Abstract
Objective
To determine the impact of tumor biology on rates of breast-conserving surgery and pathologic complete response (pCR) after neoadjuvant chemotherapy.
Summary Background Data
The impact of tumor biology on the rate of breast-conserving surgery after neoadjuvant chemotherapy has not been well studied.
Methods
We used data from ACOSOG Z1071, a prospective, multicenter study assessing sentinel node surgery after neoadjuvant chemotherapy in patients presenting with node-positive breast cancer from 2009 through 2011, to determine rates of breast-conserving surgery and pCR after chemotherapy by approximated biologic subtype.
Results
Of the 756 patients enrolled on Z1071, 694 had findings available from pathologic review of breast and axillary specimens from surgery after chemotherapy. Approximated subtype was triple-negative in 170 (24.5%) patients, HER2-positive in 207 (29.8%), and hormone-receptor-positive, HER2-negative in 317 (45.7%). Patient age and clinical tumor and nodal stage at presentation did not differ across subtypes. Rates of breast-conserving surgery were significantly higher in patients with triple-negative (46.8%) and HER2-positive tumors (43.0%) than in those with hormone-receptor-positive, HER2-negative tumors (34.5%) (P = 0.019). Rates of pCR in both the breast and axilla were 38.2% in triple-negative, 45.4% in HER2-positive, and 11.4% in hormone-receptor-positive, HER2-negative disease (P < 0.0001). Rates of pCR in the breast only and the axilla only exhibited similar differences across tumor subtypes.
Conclusions
Patients with triple-negative and HER2-positive breast cancers have the highest rates of breast-conserving surgery and pCR after neoadjuvant chemotherapy. Patients with these subtypes are most likely to be candidates for less invasive surgical approaches after chemotherapy.
Keywords: breast cancer, neoadjuvant chemotherapy, tumor subtype, breast conservation
INTRODUCTION
For patients with newly diagnosed, operable breast cancer who have an indication for systemic chemotherapy at the time of presentation based on primary tumor factors, chemotherapy is increasingly given before surgery.1-4 The rationale for neoadjuvant chemotherapy is that, in addition to allowing assessment of response to therapy, it can decrease the size or extent of the tumor prior to surgical resection, decreasing the amount of tissue that needs to be resected and potentially allowing breast-conserving surgery in patients who without such chemotherapy would have required mastectomy.5-7 Breast-conserving surgery is considered the preferred approach in early-stage breast cancer, and multiple prospective studies have shown the oncologic safety of breast-conserving surgery followed by adjuvant radiation therapy.8-13 Breast-conserving surgery after neoadjuvant chemotherapy has been shown to result in local-regional control rates similar to those in patients undergoing up-front breast surgery. 14
In addition to reducing the size of primary tumors, neoadjuvant chemotherapy can eradicate disease in the regional lymph nodes and can convert node-positive disease to nodenegative. Recent studies have shown nodal pathologic complete response (pCR) rates of approximately 40% after neoadjuvant chemotherapy, with some variation based on tumor biologic subtype.15-17 The finding of pCR in the breast and axillary lymph nodes after neoadjuvant chemotherapy has been shown in multiple studies to be associated with improved outcomes and is a surrogate marker for survival.18, 19
Breast cancer is currently classified into biological subtypes with distinct behavior, response to treatment, and outcomes. 20-22 Molecular subtyping provides the most accurate categorization but often is not available for clinical use. In clinical practice, the approximated tumor subtype derived from the status of receptors [estrogen and progesterone receptors (hormone receptors) and human epidermal growth factor receptor 2 (HER2)] is readily available and can be used to classify tumor types. Approximated biologic subtype is associated with rates of clinical and pathologic response to chemotherapy, but the impact of on surgical procedure has not been well studied.
The American College of Surgeons Oncology Group (ACOSOG) Z1071 study was a prospective clinical trial enrolling patients with node-positive breast cancer receiving neoadjuvant chemotherapy between 2009 and 2011 to evaluate the accuracy of sentinel node surgery. In the study reported here, we sought to determine the impact of tumor biology on rates of breast-conserving surgery as well as on pCR rates in the breast and axillary lymph nodes in patients enrolled on Z1071.
METHODS
ACOSOG Z1071 (clinicaltrials.gov identifier NCT00881361) was a prospective clinical trial enrolling women with histologically proven clinical T0-T4 N1-N2 M0 primary invasive breast cancer who had completed or were planning to undergo neoadjuvant chemotherapy. Patients were enrolled from July 2009 through July 2011. All patients had node-positive disease at presentation confirmed by either fine-needle aspiration biopsy or core needle biopsy of ipsilateral axillary lymph nodes. All patients received chemotherapy prior to definitive breast and axillary surgery. The current analysis includes all patients who met the protocol eligibility criteria, completed neoadjuvant chemotherapy, and underwent breast and axillary surgery. The institutional review boards of all participating institutions approved the Z1071 study, and written informed consent was obtained from each patient prior to study entry.
After completion of neoadjuvant chemotherapy, all patients underwent breast and axillary surgery, and surgical specimens were evaluated by a pathologist. The extent of residual disease in the breast and axillary lymph nodes was determined. Operative reports and pathology reports were submitted for central review. Surgical procedure(s) and pathologic response data were entered into a central database.
All patients had estrogen receptor, progesterone receptor, and HER2 status tested on the diagnostic core biopsy sample prior to neoadjuvant chemotherapy. Approximated biologic subtypes were assigned on the basis of estrogen receptor, progesterone receptor, and HER2 status, which was reported by the treating institution. HER2-positive disease was defined as disease with 3+ HER2 overexpression by immunohistochemistry or HER2 amplification by fluorescence in situ hybridization. Tumors that were negative for estrogen receptor, progesterone receptor, and HER2 were classified as triple-negative; HER2-positive tumors were classified as HER2-positive; and the remaining tumors were classified as hormone-receptorpositive, HER2-negative.
Clinical response to neoadjuvant chemotherapy was determined by comparing the largest single tumor diameter on clinical examination and imaging (mammography, ultrasonography, and magnetic resonance imaging, when performed) at baseline to the largest single tumor diameter on clinical examination and imaging after neoadjuvant chemotherapy. Response was classified according to the Response Evaluation Criteria in Solid Tumors. 23 Complete response was defined as complete or near-complete resolution of the lesion, partial response as a 30% or greater decrease in the size of the lesion, and disease progression as a 20% or greater increase in the size of the lesion; all other responses were defined as stable disease.
Breast surgery was performed within 6 weeks of completion of neoadjuvant chemotherapy. The type of breast surgery was chosen according to surgeon and patient preference and was not mandated by the study protocol. Axillary staging was performed with both sentinel lymph node surgery and completion axillary lymph node dissection. Pathologic response was calculated using the largest single dimension of the tumor in the surgical specimen. We examined 3 types of pCR: (1) pCR defined according to the most widely used definition—i.e., no residual invasive disease in the breast or axilla 19; (2) pCR in the breast regardless of axillary response; and (3) pCR in the axilla regardless of breast response.
Statistical Analysis
We evaluated the association of tumor biology, using approximated molecular subtypes, with surgery type and with pCR. Continuous patient and tumor characteristics were compared among the tumor groups with an ANOVA test or Kruskal-Wallis test, whichever was more appropriate. In the case of comparisons between 2 groups, a 2-sample t-test or rank-sum test, whichever was appropriate, was used. Categorical variables were compared among/between groups using a chi-squared test. To determine factors associated with breast-conserving surgery after neoadjuvant chemotherapy, univariable and multivariable logistic regression models were used. Point estimates and 95% CIs of the odds ratios are presented. All tests were 2-sided, and a P < 0.05 was considered statistically significant. The database used for these analyses was locked April 8, 2014. The statistical analyses were performed by the Alliance Statistics and Data Center using SAS version 9.3.
RESULTS
Patient characteristics
Seven hundred fifty-six patients with T0-T4 N1-N2 M0 breast cancer across 136 institutions were enrolled in the ACOSOG Z1071 study. Twenty one women were ineligible and 34 withdrew from the study prior to surgery. Six hundred and ninety four patients were eligible and had findings available from pathology review of breast and axillary specimens from surgery after neoadjuvant chemotherapy. These 694 patients made up the cohort for this study. Approximated tumor subtype was triple-negative in 170 (24.5%) patients, HER2-positive in 207 (29.8%) patients, and hormone-receptor-positive, HER2-negative in 317 (45.7%) patients. Baseline patient and tumor characteristics for the 694 patients by tumor subtype are shown in Table 1. Patient age, clinical T stage and tumor size at presentation, and clinical nodal stage at presentation (N1 vs N2) did not differ across the subtypes.
Table 1.
Baseline patient and tumor characteristics of 694 patients treated with neoadjuvant chemotherapy by tumor subtype.
| Characteristic | All Patients (n = 694) | Triple- Negative (n = 170) | HER2-Positive (n = 207) | Hormone- Receptor- Positive, HER2- Negative (n = 317) | P Value |
|---|---|---|---|---|---|
| Patient age, years | 0.24 | ||||
| <50 | |||||
| 50+ | 349 (50.3) | 77 (45.3) | 103 (49.8) | 169 (53.3) | |
| 345 (49.7) | 93 (54.7) | 104 (50.2) | 148 (46.7) | ||
| T category at presentation | 0.46 | ||||
| T0 | 9 (1.3) | 3 (1.8) | 4 (1.9) | 2 (0.6) | |
| Tis | 1 (0.1) | 0 | 1 (0.5) | 0 | |
| T1 | 90 (13.0) | 24 (14.1) | 23 (11.1) | 43 (13.6) | |
| T2 | 383 (55.3) | 100 (58.8) | 108 (52.2) | 175 (55.4) | |
| T3 | 178 (25.7) | 37 (21.8) | 61 (29.5) | 80 (25.3) | |
| T4 | 32 (4.6) | 6 (3.5) | 10 (4.8) | 16 (5.1) | |
| Clinical tumor size at baseline, median (range), cm | 4.0 (0.8-15.1) | 4.0 (0.8-15.0) | 4.5 (0.8-15.1) | 4.0 (0.9-15.0) | 0.077 |
| Clinical tumor size at baseline, cm | 3 (0.4) | 1 (0.3) | 0.44 | ||
| 37 (5.5) | 1 (0.6) | 1 (0.5) | 17 (5.4) | ||
| <1.0 | 111 (16.3) | 11(6.7) | 9 (4.4) | 50 (16.0) | |
| 1.0-1.9 | 126 (18.5) | 33 (20.1) | 28 (13.7) | 63 (20.1) | |
| 2.0-2.9 | 132 (19.4) | 24 (14.6) | 39 (19.1) | 60 (19.2) | |
| 3.0-3.9 | 272 (39.9) | 38 (23.2) | 34 (16.7) | 122 (39.0) | |
| 4.0-4.9 | 13 | 57 (34.8) | 93 (45.6) | 4 | |
| ≥ 5.0 | 6 | 3 | |||
| Unknown | |||||
| Nodal category at presentation | |||||
| N1 | 658 (94.8) | 159 (93.5) | 196 (94.7) | 303 (95.6) | |
| N2 | 36 (5.2) | 11 (6.5) | 11 (5.3) | 14 (4.4) | |
| Chemotherapy received | <0.0001 | ||||
| Anthracycline + taxane | 520 (74.9) | 135 (79.4) | 117 (56.5) | 268 (84.5) | |
| Anthracycline, no taxane | 43 (6.2) | 11 (6.5) | 11 (5.3) | 21 (6.6) | |
| Taxane, no anthracycline | 119 (17.2) | 18 (10.6) | 76 (36.7) | 25 (7.9) | |
| Other | 12 (1.7) | 6 (3.5) | 3 (1.4) | 2 (1.0) |
For the study group overall, the median tumor diameter was 4.0 cm (range, 0.8-15.0 cm). The majority of patients had T2 tumors (55%) and 59% of patients had tumors 4 cm or larger in diameter. Chemotherapy regimens included both an anthracycline and a taxane in 520 patients (74.9%), a taxane but not an anthracycline in 119 patients (17.2%), an anthracycline but not a taxane in 43 patients (6.2%), and other regimens in 12 patients (1.7%). Of the 207 patients with HER2-positive disease, 184 (88.9%) received trastuzumab with their neoadjuvant chemotherapy.
Breast surgery
Overall, 60.0% of patients underwent mastectomy, and 40.0% underwent breastconserving surgery (Table 2). The rates of breast-conserving surgery were higher in patients with triple-negative and HER2-positive breast cancer (46.8% and 43.0%, respectively) than in patients with hormone-receptor-positive, HER2-negative disease (34.5%; P = 0.019) (Table 2, Figure 1).
Table 2.
Breast surgical procedure and clinical and pathologic response to neoadjuvant chemotherapy by tumor subtype
| Characteristic | All Patients (n = 694) | Triple- Negative (n = 170) | HER2-Positive (n = 207) | Hormone- Receptor- Positive, HER2- Negative (n = 317) | P Value |
|---|---|---|---|---|---|
| Clinical tumor size after chemotherapy, median (range), cm | 1.4 (0-15.8) | 1.2 (0-11.0) | 1.2 (0-12.5) | 1.5 (0-15.8) | 0.10 |
| Clinical response | 0.48 | ||||
| Complete response | 166 (27.4) | 36 (24.0) | 58 (33.3) | 72 (25.4) | |
| Partial response | 324 (53.4) | 83 (55.3) | 89 (51.2) | 152 (53.7) | |
| Stable disease | 79 (13.0) | 20 (13.3) | 19 (10.9) | 40 (14.1) | |
| Disease progression | 38 (6.3) | 11 (7.3) | 8 (4.6) | 19 (6.7) | |
| Unknown | 87 | 20 | 33 | 34 | |
| Pathologic response, breast tumor | <0.0001 | ||||
| Complete response | 233 (33.8) | 81 (47.9) | 103 (50.2) | 49 (15.5) | |
| Partial response | 318 (46.1) | 60 (35.5) | 80 (39.0) | 178 (56.3) | |
| Stable disease | 96 (13.9) | 22 (13.0) | 15 (7.3) | 59 (18.7) | |
| Disease progression | 43 (6.2) | 6 (3.6) | 7 (3.4) | 30 (9.5) | |
| Unknown | 4 | 1 | 2 | 1 | |
| Pathologic tumor size in cases with residual disease, median (range), cm | 1.8 (0.1-22.5) | 1.8 (0.1-9.0) | 1.7 (0.1-9.0) | 2.0 (0.1-22.5) | 0.026 |
| Pathologic N category | <0.0001 | ||||
| N0 | 285 (41.1) | 84 (49.4) | 134 (64.7) | 67 (21.1) | |
| N1 | 241 (34.7) | 55 (32.4) | 53 (25.6) | 133 (43.0) | |
| N2 | 129 (18.6) | 26 (15.3) | 16 (7.7) | 87 (27.4) | |
| N3 | 39 (5.6) | 5 (2.9) | 4 (1.9) | 30 (9.5) | |
| Unknown | 0 | 0 | 0 | 0 | |
| Largest lymph node metastasis in cases with residual positive nodes, median (range), cm | 0.9 (0.01-8.0) | 1.0 (0.01-4.5) | 0.7 (0.04-4.0) | 0.9 (0.01-8.0) | 0.056 |
| Number of breast operations | 0.039 | ||||
| 1 | 643 (92.7) | 164 (96.5) | 193 (93.2) | 286 (90.2) | |
| 2 or more | 51 (7.3) | 6 (3.5) | 14 (6.8) | 31 (9.8) | |
| Final breast surgery | 0.019 | ||||
| Breast-conserving surgery | 277 (40.0) | 79 (46.8) | 89 (43.0) | 109 (34.5) | |
| Mastectomy | 415 (60.0) | 90 (53.2) | 118 (57.0) | 207 (65.5) | |
| pCR (breast and axilla) | 195 (28.1) | 65 (38.2) | 94 (45.4) | 36 (11.4) | <0.0001 |
| pCR in breast only | 233 (33.6) | 81 (47.6) | 103 (49.8) | 49 (15.5) | <0.0001 |
| pCR in axilla only | 285 (41.1) | 84 (49.4) | 134 (64.7) | 67 (21.1) | <0.0001 |
Figure 1.
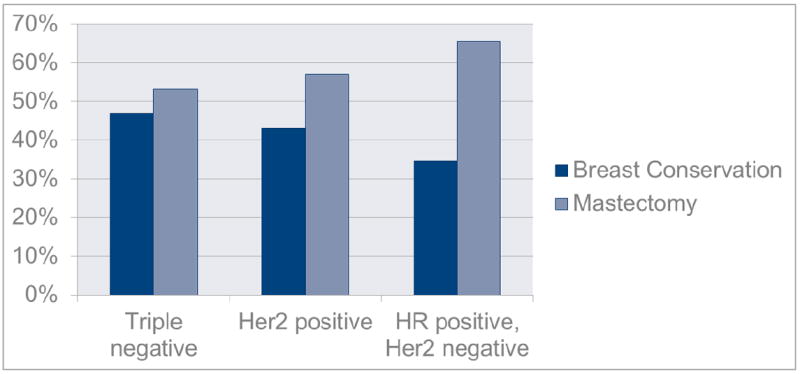
Breast surgery by approximated biologic subtype
The overall reoperation rate was 7.3%. The reoperation rate was significantly higher in patients with hormone-receptor-positive, HER2-negative disease (9.8%) than in patients with triple-negative or HER2-positive disease (3.5% and 6.8%, respectively; P = 0.039). Of the 51 patients who required more than 1 breast operation, 31 patients (60.8%) underwent re-excision of the lumpectomy cavity and completed breast conservation, 14 patients (27.5%) converted from breast-conserving surgery to mastectomy, and 6 patients (11.8%) had re-excision of a margin after initial mastectomy. Within the group of patients with hormone-receptor-positive, HER2- negative disease, the requirement for more than 1 breast operation was significantly higher in lobular tumors than ductal tumors (25% versus 9%, p=0.044).
Breast-conserving surgery was more common in patients who presented with T0-T2 tumors than in those who presented with T3-T4 tumors (Table 3). However, 52% of patients who presented with clinical T0-T2 disease underwent mastectomy. Of the patients who presented with clinical T3-T4 disease, 23% underwent breast-conserving surgery. Reoperation after initial breast-conserving surgery was more common in patients with T2/T3 tumors than in those with T0/T1 tumors.
Table 3.
Breast surgery by clinical T category at presentation
| T0/T1 (n = 100) |
T2 (n = 383) |
T3 (n = 178) |
T4 (n = 32) |
P Value | |
|---|---|---|---|---|---|
| Surgery | <0.0001 | ||||
| BCS | 41 (41.8) | 188 (49.1) | 44 (24.7) | 4 (12.5) | |
| Mastectomy | 57 (58.2) | 195 (50.9) | 134 (75.3) | 28 (87.5) | |
| Surgery | <0.0001 | ||||
| BCS | 41 (41.8) | 188 (49.1) | 44 (24.7) | 4 (12.5) | |
| BCS converted to mastectomy | 1 (1.0) | 7 (1.8) | 6 (3.4) | 0 | |
| Mastectomy | 56 (57.1) | 188 (49.1) | 128 (71.9) | 28 (87.5) | |
| Number of surgeries (all patients) | 0.040 | ||||
| 1 | 98 (98.0) | 350 (91.4) | 162 (91.0) | 32 (100.0) | |
| 2 or more | 2 (2.0) | 33 (8.6) | 16 (9.0) | 0 | |
| Number of surgeries (patients with BCS as first surgery) | 0.038 | ||||
| 1 | |||||
| 2 or more | 40 (95.2) | 165 (84.6) | 37 (74.0) | 4 (100.0) | |
| 2 (4.8) | 30 (15.4) | 13 (26.0) | 0 | ||
| pCR in breast only | 41 (41.0) | 138 (36.0) | 52 (29.2) | 2 (6.3) | 0.001 |
| pCR in axilla only | 44 (44.0) | 157 (41.0) | 76 (42.7) | 8 (25.0) | 0.27 |
BCS, breast-conserving surgery.
Predictors of successful breast conservation
The univariable and multivariable evaluation of factors associated with rates of successful breast conservation included patient age, clinical T stage at presentation, and tumor subtype (Table 4). On univariable analysis, variables associated with a higher likelihood of breast conservation were older age, lower T category at presentation, and HER2-positive and triple-negative tumor subtypes.
Table 4.
Univariable and multivariable models for breast-conserving surgery
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age | 1.02 | 1.01-1.04 | 0.002 | 1.02 | 1.01-1.04 | 0.001 |
| Baseline clinical T category | <0.0001 | <0.0001 | ||||
| T0/T1 | --- | --- | --- | --- | ||
| T2 | 1.34 | 0.86-2.10 | 1.30 | 0.82-2.06 | ||
| T3 | 0.46 | 0.27-0.77 | 0.43 | 0.25-0.74 | ||
| T4 | 0.20 | 0.06-0.61 | 0.17 | 0.06-0.54 | ||
| Baseline clinical N category | 0.22 | 0.62 | ||||
| N1 | --- | --- | --- | --- | ||
| N2 | 0.63 | 0.31-1.31 | 0.82 | 0.37-1.79 | ||
| Tumor subtype | 0.02 | 0.04 | ||||
| Hormone-receptor-positive, | --- | --- | ||||
| HER2-negative | --- | --- | ||||
| HER2-positive | 1.43 | 0.99-2.05 | 1.48 | 1.02-2.16 | ||
| Triple-negative | 1.67 | 1.14-2.44 | 1.54 | 1.03-2.29 | ||
On multivariable analysis, older age, lower tumor stage at presentation, and HER2- positive and triple-negative tumor subtypes (P = 0.04) remained significant predictors of breast conservation (Table 4).
Clinical breast tumor response to neoadjuvant chemotherapy
Clinical assessment of changes in tumor size in the breast between baseline and completion of neoadjuvant chemotherapy showed that 166 patients (27.4%) had a complete response, 329 (54.2%) had a partial response, 79 (13.0%) had stable disease, and 33 (5.4%) had disease progression. These clinical response rates appeared to be similar across all tumor subtypes (P = 0.42) (Table 2). Clinical tumor size after neoadjuvant chemotherapy was also similar across all tumor subtypes (P = 0.12) (Table 2).
Pathologic breast tumor response to neoadjuvant chemotherapy
Overall, pathologic response rates in the breast were as follows: complete response, 33.8%; partial response, 46.1%; stable disease, 13.9%; and disease progression, 6.2% (Table 2). The breast pCR rates were significantly higher in patients with triple-negative tumors and HER2- positive tumors (47.9% and 50.2%, respectively) than in patients with hormone-receptorpositive, HER2-negative tumors (15.5%; P < 0.0001). Additionally, among patients with residual disease, pathologic tumor size after neoadjuvant chemotherapy was larger in patients with hormone-receptor-positive, HER2-negative disease than in patients with the other tumor subtypes (P= 0.026).
Pathologic nodal response to neoadjuvant chemotherapy
All patients enrolled in ACOSOG Z1071 had biopsy-proven nodal disease at presentation; 94.8% of patients had clinical N1 and 5.2% of patients had clinical N2 disease. After completion of neoadjuvant chemotherapy, the nodal pCR rate was 41.1% (Table 2). The nodal pCR rate was significantly higher in patients with triple-negative and HER2-positive disease (49.4% and 64.7%, respectively) than in those with hormone-receptor-positive, HER2- negative disease (21.1%; P < 0.0001). Overall, the burden of disease in the nodes after neoadjuvant chemotherapy was higher in patients with hormone-receptor-positive, HER2-negative disease (Figure 2). Among patients with residual nodal disease, the mean number of positive nodes was higher in patients with hormone-receptor-positive, HER2-negative disease (5.0 nodes) than in those with triple-negative and HER2-positive disease (3.5 nodes and 3.3 nodes, respectively; P = 0.001). Although the size of the largest residual lymph node metastasis in patients with residual positive nodes differed across tumor subgroups (Table 2), this difference did not achieve statistical significance (P = 0.06).
Figure 2.
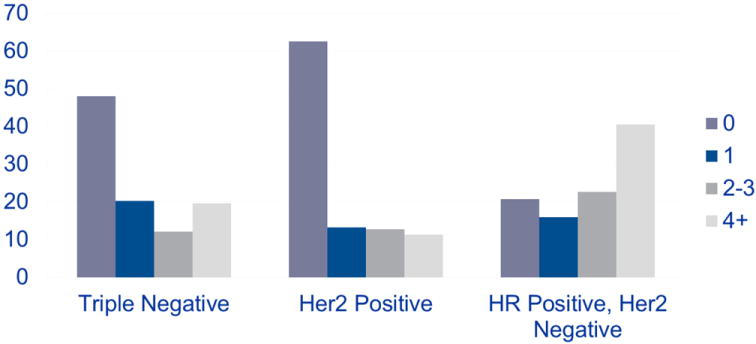
Pathologic nodal disease burden by approximated biologic subtype
Rates of pCR in the breast and axilla
Overall, the rate of pCR in both the breast and axilla was 28%. The rate of pCR in the breast and axilla was significantly higher in patients with triple-negative disease and HER2- positive disease (38.2% and 45.4%, respectively) than in patients with hormone-receptorpositive, HER2-negative disease (11.4%; P < 0.0001).
DISCUSSION
Determination and stratification of breast cancer by molecular subtypes has improved understanding and treatment of the disease. The subtype influences the choice of chemotherapeutic agent, response to chemotherapy and risk of recurrence. In this retrospective review of data from a prospective multicenter study of 694 women treated with neoadjuvant chemotherapy, we found that approximated tumor subtype was associated with type of surgical procedure after neoadjuvant chemotherapy as well as with the pathologic response in the breast and regional nodes. Specifically, patients with triple-negative or HER2-positive breast cancer were more likely to undergo breast-conserving surgery and more likely to achieve a pCR.
The response rates to neoadjuvant chemotherapy in this study are in keeping with those in prior studies, which have shown that pCR rates in the breast and lymph nodes are higher in patients with triple-negative or HER2-positive disease. For triple-negative tumors, the reported rates of pCR in the breast and axilla include 35.8% in the German Breast Group pooled analysis, 35% in the ISPY1 trial, and 38% in a report from The University of Texas MD Anderson Cancer Center, similar to the rate of 38% seen in this study. 15, 24, 25 Additionally, all patients on Z1071 had node positive disease, whereas the other studies mentioned had a mixture of clinically node negative and node positive disease at presentation which accounts for some of the differences in pCR rates reported here.
The rate of pCR in the breast and axilla for patients with HER2-positive tumors in the current study was 45%. This is similar to the 48.3% and 46.7% rates of breast and axillary pCR seen in the ACOSOG Z1041 study.26 For HER2-positive tumors, the German pooled analysis reported pCR rates of 32% in luminal B/HER2-positive and 51% in non-luminal HER2-positive disease treated with trastuzumab.15 The ISPY1 investigators reported a pCR rate of 54% in patients with HER2-positive disease; however, this was based on only 13 patients.24 The fact that not all patients with HER2-positive tumors in the Z1071 trial received trastuzumab with their chemotherapy may account for some of the differences between our study and prior studies regarding pCR rates in patients with HER2-positive disease.
In the current study, we found a breast conservation rate of 43% in women with HER2-positive breast cancer, which is higher than rates in the NOAH study, in which 23% of patients with HER2-positive disease who were treated with neoadjuvant trastuzumab from 2002 to 2005 completed breast-conserving surgery, compared to 13% of those who did not receive trastuzumab.27 Our rate of 43% is similar to the 38% rate of breast-conserving surgery in HER2-positive patients in the Z1041 trial, in which surgery type was also dictated by surgeon and patient preference.26
We found that the pCR rate in patients with hormone-receptor-positive, HER2-negative disease was only 11%, which is similar to the previously reported pCR rates of 9% in ISPY124 and of 8.9% in luminal A and 15.4% in luminal B/HER2-negative disease in the German studies. 15 In a series from MD Anderson Cancer Center, the pCR rate in patients with hormonereceptor-positive, HER2-negative disease was only 9%. These patients had excellent local recurrence-free survival regardless of tumor response to neoadjuvant chemotherapy, reflecting the overall favorable biology of hormone-receptor-positive disease and the effectiveness of endocrine therapy. 25 The German pooled analysis showed that pCR correlates with survival in estrogen-receptor-negative, HER2-positive disease and triple-negative disease but not in luminal tumors.15 Although lower rates of pCR to neoadjuvant chemotherapy in patients with hormonereceptor-positive, HER2-negative tumors have been previously reported, the current study is one of the first to show lower rates of breast-conserving surgery in these patients. The ACOSOG Z1031 trial prospectively evaluated patients with estrogen-receptor-positive disease and demonstrated that neoadjuvant endocrine therapy could decrease tumor size, such that 65% of patients thought to be marginal candidates for breast conservation and 38% of patients though to be ineligible for breast conservation were able to preserve their breast.28 For patients with luminal A tumors, neoadjuvant endocrine therapy may be more likely to provide reduction in tumor size and successful breast conservation than is neoadjuvant chemotherapy.
Because our study was a retrospective analysis of data from a prospective clinical trial, we do not know how many patients were not candidates for breast-conserving surgery at initial presentation but were converted to breast conservation as a result of neoadjuvant chemotherapy. However, in this study, 23% of women who presented with T3-T4 tumors had breast conservation, and it is likely that neoadjuvant chemotherapy altered their surgical options as it would be anticipated that most women with T3-T4 tumors (tumors larger than 5 cm) would require mastectomy. This is similar to the National Surgical Adjuvant Breast and Bowel Project B-18 study in which for patients with tumors ≥ 5cm lumpectomy was proposed at presentation in 3%, but performed in 22% after neoadjuvant chemotherapy. 29
Additionally, because of the retrospective nature of our study, we do not know how many women were potential candidates for breast conservation after neoadjuvant chemotherapy but elected mastectomy. However, 52% of patients with clinical T0-T1 disease at presentation elected mastectomy after chemotherapy, and as rates of disease progression were very low, this finding suggests that overall, significantly more women were candidates for breast conservation than elected breast conservation.
Another limitation of our study is the use of approximated tumor subtype and inability to separate the hormone-receptor-positive, HER2-negative tumors into luminal A and luminal B as we did not perform gene profiling. Furthermore, because of the small sample size, we did not separate the HER2-positive tumors by hormone receptor status.
This is one of a few studies to evaluate the impact of tumor subtype on surgical procedure. This information is important when setting patient expectations at the initiation of chemotherapy and can guide patient education as well as the selection of surgical procedure after completion of chemotherapy. Our findings indicate that women with HER2-positive disease or triple-negative disease have a high likelihood of pCR and are likely to successfully complete breast-conserving surgery. However, women with hormone-receptor-positive, HER2-negative disease are significantly less likely to achieve a pCR and in this study had a higher rate of mastectomy. This may be explained by the higher proportion of lobular tumors in the hormone receptor positive, HER2 negative group, as the lobular tumors in this group had higher rates of second breast procedures compared with the ductal tumors in the same subgroup.
The differences in breast surgery observed in this study reflect the surgical choice of patients and their treating surgeons. Almost half the patients that achieved a pathological complete response elected to undergo mastectomy. Perhaps this identifies an opportunity to improve patient counselling and surgeon education regarding the option of breast conservation surgery after neoadjuvant chemotherapy. Although pathologic complete response rates did vary by tumor type, pCR is not a prerequisite for breast conservation and clinical tumor sizes were not different across the approximated tumor type groups. We are not recommending that patients with HR+Her2- disease are discouraged from breast conservation when clinical response is appropriate for breast conservation.
In addition to decreasing the size of the primary tumor, neoadjuvant chemotherapy is effective in reducing the burden of disease in the regional lymph nodes. Our findings show that the likelihood of conversion from node-positive to node-negative disease is higher in women with HER2-positive or triple-negative breast cancer than in those with hormone-receptorpositive, HER2-negative disease and that the overall residual nodal burden is higher in hormonereceptor- positive, HER2-negative disease. Neoadjuvant chemotherapy provides opportunities to minimize the extent of surgery both to the breast and the axilla. With conversion of node-positive disease to node-negative, axillary staging with sentinel lymph node surgery allows patients who have converted to node-negative to potentially avoid axillary lymph node dissection.30
In conclusion, patients with triple-negative or HER2-positive breast cancer achieve the highest rates of breast-conserving surgery and pCR after neoadjuvant chemotherapy (when trastuzumab is included for HER2-positive tumors). Patients with these tumor subtypes are more likely to be candidates for less invasive surgical approaches following neoadjuvant chemotherapy.
Acknowledgments
We thank the patients with breast cancer who participated in the study and their caregivers, and we thank the investigators and their research teams who participated in the ACOSOG Z1071 study. We thank Amy Oeltjen (Mayo Clinic) for her work with data quality. We thank the ACOSOG and Alliance for Clinical Trials in Oncology staff. We thank Stephanie Deming, BA (MD Anderson Cancer Center), for her assistance with critical editing of the manuscript. These contributors did not receive compensation besides their salaries.
Source Funding:
ACOSOG Z1071 (Alliance) was supported, in part, by grants from the National Cancer Institute to ACOSOG (CA076001), to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair, CA31946), and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. Dr. Boughey reports receiving travel accommodations from the Alliance for Clinical Trials in Oncology Foundation. Dr. Mittendorf reports receiving grant funding from the National Institutes of Health (NIH) and having contracts with Galena BioPharma and the Henry M. Jackson Foundation. Dr. Wilke reports receiving grant funding from NIH, the Wisconsin Partnership Program, and receiving travel accommodations from Alliance. Dr. Hunt reports receiving grant funding from the Susan G. Komen Foundation, royalties from Springer for an MD Anderson Cancer Care series book on breast cancer, and travel accommodations from the Alliance for Clinical Trials in Oncology Foundation.
Footnotes
Conflict of Interest Disclosures:
No other disclosures were reported.
References
- 1.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Powles TJ, Hickish TF, Makris A, et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J Clin Oncl. 1995;13:547–552. doi: 10.1200/JCO.1995.13.3.547. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham JD, Weiss SE, Ahmed S, et al. The efficacy of neoadjuvant chemotherapy compared to postoperative therapy in the treatment of locally advanced breast cancer. Cancer Invest. 1998;16:80–86. doi: 10.3109/07357909809039761. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 5.Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22:2303–2312. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 6.Cance WG, Carey LA, Calvo BF, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg. 2002;236:295–302. doi: 10.1097/01.SLA.0000027526.67560.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93–100. doi: 10.1200/JCO.1998.16.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Arriagada R, Le MG, Rochard F, et al. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558–1564. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 9.Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr. 1992;11:19–25. [PubMed] [Google Scholar]
- 10.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 11.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98:697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 12.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 13.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1132. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 14.Mittendorf EA, Buchholz TA, Tucker SL, et al. Impact of chemotherapy sequencing on local-regional failure risk in breast cancer patients undergoing breast-conserving therapy. Ann Surg. 2013;257:173–179. doi: 10.1097/SLA.0b013e3182805c4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 16.Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–9311. doi: 10.1200/JCO.2005.02.5023. [DOI] [PubMed] [Google Scholar]
- 17.Dominici LS, Negron Gonzalez VM, Buzdar AU, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116:2884–2889. doi: 10.1002/cncr.25152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuerer HM, Newman LA, Buzdar AU, et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg. 1998;176:502–509. doi: 10.1016/s0002-9610(98)00253-0. [DOI] [PubMed] [Google Scholar]
- 19.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014 Feb 13; doi: 10.1016/S0140-6736(13)62422-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyers MO, Klauber-Demore N, Ollila DW, et al. Impact of breast cancer molecular subtypes on locoregional recurrence in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 2011;18:2851–2857. doi: 10.1245/s10434-011-1665-8. [DOI] [PubMed] [Google Scholar]
- 22.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2012;132:1049–1062. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caudle AS, Yu TK, Tucker SL, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14:R83. doi: 10.1186/bcr3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buzdar AU, Suman VJ, Meric-Bernstam F, et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1317–1325. doi: 10.1016/S1470-2045(13)70502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semiglazov V, Eiermann W, Zambetti M, et al. Surgery following neoadjuvant therapy in patients with HER2-positive locally advanced or inflammatory breast cancer participating in the NeOAdjuvant Herceptin (NOAH) study. Eur J Surg Oncol. 2011;37:856–863. doi: 10.1016/j.ejso.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Olson JA, Jr, Budd GT, Carey LA, et al. Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: results from a multicenter phase II trial. J Am Coll Surg. 2009;208:906–914. doi: 10.1016/j.jamcollsurg.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 30.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]


