Abstract
Lymphoma is an important disease in dogs and people, with similar biological characteristics. We tested the binding affinity of a peptidomimetic LLP2A, previously shown to bind the alpha4–beta1 integrin on human lymphoma cell lines, to lymphocytes of dogs with spontaneously occurring lymphoma. Fine needle aspirates of lymph nodes from 32 dogs with B-cell lymphoma and 7 dogs with T-cell lymphoma were evaluated using flow cytometry. For B cells, the lowest MFI levels were in unlabeled, non-neoplastic lymphocytes. The highest median fluorescent intensity (MFI) levels occurred in LLP2A-labeled lymphoma cells from dogs that had not received chemotherapy followed by labeled lymphoma cells from dogs that had received chemotherapy. The fluorescence profile of the T-cell samples was similar although many of the differences were not statistically significant, likely due to low sample number. Specifically, LLP2A-labeled T-cell lymphoma cells had a significantly higher MFI compared to unlabeled non-neoplastic lymphocytes. LLP2A affinity was not significantly different in unlabeled and labeled T-cell lymphoma cells, and labeled non-neoplastic lymphocytes. For both B and T cells, labeling with LLP2A tended to increase MFI in both normal and lymphoma cells. Lymphoma cells had higher mean MFI levels than non-neoplastic lymphocytes, and chemotherapy acted to decrease MFI. In summary, these data demonstrate that LLP2A has affinity to canine lymphoma cells and indicates expression of the alpha4–beta1 integrin on these cells. In fact, LLP2A preferentially binds neoplastic B-cells, suggesting that this small molecule may be of use in cross-species clinical trials of targeted therapeutics.
Keywords: Canine, Lymphoma, Peptidomimetic, Non-Hodgkin’s, LLP2A
1. Introduction
Lymphoma is an important and common disease in dogs and people. The incidence rate of lymphoma in dogs is estimated to be 13–24 cases per 100,000 dogs, which is similar to that in people (Dorn et al., 1967; Hahn et al., 1994; Hansen and Khanna, 2004). Lymphoma accounts for 7–24% of cancers in pet dogs, and the incidence appears to be increasing. The most common form of lymphoma in dogs is non-Hodgkin’s lymphoma (NHL). Specifically the most common histological type is a diffuse large B-cell lymphoma (Vezzali et al., 2010). Non-Hodgkin lymphoma results in 5% of new cancer cases and 3% of annual cancer deaths in people in the United States (Jemal et al., 2008). Furthermore, the intratumoral heterogeneity of canine tumors mimics that in human NHL, with additional similarities including resistance to therapy, recurrence, and staging. The similarities in the diseases, between the two species, make spontaneously occurring canine lymphoma a good model of human disease which can be exploited to find and test new targeting agents (Hansen and Khanna, 2004; Rowell et al., 2011). Despite the availability of treatment options for human and canine lymphoma, disease recurrence and development of resistant disease warrants improved therapies.
Peptidomimetics are molecules designed to mimic peptides using natural and unnatural amino acids, which may provide increased biological stability and reduced susceptibility to proteolysis. LLP2A is a tripeptide-based peptidomimetic ligand that was developed to have high affinity (IC50 = 2 pM) to the activated form of the alpha4–beta1 integrin expressed on human leukemia and lymphoma cells and to be used as a targeted therapeutic carrier (Denardo et al., 2009; Peng et al., 2006; Xiao et al., 2009). The alpha4–beta1 integrin (very late antigen 4, VLA 4, CD49d) binds to vascular cell adhesion molecule-1 (VCAM-1) and fibronectin and is expressed on the cell surface of all circulating leukocytes except neutrophils (Yusuf-Makagiansar et al., 2002). It regulates trafficking and homing of lymphocytes and is expressed in cancers such as leukemia, lymphoma, melanoma, and sarcoma (Holzmann et al., 1998). The integrin has been associated with tumorigenicity and metastatic potential in models of melanoma (Rebhun et al., 2010). Expression of alpha4 and beta1 subunits has also been associated with increased incidence of bone marrow infiltration, an advanced stage of disease, and with extranodal involvement in human non-Hodgkin lymphoma (Terol et al., 1999).
Canine lymphoma is an excellent model for human non-Hodgkin lymphoma, having a similar presentation and response to chemotherapy (Rowell et al., 2011). This study evaluates the therapeutic efficacy of a peptidomimetic ligand for an integrin thought to be expressed in both canine and human lymphomas, using spontaneously occurring canine lymphomas. In contrast to monoclonal antibodies, peptides and peptidomimetics represent a class of targeting agents that can be used across species. The combination of the similar expression of disease between humans and canines and the ability to utilize the same targeting agent in the two species make this an excellent opportunity to evaluate the affinity of a new targeting agent to lymphoma.
We hypothesized that the alpha4–beta1 integrin would be expressed on canine lymphoma cells, and that the cells would exhibit affinity to LLP2A. We used spontaneously occurring canine lymphoma lymph node samples labeled with LLP2A and a fluorescent marker to evaluate the expression of the alpha4–beta1 integrin. The affinity of canine lymphoma to LLP2A may serve as a translational model to facilitate early clinical trials in people.
2. Materials and methods
2.1. Reagents
Mouse-anti-canine antibodies for CD3 (CA17.6F9-IgG2b-Azide) and CD21 (CA2.1D6-IgG1-Azide) (courtesy of Dr. P.F. Moore, UC Davis) were used as labels for T and B lymphocytes, respectively (Vernau and Moore, 1999). The secondary antibody was conjugated to the fluorochrome, R-Phycoerythrin anti-mouse IgG (H + L) (Vector Laboratories, Inc., Burlingame, CA). LLP2A-biotin (Peng et al., 2006), courtesy of Dr. Kit Lam, UC Davis, interacted with streptavidin-Alexa Fluor®-488 (Invitrogen Corporation, Carlsbad, CA). The Jurkat cell line (American Type Culture Collection Rockville, MD), used for positive controls, was maintained by the addition or replacement of fresh RPMI-1640 medium (ATCC, Cat. 30-2001) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin. The cultures were incubated at 37 °C in a humidified atmosphere of 5% CO2.
2.2. Patient population
Dogs presenting to the Veterinary Medical Teaching Hospital for evaluation and treatment of lymphoma were sequentially enrolled in the study between 2007 and 2009. Dogs were included if they had enlarged lymph nodes that were accessible for fine needle aspiration and a confirmed cytologic or pathologic diagnosis of lymphoma. Owner consent for the procedure was obtained prior to sampling.
2.3. Labeling canine lymphoma cells with LLP2A
Bio-1211 (N-[[4-[[[(2-methylphenyl)amino]carbonyl] amino]-phenyl]acetyl]-L-leucyl-L-α-aspartyl-L-valyl-L-proline), (Tocris Bioscience, Ellisville, MO), a peptidomimetic previously shown to bind to the alpha4–beta1 integrin, was used to inhibit binding of LLP2A to the cell surface. Bio-1211 was incubated with 5 canine B cell lymphoma cell lines (UCDK9B1–UCDK9B5) (Zwingenberger et al., in press) at concentrations of 1 μM, 5 μM, and 50 μM per 100 μL of cell suspension in flow buffer. Samples were incubated for 30 min at room temperature. 1 μL of LLP2A-biotin (10 μM) was added to each sample. Control samples were prepared with 100 μL cell suspension with 1 μL DMSO (negative), and 1 μL LLP2A-biotin dissolved in DMSO (10 μM) (positive). All samples were incubated for 30 min at room temperature and washed twice with flow buffer, and then centrifuged for 5 min at 200 × g. The supernatant was removed, and pellets resuspended. 25 μL of streptavidin-Alexa 488 (diluted 1:100 in TBS buffer) was added to each sample, and flow buffer was added to create a final volume of 100 μL. Samples were incubated for 30 min at room temperature in the dark, and washed twice with flow buffer. They were then centrifuged, the supernatant was removed, and the cells were resuspended in 500 μL of flow buffer for flow cytometry.
2.4. Canine lymphoma sample collection and cell preparation
All fine needle aspirate (FNA) samples were obtained from live animals. A 23-gauge needle was inserted into the lymph node and redirected through the node 2 to 3 times. The needle then was removed from the lymph node, attached to a 12 mL syringe, and the cells of the needle were expelled into a tube containing 1 mL of flow buffer (1 mM Mg2+ in 1× TBS, 1% horse serum). A second needle was introduced into the same lymph node, and the sampling procedure was repeated as described above. The cells from the 2nd sample were expelled into the same tube, pooling the 2 samples. All samples were stored at 4 °C until analyzed, within 24 h of collection.
The aspirated cells were suspended in flow buffer (TBS, 1 mM Mg2+, 1% Horse Serum) and washed twice. 3 mL of red cell lysis buffer (155 mM ammonium chloride, 12 mM potassium bicarbonate in purified water (Millipore Corporation, Billerica, MA), pH 7.2) were added, and the sample was incubated for 5 min at room temperature until clear. The sample was then centrifuged for 5 min at 220 × g. The supernatant was removed, and the cells were resuspended in 100 μL of flow buffer.
2.5. Labeling canine lymphoma with LLP2A
Multiple double-labeled tubes (LLP2A and canine antibody) were used to assess the affinity of LLP2A to canine lymphoma cells using two-color flow cytometry. To prepare the double-labeled samples, 1 μL LLP2A-biotin (10 μM) and 25 μL primary antibody (tissue culture fluid) were added into 100 μL of sample cells, and incubated on ice for 1 h or at room temperature for 30 min. Thereafter, 0.5 mL of flow buffer was added, and the cells were washed as described above. Then 27 μL streptavidin-Alexa Fluor®-488 (prediluted 1:100 in flow buffer) and 50 μL of secondary antibodies conjugated to the fluorochrome, rabbit-anti-mouse-phycoerythrin [PE] (prediluted 1:100 in flow buffer), were added to each tube, and samples again were incubated on ice for 1hr. After a final wash, the cells were resuspended in 0.5 mL of flow buffer, protected from light, and analyzed immediately. Negative control samples consisted of cells incubated with secondary antibodies alone, and positive controls were Jurkat cell lines which are known to have alpha4–beta1 integrin expression and affinity to LLP2A (Peng et al., 2006).
2.6. Flow cytometry
Two-color flow cytometry (FACScan, Becton Dickinson, Inc.) was performed on each tube. Samples were collected to 20,000 events or 10 min. Samples were analyzed with commercial software (Flowjo, Treestar Inc., Ashland, OR) by first displaying events on an SSC/FSC scatterplot and creating a lymphocyte gate. Cells within the lymphocyte gate were displayed on a SSC/CD3 or SSC/CD21 scatterplot and gates were created for CD3 positive (T-cells) and CD21 positive (B-cells) cells. The median fluorescence intensity (MFI) for LLP2A in the FL1 channel was recorded for these populations (Fig. 1). Non-neoplastic T-cells or B-cells within the samples were used as normal controls. The same analysis was performed for the negative control samples. Positive control samples were evaluated to ensure the assay performed as expected.
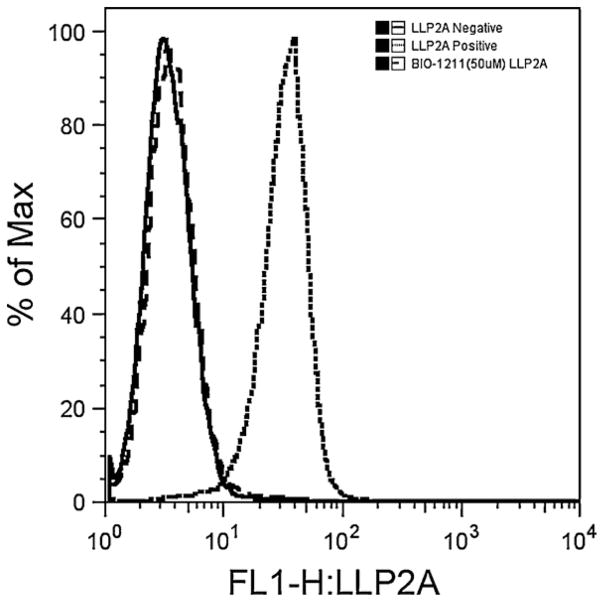
Fig. 1.
Flow cytometry histograms performed with canine B-cell lymphoma cell lines. LLP2A shows strong affinity at 100 nM to the cells in the positive sample. The negative control sample has the same median fluorescent intensity (FL1-H) as the sample blocked with 50 μM Bio-1211 prior to adding LLP2A, indicating that LLP2A binds to the alpha4–beta1 integrin on canine lymphoma cell lines.
2.7. Stastistical analysis
The effect of LLP2A labeling on mean MFI was evaluated with respect to lymphoma status, and whether the dog had received prior chemotherapy, with a hierarchical, mixed effect model. Mean MFI was modeled as a function of LLP2A labeling (labeled or not), lymphoma status (non-neoplastic or lymphoma cells), whether the dog received chemotherapy (Yes or No) and all two and three-way interactions between these fixed effects. Because B and T-cell lymphomas have different biologic behaviors in dogs (Fournel-Fleury et al., 1997), B and T cells were evaluated separately. Each subject was included in the model as a random factor to account for the potential correlation of MFI values of cells from the same dog. Further, the cell type from each dog was included as a second random factor to account for the correlation between measurements on the same cell type with and without LLP2A labeling. A significance level of 0.05 was used in hypothesis testing. Pairwise comparisons of all combinations of lymphoma status, LLP2A labeling and chemotherapy were evaluated with Tukey’s Honestly Significance Difference procedure to maintain the overall significance level at 0.05. MFI values were log transformed prior to analysis to meet model assumptions, and the modeling was conducted using PROC MIXED in SAS/STAT software, Version 9.2 for Windows (SAS Institute Inc., Cary, NC).
3. Results
3.1. Confirmation of alpha4–beta1 expression on canine lymphoma cells
Since there is no confirmed antibody to canine alpha4–beta1, we performed a blocking experiment with a peptide known as alpha4–beta1 affinity, or Bio-1211 (Chen et al., 1999), used in human cell line studies to confirm the expression of alpha4–beta1, on canine B cell lymphoma cell lines. These B-cell lines had previously shown affinity to canine CD49d antibody, which binds to the alpha subunit of the alpha4–beta1 integrin, indicating its expression on these canine lymphoma cells.
As expected, LLP2A was prevented from binding to UCDK9B1-5 cell lines by competitive binding of the alpha4–beta1 adherent Bio-1211. 20,000 counts were collected from each sample. Gates were created for each cell sample and displayed on overlaid histograms. Blocking lymphoma cells with Bio-1211 at 10 μM greatly decreased the binding affinity of LLP2A compared to positive controls (data not shown). A concentration of 50 μM Bio-1211 completely blocked affinity of LLP2A (100 nM) to lymphoma cells (Fig. 1). The ability of Bio-1211 to block the affinity of the peptidomimetic ligand LLP2A to lymphoma cells confirms the specific binding of LLP2A to alpha4–beta1 integrins on the surface of these cells.
3.2. Patient population
Samples were obtained from lymph nodes of 32 dogs with B cell lymphoma and 7 dogs with T cell lymphoma. The mean age of the dogs was 8.4 years (SD 2.7) with a range of 2.9–13 years. The sex distribution was 1 female, 14 female neutered, 4 male, and 20 male neutered. Six dogs with B cell lymphoma and 4 dogs with T cell lymphoma had been treated with chemotherapy before the sample was taken, and the remaining 30 dogs were naive to treatment. Treatments included prednisone alone (2 dogs; not yet in remission), prednisone and vincristine (1 dog; not yet in remission), Elspar and dexamethasone (1 dog; not yet in remission), GCP (nutritional soy supplement, 1 dog; not yet in remission), and combinations of multiple drugs including Cytoxan, doxorubicin, vincristine, Leukeran, Elspar, lomustine, melphalan, Madison–Wisconsin protocol (vin-cristine, doxorubicin, cyclophosphamide, l-asparaginase, prednisone) (Chun et al., 2000; Rebhun et al., 2011), and MOPP (mechlorethamine, vincristine, prednisone, procar-bazine) (5 dogs; 4 relapse, 1 multidrug resistant with progressive disease) (Northrup et al., 2009; Rassnick et al., 2002).
3.3. Lymphoma cells exhibit affinity to LLP2A
In both non-neoplastic and lymphoma B and T cells, LLP2A labeling significantly increased mean MFI compared to negative controls (unlabeled cells) (B cells: F1,63 = 276.0, p < 0.0001; T cells: F1,31 = 17.6, p = 0.0002), suggesting the presence of the alpha4–beta1 integrin on these cells. In all B cells, LLP2A labeling increased mean MFI to 7.23 ± 0.52 relative to 2.97 ± 0.21 in unlabeled cells (Fig. 2). In T cells, mean MFI were 4.28 ± 1.20 and 1.84 ± 0.11 in LLP2A labeled and unlabeled cells, respectively. Mean MFI also differed significantly between non-neoplastic and lymphoma cells (B cells: F1,104 = 123.0, p < 0.0001; T cells: F1,31 = 9.7, p = 0.0040) with greater affinity of LLP2A to lymphoma cells than non-neoplastic lymphocytes. Non-neoplastic lymphocytes had similar mean MFI levels in B cells (3.02 ± 0.24) and T cells (2.91 ± 0.76). But, interestingly, B cell lymphomas (6.66 ± 0.50) had much higher MFI levels than T cell lymphomas (3.56 ± 0.44).
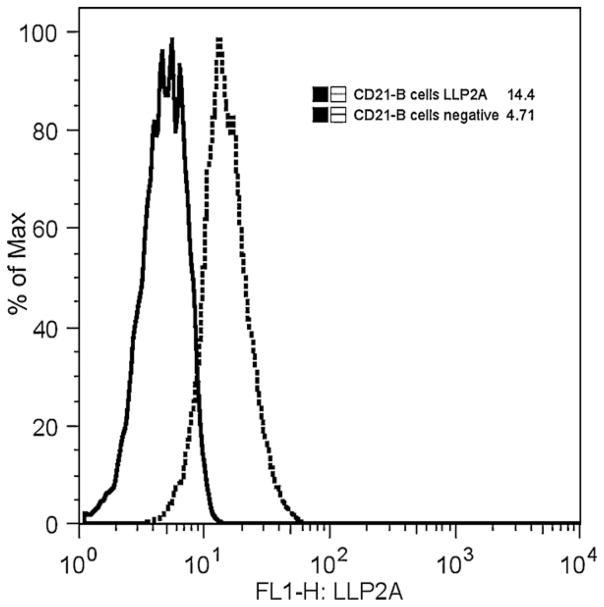
Fig. 2.
Gated populations of a canine lymphoma patient’s CD21 positive B cell lymphoma cells labeled with LLP2A-Alexa 488. The MFI of unlabeled lymphoma cells was 4.71 (solid line). LLP2A labeled B-cell lymphoma cells had a significantly higher MFI (14.4, dotted line).
The MFI of neoplastic B cells labeled with LLP2A were significantly affected by whether the dog had received chemotherapy (F1,63 = 4.6, p = 0.0354) In LLP2A-labeled cells (normal and lymphoma cells combined), the mean (±SE) MFI for dogs treated with chemotherapy was 5.89 ± 1.04 while for dogs that had not received chemotherapy the mean MFI was much higher (7.56 ± 0.60) In contrast, for cells not labeled with LLP2A, mean MFI levels were similar between dogs that did (2.85 ± 0.43) and did not (3.00 ± 0.24) receive chemotherapy. Potentially because of the small number of dogs with T cell lymphomas, the interaction between chemotherapy and LLP2A labeling was not significant in T cells (F1,31 = 0.93, p = 0.3430). Mean MFI in LLP2A-labled T cells was higher in dogs that did not receive chemotherapy (chemotherapy: 2.72 ± 0.25; no chemotherapy: 4.93 ± 1.69). Chemotherapy did not affect unlabeled cells’ MFI (chemotherapy: 1.88 ± 0.19; no chemotherapy: 1.82 ± 0.14). Median and interquartile range (IQR) for normal and lymphoma cells with and without LLP2A labeling are shown in Table 1 for dogs that did not receive chemotherapy and in Table 2 for those that were treated with chemotherapy.
Table 1.
Median, interquartile range (IQR) and range of MFI for non-neoplastic and lymphoma cells labeled and not labeled with LLP2A for dogs that did not receive chemotherapy.
| LLP2A labeled
|
Not LLP2A labeled
|
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Median | IQR | Range | n | Median | IQR | Range | |
| Non-neoplastic lymphocytes | ||||||||
| B cells | 19 | 4.09 | (3.11, 5.69) | (2.61, 7.09) | 19 | 1.90 | (1.23, 2.01) | (1.04, 3.19) |
| T cells | 21 | 2.69 | (2.22, 3.41) | (1.58, 43.10) | 22 | 1.51 | (1.32, 1.92) | (1.04, 2.70) |
| Lymphoma cells | ||||||||
| B cells | 26 | 9.04 | (7.63, 11.1) | (4.90, 21.80) | 26 | 3.59 | (2.98, 4.62) | (1.05, 8.85) |
| T cells | 3 | 5.46 | (4.25, 6.95) | (3.05, 8.43) | 3 | 2.90 | (2.88. 3.36) | (2.85, 3.82) |
Table 2.
Median, interquartile range (IQR) and range of MFI for non-neoplastic and lymphoma cells labeled and not labeled with LLP2A for dogs that received chemotherapy.
| LLP2A labeled
|
Not LLP2A labeled
|
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Median | IQR | Range | n | Median | IQR | Range | |
| Non-neoplastic lymphocytes | ||||||||
| B cells | 5 | 3.63 | (3.38, 4.24) | (3.13, 5.28) | 5 | 2.08 | (1.34, 2.18) | (1.04, 2.53) |
| T cells | 6 | 2.28 | (1.88, 2.64) | (1.72, 2.80) | 6 | 1.37 | (1.27, 1.68) | (1.23, 2.09) |
| Lymphoma cells | ||||||||
| B cells | 6 | 7.83 | (3.88, 10.13) | (3.25, 12.80) | 6 | 3.98 | (2.79, 4.53) | (1.66, 5.42) |
| T cells | 4 | 3.38 | (3.00, 3.76) | (2.57, 4.23) | 4 | 2.35 | (2.10, 2.68) | (2.09, 2.94) |
3.4. Differential LLP2A labeling reveals different subsets of B and T cells
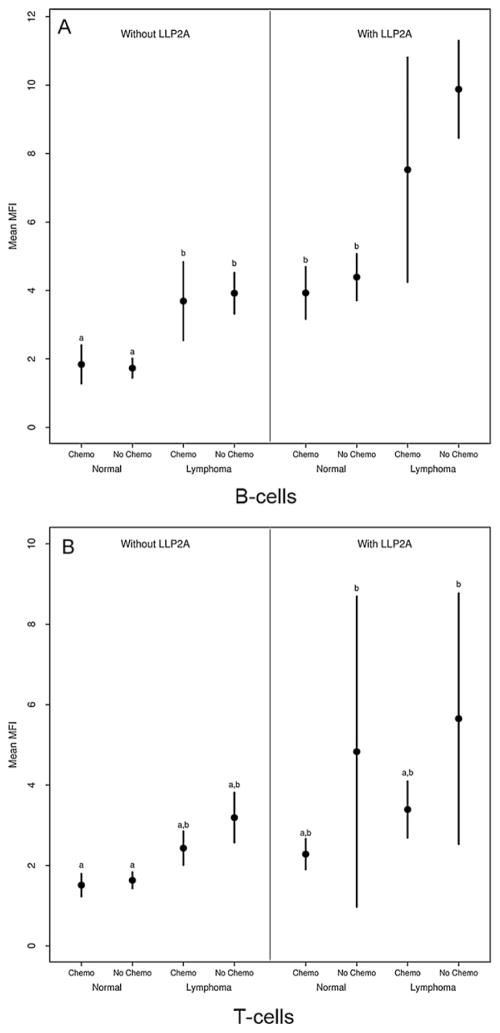
For B cells, pairwise comparisons of MFI levels from different samples revealed four non-overlapping groups. The lowest MFI levels were in unlabeled, non-neoplastic lymphocytes. The highest levels occurred in LLP2A-labeled lymphomas cells from dogs that had not received chemotherapy followed by those that had received chemotherapy (Fig. 3A). The largest group had intermediate MFI levels and was comprised of the remaining groups, to include non-neoplastic, labeled lymphocytes and unlabeled lymphocytes. In T cells, the pairwise comparisons identified only two overlapping groups. LLP2A-labeled T-cell lymphoma cells had a significantly higher MFI compared to unlabeled non-neoplastic lymphocytes. LLP2A affinity was not significantly different in unlabeled and labeled T-cell lymphoma cells, and labeled non-neoplastic lymphocytes. However, the pattern in mean MFI values was generally similar to that in B cells with respect to LLP2A labeling, lymphoma status and chemotherapy use (Fig. 3B). For both B and T cells, labeling with LLP2A increased MFI in both normal and lymphoma cells. Lymphoma cells had higher mean MFI levels than non-neoplastic lymphocytes, and chemotherapy acted to decrease MFI.
Fig. 3.
Mean ± 2 SE median fluorescence intensity (MFI) in B-cells (A) and T-cells (B) with respect to LLP2A labeling, lymphoma status (normal or lymphoma) and whether the dog received chemotherapy (chemo, no chemo). Groups with the same letter do not differ significantly (p > 0.05). B-cell lymphoma showed higher affinity to LLP2A than non-neoplastic lymphocytes. Both of these groups had higher MFI than unlabeled lymphoma cells. Chemotherapy significantly decreased the mean MFI in B-cell lymphoma. There was increased affinity of LLP2A to T-cell lymphoma although this was not significant.
4. Discussion
LLP2A showed significantly higher affinity to lymphoma cells than non-neoplastic lymphocytes, although both populations showed expression of activated alpha4–beta1 integrin. B-cell lymphoma cells also had higher affinity to LLP2A than T-cell lymphomas in dogs. Previous treatment with chemotherapy decreased the binding affinity of LLP2A to lymphoma cells (Tables 1 and 2).
The affinity of LLP2A to canine lymphoma cells was greatest in untreated lymphoma, with B-cell lymphoma having the highest affinity. Chemotherapy affected the expression of alpha4–beta1 negatively as evidenced by decreased MFI in both B-cell and T-cell populations. Specifically, there was a 2.25-fold increase in mean MFI of LLP2A in B-cell lymphoma untreated with chemotherapy compared with normal B-cells.
There is some evidence that cancer therapeutics such as histone deacetylase inhibitors downregulate adhesion due to VLA4 in people with leukemia (Mahlknecht and Schonbein, 2008). Several of the dogs underwent multiple courses of chemotherapy including different protocols prior to sampling for the study. It is possible that chemotherapy drugs used in these dogs could have caused reduced the expression of alpha4–beta1, leading to decreased affinity to LLP2A. This finding may be important when selecting patients for targeted therapeutics since there may be less binding to LLP2A after exposure to one or multiple types of chemotherapy. This will also need to be explored in human studies.
There was an increase in MFI between unlabeled normal lymphocytes and unlabeled lymphoma cells, which indicates increased autofluorescence. Autofluorescence of B-cell lymphoma compared to normal T-cells has been demonstrated when the cells were excited by 488 nm light (Pantanelli et al., 2009), and emitted light at 530–540 nm. Alexa-488 was used to evaluate LLP2A, and emits light from 400 to 650 nm, detected through the 542–585 filter, which was in a similar range. This autofluoresence may be caused by increased aerobic energy metabolism due to differing levels of NAD(P)H and flavin coenzymes between normal and neoplastic lymphocytes (Pantanelli et al., 2009). Lymphoma cells are generally larger than non-neoplastic lymphocytes, and contain cytoplasmic granules, which may also contribute to autofluorescence (Zoli et al., 2002).
Non-neoplastic lymphocytes increased MFI after LLP2A labeling. The increase in fluorescence was likely due to mild expression of activated alpha4–beta1 integrin on the surface of normal lymphocytes. The degree of LLP2A affinity to non-neoplastic lymphocytes was significant, but also significantly lower than lymphoma cells labeled with LLP2A. The degree of increased MFI of LLP2A-labeled, normal B-cells was similar to the autofluorescence of the unlabeled lymphoma cells. Interestingly, this differential affinity of LLP2A allows separation of non-neoplastic lymphocytes and B-cell lymphoma cells using flow cytometric analysis. This finding could be of interest in developing flow cyto-metric tests for lymphoma. Furthermore, these findings confirm that the alpha4–beta1 integrin is a good receptor for peptidomimetic targeting strategies that spare normal lymphocytes.
The number of T-cell lymphoma samples was low. The small increase in LLP2A affinity between labeled normal and lymphoma cells that was not statistically significant may indicate a type II statistical error. There is a lesser incidence of T-cell lymphoma in the canine cancer population, making sample collection in large numbers challenging. This increase in MFI indicates that alpha4–beta1 is expressed on canine T-cell lymphoma, and may have significantly more expression than on normal T-cells, given a larger sample size.
In conclusion, canine lymphoma expresses alpha4–beta1 integrin on the cell surface. LLP2A is able to bind B-cell lymphoma cells with greater affinity than normal lymphocytes, indicating a differential effect with targeted therapy aimed at sparing normal lymphocytes. Prior chemotherapy negatively affected the affinity of LLP2A to lymphoma cells. Spontaneously occurring canine lymphoma is a promising model to evaluate the efficacy of LLP2A-labeled chemotherapeutics in humans.
Acknowledgments
Funding source
Funding was supplied by NCDDG grant U19CA113298.
This publication was made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
References
- Chen LL, Whitty A, Lobb RR, Adams SP, Pepinsky RB. Multiple activation states of integrin alpha4beta1 detected through their different affinities for a small molecule ligand. J Biol Chem. 1999;274:13167–13175. doi: 10.1074/jbc.274.19.13167. [DOI] [PubMed] [Google Scholar]
- Chun R, Garrett LD, Vail DM. Evaluation of a high-dose chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med/Am Coll Vet Intern Med. 2000;14:120–124. doi: 10.1892/0891-6640(2000)014<0120:eoahcp>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Denardo SJ, Liu R, Albrecht H, Natarajan A, Sutcliffe JL, Anderson C, Peng L, Ferdani R, Cherry SR, Lam KS. 111In-LLP2A-DOTA polyethylene glycol-targeting {alpha}4{beta}1 integrin: comparative pharmacokinetics for imaging and therapy of lymphoid malignancies. J Nucl Med. 2009;50:625–634. doi: 10.2967/jnumed.108.056903. [DOI] [PubMed] [Google Scholar]
- Dorn CR, Taylor DO, Hibbard HH. Epizootiologic characteristics of canine and feline leukemia and lymphoma. Am J Vet Res. 1967;28:993–1001. [PubMed] [Google Scholar]
- Fournel-Fleury C, Magnol JP, Bricaire P, Marchal T, Chabanne L, Delverdier A, Bryon PA, Felman P. Cytohistological and immunological classification of canine malignant lymphomas: comparison with human non-Hodgkin’s lymphomas. J Comp Pathol. 1997;117:35–59. doi: 10.1016/s0021-9975(97)80065-5. [DOI] [PubMed] [Google Scholar]
- Hahn KA, Bravo L, Adams WH, Frazier DL. Naturally occurring tumors in dogs as comparative models for cancer therapy research. In Vivo. 1994;8:133–143. [PubMed] [Google Scholar]
- Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–880. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Holzmann B, Gosslar U, Bittner M. alpha 4 integrins and tumor metastasis. Curr Top Microbiol Immunol. 1998;231:125–141. doi: 10.1007/978-3-642-71987-5_8. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Mahlknecht U, Schonbein C. Histone deacetylase inhibitor treatment downregulates VLA-4 adhesion in hematopoietic stem cells and acute myeloid leukemia blast cells. Haematologica. 2008;93:443–446. doi: 10.3324/haematol.11796. [DOI] [PubMed] [Google Scholar]
- Northrup NC, Gieger TL, Kosarek CE, Saba CF, LeRoy BE, Wall TM, Hume KR, Childress MO, Keys DA. Mechlorethamine, pro-carbazine and prednisone for the treatment of resistant lymphoma in dogs. Vet Comp Oncol. 2009;7:38–44. doi: 10.1111/j.1476-5829.2008.00170.x. [DOI] [PubMed] [Google Scholar]
- Pantanelli SM, Li Z, Fariss R, Mahesh SP, Liu B, Nussenblatt RB. Differentiation of malignant B-lymphoma cells from normal and activated T-cell populations by their intrinsic autofluorescence. Cancer Res. 2009;69:4911–4917. doi: 10.1158/0008-5472.CAN-08-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha(4)beta(1) integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2:381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- Rassnick KM, Mauldin GE, Al-Sarraf R, Mauldin GN, Moore AS, Mooney SC. MOPP chemotherapy for treatment of resistant lymphoma in dogs: a retrospective study of 117 cases (1989–2000) J Vet Intern Med/Am Coll Vet Intern Med. 2002;16:576–580. doi: 10.1892/0891-6640(2002)016<0576:mcftor>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Rebhun RB, Cheng H, Gershenwald JE, Fan D, Fidler IJ, Langley RR. Constitutive expression of the alpha4 integrin correlates with tumorigenicity and lymph node metastasis of the B16 murine melanoma. Neoplasia. 2010;12:173–182. doi: 10.1593/neo.91604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhun RB, Kent MS, Borrofka SA, Frazier S, Skorupski K, Rodriguez CO. CHOP chemotherapy for the treatment of canine multicentric T-cell lymphoma. Vet Comp Oncol. 2011;9:38–44. doi: 10.1111/j.1476-5829.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med. 2011;17:380–388. doi: 10.1016/j.molmed.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terol MJ, Lopez-Guillermo A, Bosch F, Villamor N, Cid MC, Campo E, Montserrat E. Expression of beta-integrin adhesion molecules in non-Hodgkin’s lymphoma: correlation with clinical and evolutive features. J Clin Oncol: Off J Am Soc Clin Oncol. 1999;17:1869–1875. doi: 10.1200/JCO.1999.17.6.1869. [DOI] [PubMed] [Google Scholar]
- Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet Immunol Immunopathol. 1999;69:145–164. doi: 10.1016/s0165-2427(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Vezzali E, Parodi AL, Marcato PS, Bettini G. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet Comp Oncol. 2010;8:38–49. doi: 10.1111/j.1476-5829.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- Xiao K, Luo J, Fowler WL, Li Y, Lee JS, Xing L, Cheng RH, Wang L, Lam KS. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30:6006–6016. doi: 10.1016/j.biomaterials.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–167. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- Zoli W, Barzanti F, Dal Susino M, De Paola F, Tesei A, Ricotti L, Padovani F, Reno F, Amadori D. Flow-cytometric determination of tumor cells in lymph nodes. Oncology. 2002;62:128–135. doi: 10.1159/000048258. [DOI] [PubMed] [Google Scholar]
- Zwingenberger A, Vernau W, Shi C, Gordon IK, Kent MS. Development and characterization of 5 canine B-cell lymphoma cell lines. Leukemia Res. doi: 10.1016/j.leukres.2011.11.004.. [DOI] [PMC free article] [PubMed] [Google Scholar]