Abstract
Lymph flow is the primary mechanism for returning interstitial fluid to the blood circulation. Currently, the adaptive response of lymphatic vessels to mesenteric venous hypertension is not known. This study sought to determine the functional responses of postnodal mesenteric lymphatic vessels. We surgically occluded bovine mesenteric veins to create mesenteric venous hypertension to elevate mesenteric lymph flow. Three days after surgery, postnodal mesenteric lymphatic vessels from mesenteric venous hypertension (MVH; n = 7) and sham surgery (Sham; n = 6) group animals were evaluated and compared. Contraction frequency (MVH: 2.98 ± 0.75 min−1; Sham: 5.42 ± 0.81 min−1) and fractional pump flow (MVH: 1.14 ± 0.30 min−1; Sham: 2.39 ± 0.32 min−1) were significantly lower in the venous occlusion group. These results indicate that postnodal mesenteric lymphatic vessels adapt to mesenteric venous hypertension by reducing intrinsic contractile activity.
Keywords: lymphangion, lymph flow, interstitial fluid, postnodal lymphatic vessels
the lymphatic system drains fluid from the interstitial space and transports it as lymph to the venous system. On the one hand, lymphatic vessels have a very different structure than blood vessels. Unlike blood vessels with primarily circumferential arrangement of smooth muscle, lymphatic vessels also have smooth muscle layers that are oriented longitudinally (38). On the other hand, lymphatic vessels are similar to veins. Lymphatic vessels are relatively thin-walled and can collapse, have muscle allowing phasic and tonic contraction, contain unidirectional valves, and have a tone modulated by a number of vasoactive mediators (14, 23, 46). Gaining far more attention in the last decade, however, is the observation that lymphangions, the segments of lymphatic vessels bound by valves, form units that function like cardiac ventricles. Lymphangions can act as chambers that cyclically contract, and are capable of actively pumping lymph against an axial pressure gradient (35). Similar to ventricles (21), increases in transmural pressure can cause lymphangions to increase stroke volume and contraction frequency (22, 31). Not only does this property allow lymph to be propelled from the low-pressure interstitial space to the higher-pressure venous system, it also allows lymphatic vessels to respond to increases in interstitial fluid pressure by pumping more fluid out of the interstitium (3, 18, 43).
Mesenteric venous hypertension causes persistent intestinal edema.
Intestinal microvascular hypertension and resulting interstitial edema may occur with portal venous thrombosis, hepatic fibrosis, or mesenteric venous thrombosis. Acute venous hypertension leads sequentially to increased intestinal microvascular pressure, increased microvascular filtration, increased interstitial fluid volume and pressure, and finally, a several-fold increase in mesenteric lymph flow (13, 20, 27). The increase in lymph flow is particularly pronounced when hypertension is induced regionally by increased resistance of regional veins (13, 36, 45), since, unlike central venous hypertension, regional venous hypertension does not raise the outlet pressure of the lymphatic system (10, 28). Gashev et al. (17) reported that increased luminal flow in vitro decreases the rate and magnitude of lymphangion contraction in mesenteric lymphangions. Like blood vessels, this response is due to increased endothelial shear stress and is mediated, in part, by the production of nitric oxide (17). Although regional venous hypertension has been used by numerous investigators to study edema formation and lymphatic function (12, 13, 45), all studies up to this point have been acute. Investigators have yet to report that persistent intestinal edema secondary to increased microvascular pressure involves adaptive responses by lymphangions. Recent studies have reported that postnodal bovine mesenteric lymphangions adapt to altered pressures in vivo in as little as 3 days (8). Therefore, we used a mesenteric venous hypertension model to test the hypothesis that postnodal bovine mesenteric lymphatic vessels adapt to become weaker pumps within 3 days.
METHODS
Experimental groups.
Experimental procedures and animal care were performed in compliance with protocols approved by the Texas A&M University Institutional Animal Care and Use Committee and were consistent with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Holstein cows used for this study were randomly divided into two experimental groups: mesenteric venous hypertension (MVH) and sham surgery (Sham). Functional and histological analyses of lymphatic vessels were performed to determine their response to MVH.
Surgical preparation.
An intravenous catheter was placed in the jugular vein for drug administration. Oxytetracycline (20 mg/kg) was administered subcutaneously. Anesthesia was induced using xylazine (0.1 mg/kg iv), followed by diazepam (0.01 mg/kg iv) and ketamine (2 mg/kg). Animals were intubated, positioned in left lateral recumbency, and maintained using mechanical ventilation with 1–3% isoflurane in oxygen sufficient to maintain a surgical plane of anesthesia. Arterial oxygen saturation was monitored (Cardell Veterinary Monitor-model MAX-12 HD; CAS Medical Systems, Branford, CT) and maintained >95% throughout the procedure. The right flank was aseptically prepared and draped. A 25-cm incision was made midway between the tuber coxae and the last rib. The small intestine was exteriorized and kept moist using warm (37°C) 0.9% saline.
MVH.
The veins draining the small intestine are arranged in arcades, such that complete obstruction of one mesenteric vein does not stop blood flow, but rather redirects flow, and increases local venous pressure (Fig. 1). The proximal jejunal venous arcade was identified. The vein draining one end of this arcade was carefully isolated by blunt dissection and ligated. The vein draining the other end of the arcade was isolated in a similar fashion and fitted with an appropriately sized vascular occluder (Kent Scientific, Torrington, CT). The occluder was fixed to the mesentery with a piece of suture (2-0 polyglactin 910) and filled with 50% dextrose solution. To measure pressure within the arcade, an upstream vein draining the proximal jejunal venous arcade was isolated by blunt dissection, and 1/8"; O.D. polyvinyl chloride tubing (VWR Scientific, Suwanee, GA) filled with heparinized saline was inserted into the vein and secured with 2-0 polyglactin 910 suture. The PVC tubing and the tail of the vascular occluder were exteriorized through a small skin incision dorsal to the abdominal incision. The jejunum was replaced in the abdomen, and the abdominal incision was closed in multiple layers. The animals then recovered from anesthesia.
Fig. 1.
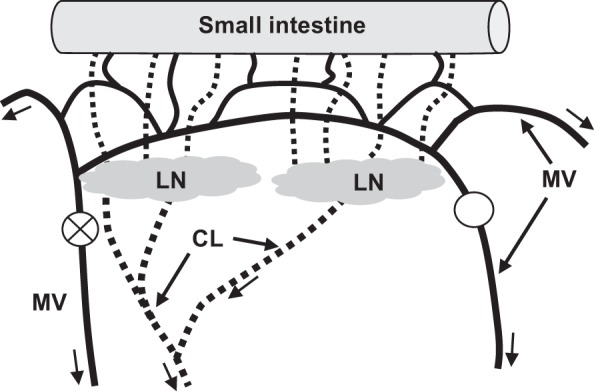
Experimental induction of mesenteric venous hypertension. The veins (solid lines) draining the small intestine are arranged in arcades so that complete obstruction (○) of one mesenteric vein (MV) and constriction (represented as circle with X) of an adjacent MV does not stop blood flow, but rather redirects flow and increases local venous pressure. The resulting increase in intestine microvascular pressure causes microvascular filtration and interstitial water volume to increase. As a result, lymph flow from the intestine and through the lymph nodes (LN) and postnodal collecting lymphatic vessels (CL) increases. Arrows indicate direction of flow for venous blood and lymph.
Sham surgery group.
The same surgical procedures were performed in the sham-treated animals. Sham-treated animals, however, were only instrumented with the intravenous tubing for measurement of intestinal venous pressure. The mesenteric vein was not ligated, and the vascular occluder was not applied.
Measurement of venous pressure.
The day after surgery, mesenteric venous pressure was measured by placing the cow in a restraining chute. The venous catheter was flushed with heparinized saline to ensure patency. A water manometer was used to measure venous pressure in a fashion adapted from a common clinical technique for measuring central venous pressure (49). The manometer was attached to the venous catheter and fixed with the zero-point at the level of the point of the shoulder. The equilibrium column height was recorded as venous pressure. The system was zeroed, and pressures were recorded three times to ensure repeatability. If pressure in the MVH group was less than 30 cmH2O, dextrose solution was injected into the vascular occluder until that pressure was attained. This was done to ensure that the elevated mesenteric venous pressure obtained during surgery was maintained postoperatively. Venous pressure data were compared using the Mann-Whitney rank sum test.
Sample collection.
Three days following surgery, animals were euthanized by captive bolt and exsanguination. Study samples were collected immediately following euthanasia. The small intestine was exposed for tissue sample collection. Postnodal mesenteric lymphatic vessels draining the affected segment were ligated at the downstream end. After carefully dissecting free from surrounding tissue, vessels used for functional and structural analysis were placed in a thermos containing warm (37°C) 1% albumin physiological saline solution (APSS, pH 7.4) containing (in mM) 145 NaCl, 4.70 KCl, 2.00 CaCl2, 1.17 MgSO4, 1.20 NaH2PO4, 5.00 dextrose, 2.00 sodium pyruvate, 0.020 EDTA, and 3.00 MOPS. All the tissue samples were transported a short distance to the laboratory.
Determination of intestinal water content.
Intestinal water content was measured to characterize the effects of the preparation on interstitial fluid balance. A jejunal segment was rinsed with saline to remove the luminal contents, patted dry, and weighed. The segment was placed in a drying oven and dried at 60°C to a constant weight. Percent water content of the intestinal segment was calculated from 100 × (wet weight-dry weight)/wet weight. Data were compared using a Student's t-test.
Preparation of postnodal vessels.
Experimental procedures were similar to those reported previously (39, 48). Lymphatic vessels were carefully cleared of all adipose tissue. Vessel segments ∼3 cm in length were isolated and instrumented in an isolated vessel bath in which inlet and outlet pressures could be independently manipulated and measured. Axial tension on the vessels was applied to a degree just sufficient to raise the center portion of the vessel segment from the bottom of the bath. Using vessel segments between valves, we could ensure that there was no potential for valve closure to isolate the pressure transducers from the lumen of the vessel. The vessels were perfused and bathed with APSS with pH of 7.4 and warmed to 37°C. Normal rectal temperature in beef cows is 36.7–39.1°C and in dairy cows is 38.0–39.3°C (1). Previous studies have set bath temperatures between 35°C and 38°C (15, 29, 32, 33). We chose 37°C for the bath temperature so that our results would be more comparable to those of prior studies of bovine mesenteric lymphatic vessels (26, 30, 42, 51). External pressure was set to 1 cmH2O by setting the bath solution height 1 cm above the center line of the vessel. Transmural pressure was determined as the difference between inlet and external pressures. Flow through the vessel was minimized by maintaining inlet and outlet pressures at equal levels throughout the measurement process. Transmural pressure was adjusted by increasing or decreasing both inlet and outlet pressures in tandem. Instantaneous vessel outer diameter was measured using custom video caliper software (IMAQ, National Instruments, Austin, TX). Vessels were allowed to equilibrate for 15 min. Lymphatic vessel segments failing to exhibit stable spontaneous contractions were discarded.
Functional analysis of postnodal lymphatic vessels.
Postnodal lymphatic vessels were exposed to five transmural pressures (3, 6, 9, 12, and 15 cmH2O). The instrumented vessel was allowed to equilibrate for 5 min at each transmural pressure setting followed by a 5-min recording period. Following analysis of the active vessel properties, the bath and lumen were perfused with Ca2+-free APSS for 30 min until the vessel was fully relaxed. Diameters of the passive vessel were recorded for 1 min at each transmural pressure level (i.e., 3, 6, 9, 12, and 15 cmH2O).
Analysis of passive properties.
To detect changes in passive stiffness, passive pressure-diameter data were fit to an exponential function commonly used to characterize biomechanical properties (i.e., D = αeβx) (34). The values of α and β were compared using the Mann-Whitney rank sum test.
Functional data analysis.
The functional data from postnodal vessels were analyzed using two-way repeated-measures ANOVA comparing the experimental groups (MVH vs. Sham) with transmural pressure as the repeated measure. The Holm-Sidak method was used for post hoc multiple-comparison tests. All volume measurements were expressed in terms of cross-sectional area (i.e., volume normalized by length assuming uniform cylindrical contraction). The functional parameters compared were diastolic tone (100 × [passive diameter − diastolic diameter]/passive diameter), maximum (diastolic) volume, minimum (systolic) volume, stroke volume, ejection fraction (stroke volume/diastolic volume), contraction frequency, and fractional pump flow (contraction frequency × stroke volume/diastolic volume). A calculated P value of less than 0.05 was considered significant. In all cases, each cow contributed one vessel for analysis.
Histological analysis of postnodal lymphatic vessels.
Changes in the structural components of the postnodal lymphatic vessels were determined using histological analysis. Each vessel was dehydrated using a series of graded alcohol (70%, 80%, 90%, and 95% EtOH) and then embedded with paraffin. Five-micrometer-thick cross sections of the vessels were then obtained using a rotary microtome (model no. RM2255, Leica Microsystems, Bannockburn, IL). The sections were mounted on plus-coated slides and stained with Masson's Trichrome and hematoxylin and eosin (H&E). Images of each vessel cross section were obtained, and their contrast and saturation increased using image enhancement software (Picasa, Google, Mountain View, CA). Images were then analyzed with a custom application developed with MATLAB (R2008a; MathWorks, Natick, MA). Lymphatic muscle cells (stained pink) and collagen (stained blue) were identified from Trichrome-stained images, and fibrous structures of the media were identified from H&E-stained images. Using the filtered images, we estimated the area occupied by each constituent.
Histological data analysis.
Cross-sectional area occupied by smooth muscle cells (SMCA), total cross-sectional area occupied by collagen (TCA), and cross-sectional area occupied by medial collagen were determined and normalized by total cross-sectional area (TA) and medial area (MA) and were reported as means ± SE. A calculated P value less than 0.05 was considered significant. Each cow contributed one lymphatic vessel for analysis.
RESULTS
Fluid balance parameters.
Mesenteric venous pressure measured one day after surgery was significantly higher in MVH (31.4 ± 2.2 cmH2O; n = 12) than Sham (11.6 ± 2.8 cmH2O; n = 5). Although the intestinal water content determined post mortem 3 days after surgery in MVH (77.9 ± 1.6%) was elevated, it was not significantly different from the Sham group (73.3 ± 2.6%).
Functional analysis.
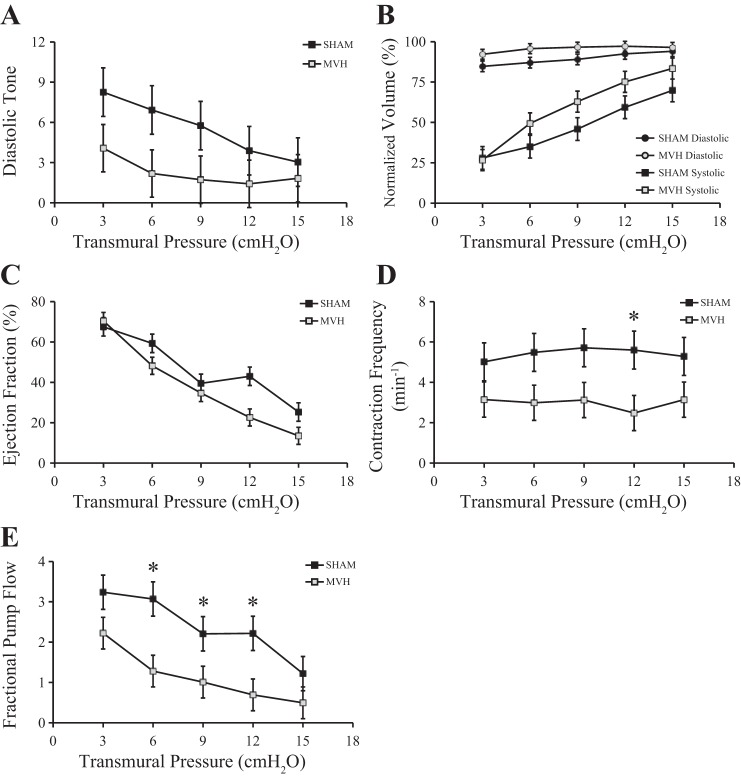
There was no difference in passive stiffness between the MVH and Sham group in the pressure range of interest (Fig. 2). Passive diameter, diastolic diameter, and systolic diameter at five transmural pressure levels (Sham, n = 6; MVH, n = 7) are listed in Table 1. Diastolic tone, normalized diastolic volume, normalized systolic volume, normalized stroke volume, ejection fraction, contraction frequency, and fractional pump flow for postnodal lymphatic vessels (Sham, n = 6; MVH, n = 7) pooled over all transmural pressure levels are listed in Table 2. Figure 3 depicts these functional variables at each transmural pressure level. There were no significant differences in normalized systolic volume, normalized diastolic volume, or ejection fraction of the lymphatic vessels between the two groups (Table 2). However, contraction frequency and fractional pump flow were significantly lower in the MVH group compared with the Sham group (Table 2). Post hoc testing showed that contraction frequency and fractional pump flow were significantly lower at particular pressures (Fig. 3).
Fig. 2.
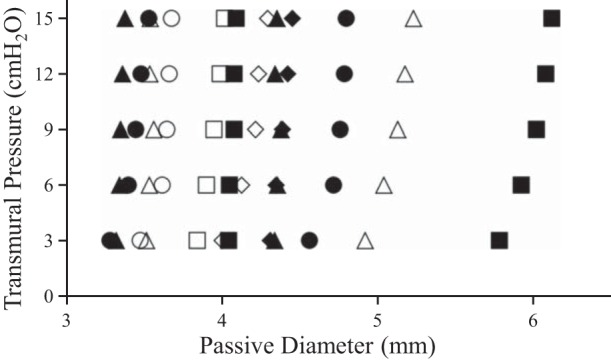
Passive pressure-diameter relationship of MVH (solid symbols) and Sham (open symbols). Passive stiffness for each vessel was estimated from the parameters estimated using nonlinear regression (D = αeβx). There was no significant change in passive stiffness in the pressure range of interest.
Table 1.
Comparison of passive, diastolic, and systolic diameters of bovine mesenteric postnodal lymphatic vessels in Sham and MVH groups
| Transmural Pressure, cmH2O | Passive Diameter, mm | Diastolic Diameter, mm | Systolic Diameter, mm |
|---|---|---|---|
| Sham Group | |||
| 3 | 3.64 ± 0.37 | 3.38 ± 0.39 | 1.87 ± 0.27 |
| 6 | 3.74 ± 0.37 | 3.51 ± 0.39 | 2.15 ± 0.29 |
| 9 | 3.79 ± 0.38 | 3.59 ± 0.39 | 2.49 ± 0.29 |
| 12 | 3.80 ± 0.39 | 3.66 ± 0.38 | 2.89 ± 0.30 |
| 15 | 3.84 ± 0.40 | 3.71 ± 0.36 | 3.14 ± 0.30 |
| MVH Group | |||
| 3 | 4.23 ± 0.32 | 4.06 ± 0.31 | 2.15 ± 0.26 |
| 6 | 4.30 ± 0.32 | 4.21 ± 0.31 | 2.93 ± 0.30 |
| 9 | 4.34 ± 0.34 | 4.27 ± 0.33 | 3.40 ± 0.35 |
| 12 | 4.36 ± 0.35 | 4.30 ± 0.35 | 3.77 ± 0.35 |
| 15 | 4.39 ± 0.34 | 4.31 ± 0.35 | 4.00 ± 0.34 |
Data are presented as means ± SE.
MVH, microvascular hypertension.
Table 2.
Comparison of critical variables characterizing the response of bovine mesenteric postnodal lymphatic vessels pooled over all transmural pressure levels
| Group | Diastolic Tone | Diastolic Volume, % of PV | Systolic Volume, % of PV | Stroke Volume, % of PV | Ejection Fraction | Frequency, min−1 | Fractional Pump Flow, min−1 |
|---|---|---|---|---|---|---|---|
| Sham | 5.57 ± 1.53 | 89.49 ± 2.83 | 47.61 ± 5.93 | 41.87 ± 6.88 | 0.47 ± 0.06 | 5.42 ± 0.81 | 2.39 ± 0.32 |
| MVH | 2.25 ± 1.42 | 95.64 ± 2.62 | 59.50 ± 5.49 | 36.14 ± 6.37 | 0.38 ± 0.06 | 2.98 ± 0.75* | 1.14 ± 0.30* |
Data are presented as means ± SE. Contraction frequency and fractional pump flow were significantly decreased in microvascular hypertension group (MVH; n = 7) compared to Sham (n = 6).
P < 0.05 vs. Sham.
PV, passive volume.
Fig. 3.
Functional parameters for bovine mesenteric postnodal lymphatic vessels. A: diastolic tone. B: systolic and diastolic volumes normalized by passive volume. C: ejection fraction. D: contraction frequency. E: fractional pump flow. Contraction frequency and fractional pump flow were significantly lower in MVH (n = 7) compared with Sham (n = 6). Data are presented as means ± SE. *P < 0.05 vs. Sham.
Histological analysis.
There were no significant differences detected in the SMCA/TA ratio (Sham: 0.33 ± 0.070 and MVH: 0.41 ± 0.081), TCA/TA ratio (Sham: 0.69 ± 0.069 and MVH: 0.63 ± 0.085), or MCA/TA ratio (Sham: 0.21 ± 0.087 and MVH: 0.28 ± 0.12). We also found no differences in SMCA/MA ratio (Sham: 0.61 ± 0.13 and MVH: 0.60 ± 0.097) and MCA/MA ratio (Sham: 0.39 ± 0.11 and MVH: 0.40 ± 0.097) (Fig. 4).
Fig. 4.
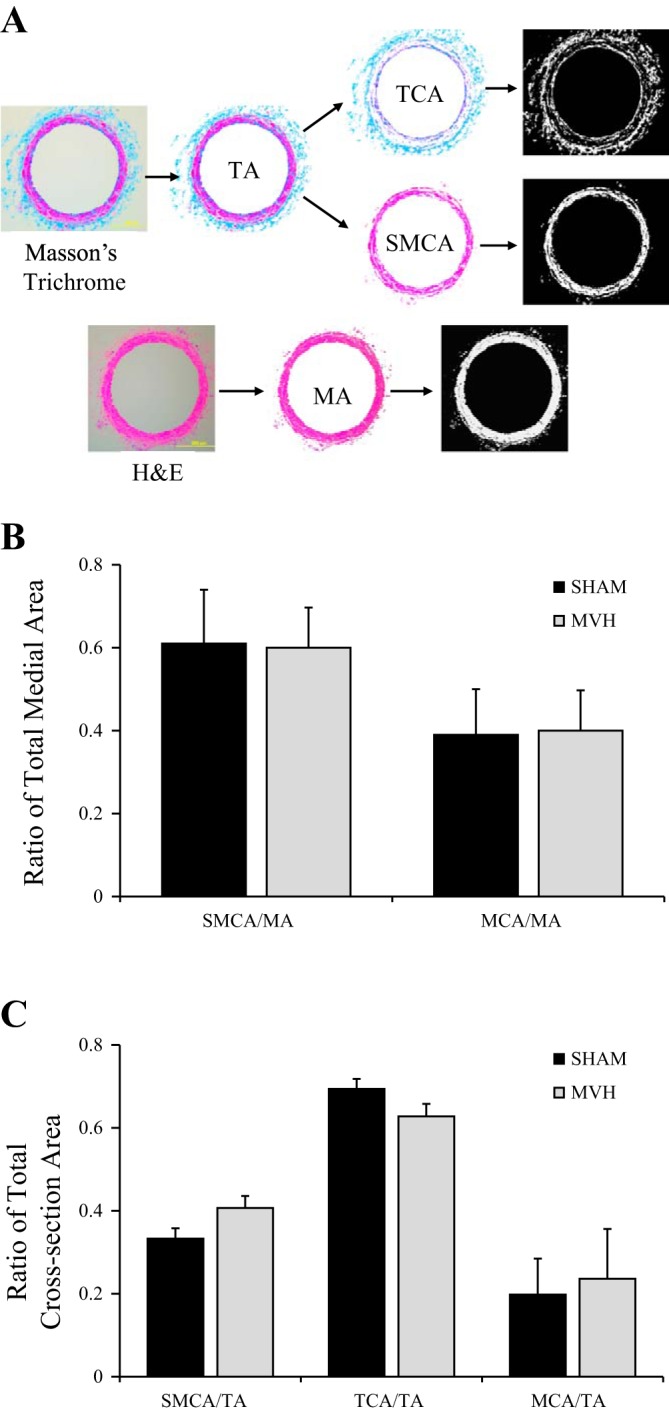
A: postnodal lymphatic vessel sections stained with Masson's Trichrome and hematoxylin and eosin (H&E) to estimate the percent area of the constituents of interest: vessel total area (TA), medial area (MA), area occupied by collagen (TCA), and smooth muscle cells (SMCA). B: between the Sham (n = 8) and MVH (n = 8) groups, there were no significant differences in the smooth muscle cell area/total area ratio (SMCA/TA), total collagen area/total area ratio (TCA/TA), medial collagen area/total area ratio (MCA/TA), smooth muscle cell area/medial area ratio (SMCA/MA), and medial collagen area/medial area ratio (MC/MA). C: Ratio of total cross-sectional area for SMC/TA, TCA/TA, and MCA/TA. Data are presented as means ± SE.
DISCUSSION
Decreased lymphatic pump function in mesenteric venous hypertension.
Within 3 days of induction of mesenteric venous hypertension, postnodal vessels demonstrated decreased contraction frequency and fractional pump flow. The fact that MVH vessels were weaker pumps was due primarily to decreases in contraction frequency. Contraction frequency in our study may not have increased with increases in transmural pressure as much as previously reported. We believe that apparent differences between our study and previous studies in contraction frequency data can be explained by differences in preparation. The classic study demonstrating the effect of transmural pressure in bovine mesenteric lymphatic vessels by McHale and Roddie (31) used transmural pressures of 0 to 6 cmH2O, while the current study used a pressure range of 3 to 15 cmH2O. If we compare the transmural pressures common to both studies, the results for the Sham group appear similar to McHale and Roddie's study. In this study, the increase in mean frequency from 3 to 6 cmH2O was ∼17%. In the current study, mean frequency in the Sham group at 6 cmH2O was 9% greater than that at 3 cmH2O. However, mean frequency in the MVH group in the present study was 5% lower at 6 cmH2O than at 3 cmH2O. This could be an aspect of the adaptive response to venous hypertension. Because the frequency change from 3 to 6 cmH2O was not statistically significant, it is difficult to make any claim as to its importance. We chose a much greater pressure range than most in vitro studies, because the particular intervention that we used could potentially raise lymphatic transmural pressure in MVH vessels in vivo above normal values. Comparing Sham and MVH vessels in the same extended pressure range in vitro allowed us to detect any differences. Given the statistical difference between Sham and MVH vessels at 9 and 12 cmH2O, this increased range is, in retrospect, justified. Because the passive properties of lymphangions have been reported to change significantly for the pressure range between 0 and 5 cmH2O (41), it may be interesting in future biomechanical studies to explore changes in passive stiffness in lower pressures than those illustrated in Fig. 2, particularly in response to interventions that are less likely to raise lymphatic transmural pressures than MVH.
Potential mechanical mechanisms for lymphatic pump inhibition.
The present work was primarily a functional study, and the changes in function were not related to particular changes in biomechanical properties. In particular, the decrease in pump function with MVH may be related to changes in either circumferential or longitudinal smooth muscle. It is possible that changes in length, due to the longitudinal arrangement of smooth muscle, leads to significant shortening in vivo. Because our preparation set length, the difference in Sham and MVH vessels manifested only as a change in radius. Similarly, the mechanical stimuli in vivo that resulted in adaptation are difficult to identify. Adaptive responses occurred as a result of our hypertension model, but it is not clear whether this was due primarily to enhanced lymph flow, elevated lymphatic pressure, or even tissue oncotic pressure changes. Some or all of these mechanisms may be contributory, and their effects may be interrelated. Changes in contractility can affect transmural pressure and changes in transmural pressure can affect contractility (31). Elevated lymph flow increases shear stress, and it is known to acutely inhibit lymphangion pumping (17, 34). Since, in vivo, lymph flow is increased by increasing microvascular fluid filtration, it is not possible to increase lymph flow without altering interstitial protein concentration (44) and thus the content of lymph. Now that functional adaptations have been identified, further biomechanical study is warranted, including quantifying maximum tension generated (19, 52) or alterations of sensitivity to shear stress (17).
Potential inflammatory mechanisms.
The alterations in lymphatic function observed in this study may be the result of an inflammatory response induced by the mesenteric venous hypertension. We did not seek to detect differences in immune cell populations or indicators of inflammation within the sections of the vessel walls or surrounding tissue. Inflammatory signaling molecules could originate in or near the intestinal interstitium and travel via lymph to affect the mesenteric lymphatic vessels. Using a model of bacterial tripeptide-induced ileitis in rats, Benoit and Zawieja (4) demonstrated that mesenteric lymphatic vessels examined in vivo increased stroke volume within the first hour. In that study, lymphatic contraction frequency was not significantly affected. However, Wu et al. (50) examined contractile behavior of mesenteric lymphatic vessels in vivo and in vitro 3 and 6 days after 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced ileitis in guinea pigs. They reported that intestinal inflammation was associated with decreased contraction frequency, decreased stroke volume, and increased diastolic diameter—results broadly consistent with the current study. The inflammation-related decrease in contraction frequency reported by Wu et al. (50) was moderated by application of a nonselective COX inhibitor (indomethacin), as well as by combined COX 1 and COX 2 inhibitors. Numerous inflammatory mediators have been demonstrated to affect lymphatic contractile behavior, including prostaglandins, bradykinin, histamine, and substance P (2, 6, 16, 23, 25). Recently, substance P has been shown to activate both inflammatory signaling pathways and contractile activity in lymphatic vessels (5). However, because substance P acutely stimulates lymphatic contraction, it may not be a good candidate for inducing the behavior observed in the present study. The finding by Wu et al. that COX inhibition partially reversed the effect of intestinal inflammation on lymphatic pumping (50) provides support for the possible role of prostacyclin.
Novel bovine model for the study of lymphatic vessel adaptation.
The present work is complementary to a recent study by Dongaonkar et al. (8), which reported changes in postnodal bovine mesenteric lymphatic pumping in response to altered transmural pressures. In that study, lymphatic vessels were partially constricted for 3 days, causing upstream pressure to be higher than downstream pressure in the same vessels. Although no change in contraction frequency was observed, systolic and diastolic diameters and the pump index (variation of fractional pump flow) of upstream vessel segments were higher than those of downstream segments. In effect, lymphangions adapted to higher pressures by becoming stronger pumps. In the present study, the primary stimulus was mesenteric venous hypertension, which is more clinically relevant. It is suspected that MVH results in higher flows, which could explain the fundamentally different result. Taken together, these two studies help establish the bovine model as a novel model for studying lymphatic vessel adaptation. Historically, the postnodal bovine mesenteric lymphatic vessel has been a well-established in vitro model of lymphatic function (30, 31, 37). The animal is large enough, not only to surgically create mesenteric venous hypertension, but also to measure and manipulate mesenteric venous pressure postoperatively. In developing this animal model to study lymphatic adaptation, we chose a conservative 3-day time frame for two reasons. First, this is the earliest time period that we could expect to document significant changes in function. Second, we wanted to initially avoid longer adaptation periods to minimize the potential confounding adaptive changes in the microvascular, interstitial (24), and serosal barriers. Dongaonkar et al. (7) identified no less than seven parameters affecting interstitial fluid volume. Changes in any of these seven could act to blunt the effects of mesenteric venous hypertension. The failure to demonstrate a significant difference in intestinal water content may be due, in part, to the effectiveness of these multiple adaptive processes. In particular, decreases in the microvascular filtration coefficient have been demonstrated with chronic increases in microvascular filtration pressure following venous hypertension (24, 47). Now that we have established adaptive changes in 3 days to a clinically relevant perturbation, we can suggest that there is value in repeating this complex, resource-intensive experiment for longer adaptive periods using this novel animal model.
Perspectives and Significance
The results of the present study are the first to suggest that postnodal lymphatic vessels can respond to edemagenic conditions by becoming weaker pumps. Given the great complexity of microvascular-interstitial-lymphatic interaction, mathematical models have been used to explore how changes in lymphatic pumping affects interstitial fluid balance. Quick et al. (40) developed a relatively simple model of a lymphangion that suggests that decreases in lymphangion pumping frequency raises lymphangion effective lymphatic resistance, first characterized by Drake et al. (11). Dongaonkar et al. (7) illustrated with a system-level model that increased effective lymphatic resistance will decrease lymph flow, increase interstitial volume, as well as “edemagenic gain” (i.e., the sensitivity of interstitial volume to increases in microvascular pressure) (9). These modeling insights suggest persistent adaptive responses manifesting as “lymphatic pump failure,” therefore, are either homeostatic, ameliorating elevated interstitial flow, or maladaptive, exacerbating interstitial edema.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL-092916 (E. Wilson and R. H. Stewart) and R01HL070308 (A. A. Gashev) and National Science Foundation Grant CMMI-1063954 (C. M. Quick).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.M.Q., J.C., A.A.K., J.H., A.A.G., and R.H.S. conception and design of research; C.M.Q., J.C., A.A.K., R.M.D., and R.H.S. analyzed data; C.M.Q., J.C., R.M.D., J.H., E.W., A.A.G., G.A.L., and R.H.S. interpreted results of experiments; C.M.Q. drafted manuscript; C.M.Q., R.M.D., E.W., A.A.G., G.A.L., and R.H.S. edited and revised manuscript; C.M.Q., J.C., A.A.K., R.M.D., J.H., E.W., A.A.G., G.A.L., and R.H.S. approved final version of manuscript; A.A.K., J.H., and R.H.S. performed experiments; R.M.D. and R.H.S. prepared figures.
ACKNOWLEDGMENTS
The authors thank Dr. David C. Zawieja for careful review of the manuscript.
REFERENCES
- 1.Aiello SE, Moses MA., Eds The Merck Veterinary Manual Online. Whitehouse Station, NJ: Merck Sharp and Dohme Corp, 2012 [Google Scholar]
- 2.Amerini S, Ziche M, Greiner ST, Zawieja DC. Effects of substance P on mesenteric lymphatic contractility in the rat. Lymphat Res Biol 2: 2–10, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 73: 1–78, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Benoit JN, Zawieja DC. Effects of F-Met-Leu-Phe-induced inflammation on intestinal lymph-flow and lymphatic pump behavior. Am J Physiol Gastrointest Liver Physiol 262: G199–G202, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty S, Nepiyushchikh Z, Davis MJ, Zawieja DC, Muthuchamy M. Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation 18: 24–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobbins DE, Buehn MJ, Dabney JM. Constriction of perfused lymphatics by acetylcholine, bradykinin and histamine. Microcirc Endothelium Lymphatics 6: 409–425, 1990 [PubMed] [Google Scholar]
- 7.Dongaonkar RM, Laine GA, Stewart RH, Quick CM. Balance point characterization of interstitial fluid volume regulation. Am J Physiol Regul Integr Comp Physiol 297: R6–R16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dongaonkar RM, Nguyen TL, Quick CM, Hardy J, Laine GA, Wilson E, Stewart RH. Adaptation of mesenteric lymphatic vessels to prolonged changes in transmural pressure. Am J Physiol Heart Circ Physiol 305: H203–H210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dongaonkar RM, Quick CM, Stewart RH, Drake RE, Cox CS, Jr, Laine GA. Edemagenic gain and interstitial fluid volume regulation. Am J Physiol Regul Integr Comp Physiol 294: R651–R659, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Drake RE, Abbott RD. Effect of increased neck vein pressure on intestinal lymphatic pressure in awake sheep. Am J Physiol Regul Integr Comp Physiol 262: R892–R894, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Drake RE, Allen SJ, Katz J, Gabel JC, Laine GA. Equivalent circuit technique for lymph flow studies. Am J Physiol Heart Circ Physiol 251: H1090–H1094, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Drake RE, Gabel JC. Effect of outflow pressure on intestinal lymph flow in unanesthetized sheep. Am J Physiol Regul Integr Comp Physiol 260: R668–R671, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Dunbar BS, Elk JR, Drake RE, Laine GA. Intestinal lymphatic flow during portal venous hypertension. Am J Physiol Gastrointest Liver Physiol 257: G94–G98, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Ferguson MK, Shahinian HK, Michelassi F. Lymphatic smooth muscle responses to leukotrienes, histamine and platelet activating factor. J Surg Res 44: 172–177, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Ferguson MK, Williams U. Measurement of flow characteristics during individual contractions in bovine mesenteric lymphatic vessels. Lymphology 33: 36–42, 2000 [PubMed] [Google Scholar]
- 16.Fox JL, von der Weid PY. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol 136: 1210–1218, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gashev AA, Zawieja DC. Physiology of human lymphatic contractility: a historical perspective. Lymphology 34: 124–134, 2001 [PubMed] [Google Scholar]
- 19.Gashev AA, Zhang RZ, Muthuchamy M, Zawieja DC, Davis MJ. Regional heterogeneity of length-tension relationships in rat lymph vessels. Lymphat Res Biol 10: 14–19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granger DN, Taylor AE. Permselectivity of intestinal capillaries. Physiologist 23: 47–52, 1980 [PubMed] [Google Scholar]
- 21.Hall JE. Guyton and Hall Textbook of Medical Physiology. Philadelphia, PA: Saunders, 2007 [Google Scholar]
- 22.Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. Am J Physiol Heart Circ Physiol 233: H57–H65, 1977 [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto S, Kawai Y, Ohhashi T. Effects of vasoactive substances on the pig isolated hepatic lymph vessels. J Pharmacol Exp Ther 269: 482–488, 1994 [PubMed] [Google Scholar]
- 24.Huang WX, Kingsbury MP, Turner MA, Donnelly JL, Flores NA, Sheridan DJ. Capillary filtration is reduced in lungs adapted to chronic heart failure: morphological and haemodynamic correlates. Cardiovasc Res 49: 207–217, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Johnston MG. Interaction of inflammatory mediators with the lymphatic vessel. Pathol Immunopathol Res 6: 177–189, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Johnston MG, Feuer C. Suppression of lymphatic vessel contractility with inhibitors of arachidonic acid metabolism. J Pharmacol Exp Ther 226: 603–607, 1983 [PubMed] [Google Scholar]
- 27.Korthuis RJ, Kinden DA, Brimer GE, Slattery KA, Stogsdill P, Granger DN. Intestinal capillary filtration in acute and chronic portal hypertension. Am J Physiol Gastrointest Liver Physiol 254: G339–G345, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Laine GA, Allen SJ, Katz J, Gabel JC, Drake RE. Effect of systemic venous pressure elevation on lymph flow and lung edema formation. J Appl Physiol 61: 1634–1638, 1986 [DOI] [PubMed] [Google Scholar]
- 29.McHale NG, Allen JM. The effect of external Ca2+ concentration on the contractility of bovine mesenteric lymphatics. Microvasc Res 26: 182–192, 1983 [DOI] [PubMed] [Google Scholar]
- 30.McHale NG, Meharg MK. Co-ordination of pumping in isolated bovine lymphatic vessels. J Physiol 450: 503–512, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261: 255–269, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHale NG, Roddie IC. The effects of catecholamines on pumping activity in isolated bovine mesenteric lymphatics. J Physiol 338: 527–536, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHale NG, Roddie IC, Thornbury KD. Nervous modulation of spontaneous contractions in bovine mesenteric lymphatics. J Physiol 309: 461–472, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meisner JK, Stewart RH, Laine GA, Quick CM. Lymphatic vessels transition to state of summation above a critical contraction frequency. Am J Physiol Regul Integr Comp Physiol 293: R200–R208, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Mislin H. Active contractility of the lymphangion and coordination of lymphangion chains. Experientia 32: 820–822, 1976 [DOI] [PubMed] [Google Scholar]
- 36.Moore-Olufemi SD, Xue H, Allen SJ, Moore FA, Stewart RH, Laine GA, Cox CS., Jr Effects of primary and secondary intra-abdominal hypertension on mesenteric lymph flow: implications for the abdominal compartment syndrome. Shock 23: 571–575, 2005 [PubMed] [Google Scholar]
- 37.Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol 239: H88–H95, 1980 [DOI] [PubMed] [Google Scholar]
- 38.Ohhashi T, Fukushima S, Azuma T. Vasa vasorum within the media of bovine mesenteric lymphatics. Proc Soc Exp Biol Med 154: 582–586, 1977 [DOI] [PubMed] [Google Scholar]
- 39.Quick CM, Ngo BL, Venugopal AM, Stewart RH. Lymphatic pump-conduit duality: contraction of postnodal lymphatic vessels inhibits passive flow. Am J Physiol Heart Circ Physiol 296: H662–H668, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quick CM, Venugopal AM, Dongaonkar RM, Laine GA, Stewart RH. First-order approximation for the pressure-flow relationship of spontaneously contracting lymphangions. Am J Physiol Heart Circ Physiol 294: H2144–H2149, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Rahbar E, Weimer J, Gibbs H, Yeh AT, Bertram CD, Davis MJ, Hill MA, Zawieja DC, Moore JE., Jr Passive pressure-diameter relationship and structural composition of rat mesenteric lymphangions. Lymphat Res Biol 10: 152–163, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai H, Ikomi F, Ohhashi T. Effects of endothelin on spontaneous contractions in lymph vessels. Am J Physiol Heart Circ Physiol 277: H459–H466, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev 70: 987–1028, 1990 [DOI] [PubMed] [Google Scholar]
- 44.Stewart RH, Geissler HJ, Allen SJ, Laine GA. Protein washdown as a defense mechanism against myocardial edema. Am J Physiol Heart Circ Physiol 279: H1864–H1868, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Stewart RH, Laine GA. Flow in lymphatic networks: interaction between hepatic and intestinal lymph vessels. Microcirculation 8: 221–227, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Takahashi N, Kawai Y, Ohhashi T. Effects of vasoconstrictive and vasodilative agents on lymphatic smooth muscles in isolated canine thoracic ducts. J Pharmacol Exp Ther 254: 165–170, 1990 [PubMed] [Google Scholar]
- 47.Townsley MI, Fu Z, Mathieu-Costello O, West JB. Pulmonary microvascular permeability. Responses to high vascular pressure after induction of pacing-induced heart failure in dogs. Circ Res 77: 317–325, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Venugopal AM, Stewart RH, Laine GA, Dongaonkar RM, Quick CM. Lymphangion coordination minimally affects mean flow in lymphatic vessels. Am J Physiol Heart Circ Physiol 293: H1183–H1189, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Weinstein SM. Central venous access. In: Plumer's Principles and Practice of Intravenous Therapy. Philadelphia, PA: Lippincott Williams & Wilkins, 2007, chapt. 14 [Google Scholar]
- 50.Wu TF, Carati CJ, MacNaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol 291: G566–G574, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama S, Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol 264: H1460–H1464, 1993 [DOI] [PubMed] [Google Scholar]
- 52.Zhang RZ, Gashev AA, Zawieja DC, Davis MJ. Length-tension relationships of small arteries, veins, and lymphatics from the rat mesenteric microcirculation. Am J Physiol Heart Circ Physiol 292: H1943–H1952, 2007 [DOI] [PubMed] [Google Scholar]