existence of peptides and differences of nerve fibre types associated with noradrenergic and non-noradrenergic (putative cholinergic) neurons in the major pelvic ganglion of the male rat. Cell Tissue Res 266: 405–415, 1991 [DOI] [PubMed] [Google Scholar]
- 74.Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol 319: 615–623, 1992 [DOI] [PubMed] [Google Scholar]
- 75.Kim JC, Kim DB, Seo SI, Park YH, Hwang TK. Nerve growth factor and vanilloid receptor expression, and detrusor instability, after relieving bladder outlet obstruction in rats. BJU Int 94: 915–918, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Kim JC, Park EY, Hong SH, Seo SI, Park YH, Hwang TK. Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol 12: 875–880, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Kingsley RE, Gable SR, Kingsley TR, Saint Joseph Medical Center (South Bend Ind.), Magnetic Resonance Imaging Center. Concise text of Neuroscience. Baltimore, MD: Williams & Wilkins, 1996, p. viii, 564 p [Google Scholar]
- 78.Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol 296: F892–F901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuru M. Nervous control of micturition. Physiol Rev 45: 425–494, 1965 [DOI] [PubMed] [Google Scholar]
- 80.Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain 5: 150–156, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Lapchak PA, Araujo DM, Hefti F. Neurotrophins in the central nervous system. Rev Neurosci 3: 1–10, 1992 [DOI] [PubMed] [Google Scholar]
- 82.Lazzeri M, Vannucchi MG, Zardo C, Spinelli M, Beneforti P, Turini D, Faussone-Pellegrini MS. Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur Urol 46: 792–798, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol 163: 2002–2007, 2000 [PubMed] [Google Scholar]
- 84.Lewin GR, Mendell L. Nerve growth factor and nociception. Trends Neurosci 16: 353–359, 1993 [DOI] [PubMed] [Google Scholar]
- 85.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of the rat sciatic nerve. Nature 330: 658–659, 1987 [DOI] [PubMed] [Google Scholar]
- 87.Lindsay RM, Shooter EM, Radeke MJ, Misko TP, Dechant G, Thoenen H, Lindholm D. Nerve growth factor regulates expression of the nerve growth factor gene in adult sensory neurons. Eur J Neurosci 2: 389–396, 1990 [DOI] [PubMed] [Google Scholar]
- 88.Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor level could be a biomarker in the differential diagnosis of mixed urinary incontinence in women. BJU Int 102: 1440–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol 56: 700–706, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Liu HT, Kuo HC. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology 70: 463–468, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Liu HT, Kuo HC. Urinary nerve growth factor level could be a potential biomarker for diagnosis of overactive bladder. J Urol 179: 2270–2274, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology 72: 104–108, 2008 [DOI] [PubMed] [Google Scholar]
- 93.Lowe EM, Anand P, Terenghi G, Willimans-Chestnut RE, Sinicropi DV. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79: 572–577, 1997 [DOI] [PubMed] [Google Scholar]
- 94.MacInnis BL, Campenot RB. Retrograde support of neuronal survival without retrograde transport of nerve growth factor. Science 295: 1536–1539, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol 389: 584–602, 1997 [PubMed] [Google Scholar]
- 96.May V, Vizzard MA. Bladder dysfunction and altered somatic sensitivity in PACAP−/− mice. J Urol 183: 772–779, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mazieres L, Jiang C, Lindstrom S. The C fibre reflex of the cat urinary bladder. J Physiol 513: 531–541, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron 12: 1161–1171, 1994 [DOI] [PubMed] [Google Scholar]
- 99.Meller ST, Cummings CP, Traub RJ, Gebbhart GF. The role of nitric oxide in the development and maintenance of the hyperalgesia produced by intraplantar injection of carrageenan in the rat. Neuroscience 60: 367–374, 1994 [DOI] [PubMed] [Google Scholar]
- 100.Middleton JW, Keast JR. Artificial autonomic reflexes: using functional electrical stimulation to mimic bladder reflexes after injury or disease. Auton Neurosci 113: 3–15, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284: 21257–21264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moro C, Tajouri L, Chess-Williams R. Adrenoceptor function and expression in bladder urothelium and lamina propria. Urology 81: 211.e1–e7, 2013 [DOI] [PubMed] [Google Scholar]
- 103.Nilius B. Transient receptor potential (TRP) cation channels: rewarding unique proteins. Bull Mem Acad R Med Belg 162: 244–253, 2007 [PubMed] [Google Scholar]
- 104.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Nishiguchi J, Hayashi Y, Chancellor MB, de Miguel F, de Groat WC, Kumon H, Yoshimura N. Detrusor overactivity induced by intravesical application of adenosine 5′-triphosphate under different delivery conditions in rats. Urology 66: 1332–1337, 2005 [DOI] [PubMed] [Google Scholar]
- 106.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 107.Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 161: 438–442, 1999 [PubMed] [Google Scholar]
- 108.Oppenheim RW, Prevette D, Qin-Wei Y, Collins R, MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science 251: 1616–1618, 1991 [DOI] [PubMed] [Google Scholar]
- 109.Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol 168: 1230–1234, 2002 [DOI] [PubMed] [Google Scholar]
- 110.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–538, 2006 [DOI] [PubMed] [Google Scholar]
- 111.Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol 454: 200–211, 2002 [DOI] [PubMed] [Google Scholar]
- 112.Qiao LY, Vizzard MA. Up-regulation of tyrosine kinase (Trka, Trkb) receptor expression and phosphorylation in lumbosacral dorsal root ganglia after chronic spinal cord (T8-T10) injury. J Comp Neurol 449: 217–230, 2002 [DOI] [PubMed] [Google Scholar]
- 113.Ruan HZ, Birder LA, de Groat WC, Tai C, Roppolo J, Buffington CA, Burnstock G. Localization of P2X and P2Y receptors in dorsal root ganglia of the cat. J Histochem Cytochem 53: 1273–1282, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol 120: 415–426, 2003 [DOI] [PubMed] [Google Scholar]
- 115.Ruda MA, Iadarola MJ, Cohen LV, Yound IIWS. In situ hybridization, histochemistry and immunocyctochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci USA 85: 622–626, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Save S, Persson K. Extracellular ATP and P2Y receptor activation induce a proinflammatory host response in the human urinary tract. Infect Immun 78: 3609–3615, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smet PJ, Moore KH, Jonavicius J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest 77: 37–49, 1997 [PubMed] [Google Scholar]
- 119.Steers WD, Ciambotti J, Etzel B, Erdman S, de Groat WC. Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol 310: 1–10, 1991 [DOI] [PubMed] [Google Scholar]
- 120.Steers WD, de Groat WC. Effect of bladder outlet obstruction on micturition reflex pathways in the rat. J Urol 140: 864–871, 1988 [DOI] [PubMed] [Google Scholar]
- 121.Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest 88: 1709–1715, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Su HC, Polak JM, Mulderry PK, Ghatei MA, Gibson SJ, Terenghi G, Morrison JFB, Ballesta J, Bloom SR. Calcitonin gene-related peptide immunoreactivity in afferent neurons supplying the urinary tract: combined retrograde tracing and immunohistochemistry. Neuroscience 18: 727–747, 1986 [DOI] [PubMed] [Google Scholar]
- 123.Sui GP, Wu C, Fry CH. Characterization of the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. BJU Int 97: 1327–1331, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Sui GP, Wu C, Roosen A, Ikeda Y, Kanai AJ, Fry CH. Modulation of bladder myofibroblast activity: implications for bladder function. Am J Physiol Renal Physiol 295: F688–F697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun Y, Keay S, De Deyne PG, Chai TC. Augmented stretch activated adenosine triphosphate release from bladder urothelial cells in patients with interstitial cystitis. J Urol 166: 1951–1956, 2001 [PubMed] [Google Scholar]
- 126.Szallasi A, Conte B, Goso C, Blumberg PM, Manzini S. Characterization of a peripheral vanilloid (capsaicin) receptor in the urinary bladder of the rat. Life Sci 52: PL221–PL226, 1993 [DOI] [PubMed] [Google Scholar]
- 127.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 6: 357–372, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int 93: 1344–1348, 2004 [DOI] [PubMed] [Google Scholar]
- 129.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropa noyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamid e (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity. Part I. J Pharmacol Exp Ther 326: 432–442, 2008 [DOI] [PubMed] [Google Scholar]
- 130.Tuttle JB, Steers WD. Nerve growth factor responsiveness of cultured pelvic ganglion neurons from the adult rat. Brain Res 588: 29–40, 1992 [DOI] [PubMed] [Google Scholar]
- 131.Tuttle JB, Steers WD, Albo M, Nataluk E. Neural input regulates tissue NGF and growth of the adult rat urinary bladder. J Auton Nerv Syst 49: 147–158, 1994 [DOI] [PubMed] [Google Scholar]
- 132.Uckert S, Stief CG, Lietz B, Burmester M, Jonas U, Machtens SA. Possible role of bioactive peptides in the regulation of human detrusor smooth muscle—functional effects in vitro and immunohistochemical presence. World J Urol 20: 244–249, 2002 [DOI] [PubMed] [Google Scholar]
- 133.Vantini G, Skaper SD. Neurotrophic factors: from physiology to pharmacology. Pharmacol Res 26: 1–15, 1992 [DOI] [PubMed] [Google Scholar]
- 134.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 76: 387–417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vial C, Evans RJ. P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol 131: 1489–1495, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001 [DOI] [PubMed] [Google Scholar]
- 137.Vizzard MA. Alterations in spinal Fos protein expression induced by bladder stimulation followng cystitis. Am J Physiol Regul Integr Comp Physiol 278: R1027–R1039, 2000 [DOI] [PubMed] [Google Scholar]
- 138.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000 [DOI] [PubMed] [Google Scholar]
- 139.Vizzard MA. Increased expression of neuronal nitric oxide synthase in bladder afferent and spinal neurons following spinal cord injury. Dev Neurosci 19: 232–246, 1997 [DOI] [PubMed] [Google Scholar]
- 140.Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol 279: R295–R305, 2000 [DOI] [PubMed] [Google Scholar]
- 141.Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol 420: 335–348, 2000 [PubMed] [Google Scholar]
- 142.Vizzard MA, de Groat WC. Increased expression of neuronal nitric oxide synthase (NOS) in bladder afferent pathways following chronic bladder irritation. J Comp Neurol 370: 191–202, 1996 [DOI] [PubMed] [Google Scholar]
- 143.Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase (NOS) in visceral neurons after nerve injury. J Neurosci 15: 4033–4045, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase in dorsal root ganglion neurons after systemic capsaicin administration. Neuroscience 67: 1–5, 1995 [DOI] [PubMed] [Google Scholar]
- 145.Vizzard MA, Erdman SL, de Groat WC. Localization of NADPH-diaphorase in bladder afferent and postganglionic efferent neurons of the rat. J Auton Nerv Syst 44: 85–90, 1993 [DOI] [PubMed] [Google Scholar]
- 146.Vizzard MA, Erdman SL, de Groat WC. Localization of NADPH-diaphorase in pelvic afferent and efferent pathways of the rat. Neurosci Lett 152: 72–76, 1993 [DOI] [PubMed] [Google Scholar]
- 147.Vizzard MA, Erdman SL, Förstermann U, de Groat WC. Differential distribution of nitric oxide synthase in neural pathways to the urogenital organs (urethra, penis, urinary bladder) of the rat. Brain Res 646: 279–291, 1994 [DOI] [PubMed] [Google Scholar]
- 148.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21: 5670–5677, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75: 1262–1279, 2009 [DOI] [PubMed] [Google Scholar]
- 150.Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115: 2412–2422, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 139: 158–167, 2008 [DOI] [PubMed] [Google Scholar]
- 152.Wanigasekara Y, Kepper ME, Keast JR. Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell Tissue Res 311: 175–185, 2003 [DOI] [PubMed] [Google Scholar]
- 153.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor. Br J Pharmacol 121: 417–424, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wright DE, Snyder WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol 351: 329–338, 1995 [DOI] [PubMed] [Google Scholar]
- 155.Xiang Z, Bo X, Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett 256: 105–108, 1998 [DOI] [PubMed] [Google Scholar]
- 156.Yamada T, Ugawa S, Ueda T, Ishida Y, Kajita K, Shimada S. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J Histochem Cytochem 57: 277–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26: 10847–10855, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder following chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology 59: 61–67, 2002 [DOI] [PubMed] [Google Scholar]
- 160.Yu W, Sun X, Robson SC, Hill WG. Extracellular UDP enhances P2X-mediated bladder smooth muscle contractility via P2Y(6) activation of the phospholipase C/inositol trisphosphate pathway. FASEB J 27: 1895–1903, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol 294: F1146–F1156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7: 9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]