Abstract
Inflectional morphology lies at the intersection of phonology, syntax and the lexicon, three language domains that are differentially impacted in the three main variants of primary progressive aphasia (PPA). To characterize spared and impaired aspects of inflectional morphology in PPA, we elicited inflectional morphemes in 48 individuals with PPA and 13 healthy age-matched controls. We varied the factors of regularity, frequency, word class, and lexicality, and used voxel-based morphometry to identify brain regions where atrophy was predictive of deficits on particular conditions. All three PPA variants showed deficits in inflectional morphology, with the specific nature of the deficits dependent on the anatomical and linguistic features of each variant. Deficits in inflecting low-frequency irregular words were associated with semantic PPA, with lexical/semantic deficits, and with left temporal atrophy. Deficits in inflecting pseudowords were associated with non-fluent/agrammatic and logopenic variants, with phonological deficits, and with left frontal and parietal atrophy.
Keywords: inflectional morphology, primary progressive aphasia, semantic dementia
Introduction
The goal of this study was to investigate the production of inflectional morphology in primary progressive aphasia (PPA). PPA is a neurodegenerative syndrome in which focal degeneration of language areas leads to progressive language deficits, while other cognitive domains remain relatively spared (Mesulam, 1982, 2001). Recent consensus guidelines for the diagnosis of PPA recognize three variants: non-fluent/agrammatic PPA, semantic PPA (also known as semantic dementia), and logopenic PPA (Gorno-Tempini et al., 2011). The three variants differ in terms of which language domains are impacted, distribution of atrophy (Gorno-Tempini et al., 2004) and pathological substrates (Grossman, 2010; Snowden et al., 2011).
Inflectional morphology is the part of grammar that marks words for grammatical features such as tense, aspect, mood, polarity, person, number, gender and case, by means of affixation (e.g. laugh, laughed) or other modifications of the word (e.g. come, came). Inflectional morphology lies at the intersection of three major components of language: phonology, syntax, and the lexicon (Spencer, 1991). First, inflectional morphology inherently involves phonological processes such as affixation, ablaut or reduplication. When affixes are attached to words, it is often necessary to select the appropriate allomorph based on the phonological context. For instance, the past tense forms of laugh, call and want are [læf-t], [c□l-d], and [w□nt-əd], with the past tense suffix surfacing as [-t], [-d] and [-əd] respectively, depending on the phonological features of the final phoneme of the stem. Second, syntax is relevant because it determines many of the grammatical features to be marked. For instance, tense is a syntactic feature that is often instantiated via inflectional morphology, as in the past tense suffix -ed in Yesterday I laughed. To give another example, grammatical relations such as subject and object are indicated through case marking, so we say I saw him, not *Me saw he. Finally, the lexicon is relevant to inflectional morphology, because in many languages, including English, there are irregularities in inflectional paradigms such that item-specific information about inflected forms must be stored in relation to each lexical item. For instance, an English speaker must store in the lexicon the information that the past tense of give is gave, not gived, and the plural of mouse is mice, not mouses.
Inflectional morphology lies at the intersection of phonology, syntax, and the lexicon, and these three language domains are differentially impacted in the three variants of PPA. Therefore we may expect deficits in inflectional morphology in each of the three variants. Moreover, the specific nature of these deficits would be expected to differ depending on the particular language domains that are impacted in each variant.
Inflectional morphology has been investigated most thoroughly in the semantic variant of PPA, which is characterized by deficits in lexical and semantic knowledge (Hodges, Patterson, Oxbury, & Funnell, 1992; Snowden, Goulding, & Neary, 1989; Warrington, 1975). Patients with semantic PPA show a selective deficit for inflecting irregular verbs (Patterson, Lambon Ralph, Hodges, & McClelland, 2001; Patterson et al., 2006b; Cortese, Balota, Sergent-Marshall, Buckner, & Gold, 2006; Jefferies, Rogers, Hopper, & Lambon Ralph, 2010), as well as an interaction of regularity by frequency, such that performance is disproportionately poor for low-frequency irregular verbs (Patterson et al., 2001; Patterson et al., 2006b; Jefferies et al., 2010). Interactions of regularity by frequency are characteristic of a variety of linguistic and nonlinguistic domains in semantic PPA (Patterson et al., 2006b). This pattern is thought to be indicative of lexical and/or semantic deficits, because irregular items require item-specific information, and item-specific information is progressively lost, with lower frequency items affected earlier than higher frequency items. There are some indications that patients with semantic PPA show a similar pattern with nominal inflectional morphology: they have been shown to be impaired in selecting the appropriate gender of determiners for nouns whose gender does not match their phonological form, especially for low-frequency items (Lambon Ralph et al., 2011), and noun-verb agreement and noun-adjective agreement were impaired for irregular items in a Hebrew-speaking semantic PPA patient (Kavé, Heinik, & Biran, 2012). Most semantic PPA patients are able to correctly supply regular inflections to pseudo-verbs (Patterson et al., 2001). Taken together, these findings suggest that deficits in inflectional morphology in semantic PPA follow from lexical and/or semantic impairments.
Non-fluent/agrammatic PPA is characterized by agrammatism and/or motor speech deficits (Grossman et al., 1996; Hodges & Patterson, 1996; Gorno-Tempini et al., 2011). Inflectional morphology in non-fluent/agrammatic PPA was investigated in a recent study in which six different verb forms were elicited (Thompson et al., 2013). Non-fluent/agrammatic PPA patients were impaired in producing finite verb forms (i.e. verb forms that mark tense), but they did much better with non-finite verb forms (i.e. verb forms that do not mark tense, e.g. progressive -ing). Similarly, quantitative analyses of connected speech have documented the omission and erroneous use of verbal inflectional morphology in non-fluent/agrammatic PPA (Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997; Wilson et al., 2010b; Thompson et al., 2012, 2013) and, to a lesser extent, nouns (Thompson et al., 2012). Sensitivity to the syntactic factor of finiteness suggests that deficits in inflectional morphology in non-fluent/agrammatic PPA may follow from syntactic deficits. Phonological deficits may also contribute, since non-fluent patients have been shown to produce phonemic paraphasias in connected speech (Patterson, Graham, Lambon Ralph, & Hodges, 2006a; Ash et al., 2010; Wilson et al., 2010b) and to exhibit difficulties on phonological manipulation tasks (Patterson et al., 2006a; Henry et al., in prep). Logopenic PPA is associated with core phonological and word-finding deficits (Gorno-Tempini et al., 2004, 2008). In Thompson and colleagues’ recent elicited production study, patients with logopenic PPA did not make many morphological errors with either finite or non-finite verbs (Thompson et al., 2013), and they make few morphological errors in connected speech (Wilson et al., 2010b; Thompson et al., 2012). Since phonological deficits are a core feature of logopenic PPA, they may be expected to have an impact on inflectional morphology, but there is no evidence to date that this is the case.
To our knowledge, the neural correlates of deficits in inflectional morphology in PPA have not been systematically investigated. Neuropsychological studies in other patient cohorts have provided some evidence suggesting that deficits in regular morphology are associated with frontal and basal ganglia damage, in contrast to deficits in irregular morphology, which are related to temporal lobe lesions (Marin et al., 1976; Tyler et al., 2002; Miozzo, 2003; Ullman et al., 2005). A number of neuroimaging studies in healthy controls have attempted to identify brain regions differentially involved in regular or irregular morphology, yet findings have been inconsistent (Jaeger et al., 1996; Ullman et al., 1997; see Desai et al., 2006 for review). Any robust differences between these conditions appear to be secondary to phonological, executive, attentional or decision-making factors that differ between regular and irregular items (Desai et al., 2006). Several single case studies of post-stroke aphasic patients have been reported showing clear dissociations between nominal and verbal morphology, though no conclusions were drawn regarding the relevant brain regions (Shapiro, Shelton, & Caramazza, 2000; Shapiro & Caramazza, 2003).
In this study, we sought to characterize spared and impaired aspects of inflectional morphology in the three variants of PPA using an elicited production task. We varied the factors of regularity (regular, irregular), frequency (low, high), word class (verbs, nouns), and lexicality (words, pseudowords). We hypothesized that the specific linguistic and anatomical profile of each PPA variant would impact inflectional morphology in different ways. First, we expected the lexical/semantic deficits that are most prominent in semantic variant PPA to differentially impact the inflection of low-frequency irregular words, regardless of word class, since low-frequency irregular words are most dependent on item-specific information. Second, we predicted that the different kinds of phonological deficits that are seen in non-fluent/agrammatic and logopenic PPA would lead to difficulties inflecting pseudowords, which must be inflected via a productive phonological process. Third, we anticipated that the syntactic deficits that occur in nonfluent/ agrammatic PPA would affect all words regardless of regularity, frequency or lexicality, since syntactic deficits reflect sentence- or phrase-level rather than word-level impairment. Therefore syntactic deficits should lead to problems inflecting even high-frequency regular words, which make the least demands on lexical/semantic information. We also investigated the relationships between measures of deficits on particular linguistic domains, and inflection of different types of words, and we used voxel-based morphometry to determine whether atrophy of regions involved in different domains of language impacts different aspects of inflectional morphology accordingly.
Methods
Participants
Individuals with PPA and age-matched controls were recruited through the Memory and Aging Center at the University of California, San Francisco (UCSF). All participants gave written informed consent, and the study was approved by the institutional review boards at UCSF and the University of Arizona. Patients and controls received a comprehensive multidisciplinary evaluation including neurological history and examination, neuropsychological testing, and neuroimaging.
A diagnosis of PPA required progressive deterioration of speech and/or language functions, and that deficits be largely restricted to speech and/or language for at least two years. Patients were diagnosed with non-fluent/agrammatic, semantic or logopenic variants of PPA based on recent guidelines (Gorno-Tempini et al., 2011). Neuroimaging results were not used for diagnostic purposes, but only to rule out other causes of focal brain damage.
The inclusion criteria for patients were a diagnosis of PPA, fluency in English and a Mini-Mental State Examination (MMSE) score of at least 15. Over a four-year period, 72 patients met these criteria and were considered for inclusion. For 8 patients, the experiment was not carried out due to situational factors (e.g. anxiety, fatigue, behavioral issues, lack of time). For 6 patients, the experiment was conducted but the data could not be analyzed due to problems with audio recordings. For 8 patients, the experiment was attempted, but the patient was unable to learn how to do the task. Six of these patients were diagnosed with logopenic PPA and had emerging deficits in other cognitive domains; one was diagnosed with non-fluent-agrammatic PPA and could learn the task with nouns but not verbs, and one was diagnosed with semantic PPA and could learn the task with verbs but not nouns. Finally, one patient with non-fluent/agrammatic PPA could not do the task because she was mute, and one patient was excluded because she did not meet criteria for any variant, leaving 48 patients whose data were analyzed.
The 48 patients were diagnosed with non-fluent/agrammatic PPA (N = 12), semantic PPA (N = 23) or logopenic PPA (N = 13). In addition, 13 healthy age-matched controls completed the experiment. Demographic, clinical and neuropsychological characteristics for all participants are provided in Table 1. The groups were not perfectly matched: patients with non-fluent PPA were significantly older than those with logopenic PPA; patients with logopenic PPA were less educated than controls (but not less educated than the other two patient groups); and patients with logopenic PPA had significantly lower MMSE scores than those with semantic PPA. All of these differences were small in magnitude and are unlikely to influence the findings of our study. The groups did not differ in sex, handedness, or clinical dementia rating scale.
Table 1.
Demographic, neuropsychological and language measures
| Semantic PPA | Non-fluent PPA | Logopenic PPA | Controls | |
|---|---|---|---|---|
| Demographic | ||||
| Age | 64.2 ± 6.8 | 68.7 ± 7.6c | 62.4 ± 9.6 | 67.4 ± 3.3 |
| Sex (M/F) | 9/14 | 6/6 | 7/6 | 5/8 |
| Handedness (R/L) | 20/3 | 10/2 | 10/3 | 8/5 |
| Education (years) | 17.0 ± 2.2 | 16.4 ± 3.1 | 15.1 ± 2.9* | 17.8 ± 1 |
| Clinical | ||||
| Mini Mental Status Examination (30) | 26.5 ± 2.6* | 26.6 ± 2.2* | 23.8 ± 4.3*a | 29.6 ± 0.7 |
| Clinical Dementia Rating | 0.6 ± 0.2 | 0.5 ± 0.3 | 0.5 ± 0.2 | N/A |
| Age at disease onset | 59.4 ± 7.2 | 63.4 ± 7.2 | 59.0 ± 9.3 | N/A |
| Years from first symptom | 4.8 ± 2.2 | 5.3 ± 6.1 | 3.4 ± 1.8 | N/A |
| Language production | ||||
| Confrontation naming (BNT, 15) | 6.0 ± 3.7*bc | 12.8 ± 1.5 | 10.5 ± 3.2* | 14.7 ± 0.5 |
| Phonemic fluency (D words in one minute) | 8.3 ± 5.1* | 5.3 ± 2.5* | 8.5 ± 4.5* | 17.4 ± 3.6 |
| Semantic fluency (Animals in one minute) | 8.9 ± 4.6* | 10.0 ± 5.1* | 9.5 ± 4.1* | 23.3 ± 4.3 |
| Speech fluency (WAB, 10) | 9.1 ± 0.7 | 7.7 ± 1.7ac | 9.0 ± 1.0 | |
| Apraxia of speech rating (MSE, 7) | 0.0 ± 0.0 | 1.8 ± 1.5ac | 0.3 ± 1.2 | N/A |
| Dysarthria rating (MSE, 7) | 0.0 ± 0.0 | 2.0 ± 2.0ac | 0.3 ± 0.9 | N/A |
| Repetition (WAB, 100) | 94.5 ± 5.9 | 91.8 ± 5.3 | 76.9 ± 10.0ab | |
| Language comprehension | ||||
| Auditory word recognition (PPVT,16) | 9.5 ± 4.2*bc | 15.3 ± 1.0 | 14.5 ± 1.5 | 15.5 ± 0.7 |
| Sequential commands (WAB, 80) | 78.6 ± 2.5 | 71.5 ± 13.4 | 71.6 ± 10.0 | |
| Syntactic comprehension (%) | 95.6 ± 7.6 | 90.4 ± 9.8* | 87.3 ± 8.7*a | 98.6 ± 1.7 |
| Semantic knowledge (PPT-P, 52) | 42.5 ± 7.3bc | 49.2 ± 2.6 | 49.2 ± 2.2 | |
| Visuospatial function | ||||
| Modified Rey-Osterrieth copy (17) | 15.6 ± 0.7 | 14.8 ± 1.7 | 12.8 ± 5.2a | 15.3 ± 0.7 |
| Visual memory | ||||
| Modified Rey-Osterrieth delay (17) | 7.6 ± 4.6*b | 11.3 ± 2.2 | 6.0 ± 3.7*b | 12.5 ± 2.8 |
| Verbal memory | ||||
| CVLT-MS Trials 1–4 (40) | 19.5 ± 6.6 | 21.4 ± 5.6 | 17.9 ± 8.3 | |
| CVLT-MS 30 s free recall (10) | 3.7 ± 2.5b | 6.1 ± 1.7 | 4.7 ± 2.6 | |
| CVLT-MS 10 min free recall (10) | 2.3 ± 2.0b | 5.3 ± 2.5 | 4.0 ± 2.8 | |
| Executive function | ||||
| Digit span backwards | 5.0 ± 1.3 | 3.6 ± 1.4*a | 3.2 ± 0.8*a | 5.8 ± 1.5 |
| Modified Trails (lines per minute) | 24.9 ± 9.6* | 13.1 ± 9.5*a | 14.0 ± 11.9* | 41.1 ± 16.0 |
| Calculation (WAB, 5) | 4.5 ± 0.7 | 4.4 ± 0.8 | 3.3 ± 1.3*ab | 5.0 ± 0.0 |
Values shown are mean ± standard deviation. BNT = Boston Naming Test; WAB = Western Aphasia Battery; MSE = Motor Speech Evaluation (Wertz et al., 1984); PPVT = Peabody Picture Vocabulary Test; PPT-P = Pyramids and Palm Trees, Pictures; CVLT-MS = California Verbal Learning Test -- Mental Status.
significantly impaired relative to normal controls (p < 0.05); superscript letters: significantly impaired relative to (a) semantic PPA; (b) non-fluent/agrammatic PPA; (c) logopenic PPA (p < 0.05) (Tukey’s HSD). See Kramer et al. (2003).
Materials
Participants were required to provide the past tense forms of verbs or the plural forms of nouns in ten conditions. Eight of these conditions were derived by crossing word class (verb/noun), regularity (regular/irregular) and frequency (high/low). Frequencies were calculated as the sum of stem and past tense or plural frequencies in the American National Corpus (Reppen, Ide, & Suderman, 2005). The other two conditions required the inflection of pseudowords in verb or noun contexts. There were originally eight items per condition, however two items had to be excluded from their intended conditions due to non-ceiling performance in healthy age-matched controls (the verb tread and the noun focus), leaving seven items in two of the conditions. Responses on the two excluded items are reported in the Supplementary Results. The stimuli and their important characteristics are shown in Table 2.
Table 2.
Stimuli
| Condition | Items | Log frequency | ||
|---|---|---|---|---|
| Category | Regularity | Frequency | (mean ± sd, range) | |
| Verb | Regular | High | call, laugh, look, point, start, use, want, work | 3.73 ± 0.54 (2.67 – 4.27) |
| Verb | Regular | Low | ache, blush, boast, clinch, frown, hint, loot, sew | 1.74 ± 0.25 (1.34 – 2.04) |
| Verb | Irregular | High | begin, buy, come, hurt, lose, sleep, speak, think | 3.63 ± 0.60 (2.80 – 4.69) |
| Verb | Irregular | Low | bleed, breed, creep, fling, lend, weave, weep | 1.97 ± 0.16 (1.82 – 2.30) |
| Verb | Pseudoword | N/A | [b□□□], [klid], [d□ŋk], [fip], [glo□st], [klo□], [n□□k], [t□□ŋ] | N/A |
| Noun | Regular | High | book, case, coach, day, dress, girl, kid, town | 3.72 ± 0.47 (3.00 – 4.33) |
| Noun | Regular | Low | crumb, dove, frog, maze, pause, peach, stalk, wand | 1.96 ± 0.31 (1.32 – 2.29) |
| Noun | Irregular | High | child, crisis, foot, life, mouse, tooth, wife, woman | 3.60 ± 0.48 (2.78 – 4.11) |
| Noun | Irregular | Low | calf, elf, goose, hoof, ox, sheep, wharf | 1.94 ± 0.34 (1.40 – 2.45) |
| Noun | Pseudoword | N/A | [b□lf], [dæt□], [g□d], [kl□s], [sæn], [θup], [ta□f], [we□z] | N/A |
Irregular verbs involved vowel changes (e.g. come/came), vowel changes plus suffixes (e.g. sleep/slept), one item in which the past is homophonous with the stem (hurt/hurt), and one consonant change (lend/lent). Regular verbs included all three allomorphs of the past tense suffix (e.g. want/wanted, frown/frowned, look/looked).
Irregular nouns involved vowel changes (e.g. woman/women), voicing of [f]s (e.g. elf/elves), two irregular suffixes (child/children, ox/oxen), and one homophonous form (sheep/sheep). Regular nouns included all three allomorphs of the plural suffix (e.g. case/cases, dove/doves, book/books).
For both word classes, the different types of irregular forms were approximately evenly distributed across high and low frequency items, and the different regular allomorphs were exactly matched across high and low frequency items.
The pseudo-verbs were monosyllabic, and could be regularly inflected using one of the three past tense allomorphs (e.g. feep/feeped). However most of the items were specifically selected to be particularly amenable to analogical pseudo-irregular past tense forms (e.g. feep/fept) (Prasada & Pinker, 1993; Albright & Hayes, 2003). The pseudo-nouns were also monosyllabic, and could be regularly inflected using one of the three plural allomorphs (e.g. gid/gids). There are far less irregular nouns than verbs in English, so there are few phonological neighborhoods that promote pseudo-irregular plural formation, but we did include two words ending in [f] that we thought would be amenable to voicing (e.g. belf/belves). Pseudo-irregular responses were scored quite liberally as correct if there was any plausible analogical basis for them.
Procedure
The experiment was carried out interactively with each patient by a speech-language pathologist (MLH, MB, JMO) or the first author (SMW). Age-matched controls were tested by research assistants. Each session was videotaped or audiotaped for later analysis.
Participants were presented with several examples of the form Today I say, yesterday I said, then encouraged to fill in the blank, e.g. Today I walk, yesterday I ____. Up to six practice items were used for training. The examiner explained the task to each patient in an individualized manner as the situation dictated, and most patients were able to learn the task. For some patients, written cards were used during the training component only. These cards depicted the six practice items, two with the blanks filled in with the appropriate inflected forms, and four with the blanks empty.
After training, the 32 real verbs were presented in pseudo-random order, each in the same frame. Items were repeated when requested by the participant, or when the examiner judged that the participant had not heard the item correctly. After the 32 real verbs, the examiner then informed the patient that they would now be doing the same thing with made-up words, and the 8 pseudoverbs were presented.
Then, the exact same procedure was repeated for the nouns. The frame for the nouns was This is a pen, these are ____. Again, up to six practice items were used for training as necessary.
Data analysis
Each participant’s data were transcribed and coded independently by two trained research assistants (TB, CS, LW, KP). Each response was coded as correct (e.g. speak/spoke), overregularized (e.g. speak/speaked), stem (e.g. speak/speak), other errors (e.g. speak/[spikəd]), no response (silence, or I don’t know), or excluded. The most common reasons that items were excluded were that the patient used a different word (e.g. today I won, yesterday I lost; today I lend, yesterday I loaned; this is a kid, these are children) or that the patient rephrased the prompt so as not to require the intended inflected form (e.g. today I sleep, yesterday I did sleep; this is a mouse, these are another mouse). Note that while some of these responses were erroneous or odd for various reasons, they were excluded because they were not informative with respect to inflectional morphological processing. When multiple responses were provided, the participant’s final response was coded. For each participant, the two or more independent transcriptions and codings were compared, and all discrepancies were resolved with reference to the original recordings by TB and SMW. Reaction times were also measured, as described in the Supplementary Methods.
To investigate the influence of phonological, syntactic and lexical/semantic factors on inflectional morphology production, we derived measures of deficits in each of these language domains in the 48 PPA patients (not in the controls). The phonological composite measure was derived from two scores—the repetition score from the Western Aphasia Battery, and phonemic fluency (number of words starting with [d] generated in one minute)—by performing principal components analysis and retaining the first component. The syntactic measure was percent correct on an offline version of the two-alternative forced choice sentence comprehension task described by Wilson et al. (2010a); this measure was available for 47 of the 48 patients. The lexical/semantic composite measure was derived using principal components analysis from four scores: confrontation naming (Boston Naming Test), auditory comprehension of single words (Peabody Picture Vocabulary Test), semantic fluency (number of animal names generated in one minute) and semantic associations (Pyramids and Palm Trees—Pictures).
Statistical analyses were carried out with SPSS version 20 (IBM) using repeated measures ANOVAs with Greenhouse-Geisser corrections applied where appropriate.
Neuroimaging
Voxel-based morphometry (VBM) was used to identify brain regions where degeneration was associated with deficits on specific conditions.
T1-weighted 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) images were acquired on a Siemens Trio 3 Tesla scanner with the following parameters: 160 sagittal slices; slice thickness = 1 mm; field of view = 256 × 256 mm; matrix = 230 × 256; repetition time (TR) = 2300 ms; echo time (TE) = 2.98 ms; flip angle = 9°.
The T1-weighted structural images were bias-corrected, segmented into gray matter, white matter and cerebro-spinal fluid, and initially normalized to MNI space using the unified segmentation algorithm in SPM5 (Ashburner & Friston, 2005). More anatomically precise intersubject registration was then performed with the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) toolbox (Ashburner, 2007) by warping each participant’s image to a template created from 50 additional normal control participants. Gray matter and white matter probability maps were scaled by Jacobians, smoothed with a Gaussian kernel of 12 mm full width at half maximum (FWHM), then summed together to obtain a map of brain parenchyma. The advantage of this approach is that regional atrophy typically impacts both tissue types in parallel, so summing gray matter and white matter maps reveals volume loss in either tissue type that is correlated with behavioral variables in a single analysis.
We calculated correlations between parenchymal volume and production of inflected forms of (1) low-frequency irregular words (averaged across nouns and verbs); and (2) pseudowords (averaged across nouns and verbs). These conditions were selected as most dependent on lexical/semantic and phonological processing respectively. Control participants were not included in the VBM analyses. Age, sex and total intracranial volume were included as covariates. T maps were thresholded at voxelwise p < 0.005, then corrected for multiple comparisons based on cluster size with respect to 1000 permutations in which behavioral scores were randomly reassigned (Wilson et al., 2010b), using VLSM version 2.52 (http://neuroling.arizona.edu/resources.html).
Results
The accuracy of PPA patients and controls as a function of condition is shown in Table 3 and Fig. 1. The figure also shows the breakdown between the different types of errors that were produced. Details of responses on each individual item are provided in Supplementary Tables S1 through S10. Analysis of reaction times is reported in the Supplementary Results and Supplementary Fig. S1.
Table 3.
Accuracy in producing inflected forms as a function of word class, regularity, frequency, in the three PPA variants and age-matched controls
| Condition | Accuracy by group (%) | |||||
|---|---|---|---|---|---|---|
| Category | Regularity | Frequency | Semantic PPA | Non-fluent PPA | Logopenic PPA | Controls |
| Verb | Regular | High | 98.9 ± 5.2 | 94.5 ± 5.2 | 94.2 ± 14.1 | 100.0 ± 0.0 |
| Verb | Regular | Low | 88.1 ± 21.9 | 90.2 ± 21.9 | 82.1 ± 17.7 | 100.0 ± 0.0 |
| Verb | Irregular | High | 84.7 ± 13.8* | 87.1 ± 13.8 | 84.0 ± 17.0* | 99.0 ± 3.5 |
| Verb | Irregular | Low | 48.1 ± 31.9* | 73.3 ± 31.9 | 62.6 ± 33.8* | 95.4 ± 9.2 |
| Verb | Pseudoword | N/A | 82.7 ± 17.8 | 70.0 ± 17.8* | 66.0 ± 19.0*a | 90.9 ± 10.0 |
| Noun | Regular | High | 99.3 ± 3.5 | 98.8 ± 4.1 | 98.1 ± 4.7 | 100.0 ± 0.0 |
| Noun | Regular | Low | 97.7 ± 5.2 | 92.4 ± 11.8 | 97.1 ± 7.5 | 99.0 ± 3.5 |
| Noun | Irregular | High | 84.2 ± 17.2* | 79.2 ± 15.4* | 88.0 ± 13.9 | 100.0 ± 0.0 |
| Noun | Irregular | Low | 46.1 ± 30.0*b | 69.6 ± 30.4* | 59.9 ± 19.7* | 96.7 ± 6.3 |
| Noun | Pseudoword | N/A | 85.4 ± 18.9 | 60.3 ± 28.6b | 70.9 ± 29.4 | 83.7 ± 11.8 |
Values shown are mean ± standard deviation.
significantly impaired relative to normal controls (p < 0.05); superscript letters: significantly impaired relative to (a) semantic PPA; (b) non-fluent/agrammatic PPA; (c) logopenic PPA (p < 0.05) (Tukey’s HSD).
Fig. 1.
Accuracy in producing inflected forms as a function of word class, regularity, frequency, in the three PPA variants and age-matched controls. (A) Non-fluent/agrammatic PPA. (B) Semantic PPA. (C) Logopenic PPA. (D) Age-matched controls. Error bars show standard error of the mean.
Real words
A one-way ANOVA showed that the four groups differed in overall accuracy on real words (F(3, 57) = 8.52, p < 0.001). Age-matched controls inflected real words highly accurately (98.8% ± 1.8%), whereas all three PPA variants showed deficits in inflecting real words. Post hoc tests (Tukey’s HSD) showed that semantic PPA patients (80.9% ± 12.0%; p < 0.001), non-fluent/agrammatic PPA patients (85.6% ± 10.6%; p = 0.014), and logopenic PPA patients (83.3% ± 12.4%, p = 0.002) were all less accurate than controls, and that the three PPA variants did not differ from one another (all p ≥ 0.59).
To examine effects of regularity, frequency and word class, we first carried out a four-way ANOVA with one between-subjects factor (group: semantic PPA, non-fluent/agrammatic PPA, logopenic PPA, control) and three within-subjects factors (regularity: regular/irregular; frequency: high/low; word class: verb/noun). The main effect of word class was not significant (F(1, 57) = 1.19; p = 0.28). Only one interaction involving word class was significant: the interaction of word class by regularity (F(1, 57) = 6.32, p = 0.015), however this has no apparent theoretical relevance to the present study. None of the other interactions involving word class were significant (four-way interaction: F(3, 57) = 1.89; p = 0.14; group by word class by regularity: F(3, 57) = 1.12; p = 0.35; group by word class by frequency: F(3, 57) < 1; word class by regularity by frequency: F(1, 57) = 1.85; p = 0.18); word class by group: F(3, 57) < 1; word class by frequency: F(1, 57) < 1). These results show that patterns of performance on inflectional morphology do not differ between verbs and nouns. Therefore for subsequent analyses we collapsed across the factor of word class by averaging all scores across verbs and nouns.
We hypothesized that the impact of lexical/semantic deficits on inflectional morphology would be most pronounced with low-frequency irregular words, leading to an interaction of regularity by frequency, and we expected lexical/semantic effects to be strongest in semantic PPA. The groups differed in their performance on low-frequency irregular words (F(3, 57) = 13.10, p < 0.001), with semantic PPA patients performing least accurately (47.1% ± 27.2%) as expected. Post hoc tests (Tukey’s HSD) showed that semantic PPA patients were less accurate than non-fluent/agrammatic patients (71.4% ± 22.9%; p = 0.021) and controls (96.1% ± 5.6%; p < 0.001), but were not significantly less accurate than logopenic patients (61.3% ± 24.8%; p = 0.29). Nonfluent PPA patients and logopenic PPA patients were also less accurate than controls (p = 0.045, p = 0.002 respectively) and did not differ from one another (p = 0.69). The three-way interaction of group by regularity by frequency was significant (F(3, 57) = 9.51; p < 0.001). Post hoc tests showed that the regularity by frequency interaction was greater in semantic PPA patients than any other group (p < 0.001 versus controls, p < 0.001 versus non-fluent PPA, p = 0.035 versus logopenic PPA). The regularity by frequency interaction was also greater in logopenic PPA patients than controls (p = 0.028), but did not differ between non-fluent patients and controls (p = 0.62) or between non-fluent and logopenic patients (p = 0.091). These findings support our hypothesis that lexical/semantic aspects of inflectional morphology would be impacted in semantic PPA, but also suggest a somewhat similar pattern in logopenic PPA and, though to an even lesser extent, in nonfluent PPA.
Syntactic effects on inflectional morphology would be expected to impact all words, even high-frequency regular words, which pose the least lexical/semantic demands; we expected syntactic effects to be most pronounced in non-fluent/agrammatic PPA. Contrary to this prediction, we found that most patients performed at ceiling on inflecting high-frequency regular verbs and nouns, and consequently there was no effect of group (F(3, 57) = 1.67, p = 0.18). Below we discuss why our experiment may have failed to reveal syntactic effects on inflectional morphology.
Pseudowords
A one-way ANOVA showed that the four groups differed in overall accuracy on pseudowords (F(3, 57) = 6.05, p = 0.001). Post hoc tests (Tukey’s HSD) showed that age-matched controls (87.3% ± 7.9%) and semantic PPA patients (84.1% ± 14.8%) performed equivalently well (p = 0.95), and both groups performed better (all p ≤ 0.047) than non-fluent/agrammatic PPA patients (65.1% ± 23.5%) and logopenic PPA patients (68.5% ± 19.4%), who did not differ from one another (p = 0.96).
To determine whether there were any differences in performance on pseudo-verbs versus pseudo-nouns, we performed a two-way ANOVA with one between-subjects factor (group) and one within-subjects factor (word class: verb/noun). There was no main effect of word class (F(1, 57) < 1) and no interaction of group by word class (F(3, 57) = 1.44, p = 0.24).
Past tense or plural forms of pseudowords can be produced by applying regular inflectional rules (e.g. feep/feeped), or by analogy with irregular forms (e.g. feep/fept) (Fig. 1, note the distinction between the lighter and darker blues in the pseudoword bars). We carried out a two-way ANOVA with proportion of pseudo-irregular responses (e.g. feep/fept) as the dependent variable, one between-subjects factor (group) and one within-subjects factor (word class). Only participants with at least three correct responses on each of the two pseudoword conditions were included (n = 54). There was a main effect of word class, with pseudo-verbs much more likely (40.2% ± 26.7%) than pseudo-nouns (11.9% ± 14.7%) to be inflected based on analogies to irregular words (F(1, 50) = 48.52, p < 0.001). There was no interaction of group by word class (F(3, 50) < 1). The main effect of group did not reach significance (F(3, 50) = 2.054, p = 0.12), but we also carried out an a priori contrast comparing semantic PPA patients to controls, and found that semantic PPA patients produced analogical forms significantly less often (19.9% ± 3.6%) than controls (34.3% ± 4.2%) (F(1, 33) = 6.32, p = 0.017).
Relation of semantic and phonological composite scores to inflectional morphology
To determine whether inflectional morphology in specific conditions was predicted by deficits in particular linguistic domains, we carried out multiple regression analyses in the 48 PPA patients, including semantic composite, phonological composite and syntactic scores as explanatory variables.
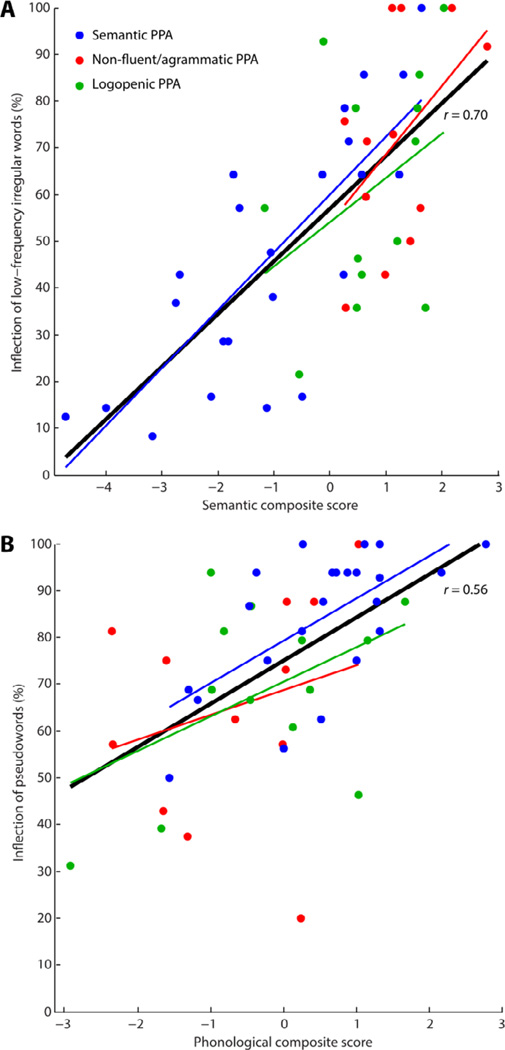
Correct inflection of low-frequency irregular words was predicted by semantic composite scores (F(1, 43) = 40.97, p < 0.001), but not by phonological (F(1, 43) = 1.26, p = 0.27) or syntactic scores (F(1, 43) < 1) (all three variables were included in the model). The correlation between semantic composite scores and correct inflection of low-frequency irregular words (Fig. 2A) was significant across all 48 PPA patients (r = 0.70, p < 0.001), was significant in the semantic group alone (r = 0.79, p < 0.001), who were expected to have deficits in the inflection of low-frequency irregular words, and was significant in the non-fluent/agrammatic and logopenic patients (r = 0.44, p = 0.027), in whom semantic deficits were less pronounced as expected.
Fig. 2.
Correlations between composite measures and inflectional morphology. (A) Correlation between semantic composite score and inflection of low-frequency irregular words. (B) Correlation between phonological composite score and inflection of pseudowords.
In contrast, correct inflection of pseudowords was predicted by phonological composite scores (F(1, 43) = 7.93, p = 0.007), but not by semantic (F(1, 43) < 1) or syntactic scores (F(1, 43) = 1.42, p = 0.24). (all three variables were included in the model). The correlation between phonological composite scores and correct inflection of pseudowords (Fig. 2B) was significant across all 48 PPA patients (r = 0.56, p = 0.002) and in the semantic PPA patients (r = 0.58, p = 0.004), in whom phonological deficits are minimal, but was only a trend in the non-fluent/agrammatic and logopenic patients (r = 0.30, p = 0.15).
Correct inflection of high-frequency regular words was not predicted by syntactic (F(1, 43) = 1.71, p = 0.20), phonological (F(1, 43) = 1.09, p = 0.30) or semantic scores (F(1, 44) < 1) (all three variables were included in the model).
Correlations between the individual measures contributing to the composite scores and the inflectional morphology measures are reported in the Supplementary Results.
Types of errors
We looked next at whether error types differed between PPA variants. First we looked at overregularization errors, which by definition could occur only on irregular words. For each patient who made at least three errors on irregular words, we calculated the proportion of errors that were over-regularizations. In semantic PPA patients, 85.6% ± 4.5% errors were over-regularizations, which was greater than non-fluent PPA (69.6% ± 7.4%) and logopenic PPA (65.2% ± 6.6%). The effect of group was significant (F(2, 36) = 3.89; p = 0.029), with post hoc tests (Tukey’s HSD) showing a significant difference between semantic PPA and logopenic PPA (p = 0.039), but non-significant differences between semantic PPA and non-fluent PPA (p = 0.17) and between non-fluent PPA and logopenic PPA (p = 0.90).
Errors comprising uninflected stem forms were slightly more prevalent in non-fluent PPA patients (20.1% ± 4.9%) than semantic PPA (15.6% ± 3.3%) or logopenic PPA (15.8% ± 4.3%), but this difference did not approach significance (F(2, 42) < 1).
Voxel-based morphometry
The items that are most dependent on lexical/semantic processing are low-frequency irregular words. Voxel-based morphometry revealed that deficits in the inflection of low-frequency irregular words were associated with parenchymal volume loss in the left anterior temporal lobe, left insula, left basal ganglia, and white matter underlying these regions, which included anterior parts of the inferior longitudinal fasciculus and the extreme capsule fiber system (Fig. 3, Table 4).
Fig. 3.
Voxel-based morphometry. Regions where parenchymal volume correlated with accuracy in inflecting low-frequency irregular words (hot) or pseudowords (blue-green) (p < 0.05, corrected for multiple comparisons).
Table 4.
Voxel-based morphometry
| MNI coordinates | Extent (mm3) |
|||||
|---|---|---|---|---|---|---|
| Brain region | x | y | z | Max t | p | |
| Low-frequency irregular words | ||||||
| Left anterior superior, middle and inferior temporal gyri, anterior fusiform gyrus, putamen, head of caudate nucleus, and underlying white matter | −36 | −1 | −22 | 7636 | 4.93 | 0.016 |
| Left inferior temporal gyrus | −48 | −16 | −36 | 4.18 | ||
| Left extreme capsule | −36 | −2 | −2 | 4.93 | ||
| Pseudowords | ||||||
| Bilateral frontal white matter, superior frontal gyri, posterior inferior frontal gyri, body of caudate nuclei, and left postcentral gyrus | −6 | 4 | 39 | 8499 | 4.44 | 0.019 |
| Left frontal white matter | −20 | 6 | 36 | 3.86 | ||
| Right frontal white matter | 34 | 20 | 22 | 3.44 | ||
| Right superior frontal gyrus | 20 | −4 | 68 | 4.44 | ||
Coordinates for clusters are centers of mass. Coordinates are shown also for prominent local maxima within clusters.
The items that are most dependent on phonological processing are pseudowords. Deficits in the inflection of pseudowords were associated with parenchymal volume loss in the white matter underlying the frontal lobe bilaterally, superior frontal gyrus bilaterally, posterior inferior frontal gyrus bilaterally, caudate body bilaterally, and the left postcentral gyrus (Fig. 3, Table 4).
Discussion
Inflectional morphology lies at the intersection of three major components of language: phonology, syntax, and the lexicon, and these three language domains are differentially impacted in the three variants of PPA. In support of our main hypothesis, we found that all three PPA variants showed deficits in inflectional morphology, and that the specific nature of the deficits depended on the anatomical and linguistic features of each variant.
Semantic variant PPA
We found that patients with semantic PPA performed particularly poorly with low-frequency irregular words, and that they showed a larger interaction of regularity by frequency than controls or the other PPA variants. This finding is consistent with several previous studies (Patterson et al., 2001; Patterson et al., 2006b; Cortese et al., 2006; Jefferies et al., 2010). Also consistent with previous research, we found that most of the errors that semantic PPA patients produced were over-regularizations. The lexical/semantic origin for these morphological deficits was supported by a strong correlation between our semantic composite measure and performance on low-frequency irregular words. Similarly, Patterson et al. (2001) reported an association between a synonym judgment task and past tense generation for irregular words in eleven semantic dementia patients.
We found that semantic PPA patients inflected pseudowords just as accurately as healthy age-matched, consistent with the findings of Patterson et al. (2001). Interestingly, although their accuracy did not differ from controls, they were more likely than controls to apply regular allomorphs of the past tense suffix (e.g. feep-feeped), and conversely less likely to inflect pseudowords by analogy to existing irregular words in the lexicon (e.g. feep-fept). The reduced propensity to analogize based on the lexicon is consistent with the lexical/semantic deficits that characterize semantic PPA, and shows that there are two mechanisms for generation of novel inflectional forms, and that when one is damaged, the other may take its place.
We found no effect of word class (verbs versus nouns) in semantic PPA or any other PPA variant. Notably, this contrasts with other domains, in which patients with semantic PPA show differences between nouns and verbs: they show more severe lexical retrieval deficits for nouns than verbs (Hillis, Oh, & Ken, 2004; Silveri & Ciccarelli, 2007) and use a lower than normal proportion of nouns relative to verbs in connected speech (Bird, Lambon Ralph, Patterson, & Hodges, 2000; Wilson et al., 2010b). Moreover, dissociations between inflectional morphology for nouns and verbs have been reported in stroke patients, including one who made more errors when inflecting nouns than compared to verbs (Shapiro, Shelton, & Caramazza, 2000), and one who showed the opposite pattern (Shapiro & Caramazza, 2003). The fact that we found no effect of word class suggests that deficits in inflectional morphology in semantic PPA are an instantiation of a domain-general loss of item-specific information (Patterson et al., 2006b), since such a mechanism would be expected to impact verbs and nouns similarly. Indeed, it has been argued that apparent noun-specific deficits in PPA are a consequence of the same general mechanism, along with the fact that nouns tend to be lower frequency than verbs (Bird et al., 2000).
Errors involving inflectional morphology are rare in the connected speech of semantic PPA patients (Meteyard and Patterson, 2009; Wilson et al., 2010b). This is presumably because if the lexical representation of a word is sufficiently degraded and/or difficult to access that it would pose a challenge to retrieve the inflected form, then the word is unlikely to be selected in the first place (Patterson et al., 2001).
Non-fluent/agrammatic variant PPA
Patients with the non-fluent/agrammatic variant of PPA were impaired in inflecting pseudowords, which we anticipated due to the phonological deficits that have been documented in this variant (Patterson et al., 2006a; Ash et al., 2010; Wilson et al., 2010b; Henry et al., in prep). Affixing a suffix to a novel word form and selecting the appropriate allomorph are phonological processes that lack lexical support and thus are challenging for patients with phonological impairments, and we found that in the PPA group as a whole, inflection of pseudowords was strongly predicted by a phonological composite measure.
We predicted that inflectional morphology in non-fluent/agrammatic PPA would be impacted not only by phonological deficits but also by syntactic deficits. Syntactic effects on inflectional morphology would arise at the level of syntactic feature specification, and thus should impact all words without respect to regularity or frequency, including regular high-frequency words. However this hypothesis was not supported: most non-fluent/agrammatic patients performed at ceiling on regular high-frequency words, as did semantic and logopenic patients. Specifically, non-fluent/agrammatic patients inflected 94.5% of regular high-frequency verbs correctly, and 98.8% of regular high-frequency nouns. This finding contrasts with previous studies, especially in regard to verbal morphology. Patients with non-fluent/agrammatic PPA have been shown to omit or make errors with verbal morphology in studies of connected speech (Thompson et al., 1997, 2012, 2013; Wilson et al., 2010b). Verb inflection rates in connected speech studies are around 80% (Thompson et al., 2012, 2013), or even lower as the disease progresses (Thompson et al., 1997). In Thompson et al.’s (2013) elicitation study, regular past tense forms were produced correctly 80.0% of the time.
We believe that the rate of correct past tense inflection in our study is artificially high because our elicitation task created the context of a “word game”. The nature of the task makes it apparent to patients that a past tense inflection is required on every trial, which may have reduced the ecological validity of our findings. Another respect in which our elicitation task was somewhat unnatural was that our stimuli included some transitive verbs (e.g. use, buy), yet there were no direct objects in the frames. Although Thompson et al. (2013) did show around 80% correct inflection in an elicitation task, it is unclear exactly how they scored responses; if responses such as “today I laugh, yesterday I did laugh” were scored as incorrect, that may explain some of the difference between their study and ours, since we excluded such trials. Alternatively, there may be differences between the composition of our non-fluent/agrammatic patient cohorts. In particular, most of our non-fluent/agrammatic patients were only mildly agrammatic when tested. The one patient we tested who was most profoundly agrammatic had to be excluded as he was unable to learn the task, most likely due to his agrammatism, since his cognitive functions were quite well preserved otherwise (his MMSE was 23). The extent to which patients diagnosed as non-fluent/agrammatic variant PPA are actually agrammatic is debated. Some researchers have argued that grammatical deficits are primary (Ash et al., 2009; Thompson et al., 2012), while others have reported that these patients are rarely frankly agrammatic (Graham et al., 2004; Patterson et al., 2006a; Wilson et al., 2010b).
Non-fluent/agrammatic patients were impaired relative to controls on inflecting irregular low-frequency words. Although they did not show a significant interaction of regularity by frequency relative to controls, performance on low-frequency irregular words was predicted by a semantic composite measure and not by the phonological or syntactic measures, including in the subgroup of non-fluent/agrammatic and logopenic patients. This suggests that the these errors are driven by lexical/semantic factors in non-fluent/agrammatic PPA, even though these patients have only mild deficits in these domains compared to the other PPA variants. In contrast, a recent study of reading aloud in non-fluent PPA found that patients were differentially impaired in reading low-frequency irregular words, but that this was driven by phonological and not semantic factors (Woollams and Patterson, 2012); note however that this cohort included both non-fluent/agrammatic and logopenic PPA patients. The different sources of differential difficulty with low-frequency irregular items between overt reading and inflectional morphology may reflect the fact that reading irregular words still involves grapheme-to-phoneme conversion, albeit involving atypical letter-sound correspondences, whereas inflecting irregular words may not pose additional phonological demands, only additional lexical/semantic demands.
Logopenic variant PPA
Patients with the logopenic variant of PPA were impaired in inflecting pseudowords, which we anticipated due to their core phonological deficit, as well as in inflecting low-frequency irregular words, which we expected due to their lexical deficits. Logopenic patients typically show lexical deficits that are less severe than those found in semantic PPA, and this was reflected in a regularity by frequency interaction on our inflectional morphology task in logopenic PPA that was greater than that seen in controls, but less than that seen in semantic PPA.
Errors involving inflectional morphology are rare in the connected speech of logopenic PPA patients (Wilson et al., 2010b; Thompson et al., 2012). This can be explained given the nature of their morphological deficits. Connected speech does not require the inflection of pseudowords, which place the greatest demands on phonological processes, and lexically derived inflectional morphological errors are unlikely for the same reason as they are unlikely in semantic PPA: that these words would be unlikely to be selected in the first place (Patterson et al., 2001). Thompson et al. (2013) did not observe deficits in inflectional morphology in logopenic patients in their elicited production study. However they did not test pseudowords, and it is unclear whether any of their irregular items were low-frequency.
Neural correlates of deficits in inflectional morphology
Deficits in the inflection of low-frequency irregular words and pseudowords were associated with atrophy of ventral and dorsal brain regions respectively. This is consistent with the view that these deficits reflect lexical/semantic and phonological problems respectively, and that these domains differentially rely on ventral and dorsal parts of the language network (Hickok and Poeppel, 2007; Saur et al., 2008; Schwartz et al., 2009, 2012; Galantucci et al., 2011).
The bilaterality of the regions correlated with deficits in pseudoword inflection was not expected. Previous research strongly suggests that phonological processing is robustly left-lateralized, so we suspect that the right hemisphere correlations reflect patterns of co-atrophy rather than a role for right hemisphere regions and tracts in phonology.
Conclusion
In sum, we found that individuals with all three variants of PPA are impaired in inflectional morphology, but that the nature of their impairments differs depending on the particular language domains impacted in each variant.
Supplementary Material
Highlights.
Elicited production task was used to investigate inflectional morphology in PPA
All three PPA variants showed deficits in inflectional morphology
Lexical and phonology contributions to morphological deficits were identified
Acknowledgments
This research was supported in part by National Institutes of Health (NIDCD R03 DC010878 to SMW, NINDS R01 NS050915 to MLGT, NIA P50 AG03006 to BLM, NIA P01 AG019724 to BLM); University of Arizona; State of California (DHS 04-35516); Alzheimer’s Disease Research Center of California (03-75271 DHS/ADP/ARCC); Larry L. Hillblom Foundation; John Douglas French Alzheimer’s Foundation; Koret Family Foundation; McBean Family Foundation; and University of Arizona. We thank Kevin Shapiro and Alfonso Caramazza for sharing their stimuli, Reva Wilheim and Jessica DeLeon for collecting control data, Alisa Berg, Ashley Chavez, Adam Gendreau, Lua Hedayati, Rachel Mueller, Leah Swanson, and Lauren Zimmerman for assistance with transcription, Karalyn Patterson and one other reviewer for constructive comments, all of the members of the UCSF Memory and Aging Center who contributed to patient evaluation and care, and all of the patients, caregivers and volunteers for their participation in our research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albright A, Hayes B. Rules vs. analogy in English past tenses: a computational/experimental study. Cognition. 2003;90:119–161. doi: 10.1016/s0010-0277(03)00146-x. [DOI] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A, Moore P, et al. Speech errors in progressive non-fluent aphasia. Brain and Language. 2010;113:13–20. doi: 10.1016/j.bandl.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Moore P, Vesely L, Gunawardena D, McMillan C, Anderson C, Avants B, Grossman M. Non-fluent speech in frontotemporal lobar degeneration. Journal of Neurolinguistics. 2009;22:370–383. doi: 10.1016/j.jneuroling.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bird H, Lambon Ralph MA, Patterson K, Hodges JR. The rise and fall of frequency and imageability: noun and verb production in semantic dementia. Brain and Language. 2000;73:17–49. doi: 10.1006/brln.2000.2293. [DOI] [PubMed] [Google Scholar]
- Cortese MJ, Balota DA, Sergent-Marshall SD, Buckner RL, Gold BT. Consistency and regularity in past-tense verb generation in healthy ageing, Alzheimer’s disease, and semantic dementia. Cognitive Neuropsychology. 2006;23:856–876. doi: 10.1080/02643290500483124. [DOI] [PubMed] [Google Scholar]
- Desai R, Conant LL, Waldron E, Binder JR. FMRI of past tense processing: the effects of phonological complexity and task difficulty. Journal of Cognitive Neuroscience. 2006;18:278–297. doi: 10.1162/089892906775783633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham N, Patterson K, Hodges J. When more yields less: speaking and writing deficits in nonfluent progressive aphasia. Neurocase. 2004;10:141–155. doi: 10.1080/13554790409609945. [DOI] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D’Esposito M, Ding X, Alavi A, et al. Progressive nonfluent aphasia: language, cognitive, and PET measures contrasted with probable Alzheimer’s disease. Journal of Cognitive Neuroscience. 1996;8:135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Grossman M. Primary progressive aphasia: clinicopathological correlations. Nature Reviews Neurology. 2010;6:88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Babiak MC, Wilson SM, Mandelli ML, Beeson PM, Miller BL, Gorno-Tempini ML. Phonological processing in primary progressive aphasia. doi: 10.1162/jocn_a_00901. In prep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Annals of Neurology. 2004;55:268–275. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. Journal of the International Neuropsychological Society: JINS. 1996;2:511–524. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Jaeger JJ, Lockwood AH, Kemmerer DL, Valin RD, van Murphy BW, Jr, Khalak HG. A positron emission tomographic study of regular and irregular verb morphology in English. Language. 1996;72:451–497. [Google Scholar]
- Jefferies E, Rogers TT, Hopper S, Lambon Ralph MA. “Pre-semantic” cognition revisited: critical differences between semantic aphasia and semantic dementia. Neuropsychologia. 2010;48:248–261. doi: 10.1016/j.neuropsychologia.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavé G, Heinik J, Biran I. Preserved morphological processing in semantic dementia. Cognitive Neuropsychology. 2012;29:550–568. doi: 10.1080/02643294.2012.759097. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Green Heredia C, Berthier ML, Martinez-Cuitino M, Torralva T, Manes F, et al. El-La: the impact of degraded semantic representations on knowledge of grammatical gender in semantic dementia. Acta Neuropsychologica. 2011;9:115–132. [Google Scholar]
- Marin OS, Saffran EM, Schwartz MF. Dissociations of language in aphasia: implications for normal function. Annals of the New York Academy of Sciences. 1976;280:868–884. doi: 10.1111/j.1749-6632.1976.tb25550.x. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Slowly progressive aphasia without generalized dementia. Annals of Neurology. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Primary progressive aphasia. Annals of Neurology. 2001;49:425–432. [PubMed] [Google Scholar]
- Meteyard L, Patterson K. The relation between content and structure in language production: An analysis of speech errors in semantic dementia. Brain and Language. 2009;110:121–134. doi: 10.1016/j.bandl.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Miozzo M. On the processing of regular and irregular forms of verbs and nouns: evidence from neuropsychology. Cognition. 2003;87:101–127. doi: 10.1016/s0010-0277(02)00200-7. [DOI] [PubMed] [Google Scholar]
- Mohr J. Broca’s area and Broca’s aphasia. In: Whitaker H, Whitaker H, editors. Studies in Neurolinguistics. Vol. 1. New York: Academic Press; 1976. pp. 201–233. [Google Scholar]
- Patterson K, Lambon Ralph MA, Hodges JR, McClelland JL. Deficits in irregular past-tense verb morphology associated with degraded semantic knowledge. Neuropsychologia. 2001;39:709–724. doi: 10.1016/s0028-3932(01)00008-2. [DOI] [PubMed] [Google Scholar]
- Patterson K, Graham N, Ralph MAL, Hodges J. Progressive non-fluent aphasia is not a progressive form of non-fluent (post-stroke) aphasia. Aphasiology. 2006a;20:1018–1034. [Google Scholar]
- Patterson K, Ralph MA, Jefferies E, Woollams A, Jones R, Hodges JR, Rogers TT. “Presemantic” cognition in semantic dementia: Six deficits in search of an explanation. Journal of Cognitive Neuroscience. 2006b;18:169–183. doi: 10.1162/089892906775783714. [DOI] [PubMed] [Google Scholar]
- Prasada S, Pinker S. Generalisation of regular and irregular morphological patterns. Language and Cognitive Processes. 1993;8:1–56. [Google Scholar]
- Reppen R, Ide N, Suderman K. American National Corpus (ANC) Second Release. Philadelphia: Linguistic Data Consortium; 2005. [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135:3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett H. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K, Caramazza A. Grammatical processing of nouns and verbs in left frontal cortex. Neuropsychologia. 2003;41:1189–1198. doi: 10.1016/s0028-3932(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Shelton J, Caramazza A. Grammatical class in lexical production and morhpological processing: evidence from a case of fluent aphasia. Cognitive Neuropsychology. 2000;17:665–682. doi: 10.1080/026432900750038281. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Ciccarelli N. Naming of grammatical classes in frontotemporal dementias: linguistic and non linguistic factors contribute to noun-verb dissociation. Behavioural Neurology. 2007;18:197–206. doi: 10.1155/2007/428191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behavioural Neurology. 1989;2:167–182. [Google Scholar]
- Snowden JS, Thompson JC, Stopford CL, Richardson AMT, Gerhard A, Neary D, Mann DMA. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011;134:2478–2492. doi: 10.1093/brain/awr189. [DOI] [PubMed] [Google Scholar]
- Spencer A. Morphological theory: An introduction to word structure in generative grammar. Oxford: Blackwell; 1991. [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M-M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–321. [Google Scholar]
- Thompson CK, Cho S, Hsu C-J, Wieneke C, Rademaker A, Weitner BB, Mesulam M-M, et al. Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology. 2012;26:20–43. doi: 10.1080/02687038.2011.584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Meltzer-Asscher A, Cho S, Lee J, Wieneke C, Weintraub S, Mesulam M-M. Syntactic and morphosyntactic processing in stroke-induced and primary progressive aphasia. Behavioural Neurology. 2013;26:35–54. doi: 10.3233/BEN-2012-110220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, deMornay-Davies P, Anokhina R, Longworth C, Randall B, Marslen-Wilson WD. Dissociations in processing past tense morphology: neuropathology and behavioral studies. Journal of Cognitive Neuroscience. 2002;14:79–94. doi: 10.1162/089892902317205348. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Corkin S, Coppola M, Hickok G, Growdon JH, Koroshetz WJ, Pinker S. A neural dissociation within language: evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of Cognitive Neuroscience. 1997;9:266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Pancheva R, Love T, Yee E, Swinney D, Hickok G. Neural correlates of lexicon and grammar: Evidence from the production, reading, and judgment of inflection in aphasia. Brain and Language. 2005;93:185–238. doi: 10.1016/j.bandl.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Quarterly Journal of Experimental Psychology. 1975;27:635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of speech in adults: the disorder and its management. New York: Grune and Stratton; 1984. [Google Scholar]
- Wilson SM, Dronkers NF, Ogar JM, Jang J, Growdon ME, Agosta F, Henry ML, et al. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. Journal of Neuroscience. 2010a;30:16845–16854. doi: 10.1523/JNEUROSCI.2547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Miller BL, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010b;133:2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollams AM, Patterson K. The consequences of progressive phonological impairment for reading aloud. Neuropsychologia. 2012;50:3469–3477. doi: 10.1016/j.neuropsychologia.2012.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.