Abstract
Germ line variation in the TP53 network genes PRKAG2, PPP2R2B, CCNG1, PIAS1 and YWHAQ was previously suggested to have an impact on drug response in vitro. Here, we investigated the effect on breast cancer survival of germ line variation in these genes in 925 Finnish breast cancer patients and further analyzed 5 SNPs in PRKAG2 (rs1029946, rs4726050, rs6464153, rs7789699) and PPP2R2B (rs10477313) for 10-year survival in breast cancer patients, interaction with TP53 R72P and MDM2-SNP309, outcome after specific adjuvant therapy, and correlation to tumor characteristics in 4701 invasive cases from four data sets. We found evidence for carriers of PRKAG2-rs1029946 and PRKAG2-rs4726050 having improved survival in the pooled data (HR 0.53, 95% CI 0.3–0.9; P = 0.023 for homozygous carriers of the rare G-allele and HR 0.85, 95% CI 0.7–0.9; P = 0.049 for carriers of the rare G allele, respectively). PRKAG2-rs4726050 showed a significant interaction with MDM2-SNP309, with PRKAG2-rs4726050 rare G-allele having a dose-dependent effect for better breast cancer survival confined only to MDM2 SNP309 rare G-allele carriers (HR 0.45, 95% CI 0.2–0.7; P = 0.001). This interaction also emerged as an independent predictor of better survival (P = 0.047). PPP2R2B-rs10477313 rare A-allele was found to predict better survival (HR 0.82, 95% CI 0.6–0.9; P = 0.018), especially after hormonal therapy (HR 0.66, 95% CI 0.5–0.9; P = 0.048). These findings warrant further studies and suggest that genetic markers in TP53 network genes such as PRKAG2 and PPP2R2B might affect prognosis and treatment outcome in breast cancer patients.
Keywords: TP53, MDM2, PRKAG2, PPP2R2B, breast cancer, hormonal therapy
Introduction
Several studies have investigated the association of single nucleotide polymorphisms (SNP)s with breast cancer risk or survival (1–4). We have previously investigated the role of TP53 and MDM2 genetic variants on breast cancer survival (5, 6). The TP53 regulatory network mediates cell cycle arrest or apoptosis in response to stress conditions such as DNA damage, hypoxia and oncogene activation (7). Recent data suggests that germ line variation in genes involved in this stress response network could affect the predisposition to cancer development or patient survival and drug response (8–10). A candidate gene study on 187 genotyped SNPs that reside in 138 genes implicated in mediating the TP53 stress response identified seven SNPs in five genes with significant genotype-drug response association in vitro by using data generated by the NCI anticancer drug screen (NCI60 screen) (9). The analysis suggested MDM2 SNP309 (rs2279744) having an effect on sensitivity to chemotherapeutic agents in cells harboring the wild type TP53 gene (9). MDM2 is a negative regulator of TP53 and MDM2 SNP309 has been shown to increase MDM2 expression (8, 11). In addition, six other SNPs, residing in five different genes CCNG1(Cyclin G1), PIAS1(Protein inhibitor of activated Stat1), PPP2R2B (PP2A, regulatory subunit B, beta isoform), PRKAG2 (AMP-activated protein kinase γ2 subunit) and YWHAQ (14-3-3ε), whose products have been shown to regulate TP53 through protein-protein interactions and post-translational modifications, were indicated for genotype-drug response association (9). The alleles of two of these SNPs, those in YWHAQ and CD44, that associate with weaker growth response to chemotherapeutics were found to associate with poorer overall survival as well as earlier age of diagnosis in patients with soft tissue sarcomas (10). Suggestive evidence was recently reported for an association of PPP2R2B (rs319217) and breast cancer risk and recurrence, especially after adjuvant endocrine therapy (12). Additionally, a recent integrated copy number aberration-expression study in primary breast tumors highlighted putative cancer genes, with deletions found in PPP2R2A (PP2A, regulatory subunit B, alpha isoform) (13). We recently reported that PRKAG2-rs1860746 within the motif sequence of a TP53 binding site in the third intron of the gene increases susceptibility to ER-negative breast cancer development (14). (13)
In the TP53 gene, a common polymorphism at a proline-rich region of TP53 (TP53 R72P, rs1042522) which results in an arginine to proline change, has been shown to exert a differential effect on apoptosis. TP53-R72 was shown to induce apoptosis faster and more efficiently than TP53-72P in patients with wild type TP53 tumor status (15, 16). 72P on the other hand, possesses an enhanced ability to induce CDKN1A (P21/Cip1) gene transcription which in turn might associate with inferior apoptotic potential and tendency to cell senescence (17). We have previously observed that among breast cancer patients with TP53-negative tumors, the TP53 rare homozygous (CC) genotype carriers had a worse survival compared to the common allele carriers (GG/GC) (6, 18). We also showed some evidence of predisposition to worse breast cancer survival for TP53 R72P in combination with the MDM2 SNP309 variant (6). We did not find evidence for an association between breast cancer survival and MDM2 SNP309 alone or by TP53 protein expression although this had been suggested in some previous studies (19). Neither the TP53 R72P nor the MDM2 SNP309, separately or in interaction, is associated with breast cancer risk in the general European population (20, 21) whereas MDM2 SNP309 has been reported to be associated with earlier (>10 years) age of onset of breast cancer in Li-Fraumeni patients (8, 22).
Altogether there is evidence that common germ line variants in the TP53 regulatory pathway may affect prognosis but there are few studies that have attempted to investigate other genes than TP53 itself and MDM2. Here, we investigated breast cancer survival effect of germ line variation in PRKAG2, PPP2R2B, CCNG1, PIAS1 and YWHAQ in 925 Finnish breast cancer patients and further analyzed 5 SNPs in PRKAG2 and PPP2R2B in a total of 4701 invasive cases from the Breast Cancer Association Consortium (BCAC) for overall survival, interaction with TP53 R72P and MDM2 SNP309 as well as outcome after specific adjuvant therapy. Additionally, we studied the effect of PRKAG2 and PPP2R2B expression in breast tumors on breast cancer survival.
Patients and Methods
Four European studies within the BCAC contributed to this study with data on altogether 4701 invasive breast cancer cases from the Helsinki Breast Cancer Study (HEBCS), Amsterdam Breast Cancer Study (ABCS), Hannover Breast Cancer Study (HABCS) and Polish Breast Cancer Study (PBCS) as described also in previous publications from the BCAC (20, 23). All contributing groups included data on conventional prognostic and predictive markers: tumor grade, size, histological type, estrogen receptor (ER) and progesterone receptor (PR) and nodal status; Her2 status was available in three out of four studies, namely ABCS, HEBCS and PBCS. Each study collected the data independently; from pathology reports and/or Tissue Micro Arrays, medical records and from tumor registries as previously described (23). In brief, ABCS obtained ER and PR status from TMAs. The status was considered positive when >10% of cells were stained. HABCS and HEBCS obtained ER and PR status from medical reports. The status was considered positive when >10% of cells were stained. PBCS obtained ER and PR status from TMAs and medical reports combined (Intensity score (0, 1, 2 and 3) X percentage of cells stained (0–100%)) ≥ 10 was considered positive (total score range 0–300). ABCS obtained HER2 status from TMAs with score 3+ being considered HER2 positive. HEBCS obtained HER2 status from TMAs using CISH-result (0–1=neg, 2–3=pos); if CISH result were not available IHC was used (0–1=neg, 3=pos; score 2 was not used). HER2 status was not available for HABCS. PBCS obtained HER2 status from TMAs with Score 3+ in ≥20% stained tumor cells being considered HER2 positive (23). The studies and tumor characteristics are described in table 1.
Table 1.
Description of characteristics of the participating studies.
| ABCS (n=1442) | HABCS (n=794) | HEBCS (n=2256) | PBCS (n=1540) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | % | n | % | n | % | n | % | |
|
| ||||||||
| Study design | Hospital-based consecutive cases | Hospital-based consecutive cases | Hospital-based consecutive cases+additional familial cases | population-based cases | ||||
| Age at Diagnosis | ||||||||
| Mean ± SD | 42.8±5.2 | 58.0±10.9 | 56.4±12.7 | 55.3±9.8 | ||||
| Grade | ||||||||
| 1 | 450 | 35.2 | 48 | 7.4 | 561 | 26.2 | 299 | 21.3 |
| 2 | 440 | 34.4 | 343 | 52.9 | 960 | 44.9 | 716 | 50.9 |
| 3 | 390 | 30.5 | 257 | 39.7 | 619 | 28.9 | 391 | 27.8 |
| missing | 162 | 146 | 116 | 134 | ||||
| Histopathological type | ||||||||
| Ductal | 1080 | 74.9 | 557 | 79.8 | 1533 | 68.0 | 885 | 73.0 |
| Lobullar | 148 | 10.3 | 74 | 10.6 | 459 | 20.3 | 247 | 20.4 |
| Medullar | 3 | 0.2 | 12 | 1.7 | 29 | 1.3 | 11 | 0.9 |
| Others | 211 | 14.6 | 55 | 7.9 | 235 | 10.4 | 70 | 5.8 |
| missing | 0 | 96 | 327 | |||||
| Tumor size | ||||||||
| 1 | 666 | 62.9 | 337 | 60.4 | 1357 | 60.8 | 693 | 50.1 |
| 2 | 373 | 35.3 | 187 | 33.5 | 717 | 32.1 | 634 | 45.8 |
| 3 | 19 | 1.8 | 15 | 2.7 | 75 | 3.4 | 57 | 4.1 |
| 4 | 0 | 0.0 | 19 | 3.4 | 84 | 3.8 | 0 | 0.0 |
| missing | 384 | 236 | 23 | 156 | ||||
| Nodal status | ||||||||
| Negative | 739 | 52.1 | 472 | 66.6 | 1217 | 54.7 | 866 | 62.3 |
| Positive | 679 | 47.9 | 237 | 33.4 | 1008 | 45.3 | 525 | 37.7 |
| missing | 24 | 85 | 31 | 149 | ||||
| Metastasis at diagnosis | ||||||||
| Negative | 1406 | 98.3 | 693 | 97.0 | 2145 | 96.7 | 1330 | 98.8 |
| Positive | 24 | 1.6 | 21 | 3.0 | 72 | 3.7 | 16 | 1.2 |
| missing | 12 | 80 | 39 | 194 | ||||
| ER Status | ||||||||
| Negative | 325 | 34.1 | 59 | 10.1 | 408 | 18.9 | 503 | 36.0 |
| Positive | 627 | 65.9 | 523 | 89.9 | 1750 | 81.1 | 895 | 64.0 |
| missing | 490 | 212 | 98 | 142 | ||||
| PR Status | ||||||||
| Negative | 451 | 47.6 | 72 | 13.0 | 744 | 34.5 | 682 | 48.9 |
| Positive | 496 | 52.4 | 483 | 87.0 | 1412 | 65.5 | 712 | 51.1 |
| missing | 495 | 239 | 100 | 146 | ||||
| Her2 | ||||||||
| Negative | 691 | 75.9 | NA | NA | 1071 | 84.9 | 828 | 82.0 |
| Positive | 220 | 24.1 | NA | NA | 190 | 15.1 | 182 | 18.0 |
| missing | 531 | 995 | 530 | |||||
| TP53 Status | ||||||||
| Negative | 542 | 69.5 | 23 | 67.6 | 1322 | 78.2 | 523 | 68.6 |
| Positive | 238 | 30.5 | 13 | 32.4 | 368 | 21.8 | 239 | 31.3 |
| missing | 662 | 758 | 566 | 778 | ||||
| years of Dig | ||||||||
| Range | 1974–1994 | 1997–2003 | 1997–1998 | 2000–2003 | ||||
| Follow up Mean ± SD | 10.2±5.3 | 7.5±4.3 | 7.4±2.1 | 4.8±1.6 | ||||
| Vital Status | ||||||||
| Alive | 914 | 63.4 | 660 | 83.1 | 1851 | 82.0 | 1251 | 84.1 |
| Deceased: all-cause | 528 | 36.6 | 134 | 16.9 | 405 | 18.0 | 236 | 15.9 |
| Deceased: BC-specific | 468 | 32.5 | 112 | 14.1 | 301 | 13.3 | 53 | 3.4 |
| References | (21, 51) | (52) | (2, 28, 29) | (53) | ||||
ABCS=Amsterdam Breast Cancer Study, HABCS=Hannover Breast Cancer Study, HEBCS=Helsinki Breast Cancer Study, PBCS=Polish Breast Cancer Study, NA=Not Available
Breast cancer tissue microarray sections were immunohistochemically stained for TP53 expression using mouse monoclonal anti-human TP53-antibody (DO-7, DAKO) as previously described (6, 24) In ABCS, HABCS, HEBCS and PBCS samples were considered positive when >10%, >10%, >20% and >10%, respectively, of the cancer cells were stained. Missing TP53 data could be attributed to missing tumor blocks, loss of cores in the sectioning or staining process or cores not containing enough tumor material (6, 24), or because just a subset of tumors was stained (HABCS). Methods and results are reported according to reporting recommendations for tumor marker prognostic study (REMARK) (25). All studies were done with informed consent from the patients where applicable and permissions from the corresponding Ethics Committee.
SNP selection and genotyping
We initially evaluated the SNPs in the regions of the five genes identified by Vazquez et al. (9), namely PRKAG2, PPP2R2B, CCNG1, PIAS1 and YWHAQ, for survival in breast cancer patients in the HEBCS patients included in BCAC and for whom samples were previously genotyped for a genome-wide case-control breast cancer risk study (26, 27). Of 925 HEBCS invasive breast cancers, 760 cases from the unselected and familial breast cancer series (described in (28–30)) were genotyped with Illumina 550k SNP array and an additional 165 ER-negative cases were genotyped with the Quad610.v1 platform (26, 27). Using Cox regression models for main survival effect as well as stratification by TP53 expression, ER status, adjuvant chemotherapy and adjuvant hormonal therapy we identified five SNPs tagging different haplotypes in PRKAG2 and PPP2R2B, i.e., PRKAG2-01 (rs1029946), PRKAG2-02 (rs4726050), PRKAG2-03 (rs6464153), PRKAG2-04 (rs7789699)) and PPP2R2B (rs10477313), which were significantly associated with overall survival in all patients (PRKAG2-02 (rs4726050), PRKAG2-04 (rs7789699), PPP2R2B (rs10477313)) or in the subgroups defined by tumor TP53 status (PRKAG2-01 (rs1029946), PRKAG2-03 (rs6464153)), tumor estrogen receptor status (PRKAG2-04 (rs7789699), PPP2R2B (rs10477313)) or adjuvant hormonal treatment outcome (PPP2R2B (rs10477313)) (Supplementary Table 1). None of the SNPs in the studied genes showed survival effect after adjuvant chemotherapy treatment.
PRKAG2-01 (rs1029946) and PRKAG2-02 (rs4726050) show low linkage disequilibrium (r2= 0.22). PRKAG2-02 (rs4726050) tags PRKAG2 (rs2727567, r2=0.89) which was originally suggested by Vazquez et al. (9), whereas PPP2R2B (rs10477313) is independent (r2=0.12) of PPP2R2B rs319217 suggested by the same study. (12). For other SNPs suggested by Vazquez et al. (9) (YWHAQ(rs6734469), PPP2R2B(rs319217 and rs319227), CCNG1(rs2069347)), or SNPs that are tagging these SNPs with a r2 > 0.80, no significant association with survival was observed. PIAS1 (rs1027154) was not represented or tagged on the SNP genotyping array.
The survival effect of the five selected SNPs in PRKAG2 (PRKAG2-01 (rs1029946), PRKAG2-02 (rs4726050), PRKAG2-03 (rs6464153), PRKAG2-04 (rs7789699)) and in PPP2R2B (rs10477313) as well as their interaction with TP53 R72P and MDM2 SNP309 was further studied in independent and pooled datasets of 1442 Dutch (ABCS), 794 German (HABCS) and 1540 Polish (PBCS) breast cancer patients. The genotyping was performed by each group separately. The TP53 R72P (rs1042522) and MDM2 SNP 309 (rs2279744) were genotyped in ABCS, HABCS and HEBCS as previously described (6); PBCS genotyped MDM2 SNP309 using the same Taqman assay as the ABCS study (6) and TP53 R72P as described previously (31). The genotyping for the PRKAG2 and PPP2R2B SNPs was carried out using Applied Biosystems TaqMan SNP genotyping assays; primer and probe sequences are available from the authors upon request. There was a higher frequency of rare homozygous genotypes and deviation from Hardy-Weinberg equilibrium for PPP2R2B (rs10477313) in the ABCS, however, the results for this SNP were in keeping with the remaining studies. Genotyping for PRKAG2-02 (rs4726050) in ABCS and PRKAG2-04 (rs7789699) in PBCS failed to produce conclusive calls therefore these genotyping results were not included in the analysis.
RNA expression profile of PRKAG2 and PPP2R2B
We studied the PRKAG2 and PPP2R2B gene expression for survival using microarray data on 187 primary breast tumors that were processed and hybridized to Illumina HumanHT-12 v3 Expression BeadChips and the raw data were quality checked and normalized as previousely described (32). The RNA expression of PRKAG2 and PPP2R2B are shown in supplementary table 2.
Statistical analyses
Statistical analyses on the HEBCS discovery data set were conducted in Anduril (data integration framework) (33) and for other analyses using SPSS v15.0.1 (SPSS, inc.). The significance limit was set at 0.05 (two-sided test), and not adjusted for multiple comparisons. P-values for evaluation of proportional differences in germ line variants by tumor characteristics were calculated using Pearson’s chi-squared tests or Fisher’s exact test (for n<5). Log-rank tests were used to assess the statistical significance of differences between Kaplan-Meier curves for survival analyses among all patients. Univariate (non-adjusted) and multivariate (adjusted) Cox’s regression analyses were used to estimate survival hazard ratios overall and in various subgroups (ER, TP53 and adjuvant hormonal treatment groups). Since TP53 status was available for only 4% of patients in HABCS, this data set was not included in the particular survival analysis by TP53. Multivariate Cox’s proportional hazards models included the common prognostic factors: grade, tumor size, nodal status, primary metastasis, estrogen receptor and progesterone receptor status, as categorical variables, as well as age of diagnosis (as a continuous variable). All pooled analyses were adjusted for study; inclusion of study as a stratifier instead of a categorical co-variate did not change the result. The RNA expression of PRKAG2 and PPP2R2B was assumed as categorical variable (median split) in Log-rank test and as a continuous variable in the univariate Cox’s regression model.
In order to run models including all patients, missing value categories were included for each separate variable with missing information. However, sensitivity analysis including only the patients with available information for all the variables was additionally performed and did not change the conclusions of the analyses. The proportional hazard assumption was verified visually evaluating log-minus-log curves. To determine interaction effects between SNPs and tumor characteristics or treatment, interaction terms were included in the Cox regression models, including the main effects (2df each) as well. In the analyses with adjuvant treatment stratifications, we excluded patients who had distant metastasis at the time of diagnosis (M=1) as they had been extensively treated for metastatic disease. Follow-up was defined as the time from diagnosis to the end of follow-up or date of death due to breast cancer (for breast cancer survival) or any reason (for overall survival), and right censored at 10-years. 96% of the cases were incident breast cancers (entering the study before or in less than 6 months from the date of diagnosis).
Results
Genotype distribution of the studied SNPs among the breast cancer patients
For all SNPs, except TP53 R72P, some heterogeneity in genotype frequencies was observed that appeared to be attributable to HEBCS, HABCS and PBCS, due to normal allele frequency variation in different populations. The frequency variation was mostly pronounced in PBCS for PRKAG2-01 (rs1029946) and HEBCS for PRKAG2-02 (rs4726050) (Table 2). Significant association was seen between genotypes for PRKAG2-01 (rs1029946) and PRKAG2-02 (rs4726050) (p ≪ 0.001), i.e. the rare alleles of both SNPs tended to segregate together.
Table 2.
Genotype distribution of the studied SNPs among the breast cancer patients by study
| ABCS (n=1442) | HABCS (n=794) | HEBCS (n=925) | PBCS (n=1540) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | % | n | % | n | % | n | % | |
| PRKAG2-01(rs1029946) | ||||||||
| AA | 1112 | 78.1 | 598 | 75.7 | 715 | 77.9 | 1035 | 70.3 |
| AG | 278 | 19.5 | 178 | 22.5 | 190 | 20.7 | 393 | 26.7 |
| GG | 33 | 2.3 | 14 | 1.8 | 13 | 1.4 | 44 | 3.0 |
| missing | 19 | 4 | 7 | 68 | ||||
| PRKAG2-02(rs4726050) | ||||||||
| AA | NA | NA | 296 | 37.5 | 468 | 50.8 | 519 | 34.9 |
| AG | NA | NA | 366 | 46.4 | 373 | 40.5 | 697 | 46.8 |
| GG | NA | NA | 127 | 16.1 | 81 | 8.8 | 272 | 18.3 |
| missing | 5 | 3 | 52 | |||||
| PRKAG2-03(rs6464153) | ||||||||
| GG | 1211 | 84.7 | 664 | 83.7 | 791 | 86.3 | 1190 | 78.5 |
| GA | 204 | 14.3 | 125 | 15.8 | 122 | 13.3 | 306 | 20.2 |
| AA | 14 | 1.0 | 4 | 0.5 | 4 | 0.4 | 19 | 1.3 |
| missing | 13 | 1 | 8 | 25 | ||||
| PRKAG2-04(rs7789699) | ||||||||
| GG | 447 | 31.3 | 191 | 24.1 | 248 | 27.0 | NA | NA |
| GA | 610 | 42.8 | 408 | 51.4 | 457 | 49.7 | NA | NA |
| AA | 369 | 25.9 | 195 | 24.6 | 215 | 23.4 | NA | NA |
| missing | 16 | 0 | 5 | |||||
| PPP2R2B(rs10477313) | ||||||||
| GG | 1041 | 73.7 | 610 | 77.0 | 747 | 81.1 | 1200 | 79.6 |
| GA | 265 | 18.8 | 170 | 21.5 | 161 | 17.5 | 287 | 19.0 |
| AA | 106 | 7.5 | 12 | 1.5 | 13 | 1.4 | 21 | 1.4 |
| missing | 30 | 2 | 4 | 32 | ||||
| MDM2(rs2279744) * | ||||||||
| TT | 483 | 41.7 | 325 | 41.6 | 776 | 34.6 | 628 | 41.9 |
| TG | 517 | 44.6 | 351 | 44.9 | 1110 | 49.4 | 702 | 46.8 |
| GG | 158 | 13.6 | 105 | 13.4 | 360 | 16.0 | 170 | 11.3 |
| missing | 284 | 13 | 10 | 40 | ||||
| TP53(rs1042522)* | ||||||||
| GG (Arg/Arg) | 651 | 53.4 | 424 | 54.2 | 807 | 53.6 | 759 | 53.2 |
| GC (Arg/Pro) | 468 | 38.4 | 297 | 37.9 | 591 | 39.2 | 558 | 39.1 |
| CC (Pro/Pro) | 101 | 8.3 | 62 | 7.9 | 109 | 7.2 | 109 | 7.6 |
| missing | 222 | 11 | 749 | 114 | ||||
n=2256 in HEBCS
SNPs in PRKAG2 and PPP2R2B associate with clinical and pathological features of the tumors in the pooled data
The association of the studied SNPs with histopathological characteristics of the tumors is presented in supplementary Table 3. Homozygous carriers of PRKAG2-01 (rs1029946) rare G-allele developed more TP53-immuno-negative tumors (p = 0.005). Homozygous carriers of PRKAG2-02 (rs4726050) rare G-allele were diagnosed with fewer high grade tumors (p = 0.005) whereas PRKAG2-04 (rs7789699) carriers had significantly more high grade tumors (p < 0.001) with a dose-dependent effect of the rare allele associating with higher grade (Chi square for trend: p = 0.002). Tumors from homozygous carriers of PPP2R2B (rs10477313) rare A-allele were more often progesterone receptor negative (p = 0.002).
PRKAG2-01 (rs1029946) and PRKAG2-02 (rs4726050) associate with breast cancer survival in the pooled data
The Hazard ratio estimates of univariate analysis by the SNPs among all patients and in applicable subgroups (as derived from discovery analysis) are shown in supplementary table 4. Among all cases, patients carrying the PRKAG2-01 (rs1029946) homozygous rare G-allele had better 10-year overall survival compared to AG and AA carriers (Figure 1a, Table 3). The Hazard ratio estimates of univariate analysis by PRKAG2-01 (rs1029946) within studies were consistent and are shown in supplementary table 5. In a multivariate Cox’s regression analysis, PRKAG2-01 (rs1029946) remained an independent variable when adjusted for established prognostic factors as well as study and age of diagnosis (HRadjusted 0.57, 95% CI 0.3–0.9; p =0.044) (Table 3); due to the PRKAG2-01 (rs1029946) association with TP53 tumor status that we found in this study the TP53 status was also included in the model as a categorical covariate. In the complete case analysis (including only the patients who had complete information for all the prognostic markers), the HR for overall survival remained the same but was no longer significant (HRadjusted 0.54, 95% CI 0.2–1.0; p = 0.137 for GG vs. AA/AG). Patients carrying the rare G-allele of PRKAG2-02(rs4726050) showed borderline significance for better overall survival compared to homozygous carriers of the common A-allele (p = 0.049), however, the effect was not significant in the multivariate model (Figure 1b, Table 3). The Hazard ratio estimate in univariate analysis by PRKAG2-02(rs4726050) showed some inconsistency between the studies (Supplementary table 6).
Figure 1. Kaplan-Meier plots of cumulative 10-year survival for breast cancer patients carrying a PRKAG2 variant.
(a) PRKAG2-01 (rs1029946), HR 0.53, 95% CI 0.3–0.9; p = 0.023 for GG vs. AA/AG and (b) PRKAG2-02 (rs1029946), HR 0.85, 95% CI 0.7–0.9; p = 0.049 for AG/GG vs. AA.
Table 3.
Univariate and multivariate Cox’s regression analysis by PRKAG2-01 (rs1029946), PRKAG2-02 (rs4726050) and PPP2R2B (rs10477313) for 10-year overall survival and by PPP2R2B (rs10477313) stratified by hormonal therapy in the pooled data.
| Variable/Analysis | HRnon-adjusted (95% CI) | p | HRadjusted (95% CI) | P |
|---|---|---|---|---|
| PRKAG2-01(rs1029946)/Overall survival | ||||
| GG vs AA/AG | 0.53 (0.3–0.9) | 0.023 | 0.57* (0.3–0.9) | 0.044 |
|
| ||||
| PRKAG2-02(rs4726050)/Overall survival | ||||
| AG/GG vs AA | 0.85 (0.7–0.9) | 0.049 | 0.85* (0.7–1.1) | 0.211 |
|
| ||||
| PPP2R2B(rs10477313)/Overall survival | ||||
| GA/AA vs. GG | 0.82 (0.6–0.9) | 0.018 | 0.83* (0.7–0.9) | 0.034 |
| PPP2R2B(rs10477313)/Hormonal treatment | ||||
| GA/AA vs. GG | 0.66 (0.5–0.9) | 0.048 | 0.48** (0.2–0.8) | 0.014 |
| PPP2R2B(rs10477313)/No Hormonal treatment | ||||
| GA/AA vs. GG | 0.87 (0.7–1.1) | 0.200 | - | - |
Adjusted for grade, tumor size, nodal status, primary metastasis, estrogen receptor, progestrone receptor, study and age of diagnosis (and for TP53 status in PRKAG2-01(rs1029946) model).
Adjusted for grade, tumor size, nodal status, adjuvant chemotherapy, study and age of diagnosis
We did not find evidence of an association with survival for PRKAG2-03(rs6464153) and PRKAG2-04(rs7789699) in the main analyses. In the pooled data, in contrast to our findings in the HEBCS study (Supplementary Table 1), none of the studied SNPs had significant effect on patient survival in the respective subgroups of TP53 positive or ER positive tumors (Supplementary table 4); the observed effect of PRKAG2-04 (rs7789699) on patient survival (p = 0.044) in the ER positive subgroup (Supplementary table 4) was driven by HEBCS data only, with no effect in the other studies.
PRKAG2-02 (rs4726050) interaction with MDM2 SNP309 (rs2279744) affects patient survival in the pooled data
For possible interaction with genetic variation in TP53 or MDM2, we stratified the analyses by the TP53 R72P or MDM2 SNP309 genotype. Of all the five SNPs, the PRKAG2-02 (rs4726050) rare G-allele was found to have a dose-dependent effect for better breast cancer survival confined only to MDM2 SNP309 heterozygous and/or rare homozygous carriers (HRnon-adjusted 0.45, 95% CI 0.2–0.7; P = 0.001 for PRKAG2-02 GG in MDM2 SNP309 TG/GG compared to common homozygous) (Figure 2). The interaction term between MDM2 SNP309 TG/GG and PRKAG2-02 (rs4726050) GG remained significant in multivariate Cox’ regression model (HRadjusted 0.59, 95% CI 0.3–0.9; P = 0.047). None of the remaining SNPs showed interaction with TP53 R72P or MDM2 SNP309.
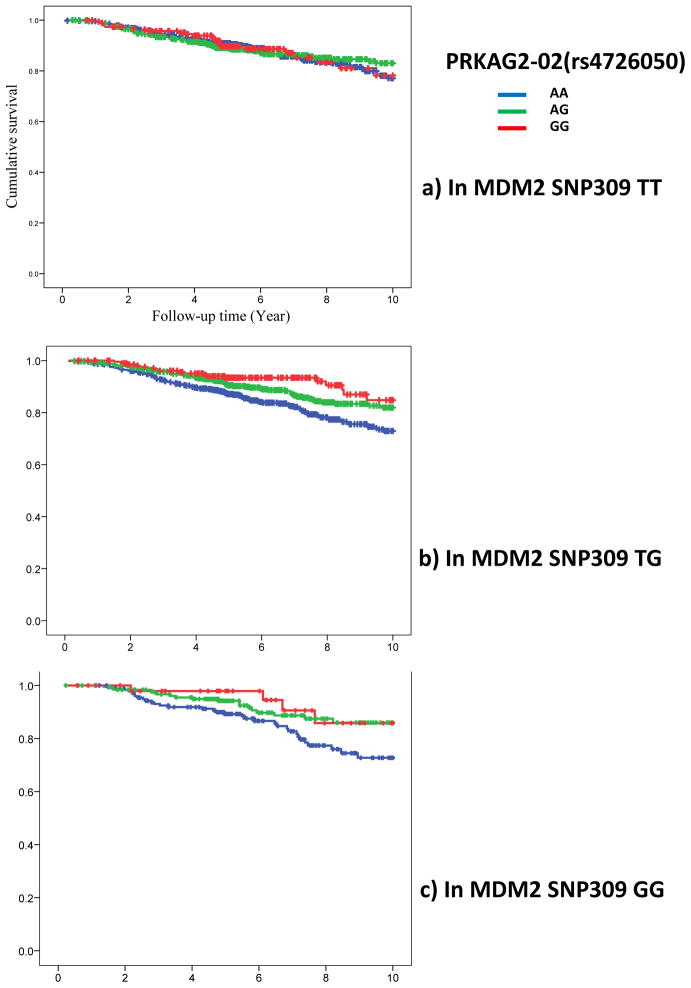
Figure 2. Kaplan-Meier plots of cumulative 10-year survival for breast cancer patients carrying PRKAG2 (rs4726050) and MDM2-SNP309 variants.
Each figure shows Kaplan-Meier breast cancer survival curves of PRKAG2 (rs4726050) genotypes within one group of MDM2-SNP309 genotype (a) MDM2-SNP309 TT genotype, p = 0.924 (b) MDM2-SNP309 TG, p = 0.002 and (c) MDM2-SNP309 GG, p = 0.038. Carriers of PRKAG2-02 (rs4726050) rare G-allele have better breast cancer survival among the MDM2 SNP309 TG/GG carriers HR 0.45, 95%CI 0.2–0.7; p = 0.001 (for b and c combined).
PPP2R2B (rs10477313) associates with patient survival after adjuvant hormonal therapy in the pooled data
In the pooled data, patients carrying PPP2R2B (rs10477313) rare A-allele (GA/AA) showed improved survival compared to homozygous carriers of the common G-allele (p = 0.018). PPP2R2B (rs10477313) also emerged as an independent prognostic factor in the multivariate Cox’s regression analysis (HRadjusted 0.83, 95% CI 0.7–0.9; p = 0.034) (Table 3). We further investigated the association between the PPP2R2B (rs10477313) and survival stratified by adjuvant hormonal therapy as suggested by the initial analysis in HEBCS study (Supplementary table 1). Among patients who had received adjuvant hormonal therapy, the PPP2R2B (rs10477313) rare A-allele (GA/AA) associated with better overall survival compared to GG genotype carriers (HRnon-adjusted=0.66, 95%CI 0.5–0.9; P = 0.048 for GA/AA vs. GG) whereas no such effect was observed in patients who had not received hormonal therapy (HRnon-adjusted 0.87, 95% CI 0.7–1.1; P = 0.200) (Figure 3, Table 3). While nearly all of the 1278 cases treated with hormonal therapy had hormone receptor positive tumors (98%), no significant survival effect was seen among all ER positive patients as such (1597 cases, Supplementary table 4), nor among patients with ER positive tumor who did not receive hormonal therapy (n=218, HRnon-adjusted=0.97, 95%CI 0.7–1.2; P = 0.813 for GA/AA vs. GG). These data suggest that the observed survival effect is likely due to improved response to hormonal treatment. The Hazard ratio estimates of univariate analysis by PPP2R2B (rs10477313) within studies are shown in supplementary table 7. Furthermore, in the multivariate model, the SNP remained as an independent predictor of survival after hormonal therapy (HRadjusted 0.48, 95% CI 0.2–0.8; p = 0.014) (Table 3). Interaction analysis in all patients indicated that the interaction term between adjuvant hormonal therapy and PPP2R2B (rs10477313) GA/AA genotype was significant in multivariate Cox’ regression model (p = 0.039). We did not observe any significant effect of MDM SNP309 or TP53 R72P on survival after adjuvant hormonal or chemotherapy treatment.
Figure 3. Kaplan-Meier plots of cumulative 10-year survival for breast cancer patients carrying PPP2R2B (rs10477313) variant in.
(a) all breast cancer patients, HR 0.82, 95%CI 0.6–0.9; p = 0.018 for GA/AA vs.GG (b) those who received hormonal therapy, HR 0.66, 95%CI 0.5–0.9; p = 0.048 for GA/AA vs.GG and (c) those who did not receive hormonal therapy, HR 0.87, 95%CI 0.7–1.1; p = 0.200 for GA/AA vs.GG. Carriers of PPP2R2B (rs10477313) rare A-allele (GA/AA) show better overall survival especially after hormonal therapy compared to GG genotype.
RNA expression of PRKAG2 and PPP2R2B associates with patient survival
In the HEBCS data set studied for gene expression, we did not find any significant correlation between the studied PRKAG2 and PPP2R2B SNPs and RNA expression of these genes. In the gene expression analysis per se, however, higher PRKAG2 expression correlated with poor breast cancer outcome (HRunivariate 8.6, 95%CI 1.3 to 56.1; P = 0.024) (Supplementary figure 1). The PPP2R2B expression also associated with a significant effect on breast cancer survival: patients with higher expression of PPP2R2B had better prognosis (HRunivariate 0.03, 95%CI 0.002 to 0.565; P = 0.019)(Supplementary figure 2). There was insufficient statistical power for survival analysis by PPP2R2B gene expression in the hormone treated subgroup of patients with ER or PR positive tumors
Discussion
We have here investigated germ line variants in the TP53 network genes PRKAG2 and PPP2R2B for survival among breast cancer patients as well as their interaction with TP53 R72P and MDM2 SNP309 variants. SNPs in PRKAG2 and PPP2R2B were previously suggested to have significant genotype-drug response association in vitro (9). We initially identified four SNPs tagging different haplotypes in PRKAG2 and one SNP in PPP2R2B affecting patient survival by ER, somatic TP53 status on primary breast tumors, or survival after hormonal treatment in HEBCS. The effect of these variants was further tested in 4701 invasive breast cancer cases from the BCAC.
For two SNPs in the PRKAG2 gene we found suggestive evidence for improved survival in the pooled analysis: the PRKAG2-01 (rs1029946) rare homozygous GG genotype as well as the PRKAG2-02(rs4726050) rare G-allele (AG/GG) were found to predict better survival in breast cancer patients, with rare allele of PRKAG2-01 (rs1029946) having an independent effect in the multivariate analysis. PRKAG2 gene encodes the gamma-2 regulatory subunit of AMP-activated protein kinase (AMPK) which is a conserved cellular energy sensor in eukaryotes as well as a modulator of TP53 (34). In nearly every tissue type, AMPK is regulated by LKB1 (35, 36), a tumor suppressor gene whose germ line mutations predispose to the hereditary Peutz-Jeghers cancer syndrome with also 70% breast cancer risk by the age of 70 years (37). Being a highly sensitive cellular energy sensor, AMPK is directly activated by AMP or ADP upon minor increased cellular AMP/ATP ratio. Once activated, AMPK inhibits biosynthetic pathways and activates catabolic pathways in order to conserve and generate more ATP (38). In addition, when nutrients are scarce, AMPK acts as a metabolic checkpoint inhibiting cellular growth (39). AMPK negatively regulates mTORC1 by activating its inhibitor Tuberine (TSC2) or by direct inhibitory phosphorylation of the mTORC1 subunit Raptor which results in regulation of cell growth and metabolism (39). Furthermore, activated AMPK triggers the TP53 pathway under energy shortage conditions, that is, in low glucose state AMPK directly phosphorylates TP53 to induce G1/S cell-cycle arrest whereas under extensive glucose starvation, persistent activation of AMPK promotes accelerated TP53-dependent cellular senescence (40). In contrary to the metabolic tumor suppressor role of AMPK, there is evidence suggesting that under hypoxia and energy shortage, AMPK might give tumor cells a survival advantage by maintaining their energy homeostasis and protecting them from apoptosis (41–43). Although the underlying mechanisms remain poorly defined, it has been demonstrated that AMPK promotes cell survival by both up-regulating NF-κB-dependent expression of Bcl-2 and Survivin (two important anti-apoptotic proteins) and maintaining intracellular ATP levels in endothelial cells (34, 42, 43). Interestingly, our analysis by PRKAG2 RNA expression in breast cancer tumors showed significant correlation of PRKAG2 expression with patient survival indicating a role for this gene in breast cancer progression and patient outcome. However, the actual functional mechanism of how the studied variants affect PRKAG2/AMPK2 protein and patient survival is still to be explored. Both PRKAG2-01 (rs1029946) and PRKAG2-02 (rs4726050) are intronic variants with no predicted effect on the protein sequence; PRKAG2-02 (rs4726050) is tagging the PRKAG2 (rs2727567) variant previously suggested for in vitro drug response by Vazquez et al. (9). Whether the studied SNPs might have some regulatory effect on the gene or whether another variant in linkage disequilibrium with these may be the causal allele remains to be determined.
Furthermore, we observed a significant (P = 0.001) dose-dependent interactive effect of the PRKAG2-02 (rs4726050) with MDM2 SNP309 on breast cancer survival. The better survival effect by PRKAG2-02 (rs4726050) was confined to MDM2 SNP309 (TG/GG) carriers only, lending further support on the survival effect by PRKAG2-02(rs4726050). The production of MDM2 protein is increased by the MDM2 SNP309 promoter variant, leading to the attenuation of the TP53. We have previously shown a statistically significant interaction of the TP53 72Pro variant and the GG genotype of MDM2 SNP309 on worse breast cancer survival, consistent with the Pro variant having a role of enhanced TP53 DNA repair function combined with increased expression of the MDM2 protein and overall attenuation of the TP53 pathway in the tumor cells (6). The functional effects mediated by PRKAG2/AMPK are not completely understood but the results from this study could be taken to suggest that genetic variants affecting the energy metabolism and homeostasis maintenance by AMPK/PRKAG2, in combination with MDM2 SNP309, may have a differential effect on TP53 function on senescence vs. cell cycle arrest/DNA repair and thus cell survival in breast tumors especially under hypoxia and energy shortage.
We found suggestive evidence for PPP2R2B (rs10477313) rare A-allele (AA/AG) predicting better patient survival and this effect was specifically evident among ER/PR positive patients who received hormonal therapy but not among patients who had not received such treatment. The variant was an independent predictor of better outcome after hormonal therapy in multivariate model. Previously, Vasquez et al. had suggested the association of another SNP in the same gene, PPP2R2B (rs319217) with breast cancer recurrence in the group of patients who had received adjuvant hormonal therapy (12). Furthermore, our results on breast cancer survival by PPP2R2B RNA expression indicated a role also for this gene in breast cancer progression and patient outcome as such even though no result on patient survival by hormonal therapy could be obtained due to the small sample size. The results from the two studies together suggest an impact of PPP2R2B in response to hormonal therapy, with different germ line variants in the gene associating with enhanced or reduced survival of breast cancer patients after adjuvant hormonal treatment. PPP2R2B encodes the regulatory B subunit of the Ser/Thr protein phosphatase 2A (PP2A). PP2A is a tumor suppressor phosphatase that acts in a wide variety of signaling pathways associating with cancer progression (44) A recent integrated copy number aberration-expression analysis revealed somatic deletion events affecting PPP2R2A in primary breast tumors (13). PP2A has also been suggested as a determinant of ER expression in human breast cancer cells. The expression of PP2A appeared to correlate with ER expression and activity but it did not change with ER degradation suggesting PP2A might be an upstream regulator of ER (45, 46). PP2A dephosphorylates TP53 in response to DNA damage, resulting in modification of TP53 activity levels in cells (47). Likewise, cyclinG recruits PP2A in order to dephosphorylate MDM2 and thus regulate both MDM2 and TP53 (48). When PP2A is phosphorylated in its catalytic subunit at Y307 (tyrosine residue) it becomes inactivated (49). pY307-PP2A is highly increased in Her2 positive breast tumors and significantly associates with breast cancer progression (50).
In this study, we have found suggestive evidence for SNPs in two TP53 network genes affecting survival in breast cancer, especially in interaction with other TP53 network genes or after specific adjuvant therapy. A drawback of our study was that out of totally 1303 deceased patients, for 369 (28%) the cause of death were unknown. This may have caused some bias in the survival estimates with respect to mortality from breast cancer, but unlikely to the extent that this would change our results. Altogether, only one of the survival associations suggested by the discovery study (HEBCS) remained significant in the pooled analysis of the four participating studies, i.e. the association of the PPP2R2B (rs10477313) with outcome after adjuvant hormonal treatment, supporting the previous findings (12). In addition, while not individually significant, data from the four studies converged to consistent and significant association of the PRKAG2-01 (rs1029946) with better survival, with suggestive evidence also for PRKAG2-02 (rs4726050) which was especially significant in interaction with MDM2 SNP309. Taken together, these results suggest a role for the PRKAG2 gene in breast cancer prognosis. To the best of our knowledge, this is the first study investigating PRKAG2 germ line variation for breast cancer survival and further studies are needed to confirm these results. Larger materials will be needed also for a more in depth analysis of the genetic variants in the studied genes to fully assess their role in breast cancer survival, also after adjuvant chemotherapy. (12). Overall, the genetic variation in the TP53 related genes is likely to form a complex interactive network on breast cancer survival and with more in depth analyses of also other genes similar effects are likely to be discovered. In the near future, this may have also clinical significance for models of breast cancer prognosis or treatment.
Supplementary Material
Novelty & Impact Statements.
This is the first study investigating PRKAG2 germ line variation for breast cancer survival. Analysis in 4701 invasive breast cancer cases showed evidence of improved survival for carriers of rare alleles of PRKAG2-rs4726050 and PRKAG2-rs1029946, with a significant interactive effect of the PRKAG2(rs4726050) with MDM2-SNP309. We also demonstrated that PPP2R2B (rs10477313) A-allele predicts better survival after hormonal therapy, supporting also previous findings.
Acknowledgments
The research leading to these results received funding from the European Community’s Seventh Framework Programme under grant agreement 223175 (HEALTH-F2-2009-223175).
ABCS acknowledges contributors of the BOSOM study, specifically Annegien Broeks, Laura van’t Veer, Flora van Leeuwen and Rob Tollenaar. This study was funded by Dutch Cancer Society grant number NKI2009-4363 and NKI2007-3839.
HABCS gratefully acknowledges Michael Bremer, Natalia Bogdanova, Johann H. Karstens, Hans Christiansen and Peter Hillemanns for their continuous support, and Frank Papendorf for his contributions to database maintenance and management.
HEBCS thanks Drs Päivi Heikkilä, Karl von Smitten and Kirsimari Aaltonen and research nurses Hanna Jäntti and Irja Erkkilä for their help in collecting the patient samples and data. Dr. Xiaofen Dai is warmly thanked for her contribution to the RNA expression analysis. The Finnish Cancer Registry is gratefully acknowledged for the cancer diagnostic and follow-up data.. HEBCS was financially supported by the Helsinki University Central Hospital Research Fund, the Sigrid Juselius Foundation, the Finnish Cancer Society, the Nordic Cancer Union and the Academy of Finland (132473). HEBCS gratefully acknowledges the funding support from the Agency for Science, Technology and Research (A*STAR) of Singapore to Jianjun Liu.
PBCS. This study was supported by the Intramural Research Program of the U.S. National Cancer Institute, Department of Health and Human Services, USA. The PBCS would like to thank Pei Chao and Michael Stagner from Information Management Services (Silver Spring, MD) for data management support; Laurie Burdette, Stephen Chanock, Amy Hutchinson, and Jeff Yuenger from the NCI Core Genotyping facility for genotyping support; the participants, physicians, pathologists, nurses, and interviewers from participating centers in Poland for their efforts during field-work; and Drs. Louise Brinton, and Beata Peplonska for their contributions to the study design.
Abbreviations
- BCAC
Breast Cancer Association Consortium
- ABCS
Amsterdam Breast Cancer Study
- HABCS
Hannover Breast Cancer Study
- HEBCS
Helsinki Breast Cancer Study
- PBCS
Polish Breast Cancer Study
- ER
estrogen receptor
- PR
progesterone receptor
- T
tumore size
- N
nodal status
- M
metastasis at time of diagnosis
Footnotes
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fasching P, Ekici A, Adamietz B, Wachter D, Hein A, Bayer C, Haberle L, Loehberg C, Jud S, Heusinger K, Rubner M, Rauh C, et al. Breast cancer risk: Genes, environment and clinics. 2011;71:1056–66. doi: 10.1055/s-0031-1280437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagerholm R, Hofstetter B, Tommiska J, Aaltonen K, Vrtel R, Syrjakoski K, Kallioniemi A, Kilpivaara O, Mannermaa A, Kosma VM, Uusitupa M, Eskelinen M, et al. NAD(P)H:Quinone oxidoreductase 1 NQO1*2 genotype (P187S) is a strong prognostic and predictive factor in breast cancer. Nat Genet. 2008 Jul;40(7):844–53. doi: 10.1038/ng.155. [DOI] [PubMed] [Google Scholar]
- 3.Azzato EM, Pharoah PD, Harrington P, Easton DF, Greenberg D, Caporaso NE, Chanock SJ, Hoover RN, Thomas G, Hunter DJ, Kraft P. A genome-wide association study of prognosis in breast cancer. Cancer Epidemiol Biomarkers Prev. 2010 Apr;19(4):1140–3. doi: 10.1158/1055-9965.EPI-10-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasching PA, Pharoah PD, Cox A, Nevanlinna H, Bojesen SE, Karn T, Broeks A, van Leeuwen FE, van’t Veer LJ, Udo R, Dunning AM, Greco D, et al. The role of genetic breast cancer susceptibility variants as prognostic factors. Hum Mol Genet. 2012 Jun 20; doi: 10.1093/hmg/dds159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tommiska J, Eerola H, Heinonen M, Salonen L, Kaare M, Tallila J, Ristimaki A, von Smitten K, Aittomaki K, Heikkila P, Blomqvist C, Nevanlinna H. Breast cancer patients with p53 Pro72 homozygous genotype have a poorer survival. Clin Cancer Res. 2005 Jul 15;11(14):5098–103. doi: 10.1158/1078-0432.CCR-05-0173. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt MK, Tommiska J, Broeks A, van Leeuwen FE, Van’t Veer LJ, Pharoah PD, Easton DF, Shah M, Humphreys M, Dork T, Reincke SA, Fagerholm R, et al. Combined effects of single nucleotide polymorphisms TP53 R72P and MDM2 SNP309, and p53 expression on survival of breast cancer patients. Breast Cancer Res. 2009;11(6):R89. doi: 10.1186/bcr2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008 May;9(5):402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 8.Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, Onel K, Yip L, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004 Nov 24;119(5):591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008 Dec;7(12):979–87. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 10.Vazquez A, Grochola LF, Bond EE, Levine AJ, Taubert H, Muller TH, Wurl P, Bond GL. Chemosensitivity profiles identify polymorphisms in the p53 network genes 14-3-3tau and CD44 that affect sarcoma incidence and survival. Cancer Res. 2010 Jan 1;70(1):172–80. doi: 10.1158/0008-5472.CAN-09-2218. [DOI] [PubMed] [Google Scholar]
- 11.Levine AJ. P53, the cellular gatekeeper for growth and division. Cell. 1997 Feb 7;88(3):323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez A, Kulkarni D, Grochola LF, Bond GL, Barnard N, Toppmeyer D, Levine AJ, Hirshfield KM. A genetic variant in a PP2A regulatory subunit encoded by the PPP2R2B gene associates with altered breast cancer risk and recurrence. Int J Cancer. 2011 May 15;128(10):2335–43. doi: 10.1002/ijc.25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Graf S, Ha G, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012 Apr 18; doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Desai KV, Li Y, Banu S, Lee YK, Qu D, Heikkinen T, Aaltonen K, Muranen TA, Kajiji TS, Bonnard C, Aittomaki K, et al. Germ-line variation at a functional p53 binding site increases susceptibility to breast cancer development. Hugo J. 2009 Dec;3(1–4):31–40. doi: 10.1007/s11568-010-9138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas M, Kalita A, Labrecque S, Pim D, Banks L, Matlashewski G. Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol. 1999 Feb;19(2):1092–100. doi: 10.1128/mcb.19.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamuro D, Sabbatini P, White E, Prendergast GC. The polyproline region of p53 is required to activate apoptosis but not growth arrest. Oncogene. 1997 Aug 18;15(8):887–98. doi: 10.1038/sj.onc.1201263. [DOI] [PubMed] [Google Scholar]
- 17.Salvioli S, Bonafe M, Barbi C, Storci G, Trapassi C, Tocco F, Gravina S, Rossi M, Tiberi L, Mondello C, Monti D, Franceschi C. p53 codon 72 alleles influence the response to anticancer drugs in cells from aged people by regulating the cell cycle inhibitor p21WAF1. Cell Cycle. 2005 Sep;4(9):1264–71. doi: 10.4161/cc.4.9.1978. [DOI] [PubMed] [Google Scholar]
- 18.Tommiska J, Eerola H, Heinonen M, Salonen L, Kaare M, Tallila J, Ristimaki A, von Smitten K, Aittomaki K, Heikkila P, Blomqvist C, Nevanlinna H. Breast cancer patients with p53 Pro72 homozygous genotype have a poorer survival. Clin Cancer Res. 2005 Jul 15;11(14):5098–103. doi: 10.1158/1078-0432.CCR-05-0173. [DOI] [PubMed] [Google Scholar]
- 19.Boersma BJ, Howe TM, Goodman JE, Yfantis HG, Lee DH, Chanock SJ, Ambs S. Association of breast cancer outcome with status of p53 and MDM2 SNP309. J Natl Cancer Inst. 2006 Jul 5;98(13):911–9. doi: 10.1093/jnci/djj245. [DOI] [PubMed] [Google Scholar]
- 20.Breast Cancer Association Consortium. Commonly studied single-nucleotide polymorphisms and breast cancer: Results from the breast cancer association consortium. J Natl Cancer Inst. 2006 Oct 4;98(19):1382–96. doi: 10.1093/jnci/djj374. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt MK, Reincke S, Broeks A, Braaf LM, Hogervorst FB, Tollenaar RA, Johnson N, Fletcher O, Peto J, Tommiska J, Blomqvist C, Nevanlinna HA, et al. Do MDM2 SNP309 and TP53 R72P interact in breast cancer susceptibility? A large pooled series from the breast cancer association consortium. Cancer Res. 2007 Oct 1;67(19):9584–90. doi: 10.1158/0008-5472.CAN-07-0738. [DOI] [PubMed] [Google Scholar]
- 22.Ruijs MW, Schmidt MK, Nevanlinna H, Tommiska J, Aittomaki K, Pruntel R, Verhoef S, Van’t Veer LJ. The single-nucleotide polymorphism 309 in the MDM2 gene contributes to the li-fraumeni syndrome and related phenotypes. Eur J Hum Genet. 2007 Jan;15(1):110–4. doi: 10.1038/sj.ejhg.5201715. [DOI] [PubMed] [Google Scholar]
- 23.Broeks A, Schmidt MK, Sherman ME, Couch FJ, Hopper JL, Dite GS, Apicella C, Smith LD, Hammet F, Southey MC, Van’t Veer LJ, de Groot R, et al. Low penetrance breast cancer susceptibility loci are associated with specific breast tumor subtypes: Findings from the breast cancer association consortium. Hum Mol Genet. 2011 Aug 15;20(16):3289–303. doi: 10.1093/hmg/ddr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tommiska J, Bartkova J, Heinonen M, Hautala L, Kilpivaara O, Eerola H, Aittomaki K, Hofstetter B, Lukas J, von Smitten K, Blomqvist C, Ristimaki A, et al. The DNA damage signalling kinase ATM is aberrantly reduced or lost in BRCA1/BRCA2-deficient and ER/PR/ERBB2-triple-negative breast cancer. Oncogene. 2008 Apr 10;27(17):2501–6. doi: 10.1038/sj.onc.1210885. [DOI] [PubMed] [Google Scholar]
- 25.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006 Nov;100(2):229–35. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Humphreys K, Darabi H, Rosin G, Hannelius U, Heikkinen T, Aittomaki K, Blomqvist C, Pharoah PD, Dunning AM, Ahmed S, Hooning MJ, et al. A genome-wide association scan on estrogen receptor-negative breast cancer. Breast Cancer Res. 2010;12(6):R93. doi: 10.1186/bcr2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Humphreys K, Heikkinen T, Aittomaki K, Blomqvist C, Pharoah PD, Dunning AM, Ahmed S, Hooning MJ, Martens JW, van den Ouweland AM, Alfredsson L, et al. A combined analysis of genome-wide association studies in breast cancer. Breast Cancer Res Treat. 2011 Apr;126(3):717–27. doi: 10.1007/s10549-010-1172-9. [DOI] [PubMed] [Google Scholar]
- 28.Kilpivaara O, Bartkova J, Eerola H, Syrjakoski K, Vahteristo P, Lukas J, Blomqvist C, Holli K, Heikkila P, Sauter G, Kallioniemi OP, Bartek J, et al. Correlation of CHEK2 protein expression and c.1100delC mutation status with tumor characteristics among unselected breast cancer patients. Int J Cancer. 2005 Feb 10;113(4):575–80. doi: 10.1002/ijc.20638. [DOI] [PubMed] [Google Scholar]
- 29.Syrjakoski K, Vahteristo P, Eerola H, Tamminen A, Kivinummi K, Sarantaus L, Holli K, Blomqvist C, Kallioniemi OP, Kainu T, Nevanlinna H. Population-based study of BRCA1 and BRCA2 mutations in 1035 unselected finnish breast cancer patients. J Natl Cancer Inst. 2000 Sep 20;92(18):1529–31. doi: 10.1093/jnci/92.18.1529. [DOI] [PubMed] [Google Scholar]
- 30.Eerola H, Blomqvist C, Pukkala E, Pyrhonen S, Nevanlinna H. Familial breast cancer in southern finland: How prevalent are breast cancer families and can we trust the family history reported by patients? Eur J Cancer. 2000 Jun;36(9):1143–8. doi: 10.1016/s0959-8049(00)00093-9. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Closas M, Kristensen V, Langerod A, Qi Y, Yeager M, Burdett L, Welch R, Lissowska J, Peplonska B, Brinton L, Gerhard DS, Gram IT, et al. Common genetic variation in TP53 and its flanking genes, WDR79 and ATP1B2, and susceptibility to breast cancer. Int J Cancer. 2007 Dec 1;121(11):2532–8. doi: 10.1002/ijc.22985. [DOI] [PubMed] [Google Scholar]
- 32.Jamshidi M, Bartkova J, Greco D, Tommiska J, Fagerholm R, Aittomaki K, Mattson J, Villman K, Vrtel R, Lukas J, Heikkila P, Blomqvist C, et al. NQO1 expression correlates inversely with NFkappaB activation in human breast cancer. Breast Cancer Res Treat. 2011 Jun 25; [Google Scholar]
- 33.Ovaska K, Laakso M, Haapa-Paananen S, Louhimo R, Chen P, Aittomaki V, Valo E, Nunez-Fontarnau J, Rantanen V, Karinen S, Nousiainen K, Lahesmaa-Korpinen AM, et al. Large-scale data integration framework provides a comprehensive view on glioblastoma multiforme. Genome Med. 2010 Sep 7;2(9):65. doi: 10.1186/gm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Guan KL. AMP-activated protein kinase and cancer. Acta Physiol (Oxf) 2009 May;196(1):55–63. doi: 10.1111/j.1748-1716.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- 35.Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008 May;7(5):377–88. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006 Jul 10;203(7):1665–70. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res. 2002 Jul;8(7):2085–90. [PubMed] [Google Scholar]
- 38.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–55. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 39.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011 Sep 2;13(9):1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005 Apr 29;18(3):283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Jeon S, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012 doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu C, Liang B, Wang Q, Wu J, Zou MH. Activation of AMP-activated protein kinase alpha1 alleviates endothelial cell apoptosis by increasing the expression of anti-apoptotic proteins bcl-2 and survivin. J Biol Chem. 2010 May 14;285(20):15346–55. doi: 10.1074/jbc.M110.102491. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006 Jul;26(14):5336–47. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mumby M. PP2A: Unveiling a reluctant tumor suppressor. Cell. 2007 Jul 13;130(1):21–4. doi: 10.1016/j.cell.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 45.Keen JC, Garrett-Mayer E, Pettit C, Mack KM, Manning J, Herman JG, Davidson NE. Epigenetic regulation of protein phosphatase 2A (PP2A), lymphotactin (XCL1) and estrogen receptor alpha (ER) expression in human breast cancer cells. Cancer Biol Ther. 2004 Dec;3(12):1304–12. doi: 10.4161/cbt.3.12.1458. [DOI] [PubMed] [Google Scholar]
- 46.Gopalakrishna R, Gundimeda U, Fontana JA, Clarke R. Differential distribution of protein phosphatase 2A in human breast carcinoma cell lines and its relation to estrogen receptor status. Cancer Lett. 1999 Mar 1;136(2):143–51. doi: 10.1016/s0304-3835(98)00315-2. [DOI] [PubMed] [Google Scholar]
- 47.Li HH, Cai X, Shouse GP, Piluso LG, Liu X. A specific PP2A regulatory subunit, B56gamma, mediates DNA damage-induced dephosphorylation of p53 at Thr55. EMBO J. 2007 Jan 24;26(2):402–11. doi: 10.1038/sj.emboj.7601519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okamoto K, Li H, Jensen MR, Zhang T, Taya Y, Thorgeirsson SS, Prives C. Cyclin G recruits PP2A to dephosphorylate Mdm2. Mol Cell. 2002 Apr;9(4):761–71. doi: 10.1016/s1097-2765(02)00504-x. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992 Aug 28;257(5074):1261–4. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 50.Wong LL, Chang CF, Koay ES, Zhang D. Tyrosine phosphorylation of PP2A is regulated by HER-2 signalling and correlates with breast cancer progression. Int J Oncol. 2009 May;34(5):1291–301. [PubMed] [Google Scholar]
- 51.Schmidt MK, Tollenaar RA, de Kemp SR, Broeks A, Cornelisse CJ, Smit VT, Peterse JL, van Leeuwen FE, Van’t Veer LJ. Breast cancer survival and tumor characteristics in premenopausal women carrying the CHEK2*1100delC germline mutation. J Clin Oncol. 2007 Jan 1;25(1):64–9. doi: 10.1200/JCO.2006.06.3024. [DOI] [PubMed] [Google Scholar]
- 52.Landwehr R, Bogdanova NV, Antonenkova N, Meyer A, Bremer M, Park-Simon TW, Hillemanns P, Karstens JH, Schindler D, Dork T. Mutation analysis of the SLX4/FANCP gene in hereditary breast cancer. Breast Cancer Res Treat. 2011 Dec;130(3):1021–8. doi: 10.1007/s10549-011-1681-1. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Closas M, Egan KM, Newcomb PA, Brinton LA, Titus-Ernstoff L, Chanock S, Welch R, Lissowska J, Peplonska B, Szeszenia-Dabrowska N, Zatonski W, Bardin-Mikolajczak A, et al. Polymorphisms in DNA double-strand break repair genes and risk of breast cancer: Two population-based studies in USA and poland, and meta-analyses. Hum Genet. 2006 May;119(4):376–88. doi: 10.1007/s00439-006-0135-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.