Abstract
In April 2010, sipuleucel-T became the first anticancer vaccine approved by the United States Food and Drug Administration. Different from the traditional chemotherapy agents that produce widespread cytotoxicity to kill tumor cells, anticancer vaccines and immunotherapies focus on empowering the immune system to overcome the tumor. The immune system consists of innate and adaptive components. The CD4+ and CD8+ T cells are the most crucial components of the adaptive arm of the immune system that act to mediate antitumor responses. However, T-cell responses are regulated by intrinsic and extrinsic mechanisms, which may interfere with effective antitumor responses. Many anticancer immunotherapies use tumor-associated antigens as vaccines in order to stimulate an immune response against tumor cells. Sipuleucel-T is composed of autologous mononuclear cells incubated with a fusion protein consisting of a common prostate cancer antigen (prostatic acid phosphatase) linked to an adjuvant (granulocyte-macrophage colony-stimulating factor). It is postulated that when the vaccine is infused into the patient, the activated antigen-presenting cells displaying the fusion protein will induce an immune response against the tumor antigen. In a recent randomized, double-blind, placebo-controlled, phase III clinical trial, sipuleucel-T significantly improved median overall survival by 4.1 months in men with metastatic castration-resistant prostate cancer compared with placebo. Although overall survival was improved, none of the three phase III clinical trials found a significant difference in time to disease progression. This, along with cost and logistic issues, has led to an active discussion. Although sipuleucel-T was studied in the metastatic setting, its ideal place in therapy is unknown, and clinical trials are being conducted in patients at different stages of disease and in combination with radiation therapy, antiandrogen therapy, and chemotherapy. Various other anticancer vaccines and immunotherapies for other tumor types are currently under investigation and in clinical trials. These immunotherapies were formulated to incorporate tumor-associated antigens aimed at stimulating effector T-cell responses or to block regulatory mechanisms that suppress the function of effector T cells. Additional studies will determine how these therapies can best improve clinical outcomes in patients with cancer.
Keywords: tumor immunology, sipuleucel-T, immunotherapy, anticancer vaccines, advanced prostate cancer
In April 2010, sipuleucel-T (Provenge; Dendreon Corp., Seattle, WA) was the first vaccine therapy approved by the United States Food and Drug Administration (FDA) for the treatment of cancer. A novel active immunotherapy, sipuleucel-T is indicated for men with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC).1 Vaccine therapy represents a new class of agents compared with traditional cancer therapies such as surgery, radiation therapy, chemotherapy, and targeted agents. These traditional treatment modalities aim to cure or palliate symptoms of the disease through largely non–immune-mediated actions such as direct cytotoxic effects or by affecting cellular pathways. Although these modalities are the standard of care, their effectiveness can be limited by their toxicity on other tissues and in metastatic disease. Vaccine therapy or immunotherapies, however, aim to stimulate the immune system and activate an appropriate immune-mediated response against malignant cells.
The concept of harnessing the immune system through vaccination to fight a malignancy was first documented by Dr. William Coley, a surgeon in New York City, at the turn of the last century.2 He observed that a patient’s sarcoma regressed after becoming infected with Streptococcus pyogenes. Based on this initial observation and other reported cases, Dr. Coley began to directly infect tumors with two killed bacteria, S. pyogenes and Serratia marcescens, to induce a fever. Although he is noted to have treated hundreds of patients, his idea was not widely accepted at the time and was left unstudied for many decades.
Since the gateway to cancer vaccines and immunotherapy has been opened with the approval of sipuleucel-T, understanding the design and use of these agents will be important. Therefore, we provide a review of tumor immunology, with a focus on sipuleucel-T’s design and trials leading to its approval, and the complicated logistics surrounding the availability of the vaccine. Finally, other cancer vaccines and immunotherapies under investigation are briefly reviewed.
Tumor Immunology
The immune system consists of innate and adaptive components. The innate immune system encompasses phagocytic cells (e.g., neutrophils, monocytes, and macrophages), natural killer cells, and cells that release inflammatory mediators (e.g., basophils, mast cells, and eosinophils). Macrophages and dendritic cells function as antigen-presenting cells (APCs), which serve as a bridge from innate to adaptive immunity. The innate immune system’s response to invading pathogens is nonspecific and does not form memory. However, the adaptive immune system remembers encounters with specific pathogens and amplifies its defense in response to subsequent encounters. Therefore, immunization with vaccines takes advantage of the memory-forming ability of the adaptive arm of the immune system.3, 4
The adaptive arm of the immune system consists of B cells and T cells. The B lymphocytes produce antigen-specific antibodies and are primarily responsible for defending against extracellular microorganisms.3, 4 The T lymphocytes are responsible for killing intracellular pathogens, as well as augmenting the B cell’s ability to make antibodies. The APCs are key mediators in the activation of T lymphocytes. In general, effector T lymphocytes express either CD4 or CD8 cell-surface molecules. The CD4+ T lymphocytes are also known as T-helper cells. These can be further differentiated into T-helper 1 and T-helper 2 cells. The T-helper 1 cells are involved in cell-mediated immunity by activating macrophages and cytotoxic T cells through the release of inflammatory mediators, interleukin (IL)-2, and interferon-γ. The T-helper 2 cells secrete IL-4, IL-5, IL-6, and IL-10, which are mostly involved in facilitating B-cell production of antibodies. The CD8+ T lymphocytes, also known as cytotoxic T cells, carry out direct killing effects. Recently, CD4+ T cells were also shown to have cytotoxic effects in a mouse tumor model.5 The T cells are the most crucial component in mediating antitumor responses induced by cancer vaccines and immunotherapies.
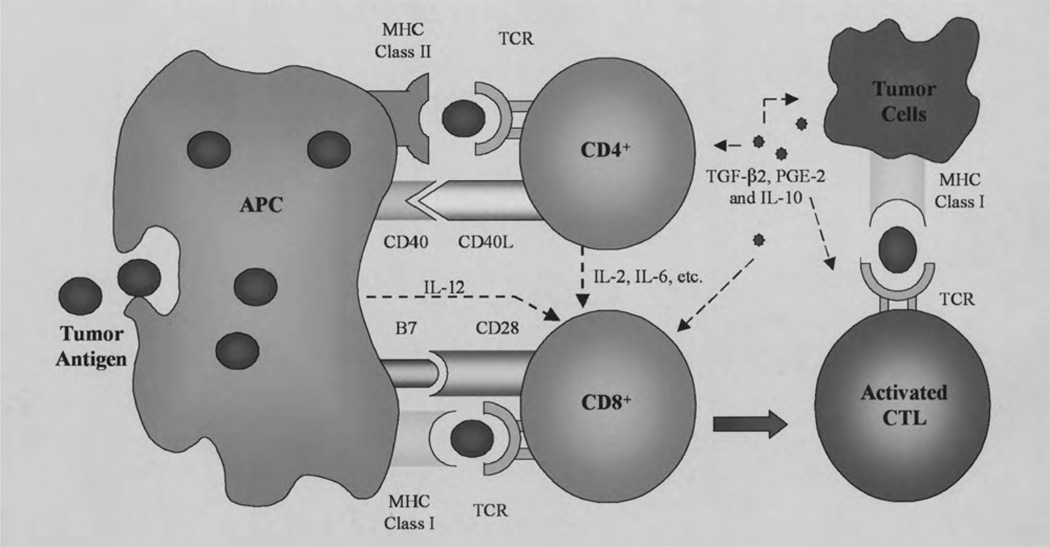
Naïve T lymphocytes, which have never encountered an antigen, are not inherently active. Instead, they must be activated by a two-step process to carry out their killing effects (Figure 1).6 The first step requires an encounter between a T lymphocyte and an APC, most commonly a primed dendritic cell. A primed dendritic cell has engulfed an antigen and processed it internally with major histocompatibility complex (MHC) proteins. The antigen-MHC complex is then presented on the dendritic cell’s surface and engages the T-cell receptor. The MHC class presented determines which type of T cell will respond: MHC class I binds CD8+ T cells and MHC class II binds CD4+ T cells. The second step in T-cell activation occurs through the binding of costimulatory molecules, B7-1 (CD80)/B7-2 (CD86) and CD28, on the surface of the APCs and T cells, respectively7 The B7:CD28 interaction is a critical costimulatory mechanism for the activation of T cells. The T cells can become anergic if either of these two signals is weak or absent.8
Figure 1.
Activation of T cells occurs through a two-step process. First, the antigen is taken up by the antigen-presenting cells (APCs), digested internally, associated with a major histocompatibility complex (MHC) molecule, and then presented on the APC’s surface. The antigen-MHC complex binds the T-cell receptor (TCR). The second step involves binding of costimulatory molecules on the APC and T cell. Cytokines such as interleukin (IL)-2 and IL-6 can help potentiate the cytotoxic T-cell response. However, the tumor can secrete its own microenvironment of cytokines and growth factors (i.e., transforming growth factor-β2, IL-10, prostaglandin E2), which could downregulate the activation of the immune response. PAP = prostatic acid phosphatase; GM-CSF = granulocyte-macrophage colony-stimulating factor. (From reference 6 with permission.)
Regulatory mechanisms come into play once a T cell has become activated through the two-step activation process. Cytotoxic T-lymphocyte antigen (CTLA)-4 is a critical intrinsic coinhibitory molecule that regulates T-cell activity9, 10 The CTLA-4 is induced and expressed on the cell surface within 2–3 days of T-cell activation.11, 12 The CTLA-4 has a 100-fold higher affinity for B7-1 and B7-2, and therefore outcompetes CD28 for B7 ligation. Through its interaction with the B7 receptor, CTLA-4 causes cell cycle arrest in the g1 phase by inhibiting the cascade of kinases within the cell and decreasing IL-2 production.12 Another coinhibitory molecule on T cells is programmed death (PD)-113; PD-1 binds to PD-L1 and PD-L2 receptors, which can be detected on activated T cells as well as other cells of the immune system. These coinhibitory molecules are discussed later as other strategies behind the design of cancer immunotherapies.
Aside from intrinsic mechanisms that regulate effector T-cell activity, extrinsic mechanisms such as regulatory T (Treg) cells act to control T-cell function. The Treg cells are a subset of CD4+ T cells with suppressive activity. Naturally occurring Treg cells are constitutively produced in the thymus gland and are responsible for maintaining self-tolerance. These cells depend on the expression of the transcription factor Foxp3 and constitutively express CTLA-4 and the α chain of the IL-2 receptor (CD25).14, 15 Inducible Treg cells can be generated from naïve T cells in the periphery under certain conditions. Conditions such as low antigenic stimulation, influence of transforming growth factor-β, or T-cell activation despite a noninflammatory environment have been identified as factors that could contribute to the induction of Treg cells.16, 17
The process by which the tumor overcomes the immune system is complex and continues to be an area of study. One model of tumor-induced immunosuppression has been described as immunoediting.18 This encompasses the three Es: elimination, equilibrium, and escape. In the first phase of immunoediting, the immune system is in a mode called immunosurveillance. During this phase, the immune system can recognize tumor-associated antigens (TAAs) and eliminate those cells expressing the TAA through natural processes, as reviewed above. The second phase is characterized by the immune system and the tumor being in a state of equilibrium. Eventually, however, resistant tumor cells will be selected for and escape immunosurveillance. This is where clinically evident disease and tumor progression come to fruition.18 Resistant tumor cells can “hide” from effector T cells through downregulation of costimulatory molecules19 and loss of TAAs or MHC class I surface molecules.20
The tumor can create its own microenvironment through the secretion of cytokines, growth factors, and signals that can downregulate the immune response.21 Release of IL-10 from tumors has been found to decrease production of stimulatory cytokines, tumor necrosis factor-α, and interferon-γ.22 Tumor-derived transforming growth factor-β can suppress various cells of the adaptive immune system through negative interaction with dendritic cells and inhibition of IL-2.23 The tumor can also release vascular endothelial growth factor, a potent blocker of dendritic cell differentiation and maturation.24 The resultant weakened dendritic cells create T-cell nonresponsiveness and indirectly promote tumor growth.
Prostate Cancer as Model for Tumor Immunology
Prostate cancer is the most common cancer diagnosis and second leading cause of cancer-related death among men, with approximately 217,730 new cases and 32,050 deaths in 2010.25 Androgen ablative therapy by bilateral orchiectomy or treatment with either a luteinizing hormone– releasing hormone agonist or antagonist is considered standard initial treatment for patients with metastatic disease or biochemical recurrence after localized therapy.26 The average response duration to androgen ablative therapy is 18–24 months before progression to a castrate-resistant disease state. Until recently, docetaxel with prednisone was the only chemotherapy shown to significantly improve overall survival in men with mCRPC, yielding an average survival of 18–20 months.27, 28 Cabazitaxel in combination with prednisone, approved in the summer of 2010, became the second agent to show improved survival in men with docetaxel-refractory CRPC.29 Limited treatment options exist, however, after failure or progression with these regimens.
Before cancer vaccines, chemotherapy in men with mCRPC had been implemented for palliation or for objective measurable disease that appeared threatening. The rationale for cancer vaccines, however, is completely different from the use of chemotherapy in these patients. Treatments such as chemotherapy and other targeted agents tend to become ineffective over time due to the development of drug resistance by tumor cells. However, in the setting of immune-based therapies, the adaptive arm of the immune system is capable of generating immune responses to the multitude of mutations within the tumor cells, thus making it likely that an activated immune response can lead to long-term control of cancer. However, to enable such an immune response will require an in-depth understanding of the many mechanisms that act to suppress immune responses and the use of combination therapies to overcome the various methods of immune suppression.
Prostate cancer is an attractive disease state for the study of immunotherapies for many reasons.30, 31 Typically, prostate cancer can be characterized as a slow-growing disease that is responsible for the prolonged disease course even among patients with mCRPC. This lengthy time frame affords the patient’s immune system time to develop an immune response that is necessary when studying the effects of novel immuno-therapies. It has been found that the prostate gland in men with prostate cancer is infiltrated with tumor-tolerant CD4+ cells, CD8+ cells, and Treg cells, suggesting a highly immune-mediated process ideal for studying immunotherapy.32, 33 As in other types of cancer, most prostate cancers are epithelial adenocarcinomas. Therefore, immunotherapies developed now for prostate cancer could be a resource for other cancer types later.
Biomarkers, such as prostate-specific antigen (PSA) and other TAAs such as prostatic acid phosphatase (PAP) and prostate-specific membrane antigen (PSMA), are specific to prostate cancer and can be useful in the study of immuno-therapies. The various TAAs in prostate cancer are well described and tend to be specific to prostate cells, providing targets for immunotherapy.34
Sipuleucel-T
Pharmacology
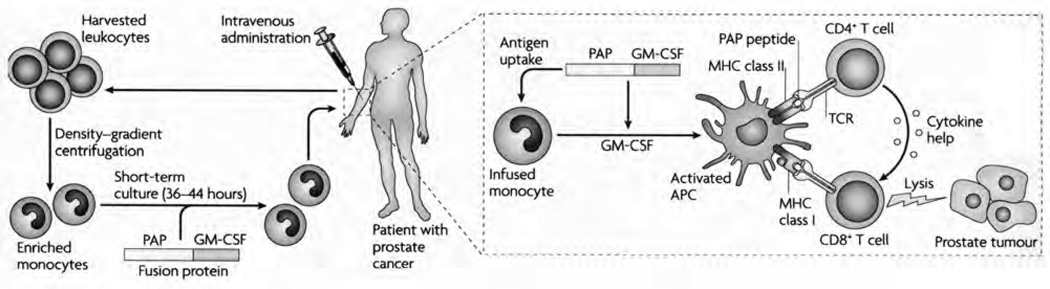
Sipuleucel-T is a personalized therapy made from the patient’s autologous peripheral blood mononuclear cells collected by leukapheresis. The erythrocytes, platelets, and low-density lymphocytes and monocytes are removed from the 1.5–2-L leukapheresis volume, leaving behind dendritic cells, T cells, B cells, and natural killer cells.35, 36 In a 36–44-hour incubation process, the patient’s monocytes are cultured ex vivo with a fusion protein, PA2024. This fusion protein is made of the whole, recombinant, human PAP fused with an adjuvant, granulocyte-macrophage colony-stimulating factor (GM-CSF). It is postulated that during incubation, the dendritic cells internally process the fusion protein and present the antigen-MHC complex on their surface.35 It is also postulated that once the vaccine is infused into the patient, the activated dendritic cells displaying the fusion protein will induce CD4+ and CD8+ immune cells against the tumor antigen, PAP, while GM-CSF enhances maturation of the dendritic cells (Figure 2).1, 31
Figure 2.
Preparation and proposed mechanism of action of sipuleucel-T. The patient’s harvested leukocytes are centrifuged to enriched monocytes and are then incubated with the fusion protein containing prostatic acid phosphatase (PAP) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Once the product is administered to the patient, the monocytes are thought to mature into activated antigen-presenting cells (APCs). These cells present PAP to the host’s immune system, activating CD4+ and CD8+ T cells against the tumor antigen, PAP. TCR = T-cell receptor; MHC = major histocompatibility complex; TGF-β2 = transforming growth factor-β2; PGE-2 = prostaglandin E2; IL = interleukin. (From reference 31 with permission.)
Clinical Trials
Phases I and II
In a phase I trial, sipuleucel-T was studied in 13 men with progressing hormone-refractory metastatic prostate cancer defined by rising PSA levels and radiographically apparent disease.37 Each patient received two infusions of APC8015 (sipuleucel-T) at weeks 0 and 4. To evaluate if humoral immunity could also be stimulated after active immunotherapy, patients were assigned to receive a subcutaneous injection of PA2024, the fusion protein without dendritic cells, in varying doses of 0.3, 0.6, or 1 mg/injection at weeks 8, 12, and 16. Twelve patients were evaluated for treatment response defined by PSA levels and T- cell reactivity. A decrease in PSA levels of 50% was found in three patients, although one patient had disease progression despite a PSA level reduction. There were no radiographic responses. After two infusions of sipuleucel-T, all evaluable patients had in vitro T-cell proliferation in response to both components of the fusion protein, GM-CSF and PAP (p=0.0004 and p=0.0001, respectively). The median time to disease progression of 135 days (range 30–274 days) was similar to that of patients treated with standard chemotherapy.
In sequential phase I and phase II trials, researchers determined the safety and efficacy of sipuleucel-T alone and its ability to induce immune responses to the fusion product of PAP and GM-CSF known as PA2024.35 Immune responses detected to PA2024 were likely due to the neoepitope that was created by the linker region used to join the PAP and GM-CSF proteins. A total of 31 patients with hormone-refractory prostate cancer with evidence of disease progression either by rising PSA level or positive radiographic results were enrolled: 12 in the phase I study and 19 in the phase II study. Patients in the phase I group had an average PSA level of 209 ng/ml (range 26.3–1007 ng/ml), were more likely to have metastatic disease, and were more heavily pretreated. In the phase I trial, each cohort consisted of three men who received escalated dose levels of sipuleucel-T (0.2 × 109, 0.6 × 109, 1.2 × 109, and 2 × 109 nucleated cells/m2) at weeks 0, 4, and 8. In addition, five of the 12 men were given doses of a control antigen, APC8017 (keyhole limpet hemocyanin [KLH]-loaded dendritic cells) to ensure specific T-cell responses to sipuleucel-T.
In the phase II trial, 19 patients without radiologic evidence of metastatic disease were enrolled with an average PSA level of 14.5 ng/ml (range 3.4–216 ng/ml).35 Patients in this trial received the maximum dose of nucleated cells that could be prepared from leukapheresis at weeks 0, 4, and 8.
Data from the two trials were pooled into one analysis. After two to three infusions of sipuleucel-T (8–12 wks), 100% of the patients had maximal T-cell responses to PA2024.35 Proliferation of T cells in response to PAP alone developed in 38% of patients who had not previously exhibited a response. Six patients experienced a greater than 25% decrease in PSA level; no patient had a radiographic improvement. Median time to disease progression was 12 weeks and 29 weeks in the phase I and phase II studies, respectively. In those who developed an immune response to PAP, median time to disease progression was 34 weeks compared with 13 weeks in those who did not develop a response (p<0.027). Those who received dendritic cell doses greater than 100 × 106 cells/infusion also had a significantly longer time to disease progression (31.7 vs 12.1 wks, p=0.013).
In 2004, another group of investigators performed a phase II study to evaluate the effect of sipuleucel-T infusions followed by PA2024 subcutaneous injections at the maximum dose of 1 mg/injection.36 Twenty-one patients with hormone-refractory prostate cancer were administered sipuleucel-T in two doses 2 weeks apart followed by three subcutaneous injections of PA2024 given monthly. Nineteen of 21 patients were evaluable. Two patients had a more than 25% decrease in PSA level, although this was transient. A third patient had a significant decrease in PSA to undetectable levels at 24 weeks, which was sustained for at least 4 years. No patient, except the patient who had undetectable levels for 4 years, had a radiographic response. Median time to disease progression was 118 days. When compared with results with sipuleucel-T used alone, these results suggest that the addition of subcutaneous PA2024 did not add significant benefit.
Phase III
One phase III, multicenter, randomized, double-blind, placebo-controlled trial (D9901) measured time to disease progression and median overall survival in patients receiving sipuleucel-T.38 Patients enrolled in the trial had mCRPC with radiographically apparent disease, serum testosterone level less than 50 ng/dl, expected survival of 3 months or longer, Eastern Cooperative Oncology group (ECOg) performance status of 0 or 1, and 25% or more of their cells positive for PAP by immunohistochemistry staining. Patients who had bone pain, used opioids for cancer- related pain, had visceral metastases, were receiving concurrent systemic corticosteroids, or who had already received an immunotherapy were not eligible. A total of 127 patients were randomly assigned in a 2:1 ratio to sipuleucel-T (82 patients) or placebo (45 patients). After disease progression, patients in the placebo group were allowed to cross over and receive sipuleucel-T and patients in either group could proceed with other anticancer treatments. Patients received sipuleucel-T or placebo at weeks 0, 2, and 4. Baseline characteristics were different between the sipuleucel-T and placebo groups only with regard to occurrence of bone-only disease (42.7% vs 26.7%) and presence of 10 or more bone metastases (40.2% vs 26.7%), although the differences were not statistically significant. Only 5–10% of the study population had received chemotherapy.
Progression was defined as radiographically apparent disease progression, new pain associated with a metastatic lesion, or other clinical events associated with progression (spinal cord compression, nerve root compression, or pathologic fracture). Of the 127 evaluable patients, 115 (90.5%) had disease progression. Time to disease progression did not differ significantly between the two groups (Table 1). In the sipuleucel-T group, 55.7% received subsequent chemotherapy compared with 62.8% in the placebo group. Although not powered for overall survival, the sipuleucel-T group had a survival advantage of 4.5 months over the placebo group (p=0.01). The estimated survival rate at 36 months was 34% for sipuleucel-T and 11% for placebo (p=0.005). Cox regression analysis revealed that when overall survival was adjusted for five different variables, the effect remained statistically significant (p<0.02, hazard ratio [HR] 2.12, 95% confidence interval [CI] 1.31–3.44).
Table 1.
Summary of Phase III Clinical Trial Results
| Parameter | D9901 Trial38 |
D9902A Trial39 |
Integrated Data (D9901, D9902A)39 |
D9902B Trial40 |
||||
|---|---|---|---|---|---|---|---|---|
| Sipuleucel-T (n=82) |
Placebo (n=45) |
Sipuleucel-T (n=65) |
Placebo (n=33) |
Sipuleucel-T (n=147) |
Placebo (n=78) |
Sipuleucel-T (n=341) |
Placebo (n=171) |
|
| Time to disease progression |
||||||||
| Median (wks) (95% CI) |
11.7 (9.1–16.6) |
10.0 (8.7–13.1) |
10.9 (9.3–17.7) |
9.9 (8.4–18.0) |
11.1 (10.0–16.3) |
9.7 (8.7–13.3) |
14.6 | 14.4 |
| Overall | ||||||||
| HR (95% CI) | 1.45 (0.99–2.11)a p=0.052c |
1.09 (0.69–1.70)a p=0.719c |
1.26 (0.95–1.68)a p=0.111c |
0.95 (0.77–1.17)b p=0.63c |
||||
| Survival | ||||||||
| Median (mo) (95% CI) |
25.9 (20.0–31.9) |
21.4 (12.3–25.8) |
19.0 (13.6–31.9) |
15.7 (12.8–25.4) |
23.2 (19.0–31.0) |
18.9 (13.5–25.3) |
25.8 | 21.7 |
| Overall | ||||||||
| HR (95% CI) | 1.7 (1.13–2.58)a p=0.01c |
1.27 (0.78–2.07)a p=0.331c |
1.5 (1.10–2.05)a p=0.011c |
0.78 (0.61–0.98)b p=0.03c |
||||
HR = hazard ratio; CI = confidence interval,
Hazard ratio is the result of the risk in patients treated with placebo divided by the risk in patients treat with sipuleucel-T. A hazard ratio > 1 shows more risk in patients treated with placebo.
Hazard ratio is the result of the risk in patients treated with sipuleucel-T divided by the risk in patients treated with placebo. A hazard ratio < 1 shows less risk in patients treat with sipuleucel-T.
Log-rank test.
While the D9901 study was being conducted, another identical phase III trial (D9902A) was under way.39 Enrollment in D9902A was closed at 98 patients of the planned 120 patients, due to probable futility of meeting its primary end point, time to disease progression. This was based on the initial time to disease progression results of D9901 and before its favorable overall survival results had been released. After the 36-month overall survival results from D9901 were released, D9902A was amended to become D9902B in which its primary end point was overall survival instead of time to disease progression.39, 40 These results are discussed later.
In the 98 patients enrolled in D9902A, several baseline characteristics, namely, the number of patients with more than 10 bone metastases, and the PSA, alkaline phosphatase, and lactate dehydrogenase levels, were less likely or lower in the placebo group.39 Time to disease progression, the primary end point, did not differ significantly between the two groups. There was a 21% risk reduction in death for patients treated with sipuleucel-T compared with those receiving placebo, although this was not statistically significant. When adjusted for baseline factors, overall survival trended in favor of patients treated with sipuleucel-T (Table 1).
Since the D9901 and D9902A trials were identical, their pooled results were analyzed to estimate an overall treatment effect.38, 39 Only five of 147 patients receiving sipuleucel-T in both studies had a greater than 50% reduction in PSA level. Pooled time to disease progression was not significant between sipuleucel-T and placebo; however, patients in the sipuleucel-T group had a median overall survival advantage of 4.3 months over placebo (Table 1).
The D9902B study, or the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial, was a randomized, double-blind, placebo-controlled, multicenter, phase III trial that studied 512 men with mCRPC as evidenced by rising PSA levels or radiographically apparent disease with an expected survival of more than 6 months.40 Different from the previous phase III studies, men with minimally symptomatic disease were included in this trial. Men were excluded if they had an ECOg performance status of 2 or more, metastases other than bone, complications from bone disease (i.e., spinal cord compression, pathologic long-bone fractures), were receiving corticosteroids, or had received two or more previous chemotherapies.
Patients were randomly assigned in a 2:1 ratio to receive sipuleucel-T (341 patients) or placebo (171 patients) given as an infusion every 2 weeks for three doses. Contrary to the other phase III trials, the primary end point for the IMPACT trial was overall survival, defined as death from any cause. The secondary end point was time to objective disease progression as monitored at specified intervals by computed tomography and bone scans. Baseline characteristics did not differ significantly between the two groups. The average patient age was 71 years, 12–15% of patients in each group had received chemotherapy, and half of patients in each group had a baseline pain score of 0.
Patients receiving active treatment had a relative risk reduction in death of 22% (p=0.03; Table 1). This was associated with a 4.1-month longer survival time (25.8 mo with sipuleucel-T vs 21.7 mo with placebo). The estimated 36-month survival was 31.7% in the sipuleucel-T group compared with 23% in the placebo group, which was similar to that found in a previous phase III trial.38 Therapy with sipuleucel-T was still favored when adjusted for over 20 baseline characteristics known to be associated with poor survival. As seen in the other phase III trials, time to objective disease progression did not differ significantly between the two groups (Table 1).
Patients whose disease progressed while taking placebo were allowed to cross over into the vaccination arm, and both groups could receive other anticancer treatment. Further anticancer treatments were administered to 81.8% of patients who received sipuleucel-T and 73.1% of patients in the placebo group. Of those, 57.2% of patients in the sipuleucel-T group and 50.3% of patients in the placebo group received docetaxel-based therapy. To measure the possibility of docetaxel confounding the results, the study investigators adjusted for the effect of docetaxel on the treatment effect of sipuleucel-T. The treatment effect of sipuleucel-T after docetaxel use remained significant over placebo (HR 0.65, 95% CI 0.47–0.90, p=0.009).
Questions Remaining from the Clinical Trial Results
Although sipuleucel-T has shown promising results, leading to its approval by the FDA in April 2010, the design and end points of the trials have been criticized. Some suggest that the trials should have chosen a better control to ensure that the actual treatment effect is from the tumor antigen alone. A reasonable placebo would include GM-CSF so that the only distinguishing feature between placebo and treatment would be the PAP antigen.41
In addition, patients in the placebo groups were allowed to cross over to receive sipuleucel-T therapy, which consisted of the patients’ autologous cells that were incubated with PA2024, then frozen until needed, and thawed before administration. Whether this frozen and thawed product represents active therapy remains questionable. Another question that remains is whether administration of the thawed cells at the time of crossover led to a delay in the administration of active chemotherapy, thus negatively impacting overall survival. Despite these unanswered questions, the median survival time of the placebo group compares with that seen with similar control groups in other randomized trials.40 Many patients received chemotherapy once their disease progressed. Although studies accounted for the effect that subsequent treatment could have on overall survival, there could again be some confounding variables influencing the outcomes, such as the duration of time that existed between treatments.41
The most perplexing finding with sipuleucel-T was how time to disease progression, defined by lack of radiographic response or change in PSA level, was not changed whereas overall survival was prolonged. This is contrary to current experience with cytotoxic chemotherapy such that an increase in overall survival would correlate with regression of disease. Perhaps there is a method by which immunotherapies interact with the cancer or some as yet unidentified variable that is influencing the results.40, 41 Clinical trials showed that an immune response was generated months after treatment with sipuleucel-T.35, 36 This could possibly allow initial tumor growth. This paradox has begun a discussion on whether the current assessment of progressive disease with use of the response evaluation criteria in solid tumors (RECIST criteria) is appropriate when measuring response to immunotherapies.42 Two principles underlie the RECIST criteria: chemotherapy shrinks tumors, and tumor shrinkage leads to patient benefit. As is seen in multiple trials, tumor shrinkage does not usually occur with immunotherapies, although a benefit cannot be excluded.38–40 To this extent, new guidelines have been proposed to measure response to immunotherapies in solid tumors.43
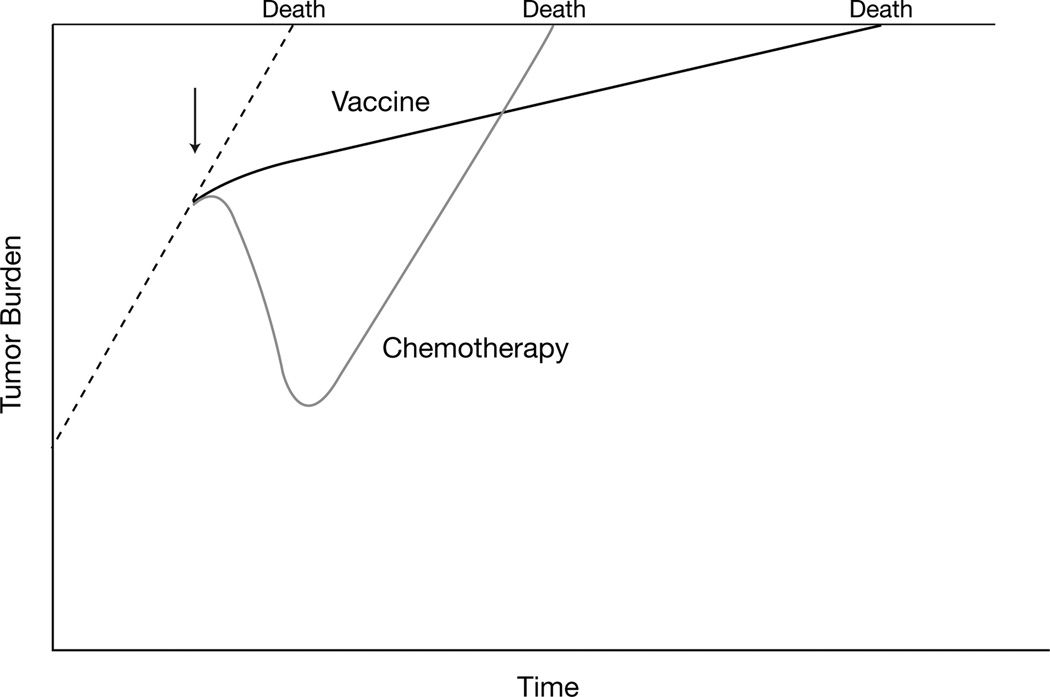
The failure to improve time to disease progression but still prolong overall survival can partially be explained through models that follow PSA level to predict death.42, 44 In general, PSA level changes can be difficult to interpret and should not determine therapy alone. However, following PSA level trends has led to interesting findings. Clinical trials using conventional chemotherapy to treat patients with mCRPC were reviewed at the National Cancer Institute. The tumor burden as inferred from PSA levels declined accordingly while patients were receiving treatment, but once treatment was stopped, PSA levels began to increase at the same rate as before chemotherapy (Figure 3).42 However, in an analysis of the PSA-TRICOM (a vaccine containing a poxvirus vector encoding for PSA and three costimulatory molecules: B7-1, intercellular adhesion molecule-1 [ICAM-1], and LFA-3), PSA levels changed independent of whether or not the patient was receiving treatment.42 This suggests that while vaccines may not alter the size of the tumor, they may reduce the rate of their growth even after therapy is completed.
Figure 3.
Effect of chemotherapy and immunotherapy (vaccine) on tumor growth. Dotted line indicates tumor burden without any treatment. Initiation of chemotherapy or immunotherapy are indicated by the arrow. Chemotherapy impacts tumor burden after administration. When it is stopped, the tumor can grow at the same rate previous to the treatment and predict time to death. Immunotherapy, theoretically, slows the progression of the tumor even when the therapy has been stopped. This may explain why overall survival can be prolonged by immunotherapies whereas time to progression is not. (From reference 42 with permission.)
Dosing and Administration
Sipuleucel-T is administered as a 60-minute infusion at weeks 0, 2, and 4.1 Leukapheresis is performed 2–3 days before the scheduled date of infusion. If a dose is not able to be given on time, another leukapheresis session is necessary. Each infusion of sipuleucel-T consists of at least 50 million autologous CD54+ cells, since clinical trials have shown that the number of CD54+ cells in each infusion correlates with improved benefit.35 The final product of cells are suspended in a cream-to-pink–colored 250-ml bag of Ringer’s lactate solution. Patients should receive pretreatment with acetaminophen and an antihistamine to reduce the risk of infusion-related reactions before each dose.1
Safety
The vaccine was well tolerated in all of the phase III trials.38–40 There were no deaths attributed to sipuleucel-T during infusion or up to 30 days afterward. The most common events occurring in greater than 20% of patients in all three trials were infusion-related reactions, including chills, fatigue, back pain, nausea, and arthralgias. Most adverse events were mild to moderate (76.2% for the combined analysis of the D9901 and D9902A studies and 65.2% in the IMPACT study).39, 40 Among serious adverse events seen in clinical trials, the occurrence of cerebrovascular events (hemorrhagic, ischemic, or embolic strokes; transient ischemic attack; or hemorrhage from metastatic brain lesion) were reported higher among the sipuleucel-T groups. In the D9901-D9902A analysis, 7.5% of patients in the sipuleucel-T group versus 2.6% in the placebo group experienced a cerebrovascular event.39 The IMPACT trial reported a 2.4% rate of cerebrovascular events in sipuleucel-T–treated patients compared with 1.8% in the placebo group.40 As a result of the serious data on cerebrovascular events, the FDA is requiring a postmarketing study to further clarify the risk of cerebrovascular events in 1500 patients who will receive sipuleucel-T. This is to be completed by 2015. In the interim, reports are required to be submitted every 6 months.45
Logistic and Economic Concerns
The cost of sipuleucel-T is $93,000 for the three infusions, which correlates to a $23,000/ month-of-survival advantage.46 In contrast, data from a retrospective registry estimated the monthly cost of care for patients with mCRPC receiving standard therapy to be $1800, although some patients in this database had not received docetaxel.41, 47 In terms of quality-adjusted life-year, the cost of sipuleucel-T is $279,000.41. The median incremental cost-effectiveness ratios per quality-adjusted life-year of breast, colorectal, and lung cancers in 2008 U.S. dollars were $27,000, $22,000, and $22,000, respectively.48 Furthermore, most men with prostate cancer are of an age that they receive Medicare benefits; sipuleucel-T has become included in the most recent National Comprehensive Cancer Network guidelines, which correlates with Medicare approval and payment. On March 30, 2011, after a year-long coverage analysis, the Centers for Medicare and Medicaid Services issued a preliminary statement “that the evidence is adequate to conclude that sipuleucel-T…is reasonable and necessary” for its indication. A final decision is pending public comments.46, 49
Another limitation for sipuleucel-T is its accessibility by patients. In the first 12 months, sipuleucel-T therapy will be limited to 2000 patients.46 This is due to the manufacturing capability of Dendreon Corporation, the sponsoring biotechnology company. Sipuleucel-T is manufactured in one facility in New Jersey. Plants in two other U.S. cities have started construction.50
Until all operating plants are fully functional, logistics can become a limiting factor. Patients undergo leukapheresis 2–3 days before the scheduled infusion.1 This collection must be sent to the company where the cells are incubated in a media containing the fusion protein for 36–44 hours.40 These cells must then be delivered back to the patient’s treatment center by courier in time for the scheduled infusion.
Place in Therapy
The current National Comprehensive Cancer Network guidelines for prostate cancer reflect the results from the phase III trials. Sipuleucel-T is a category 1 treatment recommendation for patients with evidence of metastatic disease during androgen deprivation therapy. These guidelines specify that sipuleucel-T is only recommended in patients with a good performance status, minimally symptomatic disease, and a life expectancy of more than 6 months.26
Despite the clinical studies, the ideal role of sipuleucel-T in patients with prostate cancer and the potential synergy of sipuleucel-T with other therapies have not been elucidated. These questions are being explored in patients without metastatic disease. Although sipuleucel-T has been primarily studied and approved in patients with metastatic disease, immunotherapies might be more efficacious if used earlier in the disease process or in combination with other treatment modalities. Anticancer vaccines have been studied in combination with radiation therapy.51 By design, radiation causes tumor cell death. Dying tumor cells release inflammatory mediators and TAAs, which could be processed by APCs to provoke an adaptive immune response.31 In a randomized phase II trial to study the effects of radiation on immune responses, 30 patients were randomly assigned, in a 2:1 ratio, to receive combination therapy with a poxviral-based PSA vaccine and radiation or to receive radiation alone.51 Of the 17 patients in the combination arm, 13 (76%) had at least a 3-fold increase in T cells specific for PSA compared with no increases in the placebo arm (p<0.0005).
Androgen deprivation therapy is the standard of care for patients with advanced prostate cancer.26 Studies of immune responses after androgen deprivation therapy in patients with prostate cancer have revealed that the prostate gland becomes infiltrated with activated CD4+ T cells.52 Studies in castrated mice have also noted a regeneration of thymic production of T cells.53 Therefore, combining immunotherapy with androgen deprivation therapy could enhance an already present immune process. In one trial with a crossover design, 41 patients received either a poxviral-based PSA vaccine or nilutamide, an antiandrogen agent, alone, and then at progression the patients were treated with both agents together.54 Median survival for patients who received the vaccine first versus those receiving nilutamide first trended in favor of the vaccine-first group (5.1 vs 3.4 yrs, p=0.13). However, in the 20 patients who crossed over to combination therapy, those who received vaccine first survived a median of 6.2 years versus 3.7 years in patients who received nilutamide first (p=0.045).55
Similar to radiation therapy, chemotherapy can cause tumor cell apoptosis leading to the release of TAAs, which may be taken up and presented by APCs. In addition, it has been shown that particular chemotherapies can preferentially subdue Treg cells, upregulate expression of TAAs, and decrease production of inhibitory tumor-secreted cytokines.56, 57 Preclinical studies have found that docetaxel given with TRICOM, which targets carcinoembryonic antigen, is better than either agent alone in stimulating an immune response.58 A phase II study evaluated a poxviral-PSA vaccine given either alone or in combination with low-dose docetaxel in men with mCRPC.59 Docetaxel did not inhibit T cell–specific responses. As a secondary end point, compared with a historical control group receiving docetaxel alone, the vaccine plus docetaxel group had a longer progression-free survival (6.1 vs 3.7 mo).
In the phase III clinical trials with sipuleucel-T, many patients received docetaxel-based therapy after disease progression while receiving the vaccine.38–40 Although there was still an overall survival benefit in these patients, some attributed this to the effect of the chemotherapy and not actually the vaccine. Results from studies mimicking this same design of chemotherapy after vaccine have also shown that patients who are treated with a vaccine first do better than those receiving chemotherapy alone.60, 61 This was seen in 51 patients with mCRPC who were treated with sipuleucel-T or placebo; the overall survival for patients receiving the vaccine and then chemotherapy was 34.5 months compared with 25.4 months in those who received placebo and the same chemotherapy (p=0.023).62 An ongoing clinical trial by ECOg will study the effects of either a vaccine first followed by docetaxel and prednisone, or docetaxel and prednisone first followed by vaccine.
Aside from radiation therapy and chemotherapy, there have been studies combining immuno-therapy with targeted therapies. Bevacizumab, a potent inhibitor of vascular endothelial growth factor, in combination with sipuleucel-T was studied in 22 men with mCRPC.63 Combination therapy was found to decrease PSA levels and therefore alter its doubling time in a safe manner while demonstrating an immune response against the vaccine antigen.
Other Cancer Vaccines and Immunotherapies
There are multiple cancer vaccines and immunotherapies under investigation. Cancer vaccines target TAAs to stimulate an effector T-cell response. These can be classified by subgroups, and examples of each are briefly reviewed (Table 2).64–77
Table 2.
| Immunotherapya | Targeted Cancer | Components |
|---|---|---|
| Vitespen (Oncophage) |
Renal cell carcinoma, melanoma |
Peptide-based vaccine using heat shock proteins from patient’s tumor |
| glycoprotein 100 vaccine |
Melanoma | Peptide-based vaccine using peptide from glycoprotein 100, a melanoma- associated antigen |
| gVAX | Prostate cancer | Allogeneic whole-tumor-cell–based vaccine secreting granulocyte-macrophage colony-stimulating factor |
| DCVax | Prostate cancer, glioblastoma |
Dendritic cells pulsed with prostate-specific membrane antigen for prostate cancer or tumor lysate for glioblastoma |
| ProstVac-VF | Prostate cancer | Poxviral-based vaccine encoding for prostate-specific antigen and costimulatory molecules B7-1, ICAM-1, and LFA-3 |
| BiovaxID | Non-Hodgkin’s lymphoma |
Anti-idiotype vaccine targeting B cell lymphomas |
| Ipilimumab | Melanoma, prostate cancer |
Antibody to CTLA-4 |
| Tremelimumab | Melanoma, prostate cancer |
Antibody to CTLA-4 |
| MDX-1106 | Refractory, relapsed solid tumors |
Antibody to PD-1 |
ICAM-1 = intercellular adhesion molecule-1; CTLA-4 = cytotoxic T-lymphocyte antigen 4; PD-1 = programmed death-1.
Manufacturers are as follows: Oncophage, Antigenics Inc., New York, NY; gVAX, Cell genesys, Inc., San Francisco, CA; DCVax, Northwest Biotherapeutics, Inc., Bethesda, MD; ProstVac-VF, Bavarian Nordic, Mountain View, CA; BiovaxID, Biovest International Inc., Bethesda, MD.
Peptide- or Protein-Based Vaccines
Studies with peptide- or protein-based vaccines have been completed in several types of cancer. One type of vaccine that is peptide-based includes the use of heat shock proteins (HSP), or chaperone proteins, which help fold other proteins within cells. The HSP-peptide complexes specific to tumor cells provide an antigen that can be used in cancer vaccines. Vitespen (Oncophage; Antigenics Inc., New York, NY) is a vaccine made from autologous tumor-derived HSP glycoprotein (gp)96–peptide complex (HSPPC-96). In a phase III trial, vitespen was compared with observation in 728 patients with locally advanced renal cell carcinoma after nephrectomy.64 Although there was no overall benefit in recurrence-free survival, subgroup analysis indicated a non–statistically significant benefit in patients with earlier stages of disease. In a different phase III trial, the effect of vitespen versus physician choice of treatment on overall survival was studied in 322 patients with untreated, stage IV melanoma.65 Overall survival did not differ significantly between the two groups; however, subgroup analysis indicated that patients in M1a and M1b substages who received more doses of vitespen survived longer compared with those who did not receive as many doses.
Another peptide-based vaccine uses gp100, a melanoma-associated antigen. The gp100-based vaccines have been shown to stimulate an immune response, although no reduction in tumor size with monotherapy has been found.66 As a result, gp100-based vaccines are being studied in combination with other therapies, as discussed later.
Autologous or Allogeneic Whole-Tumor-Cell Vaccines
Anticancer vaccines have been prepared from whole autologous or allogeneic tumor cell lines. Autologous whole-tumor-cell vaccines have the advantage of targeting the patient’s own TAAs. However, it is speculated that reinfusing the same tumor cells would not necessarily stimulate an immune response where one was never present. Therefore, most studies have used allogeneic whole-tumor-cell therapy, which has the potential to elicit a better immune response. Whole-tumor-cell vaccine therapy is a polyvalent approach to immunotherapy since it allows exposure to multiple antigens. This could prevent tumor cells from evading the immune systems by modifying their antigenic make-up as is possible with monovalent vaccines.31
The whole-tumor-cell vaccine gVAX (Cell genesys, Inc., San Francisco, CA) has been studied in phase III trials for prostate cancer. This prostate cancer vaccine consists of two allogeneic prostate cancer cell lines engineered to secrete GM-CSF. Efficacy in phase I and phase II trials led to the phase III trials in prostate cancer, Vaccine Immunotherapy with Allogeneic Prostate Cancer Cell Lines (VITAL) 1 and VITAL-2.67 The VITAL-2 study was terminated early due to increased deaths in the vaccine arm. Not long after, the VITAL-1 study was terminated based on a futility analysis of less than a 30% chance of meeting its end point.30
Dendritic Cell–Based Vaccines
Dendritic cell–based vaccines are another category of anticancer vaccines. The DCVax-Prostate vaccine (Northwest Biotherapeutics, Bethesda, MD) is a PSMA-loaded autologous dendritic cell vaccine being studied in prostate cancer. It is made from leukapheresed high-density monocytes incubated with PSMA peptides before infusion. Of note, DCVax-Prostate vaccine does not use the whole protein and does not contain GM-CSF. Its use in prostate cancer has been studied in phase I and phase II trials and was shown to induce an immune response.68
The DCVax-Brain vaccine (Northwest Biotherapeutics) uses the same concept as DCVax-Prostate and loads autologous dendritic cells with tumor lysate from the patient’s tumor in newly diagnosed glioblastoma multiforme.69
Gene Therapy–Based Vaccines
Vector or gene therapy–based vaccines commonly use viruses, as opposed to bacteria or yeast, as the vehicle to deliver the vaccine. Advantages to using viruses as vaccine vectors include a large genome for simple gene insertion, its ability to initiate an inflammatory response at the site of an injection, and its cost in comparison to other types of vaccines. The poxvirus family, in particular, is an attractive and popular vector since it induces a strong immune response after injection and has been safely administered to patients since the 1960s.70
The ProstVac-VF vaccine (Bavarian Nordic, Mountain View, CA) is a recombinant poxvirus vaccine that encodes for PSA and the costimulatory molecules B7-1, ICAM-1, and LFA-3 (TRICOM) to enhance T-cell activation. The vaccination schedule with ProstVac-VF is different from that of other cancer vaccines as it entails a prime-and-boost strategy.70, 71 Once the vaccinia-based vector is given, a vigorous immune response is induced, but host antibodies will usually neutralize the virus during subsequent administrations thus making the therapy ineffective. For this reason, booster shots with fowlpox-based vectors, which do not cause formation of neutralizing antibodies, are given to continue the immune response to the antigen. In a recent phase II trial, men with mCRPC were randomly assigned to receive ProstVac-VF or placebo.71 Men receiving the active treatment had an 8.5-month overall survival benefit (25.1 vs 16.6 mo with placebo) associated with a 44% reduction in the death rate (estimated HR 0.56, 95% CI 0.37–0.85, stratified log-rank p=0.0061). The ProstVac-VF vaccine has also been studied in combination with other therapies.
Idiotype Immunoglobulin–Based Immunotherapy Strategies
In lymphomas, the malignant B cells possess tumor-specific antigens called idiotypes. The BiovaxID vaccine (Biovest International Inc., Bethesda, MD) was developed as an anti-idiotype cancer vaccine toward B-cell lymphomas. Through a time- and labor-intensive process, the vaccine is made by fusing biopsy-obtained lymphoma cells with a myeloma cell line. This heterohybridoma produces antibodies similar to the tumor. The idiotypes of the antibodies are purified and conjugated to KLH (Id-KLH) to increase immunogenicity.72 A phase III randomized, placebo-controlled, double-blind clinical trial was performed in patients with follicular non-Hodgkin’s lymphoma.73 Patients who had achieved and sustained a complete response of 6 months after treatment with prednisone, doxorubicin, cyclophosphamide, and etoposide were randomly assigned to treatment with Id-KLH plus GM-CSF (76 patients) or KLH not conjugated to idiotype plus GM-CSF (placebo group, 41 patients). The primary end point of disease-free survival was 44.2 months in the Id-KLH plus GM-CSF group versus 30.6 months in the placebo group (p=0.045, HR=1.6).
Other Immunotherapies
Anticancer vaccines target tumor-specific TAAs to stimulate effector T-cell responses. Although the approval of sipuleucel-T suggests that anticancer vaccines may be effective, the improved survival of 4 months with sipuleucel-T over placebo suggests that there may be other factors at work inhibiting effector T-cell responses.40 As discussed earlier, activated T cells can be suppressed by increased expression of CTLA-4 and PD-1. The Treg cells, which constitutively express CTLA-4, can also interfere with the actions of effector T cells. Therefore, other immunotherapies such as anti-CTLA-4 antibodies and anti-PD-1 antibodies have been developed to target the regulatory mechanisms that may inhibit effector immune responses.74
Anti-CTLA-4 Therapy
Early preclinical studies with CTLA-4 blockade showed improved tumor control, yet the efficacy was limited to tumors with inherent immuno-genicity.75 This led to the use of anti-CTLA-4 therapy in combination with other immune-directed and cytotoxic approaches. Ipilimumab (MDX-101; Medarex, Princeton, NJ, and Bristol-Myers Squibb, New York, NY) and tremelimumab (Pfizer, New York, NY) are the two anti-CTLA-4 antibodies that are in clinical trials. Most of the research with anti-CTLA-4 antibodies has been focused on melanoma; however, smaller studies have been completed in other solid tumor types.74
Because of the potential synergy with other agents, ipilimumab has been studied in combination with gp100 in a phase III, randomized, double-blind, multicenter study.66 Patients with previously treated, unresectable stage III or IV melanoma were randomly assigned to receive either both ipilimumab and the gp100 vaccine, ipilimumab alone, or the gp100 vaccine alone. Median overall survival was statistically significantly improved in patients treated with ipilimumab alone or ipilimumab in combination with gp100 (10 and 10.1 mo, respectively) compared with a median overall survival of 6.4 months for patients receiving gp100 alone. Similar to findings in the phase II studies, ipilimumab was associated with immune-related adverse events. Sixty percent of the ipilimumab-treated patients compared with 32% of gp100–treated patients had immune-related adverse events, which included colitis, diarrhea, endocrine-related events, or dermatologic reactions. grade 3 or 4 immune-related adverse events occurred in 10–15% of patients treated with ipilimumab compared with 3% in the gp100 alone group. Seven deaths were attributed to immune-related adverse events.
In a phase II clinical trial, a single dose of ipilimumab plus androgen ablation was compared with androgen ablation alone in 54 patients with advanced prostate cancer.76 Fifty-five percent of the patients treated in the combination arm compared with 38% of the patients receiving androgen ablation alone achieved undetectable PSA levels. Of those patients in the combination arm, some had clinical responses and subsequent disease downstaging. The most common grade 3 or 4 immune-related adverse event seen was diarrhea or colitis, which occurred in 4.5% of patients treated with ipilimumab.
Anti-PD-1 Therapy
Like CTLA-4, PD-1 is another checkpoint molecule that can inhibit T-cell responses.10 One of the two developed anti-PD-1 antibodies, MDX-1106 (Medarex, Princeton, NJ), has been studied in a phase II trial in patients with select relapsed refractory malignancies. Clinical activity was shown in patients with renal cell carcinoma and melanoma. Similar to patients treated with anti-CTLA-4, patients treated with anti-PD-1 are also susceptible to immune-related adverse events. However, in these early trials, the occurrence of immune-related adverse events appears to be less with anti-PD-1 than with anti-CTLA-4.77
Conclusion
Prostate cancer provides an exciting model for immunotherapies, heralded by the approval of sipuleucel-T. The approval of this vaccine was based on an improvement in overall survival, although time to disease progression was unchanged. This has led to discussions about new criteria for defining disease progression with immunotherapies. Whereas many questions are left unanswered with regard to this phenomenon, as well as to sipuleucel-T’s optimal place in therapy, the approval of this vaccine has given patients another treatment option in a disease state where relatively few agents are effective at increasing overall survival. Many other anticancer vaccines and immunotherapies are under investigation, which will surely expand the available approved therapies. Understanding how these novel therapies can be integrated into the current treatment paradigm and how to possibly combine them with other agents for improved clinical benefit will be the next steps to be taken. Finally, it should be recognized that future successful immunotherapy strategies will need to address all arms of the immune system, effector and regulatory systems.
References
- 1.Dendreon Inc. Provenge (sipuleucel-T) package insert. Seattle, WA: 2010. [Google Scholar]
- 2.Starnes CO. Coley’s toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 3.Delves PJ, Roitt IM. The immune system: first of two parts. N Engl J Med. 2000;343:37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 4.Delves PJ, Roitt IM. The immune system: second of two parts. N Engl J Med. 2000;343:108–117. doi: 10.1056/NEJM200007133430207. [DOI] [PubMed] [Google Scholar]
- 5.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Ng K, Lillehei KO. Cell-mediated immunotherapy: a new approach to the treatment of malignant glioma. Cancer Control. 2003;10:138–147. doi: 10.1177/107327480301000205. [DOI] [PubMed] [Google Scholar]
- 7.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signaling costimulates murine T cells and prevents induction of anergy in T cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 8.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signaling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 9.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily: CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 10.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iida T, Ohno H, Nakaseko C, et al. Regulation of cell surface expression of CTLA-4 by secretion of CTLA-4-containing lysosomes upon activation of CD4+ T cells. J Immunol. 2000;165:5062–5068. doi: 10.4049/jimmunol.165.9.5062. [DOI] [PubMed] [Google Scholar]
- 12.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curiel TJ. Regulatory T cell development: is Foxp3 the decider? Nat Med. 2007;13:250–253. doi: 10.1038/nm0307-250. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 16.Apostolou I, Verginis P, Kretschmer K, Polansky J, Huhn J, von Boehmer H. Peripherally induced Treg: mode, stability, and role in specific tolerance. J Clin Immunol. 2008;28:619–624. doi: 10.1007/s10875-008-9254-8. [DOI] [PubMed] [Google Scholar]
- 17.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 20.Campoli M, Chang CC, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine. 2002;20(suppl 4):A40–A45. doi: 10.1016/s0264-410x(02)00386-9. [DOI] [PubMed] [Google Scholar]
- 21.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura K, Bahar R, Natsume W, Sakiyama S, Tagawa M. Secretion of interleukin-10 from murine colon carcinoma cells suppresses systemic antitumor immunity and impairs protective immunity induced against the tumors. Cancer gene Ther. 2002;9:109–115. doi: 10.1038/sj.cgt.7700418. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TgF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network. [Accessed December 28, 2010];The NCCN clinical practice guidelines in oncology prostate cancer, version 1.2011. 2010 Available from www.NCCN.org.
- 27.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 28.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 29.De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 30.Drake CG. Immunotherapy for prostate cancer: walk, don’t run. J Clin Oncol. 2009;27:4035–4037. doi: 10.1200/JCO.2009.22.2299. [DOI] [PubMed] [Google Scholar]
- 31.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller AM, Lundberg K, Ozenci V, et al. CD4+CD25 high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 34.Hurwitz AA, Yanover P, Markowitz M, Allison JP, Kwon ED. Prostate cancer: advances in immunotherapy. BioDrugs. 2003;17:131–138. doi: 10.2165/00063030-200317020-00005. [DOI] [PubMed] [Google Scholar]
- 35.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 36.Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 37.Burch PA, Breen JK, Buckner JC, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. [PubMed] [Google Scholar]
- 38.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 39.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 40.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 41.Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med. 2010;363:479–481. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 42.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolchok JD, Hoos A, O’Day S, et al. guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 44.Gulley JL, Stein WD, Schlom J, et al. A retrospective analysis of intramural NCI prostate cancer trials: progress made and insights gleaned [abstract] J Clin Oncol. 2010;28(suppl 15) abstract 4657. [Google Scholar]
- 45.U.S. Food and Drug Administration. [Accessed October 18, 2010];Approval letter: Provenge. Available from www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm210215.htm.
- 46.Brower V. Approval of Provenge seen as first step for cancer treatment vaccines. J Natl Cancer Inst. 2010;102:1108–1110. doi: 10.1093/jnci/djq295. [DOI] [PubMed] [Google Scholar]
- 47.Alemayehu B, Buysman E, Parry D, Becker L, Nathan F. Economic burden and healthcare utilization associated with castration-resistant prostate cancer in a commercial and Medicare Advantage U.S. patient population. J Med Econ. 2010;21:315–361. doi: 10.3111/13696998.2010.491435. [DOI] [PubMed] [Google Scholar]
- 48.Greenberg D, Earle C, Fang C-H, Eldar-Lissai A, Neumann PJ. When is cancer care cost-effective? A systematic overview of cost-utility analysis in oncology. J Natl Cancer Inst. 2010;102:82–88. doi: 10.1093/jnci/djp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Medicare and Medicaid Services. [Accessed May 23, 2011];Proposed decision memo for autologous celllular immunotherapy treatment of metastatic prostate cancer (CAg-00422N) Available from http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=247&ver=8&NcaName=Autologous+Cellular+Immunotherapy+Treatment+of+Metastatic+Prostate+Cancer&bc=BEAAAAAAEAAA.
- 50.Dendreon Inc. [Accessed October 18, 2010];Products: manufacturing. Available from www.dendreon.com/products/provenge/manufacturing.
- 51.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 52.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 54.Arlen PM, Gulley JL, Todd N, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 55.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gulley JL, Madan RA, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25(suppl 2):B89–B96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emens LA, Machiels JP, Reilly RT, Jaffee EM. Chemotherapy: friend or foe to cancer vaccines? Curr Opin Mol Ther. 2001;3:77–84. [PubMed] [Google Scholar]
- 58.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3344. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 61.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12(3 pt 1):878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 62.Petrylak D. Defining the optimal role of immunotherapy and chemotherapy: advanced prostate cancer patients who receive sipuleucel-T followed by docetaxel derive the greatest survival benefit; New York, NY. Presented at the Chemotherapy Symposium 14th annual meeting; 2006. Nov, pp. 8–11. [Google Scholar]
- 63.Rini BI, Weinberg V, Fong L, Conry S, Hershberg RM, Small EJ. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (Provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006;107:67–74. doi: 10.1002/cncr.21956. [DOI] [PubMed] [Google Scholar]
- 64.Wood C, Srivastava P, Bukowski R, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145–154. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 65.Testori A, Richards J, Whitman E, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100-21 study group. J Clin Oncol. 2008;26:955–962. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- 66.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Higano CS, Corman JM, Smith DC, et al. Phase 1–2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 68.Fishman M. A changing world for DCVax: a PSMA loaded autologous dendritic cell vaccine for prostate cancer. Expert Opin Biol Ther. 2009;9:1565–1575. doi: 10.1517/14712590903446921. [DOI] [PubMed] [Google Scholar]
- 69.Northwest Biotherapeutics. [Accessed October 18, 2010];DCVax Brain: phase II clinical trial. Available from www.nwbio.com/clinical_dcvax_brain.php.
- 70.Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–1011. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reinis M. BiovaxID, a personalized therapeutic vaccine against B-cell lymphomas. Curr Opin Mol Ther. 2008;10:526–534. [PubMed] [Google Scholar]
- 73.Schuster SJ, Neelapu SS, Guase BL, et al. Idiotype vaccine therapy (Biovax ID) in follicular lymphoma in first complete remission: phase III clinical trial results [abstract] J Clin Oncol. 2009;27(suppl 18) abstract 2. [Google Scholar]
- 74.Mittendorf EA, Sharma P. Mechanisms of T cell inhibition: implications for cancer immunotherapy. Expert Rev Vaccines. 2010;9:89–105. doi: 10.1586/erv.09.144. [DOI] [PubMed] [Google Scholar]
- 75.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tollefson MK, Karnes RJ, Thompson RH, et al. A randomized phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer [abstract 168]; San Francisco, California. Presented at the American Society of Clinical Oncology 2010 genitourinary cancers symposium; 2010. Mar, pp. 5–7. [Google Scholar]
- 77.Brahmer JR, Topalian SL, Powderly J, et al. Phase II experience with MDX-1106 (Ono-4538), an anti-PD-1 monoclonal antibody, in patients with selected refractory or relapsed malignancies [abstract] J Clin Oncol. 2009;27(suppl 15) abstract 3018. [Google Scholar]