Abstract
Elevated levels of circulating estrogens and androgens are linked to higher breast cancer risk among postmenopausal women; however, little is known about hormone levels within the breast. Hormone concentrations within the breast may not be reflected in the blood and are likely important contributors to breast carcinogenesis. We used a previously validated method to measure levels of estrone, estradiol, androstenedione and testosterone in adipose tissue removed as part of breast excisions performed for cancer in 100 postmenopausal women (69 ER/PR +/+ and 31 ER/PR −/−) participating in a breast cancer case-control study. We also measured the same steroid hormones, as well as estrone sulfate, and SHBG in serum from these patients and 100 controls matched on ages at blood collection and on menopause.
Overall, concentrations of serum hormones did not vary significantly between controls and cases. However, women with ER−/PR− breast cancers had lower circulating levels of all measured sex steroid hormones and higher SHBG levels than women with ER+/PR+ breast cancers and controls. Similarly, hormone concentrations in breast adipose tissue were higher among women with ER+/PR+ compared to ER−/PR− breast cancer, although differences were only significant for testosterone.
These data demonstrate that high sex steroid concentrations in both serum and adipose tissues are more strongly related to ER+/PR+ than ER−/PR− breast cancers. Measurement of sex hormones in serum and in the microenvironment may help in understanding the hormonal etiology of breast cancer, suggest methods for prevention, and have value in gauging treatment response and prognosis.
Keywords: sex steroid hormone, breast adipose, breast cancer, intratissue, hormone receptor
Introduction
Cumulative exposure of breast epithelium to elevated levels of sex steroid hormones is proposed to mediate many of the risk factors for postmenopausal breast cancer, including nulliparity, late age at first birth, early age at menarche and late age at menopause, obesity, use of hormone replacement therapy and elevated levels of circulating steroid hormones (1–6). Proposed mechanisms underlying this exposure include stimulating proliferation of breast epithelial cells, acting as a substrate for conversion into DNA- damaging metabolites and other mechanisms (7–12). Among postmenopausal women, most estrogens are produced via aromatization of androgens in adipose tissue, and data suggest that circulating hormone levels may not accurately reflect levels in the breast. Several studies of postmenopausal women demonstrate that estradiol (E2) levels are higher in the breast compared with blood (7). Others have shown higher aromatase activity in breast quadrants containing cancer than in uninvolved quadrants of the same breast (8,13–15), and higher concentrations of E2, the most biologically active estrogen, in tumor tissue than in adipose (9,16,17) or non-neoplastic tissue of the same breast (18). These results are consistent with numerous reports pointing to the importance of the tumor microenvironment, including surrounding adipocytes, in facilitating the growth and progression of breast cancer, and argue that hormone levels within breast tissues may be most relevant for understanding their role in breast carcinogenesis.
A link between obesity, high levels of circulating estrogens and increased risk for hormone receptor positive breast cancer has been established (19). Additionally, obese women have a poorer prognosis regardless of tumor hormone-receptor status, often presenting with high grade tumors and distant metastases. The underlying mechanism is not clear, although likely involves alterations in estrogen metabolism, growth factor pathways, and/or inflammatory markers. Efforts to understand the biology of breast adipose tissue may provide some clues. Benign breast epithelium and breast cancer are typically intermixed with adipose tissue, which provides both a reservoir and local site of hormone metabolism that may be particularly relevant for breast carcinogenesis in postmenopausal women. As women age, the breast undergoes progressive fatty replacement, and as this process is enhanced by obesity, the prevalence of women with predominantly fatty breasts has increased. Further, the metabolic and endocrine properties of adipose tissue differ between lean and obese women, and vary by body site, with fat in breasts of normal obese women and in tissue surrounding breast cancers exhibiting increased aromatase activity (19). Thus, studying the hormonal properties of breast adipose may provide insights into the pathogenesis of ER positive breast cancer, as well as the response to treatment or prevention with endocrine based strategies (20).
Previously, we validated a method for reliably measuring steroid hormone concentrations in breast adipose tissue (21). Herein, we used this methodology to compare within person hormone levels in breast adipose tissue and serum from breast cancer cases who participated in a population-based case-control study. For comparison, we measured hormone levels in serum from matched controls. Given data demonstrating that hormone-receptor status defines clinically and etiologically distinct forms of breast cancer, (22, 23), our aim was to evaluate whether levels of circulating and adipose hormones differed in women with ER+/PR+ compared to ER−/PR− disease. To avert complexities related to fluctuation in circulating hormones with menstrual cycling, we restricted the study to postmenopausal women.
Materials and Methods
Study Design
The Polish Women’s Health Study has been described in detail elsewhere (24). Briefly, case subjects were women aged 20–74 years with pathologically confirmed breast cancer who resided in Warsaw or Łódź, Poland from 2000 to 2003. Cases were identified through a rapid identification system in five institutions (~ 90% of cases) and cancer registries. Randomly identified population controls were frequency-matched to cases by city and age in 5-year categories. The study included 2,386 cases (79% of eligible) and 2,502 controls (69% of eligible). Informed consent was provided as required by the National Cancer Institute and Institutional Review Boards in Poland.
Information on breast cancer risk factors was obtained through a personal interview as described elsewhere (24). Women who were menstruating at the time of interview were considered premenopausal, those whose periods had stopped for 12 months or more were classified as postmenopausal, and women who began using hormone replacement therapy while still menstruating were considered to have undefined menstrual status. BMI was calculated from measured weight and standing height (kg/m2) from ~95% of subjects and estimated from self-reported information for the remainder. Information related to tumor pathology was obtained from medical records, including results for estrogen receptor (ER) and progesterone receptor (PR). Selected histologic slides were reviewed by the study pathologist (M.E.S.) to confirm tumor type and grade. For tumors with available study blocks, we repeated ER and PR immunohistochemical analyses, which yielded results that were highly correlated with those in the medical record (25).
Subject selection and tissue collection
The main aim of this study was designed to compare intra-individual hormone levels in breast adipose tissue and serum in relationship to hormone receptor status among breast cancer cases. We restricted cases to postmenopausal women residing in Warsaw who were not current users of exogenous hormones and who provided blood and frozen breast tissues (tumor and non-tumor) prior to treatment. ER/PR status was determined from hospital records. In the entire case-control study, there were 248 case subjects in Warsaw who provided blood and frozen tissues. Of these, 168 were postmenopausal at the time of surgery, including79 ER+/PR+ cases, 3 ER−/PR+, 28 ER+/PR−, 41 ER−/PR− and 17 with unknown receptor status based on medical records. Given that most tumors are ER+/PR+ or ER−/PR−, we restricted cases to these subtypes, and selected 69 ER+/PR+ (87% of available cases) and 31 (76%) of available ER−/PR− cases for study. Fresh tissue samples of adipose tissue were collected following surgery, snap frozen in liquid nitrogen and stored at −80° C. Adipose tissue was selected if it was grossly free of tumor, contained minimal non-fatty tissue and was free of blood.
Although this analysis focused on the intra-individual comparison of hormone concentrations in adipose tissue and serum among cases, we also assessed serum levels among controls for comparison with cases. Controls were frequency matched to cases by age at case diagnosis (+/− 2 years), residence, age at menopause (+/− 2 years) and were not currently using hormone supplements.
Laboratory Methods
Methods to measure steroid hormones in breast adipose tissue have been reported (21). Briefly, we separated oil from the more dense stromal cells by flotation. The minced breast adipose tissue was incubated with 1ml of 1mg/mL collagenase (type 1A; Sigma Chemical Co., St Louis, MO) in Krebs Ringer bicarbonate buffer (4% w/v BSA) (Sigma Chemical Co., St. Louis, Mo) at 37° C for 50 min in a shaking water bath. The floating oil layer was separated by centrifugation. Purification steps were undertaken prior to quantifying the following steroid hormones by RIA: E1, E2, A, and T. The tritiated forms of these steroids were used to follow procedural losses. They were added to the oil and incubated at 50° C for 10 min. The steroids were then extracted from the oil and subsequently redissolved in 1mL of isooctane. The isooctane was transferred to a Celite partition chromatography column, and E1, E2, A, and T were eluted sequentially and quantified by RIA. Results are expressed per gm of oil. Adipose tissue samples were assayed in 6 batches. For quality control (QC) purposes, a single specimen of discarded breast adipose tissue was obtained at surgery and cut into 6 pieces of equivalent weight, one of which was included in each batch. Overall CVs were as follows: A (18%), T (29%), E1 (19%), E2 (26%) and detection limits were: 12.1, 12.1, 6.1 and 3.05 pg/g oil for A, T, E1, and E2 respectively.
Serum samples were extracted and A, T, E1, and E2 were separated by Celite column partition chromatography. E1S was quantified by direct RIA (Beckman-Coulter, Minneapolis, MN). SHBG was measured by chemilumenescent immunoassay on the Immulite 2000 System analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). Overall CVs were as follows: A, 10.2%; T, 17.3%; E1,12.5%; E2, 22.7%; E1S, 6.5%; SHBG, 7.4%. The detection limits were: 40, 20, 4, 1 and 250 pg/ml for A, T, E1, E2, and E1S, respectively, and 8 nmol/L for SHBG.
Statistical Methods
The main analysis compared hormone levels in breast adipose tissue and serum of individual case subjects (within subject comparison). We also assessed serum hormone levels among controls. Levels of all hormones and SHBG were transformed to the natural logarithm scale to normalize distributions. Differences in baseline characteristics between cases and controls were compared using the t-test for continuous variables, Pearson’s correlation coefficient, and the χ2-test for categorical variables. Case–control differences and case-case differences in geometric mean hormone concentrations by hormone receptor subgroup were evaluated by analysis of covariance with adjustment for age at specimen collection, age at menopause and BMI. Further adjustment for known breast cancer risk factors, including parity, age at menarche, prior supplemental hormone and prior oral contraceptive use did not alter findings and were not included in the final models. Analyses were conducted using SYSTAT software package, Version 12 (26).
Results
Characteristics of subjects
Table 1 presents the distribution of selected demographic characteristics and breast cancer risk factors by case-control status and hormone receptor subgroup. The frequency distribution of most risk factors was similar between ER/PR +/+ and ER/PR −/− women, although the latter were more likely to have had a full term pregnancy (89.7% of ER−/PR− women v 82.9% of ER/PR +/+ women) and a history of self-reported benign breast disease (25% v 13.2%). BMI was slightly higher among ER−/PR− cases (29.5 v 28.4 in ER+/PR+ women). Compared to controls, cases overall were more likely to be nulliparous (15% of cases v 10% of controls), report a history of breast cancer in a first degree relative (16.1% of cases, 13% of controls) and a prior diagnosis of benign breast disease (16.7% cases, 9.1% controls).
Table 1.
Selected Risk Factor and Hormone Receptor Characteristics Polish Breast Cancer Study
| Controls | All Cases | ER/PR Status | ||
|---|---|---|---|---|
| +/+ | −/− | |||
| N | 100 | 100 | 70 | 30 |
| Age (mean, years) | 61.3 | 61.4 | 61.0 | 60.6 |
| BMI (mean, kg/m2) | 29.2 | 28.1 | 28.4 | 29.5 |
| Age at menopause (mean, years) | 49.7 | 49.8 | 50.1 | 49.1 |
| Parous (%) | 90.0 | 84.8 | 82.9 | 89.7 |
| Number pregnancies (mean) | 2.8 | 2.7 | 2.8 | 2.6 |
| Age at first birth (mean, years) | 24.2 | 24.3 | 24.4 | 24.0 |
| Family history of breast cancer (%) | 13.0 | 16.1 | 15.7 | 17.2 |
| Age at menarche (mean, years) | 14.0 | 13.8 | 13.8 | 13.7 |
| Benign breast disease (%) | 9.1 | 16.7 | 13.2 | 25.0 |
| Tumor size (mean, mm) | 20.7 | 21.6 | 18.7 | |
Adipose tissue hormone levels
Case-case comparisons demonstrated that unadjusted concentrations of the four hormones measured in breast adipose tissue were higher among women with ER+/PR+ breast cancer as compared to women with ER−/PR− tumors (Table 2A), despite BMI being higher in the latter group. E1S was not detected in adipose samples. After adjustment for age, BMI, age at menopause and serum concentration of the respective hormone, the largest and only significant difference between the case groups was found for T, which was almost 40% higher in women with ER+/PR+ compared to ER−/PR− tumors (p=0.002). It is notable that despite differences in the absolute levels, the relative concentrations of the adipose hormones were similar in both case groups. For instance, levels of A were approximately 17-times that of E1, while T was 4-fold higher than E2, irrespective of receptor status.
Table 2.
Polish Breast Cancer Study
| A. | Geometric Mean Sex Steroid Hormone Concentrations^ in Breast Adipose Tissue; Cases Only |
||||||
|---|---|---|---|---|---|---|---|
| All Cases |
ER/PR Status | All Cases |
ER/PR Status | ||||
| unadjusted means | adjusted means** | ||||||
| +/+ | −/− | +/+ | −/− | p value* | |||
| N | 94 | 67 | 27 | 94 | 67 | 27 | |
| E1 | 311 | 333 | 270 | 314 | 323 | 293 | 0.283 |
| E2 | 52.2 | 56.5 | 45.8 | 52.5 | 55.2 | 46.9 | 0.163 |
| T | 249 | 278 | 197 | 253 | 277 | 201 | 0.002 |
| A | 5250 | 5497 | 4741 | 5218 | 5314 | 4980 | 0.424 |
| B. | Serum Sex Steroid Hormone Concentrations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Unadjusted Geometric Means | Adjusted Geometric Means^ | |||||||
| Controls | All Cases |
ER/PR status | Controls | All Cases |
ER/PR status | ||||
| 100 | 94 | +/+ 67 |
−/− 27 |
100 | 94 | +/+ 67 |
−/− 27 |
p value* | |
| E1 | 31.2 | 29.5 | 31.8 | 25.4 | 31.1 | 29.6 | 31.8 | 24.7 | 0.106 |
| E2 | 8.8 | 7.2 | 7.4 | 6.8 | 8.7 | 7.2 | 7.5 | 6.7 | 0.48 |
| E1S | 928 | 935.4 | 984.4 | 841.3 | 929.1 | 931.3 | 985.4 | 807.2 | 0.132 |
| T | 216.7 | 219.6 | 228.1 | 203.2 | 215.2 | 220 | 230.4 | 204 | 0.473 |
| A | 507.2 | 533.3 | 558.9 | 483 | 509.2 | 521.6 | 555.9 | 435.3 | 0.526 |
| SHBG | 44.2 | 45.3 | 43.3 | 49.9 | 44.6 | 44.8 | 42.7 | 52.1 | 0.088 |
| Free E2 (%) | 2.4 | 2.4 | 2.4 | 2.3 | 2.394 | 2.4 | 2.42 | 2.3 | 0.043 |
Geometric mean concentrations expressed as picograms hormone/gram oil
Adjusted for age at specimen collection (continuous), age at menopause (continuous), BMI (continuous), and hormone level in blood (continuous).
Comparison of hormone levels between ER/PR +/+ and ER/PR −/−.
For all serum sex hormones, geometric means adjusted for age at blood collection (continuous), age at menopause (continuous) and BMI (continuous) are presented. Concentrations are expressed as pg/ml, except for SHBG, which is expressed in nmoles/L. Free E2 is the percent of unbound E2.
p value for test of differences in means between cancer subtypes
Serum hormone levels
Women with ER+/PR+ breast cancers had higher circulating levels of all measured sex steroid hormones and lower SHBG concentrations than women with ER−/PR− breast cancers or controls (Table 2B). Differences were for the most part modest, and not significant. For the cases, the greatest disparities were in A, E1S and SHBG, with women with ER+/PR+ tumors having 24% higher levels of A, (p=0.526); 20% higher E1S (p=0.132); and 20% lower SHBG (p=0.088). Concentrations of serum sex hormones did not vary significantly between controls and all cases combined. Adjustment for age at blood draw, BMI and age at menopause had little effect on these findings.
Similar to results for hormone levels in the adipose, the relative concentrations of serum hormones did not differ by receptor status. However, in constrast to adipose tissue where T was 4-fold higher than E2, in serum, T was nearly 30 times higher than E2 for both ER−/PR+ and ER−/PR− women. Although using a different metric, levels of most of the hormones in adipose were approximately 10-times greater than those in serum for both hormone receptor-defined groups of women. The notable exception was the relatively high concentration of T in serum which resulted in comparable levels in serum and adipose.
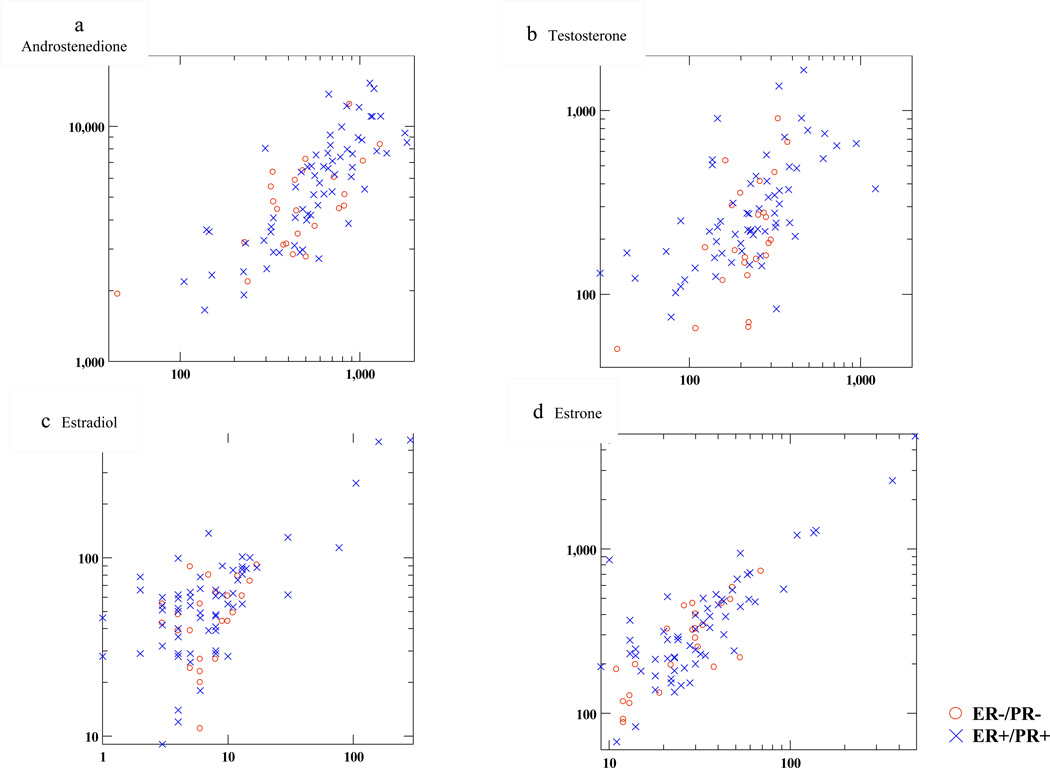
Figures 1a–1d plot serum and adipose tissue hormone concentrations by ER/PR status. Among ER+/PR+ women, adipose hormones were highly correlated with circulating levels; Pearson’s correlation coefficients were statistically significant and ranged from 0.57 for T to 0.84 for A. For ER−/PR− women, correlations were weaker (ranging from 0.35 for E2 to 0.75 for E1), but nonetheless significant for all blood/adipose tissue comparisons except E2. Women with both high serum and adipose T tended to be overwhelmingly ER+/PR+.
Figure 1.
Plots of serum (x axis) versus adipose (y axis) hormone concentration; 1a: androstenedione, 1b: testosterone, 1c: estradiol, 1d: estrone. Serum measurements are expressed in pg/ml; adipose tissue measurements are in pg/g oil (see text). Axes are scaled to log base 10 for ease of interpretation. Red circles indicate values from cases with ER−/PR− tumors; blue hatch marks are values from ER+/PR+ cases.
Correlations between serum and adipose tissue hormone levels were as follows: For ER−/PR− women, rho=0.35, 0.75, 0.44 and 0.66 for E2, E1, T and A, respectively. For ER+/PR+ women, rho=0.67, 0.74, 0.57 and 0.81 for E2, E1, T and A, respectively.
BMI influence on serum and adipose hormones
As expected, levels of T, E1 and E2 in adipose tissue, and to a lesser degree in serum, rose with increasing BMI. These associations were slightly stronger in ER−/PR− women. Among ER+/PR+ cases, correlations between BMI and adipose hormones T, E1 and E2 were 0.46, 0.35 and 0.20; for serum, correlations were 0.08, 0.25, and 0.23. In ER−/PR− women, correlations were 0.51, 0.68 and 0.43 in adipose and 0.26, 0.52, and 0.51 in serum (Table 3).
Table 3.
Pearson’s Correlation Coefficients between Body Mass Index and Circulating and Breast Adipose Sex Hormones; By ER/PR Status
| E1 | E2 | T | A | Free E2 | ||
|---|---|---|---|---|---|---|
| ER+/PR+ | serum | 0.25 | 0.23 | 0.08 | 0.04 | 0.47* |
| adipose | 0.35* | 0.20 | 0.46* | −0.12 | ||
| ER−/PR− | serum | 0.52* | 0.51* | 0.26 | −0.27 | 0.62* |
| adipose | 0.68* | 0.43 | 0.51* | −0.09 | ||
p value <0.05.
Discussion
This study suggests that analysis of breast adipose tissue may enhance our understanding of the hormonal etiology of breast cancer. Our data demonstrate that hormone levels are higher in serum and breast adipose tissues of postmenopausal women with ER+/PR+ breast cancers as compared to those with ER−/PR−tumors. In addition, although levels in serum and fat were correlated, measurements of adipose tissue were more strongly correlated with receptor status than serum concentrations, particularly for T. Nonetheless, the ratio of T to E2 in fat was lower than in serum, suggesting that adipose tissue represents a reservoir of androgens that are actively converted to estrogens in breasts containing cancer. Notably, the subset of women who had the highest levels of T in both serum and adipose tissue included a preponderance of ER+/PR+ cancers. Thus, our findings provide additional evidence for the importance of T in post-menopausal ER+/PR+ breast cancer (27) and extend findings demonstrating etiological differences between hormone receptor positive and negative tumors.
Our findings also suggest that obesity may be related to increased E2 production in the breast among postmenopausal cancer patients. Specifically, we found that elevated BMI is related to higher levels of T in breast fat, thus providing a substrate for estrogen synthesis. In turn, elevated E2 in the breast may lead to breast cancer progression or heightened aggressiveness by driving proliferation or through other mechanisms. Consistent with this view, recent studies in mice and in clinical studies found that diet-induced obesity results in inflammatory lesions within the breast, leading to elevated aromatase expression (28,). Activation of aromatase in the breast could account for our observation that the ratio of T to E2 is lower in adipose tissues than in blood. Furthermore, expression of ER by cancer cells may lead to accumulation of E2 in tumor tissue, thereby diminishing levels in fat. The translational importance of hormone metabolism in the breast adipose remains unclear. Although obesity is linked to a poorer prognosis for breast cancer, data are inconsistent as to whether elevated BMI reduces the effectiveness of anti-hormonal agents (29) and whether low mammographic density (reflecting a high percentage of breast fat) is associated with a worse prognosis independent of BMI.
Previous studies of sex hormone concentrations in breast tissue, particularly adipose, are limited in number and in size, and have focused mainly on measurement of estrogens. (7, 16–18,30,31–35). Data generally indicate that (35) E2 concentrations in breast tumor tissues are higher than in the surrounding non-neoplastic tissues, independent of menopausal status. In contrast, E1 tends to be lower in neoplastic than in the surrounding tissues, perhaps reflecting its favored conversion to E2. Limited data (36,37) suggest that dehydroepiandrosterone and A are lower, while T is comparable in breast tumors compared with the surrounding tissue (35). Although E1S is abundant in serum, it was undetectable in breast fat using our methods, presumably because of its high polarity and low fat solubility. Overall, our findings and previous studies suggest that androgen and estrogen levels are higher in breast tissues and blood of women with ER+ as compared with ER− breast cancers (30,38).
Our approach differs from previous studies with regard to technical issues and patient selection. Most previous studies analyzed adipose tissue samples identified by visual inspection and expressed hormone concentrations per gram of specimen or protein content, whereas we digested tissues prior to assaying and expressed concentrations per gram of oil. In addition, differences in sensitivity and precision of RIAs (39) may affect comparisons across studies. By including purification steps to remove potential interfering compounds prior to applying the RIAs, we achieved adequate precision, as evidenced by CVs below 20% for all analytes measured. However, CVs for the adipose tissue hormone assays were higher; probably reflecting additional variability arising from differences in the cellular composition of the samples. Despite these concerns, we are encouraged that the absolute levels of circulating estrogens and androgens in this study are similar to values reported in recent studies (10,30). Improving assay methods, including a shift to mass spectrometry assays, is a subject of much ongoing investigation (30,39).
Our finding of high correlations between adipose and serum hormones, with lower levels consistently observed in ER−/PR− women, argues that the uptake of steroids from the circulation is a primary contributor to breast adipose hormone content. However, hormone metabolism in the breast may lead to changes in local concentrations that are not evident in blood. A limitation of most studies including ours is that we studied women with prevalent invasive breast cancer, where the sequestration, synthesis and catabolism of hormones within the tumor may affect levels measured in associated non-neoplastic tissue or in serum. A more comprehensive understanding of the inter-relationships of these hormones would require testing levels in several tissues and serum simultaneously in breast cancer cases and cancer-free women. Studying these complex relationships will help to refine our understanding of breast cancer etiology and could potentially suggest methods for prevention and management.
List of Abbreviations
- E1
estrone
- E2
estradiol
- A
and rostenedione
- T
testosterone
- E1S
estrone sulfate
- SHBG
sex hormone-binding globulin
- ER/PR
estrogen receptor/progesterone receptor
- BMI
body mass index
- RIA
radioimmunoassay
- CV
coefficient of variation
Footnotes
Competing interests
The authors have no potential conflicts of interest.
References
- 1.Key T, Appleby P, Barnes I, Reeves G. Endogenous Hormones and Breast Cancer Collaborative Group Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 2.Sieri S, Krogh V, Belelli G, Abagnato CA, Grioni S, Pala V, Evangelista A, Allemani C, Micheli A, Tagliabue G, Schunemann HJ, Menard S, Berrino F, Muti P. Sex hormone levels, breast cancer risk and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:169–176. doi: 10.1158/1055-9965.EPI-08-0808. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, Lee JS, Lui LY, Stone K, Ljung BM, Cauley JA. Sex hormones, risk factors, and risk of estrogen receptor-positive breast cancer in older women: a long-term prospective study. Cancer Epidemiol Biomarkers Prev. 2005;14:1047–1051. doi: 10.1158/1055-9965.EPI-04-0375. [DOI] [PubMed] [Google Scholar]
- 4.Zeleniuch-Jacquotte A, Toniolo P, Levitz M, Shore RE, Koenig KL, Banerjee S, Strax P, Pasternack BS. Endogenous estrogens and risk of breast cancer by estrogen receptor status: a prospective study in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1995;4:857–860. [PubMed] [Google Scholar]
- 5.Missmer SA, Eliaseen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 6.Baglietto L, Severi G, English DR, Krishnan K, Hopper JL, McLean C, Morris HA, Tilley WD, Giles GG. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010 doi: 10.1158/1055-9965.EPI-09-0532. [DOI] [PubMed] [Google Scholar]
- 7.Pasqualini JR, Chetrite GS. Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J Steroid Biochem Molec Biol. 2005:221–236. doi: 10.1016/j.jsbmb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Miller WR, O’Neill J. The importance of local synthesis of estrogen within the breast. Steroids. 1987;50:537–548. doi: 10.1016/0039-128x(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 9.Miki Y, Suzuki T, Sasano H. Intracrinology of sex steroids in ductal carcinoma in situ (DCIS) of human breast: Comparison to invasive ductal carcinoma (IDC) and nonneoplastic breast. J Steroid Biochem Molec Biol. 2009;114:68–71. doi: 10.1016/j.jsbmb.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Haynes BP, Straume AH, Geisler J, A'Hern R, Helle H, Smith IE, Lønning PE, Dowsett M. Intratumoral estrogen disposition in breast cancer. Clin Cancer Res. 2010;16:1790–1801. doi: 10.1158/1078-0432.CCR-09-2481. [DOI] [PubMed] [Google Scholar]
- 11.Sasano H, Suzuki T, Nakata T, Moriya T. New development in intracrinology of breast carcinoma. Breast Cancer. 2006;13:129–136. doi: 10.2325/jbcs.13.129. [DOI] [PubMed] [Google Scholar]
- 12.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 13.Bulun SE, Lin Z, Zhao H, Lu M, Amin S, Reierstad S, Chen D. Regulation of aromatase expression in breast cancer tissue. Ann NY Acad Sci. 2009:121–131. doi: 10.1111/j.1749-6632.2009.03705.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Miki Y, Ohuchi N, Sasano H. Intratumoral estrogen production in breast carcinoma: significant of aromatase. Breast Cancer. 2008;15:270–277. doi: 10.1007/s12282-008-0062-z. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill JS, Elton RA, Miller WR. Aromatase activity in adipose tissue from breast quadrants: a link with tumour site. Br Med J. 1988;296:741–743. doi: 10.1136/bmj.296.6624.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermeulen A, Deslypere JP, Paridaens R, LeClercq G, Roy F, Heuson JC. Aromatase, 17β-hydroxysteroid dehydrogenase and intratissular sex hormone concentrations in cancerous and normal glandular breast tissue in postmenopausal women. Eur J Cancer Clin Oncol. 1986;22:515–525. doi: 10.1016/0277-5379(86)90121-5. [DOI] [PubMed] [Google Scholar]
- 17.Van Landeghem AAJ, Poortman J, Nabuurs M, Thjissen JHH. Endogenous concentration and subcellular distribution of androgens in normal and malignant human breast tissue. Cancer Res. 1985;45:2907–2912. [PubMed] [Google Scholar]
- 18.Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR. Comparison of estrogen concentrations, estrone sulfatases and aromatase activities in normal, and in cancerous, human breast tissues. J Steroid Biochem Mol Biol. 2000;72:23–27. doi: 10.1016/s0960-0760(00)00040-6. [DOI] [PubMed] [Google Scholar]
- 19.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res. 2011;4:1021.1–1029.1. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goss PE, Ingle JN, Alés-Martínez JE, Ceung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H NCIC CTG MAP.3 Study Investigators. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 21.Falk RT, Gentzschein E, Stancyzk FZ, Brinton LA, Garcia-Closas M, Ioffe OB, Sherman ME. Measurement of sex steroid hormones in breast adipocytes: methods and implications. Cancer Epidemiol Biomarkers Prev. 2008;17:1891–1895. doi: 10.1158/1055-9965.EPI-08-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, Hewitt SM, Anderson WF, Szeszenia-Dabrowska N, Bardin-Mikolajczak A, Zatonski W, Cartun R, Mandich D, Rymkiewicz G, Ligaj M, Lukaszek S, Kordek R, García-Closas M. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16:439–443. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 23.Althuis M, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–1568. [PubMed] [Google Scholar]
- 24.Garcia-Closas M, Brinton LA, Lissowska J, Chatterjee N, Peplonska B, Anderson WF, Szeszenia-Dabrowska N, Bardin-Mikolajczak A, Zatonski W, Blair A, Kalaylioglu Z, Rymkiewicz G, Mazepa-Sikora D, Kordek R, Lukaszek S, Sherman ME. Established breast cancer risk factors by clinically important tumour characteristics. Br J Cancer. 2006;95:123–129. doi: 10.1038/sj.bjc.6603207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman ME, Rimm DL, Yang XR, Chatterjee N, Brinton LA, Lissowska J, Peplonska B, Szeszenia-Dabrowska N, Zatonski W, Cartun R, Mandich D, Rymkiewicz G, Ligaj M, Lukaszek S, Kordek R, Kalaylioglu Z, Harigopal M, Charrette L, Falk RT, Richesson D, Anderson WF, Hewitt SM, García-Closas M. Variation in breast cancer hormone receptor and HER2 levels by etiologic factors: a population-based analysis. Int J Cancer. 2007;121:1079–1085. doi: 10.1002/ijc.22812. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson L. SYSTAT: The system for Statistics, version 12. Evanston IL: SYSTAT, Inc; 1986. [Google Scholar]
- 27.Secreto G, Venturelli E, Meneghini E, Greco M, Ferraris C, Gion M, Zancan M, Fabricio AS, Berrino F, Cavalleri A, Micheli A. Testosterone and biological characteristics of breast cancers in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2009 doi: 10.1158/1055-9965.EPI-09-0540. [DOI] [PubMed] [Google Scholar]
- 28.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res. 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–752. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 30.Lønning PE, Helle H, Duong NH, Ekse D, Aas T, Geisler J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Molec Biol. 2009;117:31–41. doi: 10.1016/j.jsbmb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Reed MJ, Aherne GW, Ghilchik MW, Patel S, Chakraborty J. Concentrations of oestrone and 4-hydroxyandrostenedione in malignant and normal breast tissues. Int J Cancer. 1991;49:562–565. doi: 10.1002/ijc.2910490415. [DOI] [PubMed] [Google Scholar]
- 32.Pasqualini JR, Cortes-Prieto J, Chetrite G, Talbi M, Tuiz A. Concentrations of estrone, estradiol and their sulfates, and evaluation of sulfatases and aromatase activities in patients with breast fibroadenoma. Int J Cancer. 1997;70:639–643. doi: 10.1002/(sici)1097-0215(19970317)70:6<639::aid-ijc2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 33.Blankenstein MA, van de Ven J, Maitimu-Smeele I, Donker GH, de Jong PC, Daroszewski J, Szymczak J, Milewicz A, Thijssen JH. Intratumoral levels of estrogens in breast cancer. J Steroid Biochem Molec Biol. 1999;69:293–297. doi: 10.1016/s0960-0760(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 34.Szymczak J, Milewicz A, Thijssen JHH, Blankenstein MA, Daroszewski J. Concentration of sex steroids in adipose tissue after menopause. Steroids. 1998;63:319–321. doi: 10.1016/s0039-128x(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 35.Thjissen JHH. Local biosynthesis and metabolism of oestrogens in the human breast. Maturitas. 2004;49:25–33. doi: 10.1016/j.maturitas.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Brignardello E, Cassoni P, Migliardi M. Dehydroepiandrosterone concentration in breast cancer tissue related to its plasma gradient across the mammary gland. Breast Cancer Res Treat. 1995;33:171–177. doi: 10.1007/BF00682724. [DOI] [PubMed] [Google Scholar]
- 37.Labrie F, Luu-The V, Labrie C. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other role of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 38.Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Molec Biol. 2003;86:245–253. doi: 10.1016/s0960-0760(03)00364-9. [DOI] [PubMed] [Google Scholar]
- 39.Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol. 2010;121:491–495. doi: 10.1016/j.jsbmb.2010.05.001. [DOI] [PubMed] [Google Scholar]