Abstract
Despite apparent method similarities between laboratories there appear to be confounding factors inhibiting uniform reporting and standardisation of vitamin assays. The Australasian Association of Clinical Biochemists (AACB) Vitamins Working Party, in conjunction with The Royal College of Pathologists of Australasia Quality Assurance Programs, has formulated a guideline to improve performance, reproducibility and accuracy of fat-soluble vitamin results. The aim of the guideline is to identify critical pre-analytical, analytical and post-analytical components of the analysis of vitamins A, E and carotenoids in blood to promote best practice and harmonisation. This best practice guideline has been developed with reference to the Centers for Disease Control and Prevention (CDC) “Laboratory Medicine Best Practices: Developing an Evidence-Based Review and Evaluation Process”. The CDC document cites an evaluation framework for generating best practice recommendations that are specific to laboratory medicine. These 50 recommendations proposed herein, were generated from a comprehensive literature search and the extensive combined experience of the AACB Vitamins Working Party members. They were formulated based on comparison between an impact assessment rating and strength of evidence and were classified as either: (1) strongly recommend, (2) recommend, (3) no recommendation for or against, or (4) recommend against. These best practice recommendations represent the consensus views, in association with peer reviewed evidence of the AACB Vitamins Working Party, towards best practice for the collection, analysis and interpretation of vitamins A, E and carotenoids in blood.
Introduction
In the early 1980s there was a flurry of papers attesting to the suitability of high performance liquid chromatography (HPLC) for the measurement of fat-soluble vitamins and carotenoids in serum and food. With these improved assays came an enormous increase of interest in the multiple facets of their biochemical and nutritional importance and a heightened awareness of their relationship to chronic diseases and overall population health.
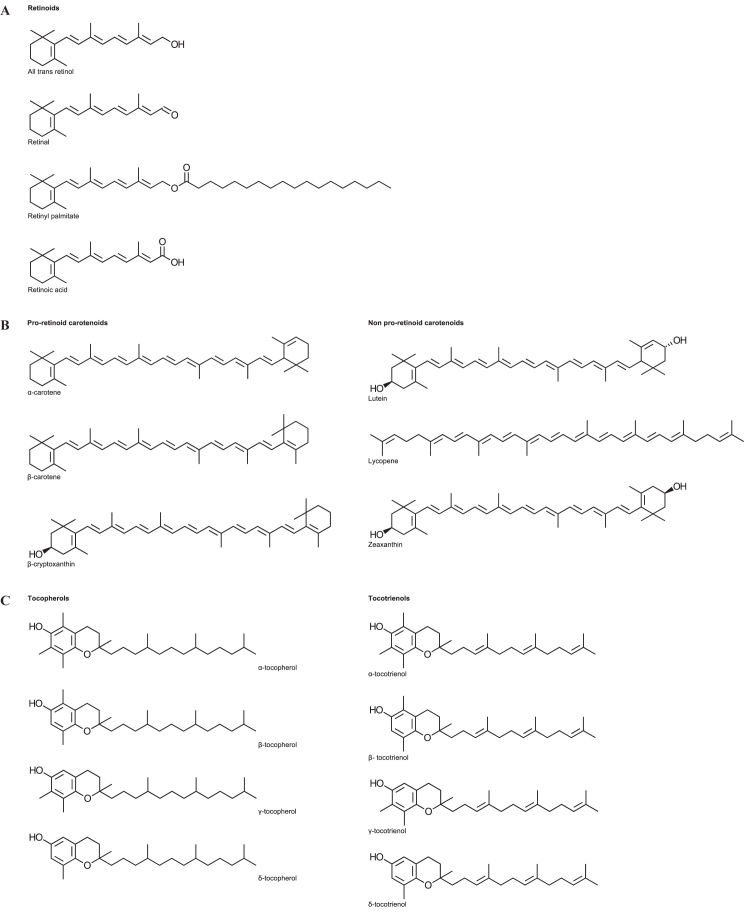
The four fat-soluble vitamins (A, D, E and K) themselves are chemically and biochemically distinct and seldom share common biological dynamics. They have different absorption, metabolism, distribution and clearance but are well characterised compounds with known structures and some common properties which facilitate their measurement (Figure 1). Of these, blood vitamin A and E are usually measured together in the same assay - which may also include the carotenoids - whereas vitamins D and K have levels in blood that are too low to be conveniently measured by the same techniques.
Figure 1.
Chemical structure of vitamin A, E and the carotenoids and analogues. (A) Chemical structure of vitamin A and related compounds. (B) Chemical structures of the common serum carotenoids. (C) Chemical structures of vitamin E and analogues.
Even though vitamin A and E are not biochemically connected, it is expedient to measure them together as their levels may both be compromised in diseases of fat malabsorption such as cystic fibrosis, inflammatory bowel disease, coeliac disease, tropical sprue, and short bowel syndrome.1 In addition, in countries with a low Gross Domestic Product, malnutrition is a common cause of fat-soluble vitamin deficiency, especially vitamin A.2 Currently there is also interest in possible anti-cancer properties of vitamin A, E and the carotenoids.3–5 Overall, the primary reason for their measurement is to avoid the consequences of deficiency or to confirm a disease state (Table 1).
Table 1.
Summary of common conditions associated with a clinical indication for measurement in blood i.e. serum / plasma.
| Deficiency | Excess | |
|---|---|---|
| Vitamin A (retinol) | Cystic fibrosis | Acute ingestion |
| Malabsorption* | Chronic ingestion | |
| Malnutrition | ||
| Pancreatic insufficiency | ||
| Carotenoids (β-carotene) | Not generally measured | Differential diagnosis of change to colouring of skin – not toxic |
| Vitamin E (α-tocopherol) | Cystic fibrosis | Generally non-toxic |
| Malabsorption* | ||
| Malnutrition† | ||
| Pancreatic insufficiency |
Malabsorption of fat may be present in obstructive liver disease (e.g. biliary atresia), and in pancreatic dysfunction (e.g. cystic fibrosis). Impaired absorption of fat may also accompany a variety of intestinal diseases such as coeliac disease, and may be a problem following surgical removal of a large part of the intestine.
The prevalence of vitamin E deficiency due to malnutrition is significantly less than for vitamin A, as vitamin E is found in a broad range of dietary sources.1
Clinically, to predict deficient from adequate or excessive vitamin intake, it is essential that the analytical method employed to measure these vitamins in blood is robust and fit for purpose. As such, accredited clinical diagnostic laboratories measuring vitamins A, E and the carotenoids routinely participate in an external quality assurance program to ascertain their performance compared to their peers. Investigations relating to these vitamins in The Royal College of Pathologists of Australasia Quality Assurance Programs (RCPAQAP) have highlighted the need for harmonisation of the analytical methods to improve consistency of results.6
Harmonisation of methods is an integral part of the pursuit of excellence in clinical biochemistry through continuous improvement in laboratory practice and the interpretation of results.7,8 As part of this endeavour, the Australasian Association of Clinical Biochemists (AACB) Vitamins Working Party in association with the RCPAQAP has directed efforts over the past decade towards the improvement of analytical methods for analysis of these vitamins.9,10 The aim of this document is to identify the critical pre-analytical, analytical and post-analytical components of the analysis of vitamins A, E and carotenoids in order to promote best practice and achieve harmonisation of results.
Vitamin A
Biochemistry
The term vitamin A (or retinoids) is a descriptor for any compound that exhibits vitamin A activity in biological systems.11 The terminology is fairly loose, particularly in a nutritional context where multiple forms may co-exist and contribute to total vitamin A intake. In the clinical environment the name ‘vitamin A’ is routinely used. Whilst the term retinoids is preferred, vitamin A is the general terminology used in clinical diagnostic laboratories. Hence for the purpose of this discussion the term ‘retinoids’ may be considered synonymous with ‘vitamin A’.
Vitamin A exists in humans in several forms and is tightly controlled (Table 2). Naturally occurring forms of vitamin A include retinol, retinol esters, retinal and retinoic acid. The alcohol form, retinol, predominates in the circulation but it is too toxic for storage. Instead, the liver stores retinol as retinyl esters - principally palmitate. The active form of vitamin A in the visual cycle is the aldehyde form, retinal. Retinoic acid is the form in tissues responsible for the biological actions of vitamin A in cellular division and differentiation.12 The most important measurand for the estimation of vitamin A status is circulating vitamin A as retinol.
Table 2.
Forms and function of vitamin A.
| Vitamin A form | Function |
|---|---|
| Retinyl esters | Storage in liver |
| All-trans retinol | Transport in serum bound to retinol binding protein (RBP) |
| Retinal | Visual cycle uniquely in retina |
| All-trans retinoic acid | Ligand for nuclear retinoic acid receptors (RAR) |
| 9-cis-retinoic acid | Putative ligand for retinoid X receptor (RXR) |
Retinoids are easily recognised in that they all have a cyclohexene ring attached to a tetra-isoprene chain (Figure 1A). Retinol has a β-ionone ring attached at one end of an alltrans unsaturated nonene chain with two methyl groups and a hydroxyl group at the opposite end. The heavy degree of unsaturation and the cis-trans isomerism dictate the properties of retinol in terms of its instability and analysis. The most predominant and bioactive form is the all-trans isomer. Conversion to cis isomers reduces the biopotency.13 Retinol is usually measured chromatographically as total retinol, which incorporates all trans retinol and cis retinol.
Status
The major intention in the measurement of vitamin A in clinical practice is to gauge whether there is sufficient abundance in the body to supply metabolic needs and prevent vitamin A related diseases. Conceptually, we are interested in vitamin A status rather than the actual levels. Vitamin A status is a paramount clinical issue because if there is insufficient total body vitamin A then the patient will exhibit vitamin A deficiency which has serious health consequences.
Serum retinol levels do not accurately reflect liver retinyl ester levels. Despite this limitation, serum retinol is still useful because the levels will diminish once the supply from the liver is diminished. The serum retinol level at which vitamin A deficiency occurs will coincide with the manifestation of night blindness, due to the interruption of the visual cycle by lack of retinal. Other more serious symptoms will occur later when retinoic acid is depleted by even less available hepatic retinyl esters.14
Supplementation of vitamin A is relatively common in specific populations. However, this may be through the purchase of over the counter vitamin products, fat-soluble vitamin supplementation coupled with pancreatic enzyme replacement or through World Health Organization (WHO) maternal and child supplementation programs in developing countries.15 It is important to note that the beneficial range of vitamin A exists within a narrow window and toxicity can be associated with both acute and chronic supplementation of vitamin A. Acute toxicity is readily assessed with the measurement of vitamin A levels in serum. Serum vitamin A measurement lacks sensitivity for the assessment of chronic toxicity because the vitamin A has increased overtime and is reflected in tissue stores rather than in serum. Nevertheless, it may still be important to monitor vitamin supplementation in association with clinical signs to prevent the effects of vitamin A toxicity.16 In practice analysis of serum vitamin A is infrequently requested to assess toxicity.
Carotenoids
Biochemistry
The term carotenoids is generally used to refer to vitamin A precursors that have no vitamin A activity themselves.17 In the diet carotenoids are sourced from plants with common examples; β-carotene being the main form in pumpkin and lycopene being the main form in tomatoes. Some carotenoids are metabolised to retinoids whilst others are not. The three most common dietary pro-retinoid carotenoids in the human diet that are present in serum are α-carotene, β-cryptoxanthin and β-carotene. Each of these has at least one β-ionone ring. The three most common non-pro-retinoid carotenoids include lutein, zeaxanthin, and lycopene; each of these are not metabolised to retinol and have no vitamin A activity but may have protective antioxidant roles (Figure 1B). Overall, β-carotene is the most abundant carotenoid in the diet and serum.1
β-carotene is converted to retinal by symmetrical cleavage of the central chain by β-carotene 15,15’-monooxygenase. Effectively β-carotene is cut in half to form two retinal molecules; with the addition of a water molecule at the noncyclical end (Figure 2). The extent and efficiency of this conversion varies between individuals and is under the control of genetic polymorphisms.18 As there is little clinical evidence to the additional value of total carotenoid measurement compared with β-carotene analysis, subsequent discussion is confined to β-carotene measurement.
Figure 2.
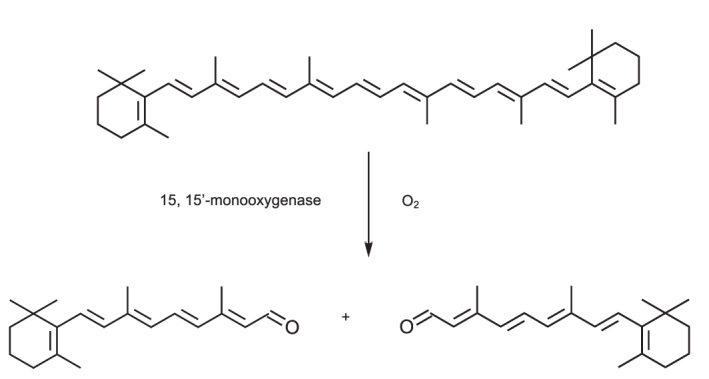
Structure of β-carotene and its conversion to retinal.
Status
The enzymatic conversion of β-carotene to retinal is rate limiting. Hence high levels of β-carotene do not confer high levels of vitamin A and are not toxic. Clinically, β-carotene is requested as part of the differential diagnosis in a patient presenting with a yellow/orange tinge. A common case example cited relates to infants who have begun solid foods such as pumpkin and have developed this tinge. β-carotene along with liver function tests may be requested to confirm the cause of this tinge is not insidious. In fact, the presence of high concentrations of carotenoids in the macula may protect against light-induced oxidative damage, which is thought to play a role in the pathology of age-related macular degeneration.19 In addition, β-carotene is also used to treat erythropoietic protoporphyria as a protection against sun sensitivity.
For those laboratories looking to assess vitamin A status to explain a possible deficiency symptom serum retinol will suffice. The measurement of β-carotene (and the other carotenoids) is an extension of this to estimate both current and prospective vitamin A status from suspected poor diet or malabsorptive state. Patients with demonstrable pro-retinoid carotenoids seldom have low retinol levels and usually preclude impending vitamin A deficiency because the primary source of dietary vitamin A is present. β-carotene can be a useful adjunct given the limitations of retinol itself but should never be measured in isolation for assessment of vitamin A status.20
In summary, individual laboratories can choose whether or not to measure total serum pro-retinoid carotenoids i.e. β-carotene, simultaneously with serum retinol or β-carotene individually.
Vitamin E
Biochemistry
The term vitamin E refers to a class of compounds which possess a substituted chromanol ring attached to a long phytyl side chain. The ring structure is necessary to confer vitamin E activity. There are four tocopherols in which the side chain is fully saturated and four equivalent tocotrienols in which the side chain is unsaturated (Figure 1C). The various tocopherol forms are not all isomeric and differ by the number and position of three methyl groups attached on the chromanol ring. They are different compounds with differing natural occurrence, bioactivities and metabolic properties. Vitamin E is an antioxidant.
Like other aqueous insoluble fat-soluble vitamins the tocopherols are transported by proteins. Vitamin E is transported by hepatic α-tocopherol transfer protein which is bio-discriminatory towards RRR-α-tocopherol in favour of other enantiomers and isomers.1,21–23 The usual chromatography columns used for analysis are not stereo-selective and are not intended to resolve RRR-α-tocopherol from other enantiomers. Hence α-tocopherol represents the sum of them all. Additionally even though γ-tocopherol is abundant in most diets it seldom occurs in high concentrations in serum. For the purpose of this discussion the term vitamin E can be taken as synonymous with α-tocopherol.
Status
Adequate vitamin E intake is thought to be protective against cardiovascular disease, cataracts, cancer, dementia and the oxidative stress associated with diabetes. It also appears to act as a cell signalling molecule, enhancing the immune response and vasodilation, and inhibiting platelet aggregation.24–26 Hypovitaminosis E leads to muscular weakness, creatinuria and fragile erythrocytes.27 In addition, in some premature infants, haemolytic anaemia has been found to be due to lack of vitamin E.28
Marked elevated levels of vitamin E are rarely found and generally not considered toxic. Data from a meta-analysis of high dose vitamin E supplementation however found an increased risk of mortality.29 In addition, data suggests that there is an increased risk of sepsis and necrotising enterocolitis in preterm infants supplemented with vitamin E and serum levels consistently >70 µmol/L.29 Hence, appropriate upper limits of reference intervals remains important to ensure patients stay within the desired therapeutic window.
Vitamin E measurements should be requested in patients with established signs of deficiency such as peripheral neuropathy and ataxia and also in individuals with pancreatic enzyme insufficiency. Most pathology requests are to examine vitamin E repletion in conditions where fat malabsorption is possible such as individuals with pancreatic insufficient cystic fibrosis.
Method: Guideline Development Rationale
Guideline Development Group
This guideline has been developed by the AACB Vitamins Working Party, which was formed in 1999 in conjunction with the introduction of a Vitamins External Quality Assurance (EQA) program provided by the RCPAQAP.6 Members of the Vitamins Working Party consist of scientists from Australia and New Zealand with analytical expertise and interest in overall method improvement. Membership of this scientific group includes scientific staff members from the RCPAQAP. This working party falls under the direction of the Scientific and Regulatory Affairs Committee of the AACB.
The goal of the Vitamins Working Party is to provide specialist expertise to the RCPAQAP and the program participants with a view to enhancing the practice of vitamin analyses within and between laboratories. This includes providing advice on the EQA; understanding methods; assessing stability of RCPAQAP material; setting target values; acting as a referral point for methodological issues; and to promote method harmonisation. The formation of this best practice document fulfills the objective of harmonisation through guideline development which is the result of ongoing work by the Vitamins Working Party.
Setting Quality Specifications
The setting of quality specifications refers to the features encompassing the total testing process, i.e. pre-analytical, analytical and post-analytical, and provides assurance that the result meets clinical expectations.30 In 1999 the meeting in Stockholm Sweden on ‘Strategies to Set Global Quality Specifications in Laboratory Medicine’ developed a hierarchy for goal setting for analytical performance.31 This has become known as the ‘Stockholm consensus hierarchy’. Together this information provides direction for setting quality specifications for many analytes including serum vitamin A and E.32 This guideline falls into Level 3 of the Stockholm Hierarchy as detailed in Table 3.33
Table 3.
Quality specifications as detailed in the Stockholm consensus hierarchy. Adapted from Kenny D, et al.33
| Level | Quality Specification |
|---|---|
| I. | Evaluation of the effect of analytical performance on clinical outcomes in specific clinical settings |
| II. | Evaluation of the effect of analytical performance on clinical decisions in general
|
| III. | Published professional recommendations
|
| IV. | Performance goals set by
|
| V. | Goals based on current state of the art
|
Formation of Recommendations and Grading Scheme
This best practice document has been developed with reference to the Centers for Disease Control and Prevention (CDC) ‘Laboratory Medicine Best Practices: Developing an Evidence-Based Review and Evaluation Process’. Specifically this document cites the Evaluation Framework for making best practice recommendations that are specific to Laboratory Medicine.34
In developing best practice recommendations the CDC approach recognises that laboratory medicine practices are unlikely to be studied as randomised control trials. In addition the evidence available to assess practices may be limited and based on developmental studies. The Vitamins Working Party over the previous decade has conducted numerous studies to assess the conduct of fat-soluble vitamin methods, particularly vitamins A, E and β-carotene. These studies in conjunction with published data are used in part to form the recommendations in this document.
The processes for developing the recommendations are:
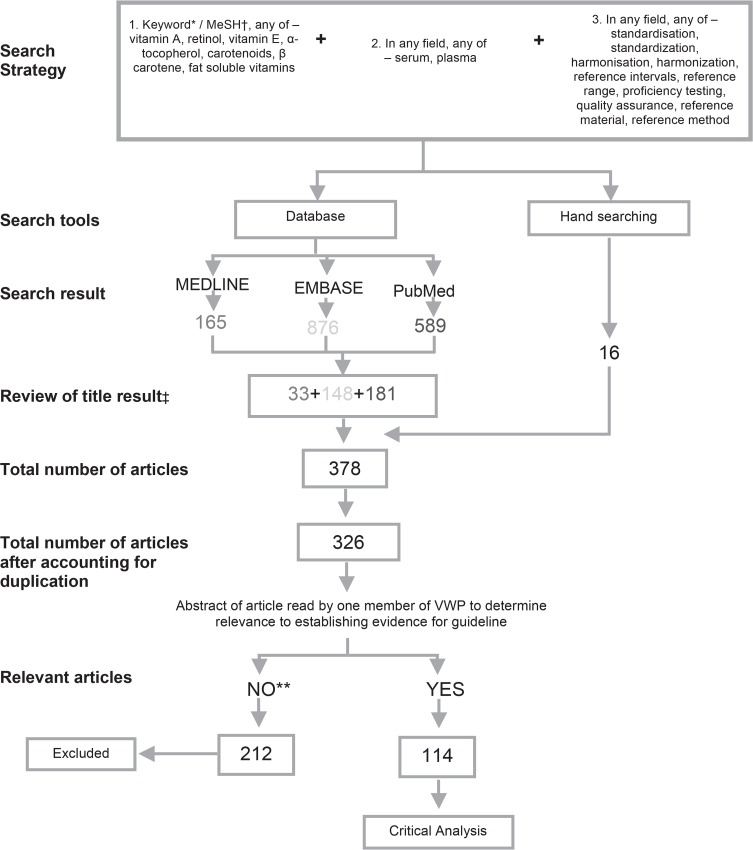
Literature search. This has been conducted on an ongoing basis since 2003 and formally conducted as part of the document preparation (Appendix 1).
Effect size assessment. The effects, or outcome variables, can be defined in terms of at least one of the following: clinical, operational process, and economic. These outcomes are measured over time and are qualitatively assessed as: substantial, moderate, minimal or none, and adverse effect.
Feasibility assessment. This involves the assessment of: cost of the intervention; applicability and sustainability; barriers to implementation; benefits which are in addition to outcomes; and potential harm which is also in addition to outcomes. The feasibility scale is either high, medium or low.
- Impact assessment. This combines the evaluation of effect size and the feasibility assessment. The impact assessment is rated as positive, neutral or negative (Table 4).
- Positive impact assessment is given when the practice or procedure will do more good than harm and is demonstrably implementable and sustainable.
- Neutral impact assessment is given when it is not clear if the practice or process will do more good than harm.
- Negative impact assessment is given when there is a clear indication that the practice or procedure does not do more good than harm.
- Strength of evidence. This is evaluated in the second stage of developing the recommendations. The categories of evidence are:
- Strong evidence, which is generally based on well designed, multisite operations, case studies or other more rigorous study designs. Such studies report the effect size in quantitative terms and use appropriate statistical analysis.
- Moderate evidence, is descriptive and relates to studies and case reports with consistent results.
- Suggestive evidence, is generally qualitative or weakly quantitative evidence. Such evidence may be based on opinion of respected authorities, descriptive studies and case reports, or reports of expert committees.
- Insufficient evidence, is the outcome when there is no evidence based support for recommendations i.e. expert opinion only with no specific case study data.
- Recommendation rating. These are the ratings given in this vitamin best practice document and are the result of the impact assessment compared to the strength of evidence (Table 4). The ratings are:
- Strongly recommend. Should be implemented.
- Recommend. Should be implemented taking into account variations in care settings.
- No recommendation for or against. Potentially favourable outcome, but is not sufficiently supported by evidence.
- Recommend against. Available evidence indicated that it is not likely to result in more good than harm.
Table 4.
CDC Best Practices in Laboratory Medicine. These are the ratings given in this vitamin best practice document and are the result of the impact assessment (Table 4a) compared to the strength of evidence (Table 4b). Reproduced with permission from CDC Laboratory Medicine Best Practices: Developing an Evidence-Based Review and Evaluation Process.34
| a. Impact assessment rating: size of effect by feasibility.
| |||
|---|---|---|---|
| Effect Size | Feasibility | ||
| High | Medium | Low | |
| Substantial | Positive | Positive | Neutral |
| Moderate | Positive | Positive | Neutral |
| Minimal or none | Neutral | Neutral | Negative |
| Adverse | Negative | Negative | Negative |
| b. Recommendation Rating: Impact Assessment (Effect/Feasibility) x Strength of Evidence.
| ||||
|---|---|---|---|---|
| Impact Assessment Rating | Strength of Evidence | |||
| Strong | Moderate | Suggestive | Insufficient | |
| Positive | Strongly recommend | Recommend | Recommend | No recommendation for or against |
| Neutral | No recommendation for or against | No recommendation for or against | No recommendation for or against | No recommendation for or against |
| Negative | Recommend against | Recommend against | Recommend against | Recommend against |
Finally, the CDC workgroup recommends “not to wait for the evidence to catch up”.34 Instead the CDC recommends that in the formation of a best practice document that a gap analysis is conducted to address future needs that can be incorporated in subsequent versions of the document.
Results: Best Practice Report
Measurement of Vitamin A, E and Carotenoids
To predict deficient from adequate or excessive vitamin intake, it is essential that the analytical method employed to measure serum/plasma vitamin concentrations is robust and fit for purpose. A 2007 external quality assurance participant questionnaire conducted by the RCPAQAP in conjunction with the AACB Vitamins Working Party demonstrated the methods commonly employed for routine clinical measurement of vitamins A, E and β-carotene. This questionnaire serves as the basis to further describe the routine clinical methods for measurement of these analytes throughout this document.9
Pre-analytical – Specimen Collection and Handling
Patient Preparation
Vitamin A, E and β-carotene are routinely collected from non-fasting patients for analysis. This is despite vitamin A levels increasing post-prandially. Ingestion of vitamin A from animal food sources is primarily in the form of retinyl esters, which are readily hydrolysed to retinol and hence blood levels can reflect recent ingestion.35,36 Whereas ingestion from plants is in the form of pro-vitamin A i.e. β-carotene, and the enzymatic conversion to vitamin A is rate limiting. The β-carotene is converted first to retinal and then to retinol. In a similar fashion, β-carotene is also increased to a small extent post-prandially, however as the conversion of β-carotene is rate-limiting, the effect of recent ingestion is less pronounced.37 Clinically, and particularly in relation to population screening, it is considered cumbersome to request fasting samples for vitamin A analysis. However, where there is doubt, vitamin A should be measured in a fasting patient to ensure there is no pre-analytical cause resulting in an artefactual increase in serum vitamin A.38 Vitamin E (measured as α-tocopherol) paradoxically does not demonstrate the same post-prandial increase as the main form ingested is γ-tocopherol.39 There is evidence that short term fasting actually causes a small but significant increase in plasma vitamin E.40 This is thought to reflect mobilization from the adipose tissue. In addition, as vitamin E is carried by lipoproteins the population reference interval has been shown to be affected by the lipid profile.41,42
Collection
Generally, two millilitres of plain or heparinised blood is sufficient to collect by venipuncture for the analysis of vitamins A, E and carotenoids. Provided sufficient sample can be collected, the equivalent may be collected by capillary or arterial phlebotomy techniques. Collection can either be in a plain, gel separator or anti-coagulated (heparin or ethylenediamine-tetra-acetic acid (EDTA)) tube.
Photosensitivity and Effects of Temperature
Vitamin A and the carotenoids are anecdotally reported to be photosensitive in blood, so samples collected for measurement of these analytes are routinely protected from light from the time of collection by, for example, wrapping samples in aluminum foil.39 Although vitamin E is not reported to be light sensitive, it is generally handled as such when requested in conjunction with vitamin A.39 Interestingly, β-carotene is not reported to require light protection for transportation or extraction.43
The most recent detailed study investigating the effects of light and temperature on a range of fat-soluble vitamins, including carotenoids, in whole blood for up to seven days was conducted by Clark and colleagues in 2004.44 Their findings suggest that there is a small but significant decrease in retinol stored under lamps but the effect was less than −1% per day. β-carotene levels were affected even less, particularly out to four days exposure. They found no effect of light exposure on vitamin E. Chilling the blood samples reduced the effects of light exposure on vitamin A and improved the stability of vitamin E which showed a small but significant increase when stored at room temperature. Their results suggest that β-carotene is relatively stable in whole blood at least to four days whether chilled or at room temperature.
Earlier studies examined the effect of light and temperature on fat-soluble vitamins in plasma. One study by Craft and colleagues in 1988 found that vitamin A, E and β-carotene were stable in plasma at room temperature for 24 hours when kept in the dark.45 Another study by Su and colleagues, conducted in 1999, looked at the effects of exposure to fluorescent lighting for 72 hours at room temperature.46 No effect was found for vitamin A and β-carotene. A small but significant effect was found for vitamin E however this was thought likely to be due to random methodological variation.
Transport
Once collected, the sample should be sent to the laboratory within one or two days if at ambient temperature (20°C). Chilling the whole blood samples is thought to extend their stability to at least four days.44 Protection from light is only necessary if the samples cannot be chilled and the time taken to reach the laboratory is extended beyond four days. Reports indicate that β-carotene can be validly shipped either refrigerated or at room temperature without protection from light.43
Storage
On receipt by the laboratory, the serum or plasma is separated by centrifugation and an aliquot is either refrigerated or frozen at −20°C or below until analysis. Vitamin A, E and β-carotene have each been demonstrated to remain stable with freeze thaw cycles.47 During these steps the sample should continue to be protected from light.46 The relevance of storage length and temperature vary for vitamin A, E and β-carotene and for long term storage data indicates that samples should be frozen at −70°C.45
Vitamin A appears to be relatively stable over time with no significant change associated with duration or increasing temperature. Retinol is reported to be stable when stored at −20°C for 15 years.48
Losses of β-carotene are reported with long term storage. Appreciable loss of carotenoids have been reported within weeks of collection when stored at −20°C, with losses of 15% reported at six months and complete loss reported when stored for 10 years.43 The very long term stability of carotenoids at −70°C beyond 15 months is yet to be determined.45
The stability of α-tocopherol seems to be intermediate compared to retinol and β-carotene with losses evident at higher temperatures and very long term storage.48 Vitamin E has demonstrated stability at −20°C for at least one year with deterioration evident past this time point.
Formal studies looking at the stability of serum retinol, β-carotene and α-tocopherol when stored at room temperature or 4°C for short periods such as one to two weeks have not been conducted.
Recommendations
-
I
Patient preparation: No recommendation for or against the patient to be fasting.
-
II
Sample collection: Recommend collection of blood into plain, gel separator, heparin or EDTA tubes for analysis of serum/plasma.
-
IIISample transport of whole blood for vitamin A, E and β-carotene:
- No recommendation for or against specific transport conditions if delivered to laboratory within 24 hours of collection.
- Recommend that samples are chilled to 4°C during transport to the laboratory if transport takes between 24 hours and four days.
- Recommend against delaying sample transport and receipt by laboratory beyond four days.
-
IV
Handling of serum/plasma samples for vitamin A, E and β-carotene:
No recommendation for or against light protection of the sample during short term receipt and processing by the laboratory (up to 24 hours under laboratory lighting).
-
VSerum/plasma storage:
- Recommend for short term storage up to one week that vitamin A and E samples are stored at ≤4°C.
- Strongly recommend for vitamin A samples to be stored at ≤−20°C for long term storage i.e. up to 15 years.
- Strongly recommend for vitamin E samples to be stored at ≤−20°C for up to six weeks and at ≤−70°C for longer term storage i.e. up to one year.
- Strongly recommend for samples awaiting β-carotene analysis to be stored at ≤−70°C until analysis.
- No recommendation for or against the long term stability of β-carotene when stored at ≤−70°C for >15 months.
Analytical - Method
Vitamins A and E may also be analysed in association with retinyl palmitate, β-carotene and other carotenoids. The sample extraction procedure, internal standards, chromatographic analysis (including mobile phase and column) and detection system selected are each important considerations for overall method performance.
Acidic conditions and oxidative destruction of retinoids, tocopherols and carotenoids is a common concern. These polyunsaturated compounds are prone to oxidative destruction by atmospheric oxygen. Retinol as an example, can undergo oxidative destruction through reactions to form inactive epoxides and furanoxides.49 In addition, retinol’s isomerisation from trans to cis is accelerated in acidic solutions hence it is therefore important analytically not to subject it to any acidic environment, including organic acids during extraction such as acetic acid.
The sample preparation described below is generally suitable for all these analytes. In addition, two worked method examples have been provided for practical information purposes by members of the Vitamins Working Party in Appendix 2.
Internal Standards
The primary role of an internal standard is to correct for losses during processing. As such internal standards should ideally behave in a similar manner to the measurand of interest and not be present in the patient sample.50 It is suggested that the concentration of the internal standard used is calculated to sit near the middle of the calibration curve or at the upper reference interval.51
Retinyl acetate and/or α-tocopheryl acetate are internal standards commonly used when assaying vitamins A and E with ultraviolet/visible detection (UV/Vis)52–54 and also for the less commonly used fluorescence and electrochemical detectors.9,55 The structure of these two internal standards is provided in Figure 3. Tocol has also been used as an alternative internal standard for assaying vitamins A and E with putative advantages of increased stability and better chromatographic separation.40,56–58 δ-Tocopherol is also used infrequently, however the selection of this internal standard is not ideal as it is present in small amounts in serum.
Figure 3.
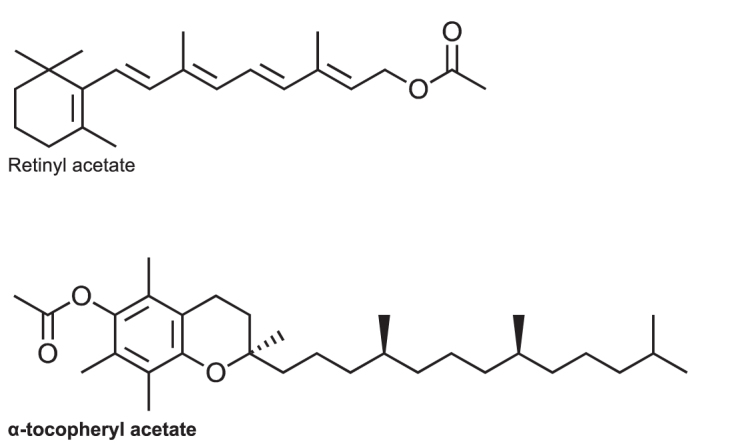
Chemical structure of the internal standards retinyl acetate and α-tocopheryl acetate. Both are suitable internal standards for HPLC analysis of Vitamin A and E with UV detection – Recommendation VII.
The internal standard commonly selected for β-carotene analysis is retinyl acetate due to the ease of availability and simultaneous analysis with vitamin A. However its performance as an internal standard is limited because during chromatography it elutes significantly earlier than β-carotene and its wavelength maximum is in the UV range (325 nm) compared to β-carotene which is in the visible range (454 nm) of the detector. Other internal standards that have been reported include echinenone,52,54,56 β-apo-8′-carotenal59 and trans-β-apo-10′carotenal oxime,60 however limited commercial availability makes them less favourable options.
The advent of liquid chromatography coupled with tandem mass spectrometry (LC-TMS) assays for these vitamins provides the opportunity to employ stable isotope derivatives of the vitamins as internal standards.61 They have the advantage of almost identical properties to the native forms but can be separated by the mass selective detector due to the difference in their mass to charge ratio (m/z).51 However, cost, availability and stability are current issues for LC-TMS matched isotopes, with only vitamin E deuterated internal standards successfully applied as matched isotopes currently.62,63
Recommendations
-
VI
Internal standard: Strongly recommend the inclusion of an internal standard.
-
VIIType of internal standard:
- Recommend retinyl acetate to be used as the internal standard for retinol with HPLC UV/Vis detection.
- Recommend α-tocopheryl acetate to be used as the internal standard for α-tocopherol with HPLC UV/Vis detection.
- Recommend, in the absence of a suitable stable carotenoid internal standard, retinyl acetate as the internal standard for β-carotene analysis.
- Strongly recommend a matched stable isotope is used for the internal standard with mass spectrometry detection.
- Recommend, where a matched stable isotope is not available, i.e. unstable or cost prohibitive, the use of the stable isotope that is closest to the expected retention time of the native vitamin is desirable to minimise variation due to ion alteration effects.
Sample Extraction
The frozen aliquot should be thawed at room temperature, protected from light and then mixed to ensure homogeneity. Between 100 and 500 μL is pipetted into either borosilicate glass or plastic tubes for sample cleanup. As is common clinical practice, calibrators and controls are treated in the same manner as the patient samples throughout the method.
Light protection of the sample during the extraction process is debated for retinol, α-tocopherol and β-carotene. In relation to β-carotene, “careful exclusion of light during the extraction process evidently is not needed. Neither protein-bound carotenoids nor pigments dissolved in petroleum ether are as sensitive to light as was once thought. Moreover, specimens can be validly shipped either under refrigeration or at room temperature. Thus, precautions for keeping specimens cold or dark will mainly depend on what other components (e.g., vitamin A) are also to be measured in a given specimen.”43
Although stabilisers, such as butylated hydroxytoluene (BHT) or ascorbate have been added, the advantages are not clear and this is not done in most clinical laboratories.6,9,45 One earlier study investigated the addition of BHT to rat and human plasma. Whilst there was an effect in the rat plasma, there was no effect for the human plasma whether BHT was added or not.64 It is felt that the addition of these antioxidants is historical and stems from the days of lower grade solvent use.
The fat-soluble vitamins are strongly bound to plasma proteins by hydrophobic interactions and a polar, water-miscible organic solvent (usually methanol or ethanol), must be added to disrupt these bonds. Addition of an internal standard is essential to correct for variable recoveries throughout the extraction. It is commonly added in combination with the polar precipitating solvent or prior to its addition.
The precipitation step is followed by liquid/liquid extraction into a non-polar solvent (usually hexane), which is lighter and immiscible with both the aqueous sample and added polar solvent. Retinol is thought not to be stable in the precipitated milieu so extraction into hexane by vortexing should occur quickly and hence a delay greater than one hour in the addition of hexane would not be recommended. Once hexane has been added the retinol becomes far more stable. After mixing and centrifugation to separate the layers, the upper organic layer is removed and dried down. Because the fat-soluble vitamins are not quantitatively extracted, but rather partitioned between the two phases, recoveries will vary with both the amount of polar solvent added to the serum/plasma sample (usually 1:1), and the volume of non-polar solvent used in the extraction, which varies between methods from 2.5 to 15 times the volume of the sample/polar solvent mix.9
A stream of nitrogen is commonly used for evaporation of the organic layer at temperatures varying from room temperature to a maximum of 40°C. Vitamin A is thought to be particularly sensitive to heat and the dry down temperature should not exceed 40°C. After evaporation the residue is commonly reconstituted in ethanol, methanol or mobile phase. In selecting a reconstitution solvent, the competing properties of extract solubility and chromatographic peak damage require consideration. Because mobile phases are methanol based it seems logical to reconstitute the dried down sample in methanol. However methanol has limited solubility for lipids and can either block the injector or worse absorb the fat-soluble vitamins. Insolubility is also reduced by low temperatures in the auto-sampler. Ethanol on the other hand has increased solubility for these vitamins. Hence, to ensure sufficient solubility of extract on reconstitution a volume of ethanol at least equal to the original plasma volume is suggested to reconstitute the sample e.g. 200 µL sample volume will need 200 µL ethanol. Note, it is possible for 500 µL sample volume to be reconstituted into 200 µL ethanol with gentle warming but the lipid may precipitate on cooling.
The reconstituted sample can then be transferred to a vial protected from light for the chromatographic separation of the sample constituents and subsequent detection (spectrophotometric or mass spectrometry). Unpublished findings by GW (member of the Vitamins Working Party) indicate that β-carotene is sensitive to light and requires light protection post reconstitution.
One simpler, alternative procedure which has been applied is to simultaneously deproteinise and extract the sample by adding a 10-fold excess of isopropanol containing an internal standard followed by centrifugation. The supernatant is dried under nitrogen, reconstituted in mobile phase, and re-centrifuged prior to analysis. This is an adaptation of a method originally applied to the extraction of ubiquinones.65 These methods omitting the liquid/liquid extraction step are not suitable for measuring carotenoids.
Recommendations
-
VIII
Lighting: No recommendation for or against processing the sample under normal laboratory lighting conditions.
-
IX
Samples: Strongly recommend that samples are co-extracted with calibrators and controls in the same batch.
-
X
Protein precipitation: Strongly recommend proteins should be precipitated out using ethanol or methanol (with or along-side the internal standard) with thorough vortex mixing.
-
XI
Extraction: Strongly recommend samples are liquid-liquid extracted using the non-polar solvent hexane; by mixing, centrifugation and then transfer of the upper hexane extract to a new tube.
-
XII
Dry-down:
Recommend the extracted sample is evaporated to dryness under either nitrogen or vacuum.
Recommend the drying down temperature should be between 20°C (i.e. room temperature) and 40°C.
Recommend against using air for evaporation.
-
XIIIReconstitution:
- Strongly recommend after drying, the residue is dissolved in either ethanol or methanol for vitamin A and E.
- Strongly recommend when β-carotene is analysed ethanol should be used for reconstitution to ensure complete dissolution.
- Recommend the reconstituted solvent should be added in sufficient volume to ensure reconstitution i.e. at least equivalent volume to the starting sample volume.
- Recommend the reconstituted sample should be protected from light for analysis.
Mobile Phases
In all instances, at least HPLC-grade solvents should be used. The majority of clinical laboratories employ isocratic separation with 90 to 100% methanol as the mobile phase; this allows for the re-circulation of the mobile phase to preserve the solvent if desired. Gradient methods are also used which allows for an increase in the percentage of methanol over the run time. The advantage of a gradient solvent program is that it removes late eluting peaks that occur in some samples which cause interference to succeeding chromatograms when an isocratic protocol is used. The disadvantage is the increased run time usually required to complete an injection. Other mobile phase constituents that are used less frequently in association with HPLC include: ethanol, ethyl acetate, isopropanol, methyl tert butyl ether, tetra-hydrofuran, chloroform, and the modifiers ammonium acetate and triethylamine.9,60 Many of these mobile phase additives are generally considered to contribute to the shortening of the life of the analytical column and unacceptable alteration in peak shape for carotenoid separation. None of these additives should be included in mobile phases if mass spectrometry detection is employed. The use of acetonitrile is also reported less frequently for vitamin A, E and β-carotene analysis.9
Mass spectrometry detection methods are currently infrequently used for clinical biochemistry diagnostic purposes of vitamins A, E and the carotenoids. Current reports support the use of positive electrospray LC-TMS assays. Therefore the addition of formic acid to the mobile phase is required i.e. the formic acid allows for a proton donor in the detection method.62,66
Recommendations
-
XIVMobile phase:
- No recommendation for or against isocratic versus gradient elution.
- Recommendation for the mobile phase to consist of a mixture of HPLC grade methanol and water. If isocratic then the mixture should be between 90 and 100%.
- Strongly recommend the addition of formic acid to the mobile phase/s for LC-TMS analysis.
- No recommendation for or against the addition of ammonium acetate, triethylamine and tetrahydrofuran to the mobile phase for β-carotene analysis with HPLC UV/Vis detection.
Column
An alkane bonded silica octadecylsilane (C18) column from a variety of manufacturers is typically used for the solid phase. C18 (with normal carbon loading 12–17%) is entirely sufficient for fat-soluble vitamins but high-load 24% C18 or (better) triacontylsilyl silica (C30) is best for separating the carotenoids.60 The addition of a matching guard column is ideal to protect the column in order to extend its life. For clinical use when all trans β-carotene is of interest a C18 column is more than adequate. Pentafluorophenyl columns have become popular for some LC-TMS applications but do not seem to offer any additional selectivity for clinical purposes for vitamin A, E and β-carotene analysis.
Recommendations
-
XV
Column: Strongly recommend C18 column and matching guard column for vitamins A, E and β-carotene.
Detectors
Routinely UV/Vis or photodiode array (PDA) detectors are used for the measurement of vitamins A, E and the carotenoids at absorbances of 325, 292 and 454 nm respectively.9 PDA detection does have advantages over UV/Vis, as it provides an additional check on analyte identity and purity via spectral library matching and peak purity algorithms within the software. Alternative methods of detection previously used by some are electrochemical and fluorescent detection.67–70 These alternative detection methods are thought to provide additional sensitivity over UV/Vis, however, clinically UV/Vis and PDA provide the level of sensitivity required clinically. Mass spectrometry (MS) detection with electrospray ionisation in either positive or negative modes has been reported but is currently used infrequently in clinical biochemistry laboratories for these analytes.62,63,66
Recommendations
-
XVIDetection:
- Strongly recommend UV/Vis (or PDA) detection for the measurement of vitamins A, E and β-carotene with programmed wavelengths at absorbances of 325, 292 and 454 nm respectively.
- Recommend mass spectrometry detection as a suitable alternative detection method.
Analytical - Standardisation
Standardisation is a fundamental concept for clinical biochemists. This is achievable when the method base is similar, which is the case with chromatographic methods routinely used for fat-soluble vitamins. With standardisation, results from one laboratory can theoretically be directly compared with another laboratory. This ultimately improves patient care.
As a result, in 2002 the Joint Committee for Traceability in Laboratory Medicine (JCTLM) was established to address the need for establishment of lists of higher order reference methods and reference materials. As part of the process, proposed reference methods are examined to ensure conformity with appropriate international documented standards and reference materials are verified by measurement institutes with demonstrated competency.71
Reference Measurement Procedure
There is no reference measurement system currently available for serum vitamin A, E or the carotenoids.72 Many reference methods listed on the JCTLM database are based on mass spectrometry detection. Although vitamin A and E are currently separated by liquid chromatography with UV/Vis detection, it is possible to detect these vitamins using mass spectrometry detectors.62,63,73 Potentially LC-TMS may develop into a reference measurement procedure for these vitamins. No recommendation for or against a reference method has been made in this best practice report, as there is no primary reference method listed on the JCTLM database at this point in time.
Primary Calibrators
There is no reference material currently listed on the JCTLM database. Previously the National Institute of Standards and Technology (NIST) (Gaithersburg, Marylands, USA) listed the standard reference material (SRM) 968c74 on this database. This listing was removed post depletion of the supply of this lot. NIST have since released two matched replacements for SRM-968c (SRM 968e and the superseded SRM 968d) neither of which is listed on the JCTLM database.71,74–76 The uncertainties around the certified values appear broad and based on consensus of participants in a related EQA program.10,70
The principal option for vitamin A, and E primary reference materials currently is either the NIST SRM or the purchase of pure substance from Sigma-Aldrich (St. Louis, Missouri, USA) or equivalent manufacturer. There appears to be some limitations with the NIST option,10,62 whilst the alternative (Sigma option) does not provide a mechanism for traceability, the approach currently promoted as one of the pillars of harmonisation in laboratory medicine. For β-carotene the option is NIST, albeit with quite low concentrations, or a novel approach proposed by one member of this working party (GW) to utilise serum from bovines and determine the concentration by means of an extinction coefficient.77
Secondary Calibrators
Secondary (or routine calibrators) are either made within the laboratory or purchased from a commercial vendor. Such calibrators are referenced back to a primary reference material. It is recommended that a primary calibrator should achieve a lower error for inaccuracy compared with the equivalent secondary calibrator.78 From the biological variation data available, the suggested maximum desirable method bias is 5.4% for vitamin A, 5.6% for vitamin E and 12.9% for β-carotene.42
The major vendors of commercial secondary serum calibrators for vitamin A and E align their material with the NIST SRM 968 SRM.79–81 Some vendors also supply values aligned with Sigma-Aldrich material. The target values assigned by these vendors differ between Sigma-Aldrich and NIST. However, even when all secondary calibrators are aligned to NIST, vitamin A and E results submitted to EQA programs indicate a relatively wide inter-laboratory dispersion.6,9 In a pilot study conducted by the AACB Vitamins Working Party, the use of a common calibrator by five laboratories demonstrated some improvement in the agreement of results.82 This highlights the need for the development of a higher order reference method plus material to provide alignment between laboratories analysing these vitamins. In addition, for all secondary calibrators, whether commercial or prepared in house, it is essential that they are commutable and do not result in a bias due to matrix differences with patient samples.83
The actual level of primary and secondary calibrators is considered by many to be important in the overall assessment of method performance. Traditionally commercial manufacturers supplied a single level secondary calibrator, now a number of commercial calibrators are supplied as multi-level sets. Whilst there is no formal recommendation for the number of calibrators used for HPLC analysis, recommendations are provided for LC-TMS methods, which state that a total of six calibrators including a zero are used to create a calibration curve.51 In addition a blank is also recommended.51 Currently such calibration sets are not available commercially for vitamin A, E or β-carotene analysis and therefore, matrix match in-house standards are prepared (often with Sigma-Aldrich pure material) for LC-TMS analysis.62
Recommendations
-
XVII
Reference method: No recommendation for or against a reference method as there is no primary reference method listed on the JCTLM database at this point in time.
-
XVIII
Primary standard: No recommendation for or against the traceability to a primary standard, as there is no primary standard currently listed on the JCTLM database at this point in time.
-
XIX
Secondary (working) calibrators: Strongly recommend that secondary calibrators are matrix matched to ensure commutability.
-
XXCalibration curve
- Recommend a multipoint calibration curve for HPLC analysis of vitamin A, E and β-carotene.
- Recommend a minimum of a six point calibration curve (including a zero standard) for LC-TMS analysis which covers the analytical range. This curve should not be forced through zero.
Analytical - Method Validation
Method validation confirms that the analytical method employed is suitable for its intended use. Studies to validate a chromatographic method for fat-soluble vitamins should include: analytical range, accuracy, imprecision, linearity, sensitivity, recovery and interference.84 If using a mass spectrometry detection method then some additional considerations may be needed.8,51 Analytical methods need to be validated or revalidated before their introduction into routine testing, whenever the conditions change (e.g. instrument or sample matrix) or whenever the method changes.
Analytical Range
Linearity and limit of quantitation (LOQ)85 are used to determine the reportable range of results. For fat-soluble vitamins the reportable range should span all decision points, such as the levels of hypovitaminosis and hypervitaminosis of vitamin A, β-carotene and vitamin E. Ideally, the LOQ of the assay should be to 0.1 μmol/L for vitamins A and β-carotene and 1 μmol/L for vitamin E. The upper limit of quantitation (without the need for dilution) should be to at least 4.0 μmol/L for vitamin A and β-carotene and at least 50 μmol/L for vitamin E.
Accuracy
Accuracy is the closeness of agreement between a test result or measurement result and the true value. Validation of the accuracy of the method should be performed using a primary or secondary calibrator (see section on Analytical – Standardisation). Validation using acceptable performance in an external quality assurance program can also be considered.
Imprecision
Imprecision is the dispersion of independent results of measurements obtained under specified conditions. It is expressed numerically as standard deviation (SD) or coefficient of variation (CV). The imprecision should be ascertained at the clinical decision points. In practice, inter-run control data generated over multiple runs (ideally >20 runs over at least a one month period or preferably longer) is used to generate this information.32
Biological Variation
Estimates of biological variation for vitamin A, E and carotenoids have been conducted in plasma and/or serum42,86,87 and have been reported on the Ricos biological variation data base.86 Of note is the variation in the within subject biological variation data for serum and plasma vitamin A levels which are reported as 13.6% and 6.2% respectively.86 From the same studies the between subject biological variation for plasma and serum was reported to be 19% and 21% percent respectively.86 Whilst the inter-individual biological variation data is consistent between these studies the within subject data does not show the same agreement. The higher value for within subject variation, as found in the study using serum, is used for the fitness for purpose calculation.30,86,87 The biological variation data and quality specifications for retinol, α-tocopherol and β-carotene from the Ricos biological variation database is summarised in Table 6.42
Table 6.
Median performance of RCPAQAP participants from 2002 compared to 2013.*
| Performance Comparison | CV % | ||
|---|---|---|---|
| 2002 | 2013 | ||
| Median | Vitamin A | 8.0 | 7.0 |
| Vitamin E | 9.4 | 6.7 | |
| β-carotene | 23.7 | 14.4 | |
Note: 2002 is the earliest data available to access from the RCPAQAP database.6
Fitness for Clinical Purpose
Total intra-individual variation (CVt), which is the combined estimate of analytical imprecision (CVa) and intra-individual biological variation (CVw) can be calculated with the equation: CVt = √[(CVa)2 + (CVw)2].30,88
The analytical goal for fitness for purpose for an assay is based on (CVw) and can be determined as optimum (CVa ≤ 0.25 x CVw); desirable (CVa ≤ 0.5 x CVw) or minimum (CVa ≤ 0.75 x CVw).30,86 Utilising the serum values (i.e. the broader specifications) from the biological variation database (Table 5) we can determine the following86:
Vitamin A - As the CVw for serum vitamin A is estimated as 13.6%, the desirable specifications for the analytical imprecision based on the above calculations must be ≤6.8%.86 Based on the RCPAQAP participant results for 2013, this is achievable as 50 % of labs could achieve a CV% ≤7.0% for vitamin A.6
Vitamin E - The desirable specifications for the analytical imprecision of serum vitamin E is <6.9%.86 Based on the RCPAQAP participant results for 2013, this is achievable as 50 % of labs could achieve a CV% ≤ 6.7%.6
β-carotene - The desirable specifications for analytical imprecision of serum β-carotene is 18%. Based on the RCPAQAP participant results for 2013, this is achievable as 50% of labs could achieve a CV% ≤14.4% for β-carotene.6
Table 5.
| Biological Variation | Desirable specification | |||||
|---|---|---|---|---|---|---|
| Matrix | Measurand | CVw | CVg | Imprecision (%) | Bias (%) | Total Error (%) |
| P- | Retinol | 6.2 | 21 | 3.1 | 5.5 | 10.6 |
| S- | Retinol | 13.6 | 19 | 6.8 | 5.8 | 17.1 |
| S- | β-Carotene | 36 | 39.7 | 18 | 13.4 | 43.1 |
| S- | α-Tocopherol | 13.8 | 15 | 6.9 | 5.1 | 16.5 |
| (B)Eryth- | Vitamin E (α-Tocopherol) | 7.6 | 21 | 3.8 | 5.6 | 11.9 |
P, plasma; S, serum; B, blood; CVw, intra-individual biological variation; CVg, inter-individual biological variation.
The tighter specifications presented in the biological variation database have been proposed by others, in particular from data developed by Talwar and colleagues.42 Based on the Talwar specifications, less than 50% of laboratories in the RCPAQAP Vitamin program can achieve this desirable imprecision target currently; although improvement in group performance is evident over time (Table 6).
Uncertainty of Measurement
Uncertainty of measurement (MU) is a “non-negative parameter characterising the dispersion of the quantity values being attributed to a measurand, based on the information used”.89 There are many components to uncertainty including the finite detail in the definition of a measurand, the inaccuracy of a calibrator’s assigned value and the imprecision associated with the method. The International Vocabulary of Metrology separately defines MU as type A and type B uncertainty.
Type A is the “evaluation of a component of measurement uncertainty by a statistical analysis of measured quantity values obtained under defined measurement conditions”.89 This type of uncertainty defines the analytical imprecision in relation to the 95% confidence interval of a result and is routinely estimated by ISO15189 accredited laboratories.8,90
Type B is the “evaluation of a component of measurement uncertainty determined by means other than a Type A evaluation of measurement uncertainty”.89 This type of uncertainty usually relates to the accuracy of measurement with reference to traceability, certified reference materials and measurement instruments. It is usually estimated by the manufacturer of the calibrator and included as part of the certificate of analysis.8
Ascertainment of the uncertainty of measurement of the fat-soluble vitamins assay is required for clinical laboratories under International Organization for Standardization (ISO) document number 15189.90 The assay imprecision can be used to calculate the Type A MU, which is the two SD range either side of a result if it were to be repeated.91
Recovery
Recovery studies should be performed prior to utilising a method for testing clinical samples. Recovery is important to ensure the efficiency of the sample extraction. Experimental design for recovery studies can be based on the criteria by the International Union of Pure and Applied Chemists (IUPAC).92 The results of the recovery experiment should ideally fall into the range of 90 to 110%.38
Interference
Efficient chromatography with adequate retention and resolution from interferences including early eluting carotenoids is attainable for fat-soluble vitamins methods. Common interferences encountered in LC-TMS methods include co-elution of isomers and ion alterations due to the presence of other compounds such as phospholipids. The presence of phospholipids is easily monitored in electrospray positive mode LC-TMS with the ion transitions 104→104 and 184→184 m/z.8
Recommendations
-
XXI
Method validation: Strongly recommend method validation experiments to include: linearity, LOQ, bias, within and between run imprecision, and relative and absolute recovery experiments.
-
XXII
Fitness for purpose: Strongly recommend that the method achieves the desirable specifications, based on comparison to intra-individual biological variation, to ensure fitness for clinical purpose.
-
XXIII
Uncertainty of measurement: Strongly recommend assessment of Type A MU and where practicable to ascertain the Type B MU of the method.
Analytical - Quality
Internal Quality Control
Assaying internal quality control (IQC) is desirable to demonstrate acceptable analytical performance and allow results from patients to be reported. Normal laboratory practice is that IQC should be included in each analytical run and assessed against the acceptability criteria which have previously been set. Running multiple levels is desirable as this validates the run at different concentration levels.93
Commercial internal quality control material for vitamin A and E are available from a variety of manufacturers including Bio-Rad Munich, Germany; Chromsystems Instruments and Chemical GmbH, Munich, Germany; and Recipe, Munich, Germany.79–81 Each of these manufacturers produce their control material as multi-level lyophilised serum vials. For β-carotene Chromsystems Instruments offers a commercial control.
An acceptable range is set by the laboratory for each level of material. This is usually determined as the mean ±2 SD of the laboratory’s results by evaluating results on a Levey Jennings plot.94–97
If the values obtained are outside the acceptable range or demonstrating a trend, then investigation is required prior to deciding whether the analytical run should be accepted or rejected. Westgard Rules can assist to determine the type of error and acceptability of the analytical run.87
Recommendations
-
XXIV
Internal quality control: Strongly recommend running at least two levels of internal quality control that cover clinical decision intervals with each analytical run.
External Quality Assurance
Participation in an external quality assurance program ensures ongoing proficiency of the analytical component of vitamin analysis. This is demonstrated in the RCPAQAP Vitamins program; for vitamin A, E and β-carotene which demonstrate an overall improvement in imprecision over time (Table 6).6 There are a number of vitamin external quality assurance programs. Some of these are detailed in Appendix 3.
As an example external quality assurance program, the RCPAQAP Vitamin Program has been in operation since 1999 and is a worldwide program, with participants from both the southern (e.g. Australia, New Zealand, Singapore and South Africa) and the northern (e.g. Israel, France and USA) hemispheres.6 Each month participating laboratories analyse two samples alongside their clinical samples. Twelve samples are analysed within a cycle, which runs for a period of six months. Participants are asked to describe their method in terms of analytical principle, measurement system, reagent source and calibrator source. Targets for this program have been set intermittently using NIST-SRM-968 for vitamin A.10 The program’s analytical performance goals are termed the allowable limits of performance.31 These have been determined for each vitamin using Level IIb (biological variation) of the Stockholm consensus hierarchy.31
RCPAQAP and other programs give participants an indication of both accuracy and precision against peers.
Recommendations
-
XXV
External quality assurance: Strongly recommend participation in an external quality assurance program.
Post Analytical
Laboratory information systems (LIS) are used extensively to report patient results.98 Consideration of significant change, MU, reporting intervals and rounding are all connected elements of trueness and interpretation. Reporting of valid significant figures avoids incorrect interpretation of results; i.e. too many decimal places cause incorrect conclusions by the requestor as to a significant change in patient’s result. There are MUs available for every test to assist with this and there is a general consensus that we do ‘over report’ accuracy especially in some areas of laboratory medicine such as therapeutic drug results. Each of these considerations requires a decision for the setup of the parameters in the LIS in order to generate timely and appropriate reports to aid the clinician in the interpretation of the result. Hence this section of the best practice report aims to support this reporting process.
Units for Reporting
This guideline recommends the Système International d’Unités (International System of Units; SI)99 as the units for reporting vitamins. Table 7 lists the compounds and their conversion factors. There is a free online unit conversion calculator to allow conversion to SI units.100
Table 7.
Conversion factor from System International (SI) units to conventional units.
| Vitamin | Compound | SI Unit | Conversion Factor | Conventional Unit |
|---|---|---|---|---|
| Vitamin A | retinol | μmol/L | 0.0349 | μg/dL |
| Carotenoids | β-carotene | μmol/L | 0.186 | μg/dL |
| Vitamin E | α-tocopherol | μmol/L | 23.22 | mg/dL |
Of note a recommendation has recently been formed by the RCPA relating to the prefix for micro (μ). The RCPA have “adopted as policy the use of u, rather than the Greek letter μ, for the expression of “micro” in units for reporting pathology results”. “This is to ensure the safe transmission of the unit to different computer systems and printers, including those with a limited character set, and also to ensure consistency with the adoption of the Unified Code for Units of Measure (UCUM) system for the unambiguous electronic transmission of units”.101 Hence for the fat-soluble vitamins in this report the use of umol/L is considered equally appropriate to the use of μmol/L.
Significant Figures
A general rule for calculating the appropriate number of decimal places for reporting results is based on the decimal place for the one SD calculation. If this SD is a whole number then no decimal place is required. However, if the SD starts with one decimal place then this is the number of places required for reporting a result.102,103 So for the measurands in this best practice report the following examples can be applied:
Vitamin A (or β carotene) of 2 μmol/L with a CV of 10%. The SD is 0.2 hence vitamin A should be reported as 2.0 μmol/L i.e. to one decimal place.
Vitamin E of 20 μmol/L with a CV of 10%. The SD is 2 hence vitamin E should be reported as 20 μmol/L i.e. to zero decimal places.
This should be checked at relevant decision levels as sometimes the decision can be different at different levels, e.g, vitamin A of 0.5 μmol/L with a CV of 10%. The SD would be 0.05 and hence the result should be reported to two decimal places using this rule. This explains why WHO report to two decimal places around this level.2,104 Hence we also need to be pragmatic in our decision. When quality control is used an extra decimal place is added for the statistical calculations.
Reference Intervals
Vitamin A, E and carotenoid reference intervals have been developed from a number of groups since the advent of their chromatographic analysis. Consistency of reference intervals between laboratories is desirable and with a common method base harmonisation of the reference intervals is conceivable. Currently the only clear attempt at a harmonised reference interval for the vitamins of this best practice report relates to the WHO decision limit of <0.7 µmol/L to define vitamin A deficiency in serum.104 Other than this, comparison of reference intervals between testing laboratories, at least for vitamin A and E, indicates that there is currently a lack of agreement.9 This inter laboratory comparison is provided in Figure 4.
Figure 4.
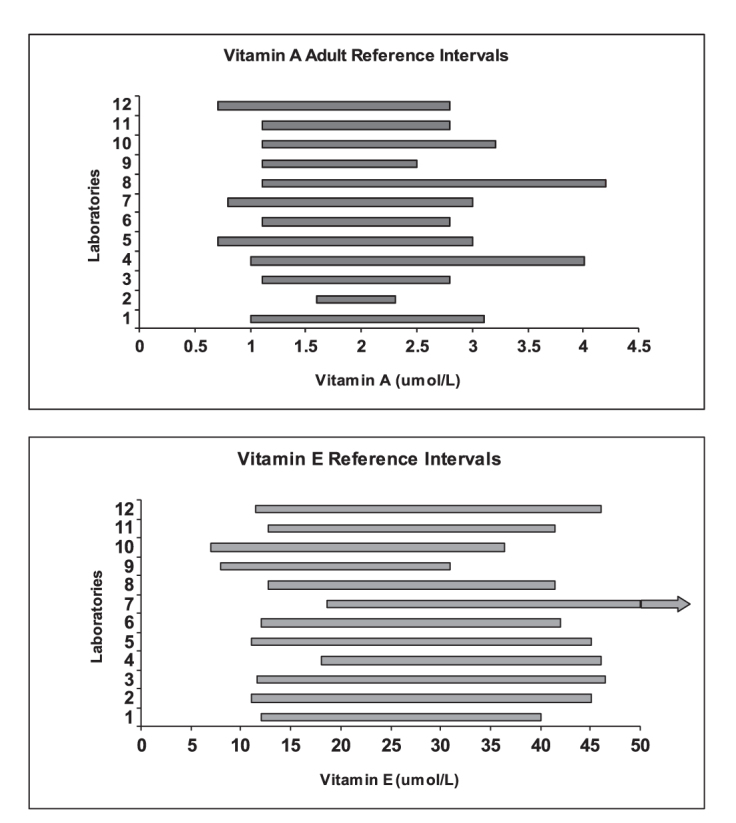
Serum vitamin A and E adult reference intervals across laboratories enrolled in the RCPA Quality Assurance Programs – Chemical Pathology Vitamin A and E Program in 2007.9
Vitamin A, E and carotenoid reference intervals vary with age. It is known that children have lower serum vitamin A and E levels than adults and also that there is a change in serum vitamin A and E reference intervals during pregnancy (Table 8).105–107 In addition, paediatric reference intervals have been reported for β-carotene from an Australian study with participants from age nine months to 62 months of age.108 Despite this evidence, laboratories differ in their inclusion of age related reference intervals. For example some laboratories only provide one reference interval for vitamin A irrespective of age and tend to have a lower limit of 0.7–0.8 µmol/L. Whilst laboratories which provide age related reference intervals appear to use a higher lower limit for both vitamin A and E for adults.9
Table 8.
Paediatric and pregnancy reference intervals for vitamin A and E in serum/plasma.106,107,108 Paediatric β-carotene data is from an Australian study (n=467) using the 2.5–97.5 percentiles to develop the reference interval.109
| Retinol (µmol/L) | α-Tocopherol (µmol/L) | β-Carotene (µmol/L) | |
|---|---|---|---|
| Preterm neonate | 0.5–1.6 | 1–8 | |
| Term neonate | 0.6–1.8 | 2–8 | |
| 1 – 6 years | 0.7–1.5 | 7–21 | 0.1–1.1* |
| 7 – 12 years | 0.9–1.7 | 10–21 | |
| 13 – 19 years | 0.9–2.5 | 13–24 | |
| Gestational Age | |||
| < 12 weeks | 0.9–2.3 | 12–37 | |
| 12 – 18 weeks: | 0.9–1.9 | 10–38 | |
| 18 – 24 weeks: | 1.1–1.8 | 15–39 | |
| 24 – 28 weeks: | 1.1–1.7 | 19–43 | |
| > 28 weeks: | 0.9–1.9 | 18–46 |
Note, reference intervals have been rounded to the nearest significant figure if required to reflect recommendation XXVId.
Age range 9 to 62 months. The ranges quoted for vitamin A and E from this study were: vitamin A 0.7–1.8 μmol/L, vitamin E 8–30 μmol/L.109
Gender, ethnicity and nutritional differences have also been proposed for vitamin A, E and the carotenoids in the literature. Some groups report no sex difference for vitamin A and E106,107 whilst others do report a difference.109,110 One vitamin A, E and β-carotene reference interval study among children found variations in intervals based on sex, ethnicity and socio economic status.108 A Vietnamese study found differing concentrations of vitamin A, E and β-carotene across three socio economic groups, with β-carotene deficiency being prevalent in all three stratifications.111 The authors hypothesise that this may represent a difference in the food consumption patterns of men and women (plant versus animal product intake).
Finally of note, an independent positive correlation has been demonstrated between retinol and α-tocopherol in association with vitamin supplementation.106,112 This is interesting as vitamin A and E are absorbed and transported in the circulation by different mechanisms.113–115
Recommendations
-
XXVIReporting results
- Strongly recommend age stratification of reference intervals and pregnancy reference interval.
- No recommendation for or against sex stratification of reference intervals.
- Recommend the units of reporting for vitamin A, E and β-carotene are µmol/L.
- Recommend reporting results to one decimal place for retinol, zero decimal places for α-tocopherol, and one decimal place for β-carotene down to 0.1 µmol/L. For β-carotene at low values below 0.1 µmol/L report to two decimal places (still only one significant figure).
Additional Analytes
In addition to reporting vitamin A and E, a minority of laboratories measure and report other compounds related to these vitamins:
For vitamin A retinol binding protein (RBP), C-reactive protein, serum albumin and retinyl esters have been reported to improve the assessment of nutritional status.116 The relative dose response test has been proposed when vitamin A results are outside the normal range to assess liver stores of vitamin A; pre and five hours post vitamin A administration.117
Vitamin E is known to be associated with circulating lipoproteins such as low-density lipoprotein hence there is a strong positive correlation between vitamin E and lipid levels (particularly cholesterol).115 As such it has been recommended to report vitamin E as a ratio to lipids.106 Certainly the expression of vitamin E as a ratio to cholesterol has been shown to eliminate the age dependence of the result in children.41 Other benefits for the measurement of the ratio of vitamin E to lipids is less certain.
Whilst there is published evidence to suggest a benefit to the analysis of additional analytes, the practice in most laboratories does not appear to support this for clinical interpretation of results.9 As there appears to be contrasting views on the requirement for the reporting of these additional analytes, no recommendation for or against is made at this point in time.
Recommendations
-
XXVII
Additional analytes: No recommendation for or against the inclusion of additional analytes.
Discussion
The presentation of this best practice report is the culmination of the work and experience of the AACB Vitamins Working Party over the last decade. The working party recognises the commonality of the routine clinical methods for the measurement of blood vitamins A, E and the carotenoids. Whilst these chromatographically based methods tend to share similar principles in terms of sample preparation and analysis, the Vitamins Working Party also recognises the significant spread of results reported between laboratories. This observation is based on the data available from review of reference intervals between laboratories, external quality assurance program studies and other comparisons. Therefore, the Vitamins Working Party has sought to harmonise the methods through the development of best practice recommendations in the first instance.
Recommendations were generated after an exhaustive search of the literature and the extensive combined experience of the nine members (GW, KH, TW, TH, SK, JG, SB, LJ, RG) of this Australasian group. The literature search entailed a common strategy applied to three databases (Medline, Embase and PubMed) as performed previously118 (details available in Appendix 1). The results of the three database searches were surprising in the uniqueness and variation in the number of manuscripts found in each database. This was further highlighted through hand-searching, which included the AACC Pre-analytical Variables textbook by Donald Young.39 Together these references have formed the recommendations in this document and demonstrated the gaps in our knowledge.
Fifty recommendations, under 27 main headings, have been formed based on this extensive review of the literature and the implementation of the CDC model for laboratory best practice.34 These recommendations cover the pre-analytical, analytical and post-analytical components relative to method performance and are summarised in Table 9. Of these recommendations: 20 are strongly recommend and should be implemented; 17 are recommend implementing taking into account the laboratory setting, 11 are no recommendationfor or against due to insufficient evidence or limited effect on performance either way; and 2 are recommend against. In developing these recommendations, the Vitamins Working Party’s mindset was based on a positive attitude towards the harmonisation of method performance, which is reflected in the overall number of affirmative recommendations for implementation; i.e. 74% of the overall recommendation list.
Table 9.
Summary of Best Practice Recommendations for the analysis of Vitamin A, E and the carotenoids in blood, with the strength of the recommendation evaluated against the CDC’s Best Practices in Laboratory Medicine process.
| Vitamins Recommendation No. | Effect Size | Feasibility | Impact Assessment Rating | Strength of Evidence | Overall Recommendation* |
|---|---|---|---|---|---|
| CDC Options | Substantial / Moderate / Minimal or None / Adverse | High/ Medium / Low | Positive / Neutral / Negative | Strong / Moderate / Suggestive / Insufficient | Strongly Recommend / Recommend / No recommendation for or against / Recommend against |
| I | Minimal or none | Medium | Neutral | Suggestive | No Recommendation for or against |
| II | Moderate | High | Positive | Suggestive | Recommend |
| III | |||||
| a. | None | High | Neutral | Moderate | No Recommendation for or against |
| b. | Moderate | Medium | Positive | Suggestive | Recommend |
| c. | Adverse | Medium | Negative | Suggestive | Recommend against |
| IV | Minimal or none | High | Neutral | Moderate | No Recommendation for or against |
| V | |||||
| a. | Moderate | Medium | Positive | Moderate | Recommend |
| b. | Moderate | Medium | Positive | Strong | Strongly Recommend |
| c. | Moderate | Medium | Positive | Strong | Strongly Recommend |
| d. | Moderate | Medium | Positive | Strong | Strongly Recommend |
| e. | Substantial | Medium | Positive | Insufficient | No Recommendation for or against |
| VI | Substantial | High | Positive | Strong | Strongly Recommend |
| VII | |||||
| a. | Substantial | High | Positive | Moderate | Recommend |
| b. | Substantial | High | Positive | Moderate | Recommend |
| c. | Moderate | High | Positive | Suggestive | Recommend |
| d. | Substantial | Medium | Positive | Strong | Strongly Recommend |
| e. | Moderate | High | Positive | Suggestive | Recommend |
| VIII | Minimal or none | Medium | Neutral | Suggestive | No Recommendation for or against |
| IX | Substantial | High | Positive | Strong | Strongly Recommend |
| X | Substantial | High | Positive | Strong | Strongly Recommend |
| XI | Substantial | High | Positive | Strong | Strongly Recommend |
| XII | |||||
| a. | Moderate | Medium | Positive | Suggestive | Recommend |
| b. | Moderate | High | Positive | Suggestive | Recommend |
| c. | Adverse | Medium | Negative | Suggestive | Recommend against |
| XIII | |||||
| a. | Substantial | High | Positive | Strong | Strongly Recommend |
| b. | Substantial | High | Positive | Strong | Strongly Recommend |
| c. | Substantial | High | Positive | Moderate | Recommend |
| d. | Moderate | High | Positive | Moderate | Recommend |
| XIV | |||||
| a. | Minimal or none | High | Neutral | Insufficient | No Recommendation for or against |
| b. | Moderate | High | Positive | Moderate | Recommend |
| c. | Substantial | High | Positive | Strong | Strongly Recommend |
| d. | Minimal or none | Medium | Neutral | Insufficient | No Recommendation for or against |
| XV | Substantial | High | Positive | Moderate | Strongly Recommend |
| XVI | |||||
| a. | Substantial | High | Positive | Strong | Strongly Recommend |
| b. | Moderate | Medium | Positive | Suggestive | Recommend |
| XVII | Substantial | Low | Neutral | Strong | No Recommendation for or against |
| XVIII | Substantial | Low | Neutral | Strong | No Recommendation for or against |
| XIX | Substantial | High | Positive | Strong | Strongly Recommend |
| XX | |||||
| a. | Moderate | Medium | Positive | Moderate | Recommend |
| b. | Moderate | Medium | Positive | Suggestive | Recommend |
| XXI | Substantial | High | Positive | Strong | Strongly Recommend |
| XXII | Substantial | High | Positive | Strong | Strongly Recommend |
| XXIII | Moderate | High | Positive | Strong | Strongly Recommend |
| XXIV | Substantial | High | Positive | Strong | Strongly Recommend |
| XXV | Moderate | High | Positive | Strong | Strongly Recommend |
| XXVI | |||||
| a. | Substantial | High | Positive | Strong | Strongly Recommend |
| b. | Moderate | High | Positive | Insufficient | No Recommendation for or against |
| c. | Substantial | High | Positive | Moderate | Recommend |
| d. | Moderate | High | Positive | Moderate | Recommend |
| XXVII | Minimal or none | High | Neutral | Moderate | No Recommendation for or against |
’Strongly Recommend’: Should be implemented; ‘Recommend’: Should be implemented taking into account variations in care settings; ‘No recommendation for or against’: Potentially favourable outcome, but is not sufficiently supported by evidence; ‘Recommend against’:Available evidence indicated that it is not likely to result in more good than harm.
During the development of the recommendations thirteen main gaps in knowledge or process were identified. These gaps are summarised in Table 10. The most significant gap was the lack of JCTLM listed reference methods and materials for vitamin A, E and β-carotene. This impacts on traceability, bias assessment, EQA target values and the development of common reference intervals. Liquid chromatography coupled with tandem mass spectrometry would appear to be a suitable approach for the development of a candidate reference method. The establishment of such a method would allow for both primary and secondary calibrators to be assigned with improved accuracy. This would effectively allow for the full transferability of results between laboratories and aid in defining upper and lower clinical decision limits for these vitamins.
Table 10.
Gap analysis. During the development of the recommendations thirteen gaps in knowledge or process were identified.
| Section of Best Practice Document | Gap Identified |
|---|---|
| Pre-analytical | The stability of serum retinol, β-carotene and α–tocopherol when stored at room temperature or 4°C for short periods such as one to two weeks have not been conducted. |
| The long term stability of β carotene when stored at ≤ −70°C. | |
| Analytical – sample preparation | Processing of the sample under normal laboratory lighting conditions. |
| The use of glass compared to plastic tubes for extraction. | |
| Effect of addition of water to protein precipitation / LLE step. | |
| Use of solid phase extraction as an alternative to liquid extraction. | |
| Availability of internal standard for β carotene. | |
| Analytical – standardisation | No reference method listed on the JCTLM database. |
| No certified reference material i.e. primary standard, currently listed on the JCTLM database. | |
| EQA target values not achievable due to the lack of reference method and certified reference material. | |
| Analytical bias difficult to establish. | |
| Sigma metrics unable to ascertain bias for calculation as there is no JCTLM listed method or standard for vitamin A, E and β-carotene. | |
| Post-analytical | Common reference intervals cannot be established as traceability to reference measurement systems and certified reference materials is not available to provide the common anchor point for their development . |
EQA, external quality assurance; LLE, liquid-liquid extraction; JCTLM, Joint Committee for Traceability in Laboratory Medicine
This best practice report provides the opportunity to harmonise blood vitamin A, E and carotenoid methods, whilst working towards filling in the gaps common robust reference intervals. This essentially means laboratories can verify that their method fits the common reference intervals using the transference approach developed by The Clinical and Laboratory Standards Institute.119 Once each laboratory implements these recommendations a review process is essential to ensure ongoing harmonisation. EQA programs provide this ongoing review and improvement mechanism.8
In conclusion, these best practice recommendations represent the consensus views, in association with peer reviewed evidence, of the AACB Vitamins Working Party towards best practice for the collection, analysis and interpretation of vitamins A, E and carotenoids in blood. This report provides an opportunity for each laboratory to advance their work towards method harmonisation. In addition, this document provides a working model, implementing the CDC guideline, for the development of future laboratory based best practice documents.
Acknowledgments
We wish to acknowledge all the participants (from across the globe) of the RCPAQAP Vitamin Program who, through their participation and interactions over the years, have given us the foundation to develop this best practice report. In addition we wish to acknowledge Ms Jill Tate (former Vice-President of the AACB - Scientific and Regulatory Affairs division) for her constant unyielding support for the work conducted by this Vitamins Working Party; which has led to the formulation of this document.
Appendix 1A. Process for guideline development.
Appendix 1B. Literature Search Strategy and outcome. Adapted from Mishra et al. 2007.118
*Keyword search used for Medline and Embase searches
†MeSH term used for PubMed search – Note: no MeSH term for fat soluble vitamins
‡Two members of the Vitamins Working Party (VWP) reviewed the titles. Title was deemed relevant if one of the two reviewers highlighted it.
**‘No’ refers to the following: 168 not relevant; 22 may be relevant; and 22 articles that could not be found for evaluation.
Appendix 2A. Example method notes provided by TW
I attach a chromatogram of a pool of “normal” patient samples.
The chromatographic conditions are
Column: Brownlie C18 100mm x 4.6mm
Mobile Phase: 93% /7% methanol water, 2mL/min at 35C.
Detection: 290nm.
Quantitation: Vitamin A and E quantitated using retinyl acetate as the internal standard.
Appendix 2B. Example method notes provided by GW.
I attach a chromatogram of a middle aged male patient with normal retinol, tocopherol and β-carotene.
The chromatographic conditions are:
Column: Prodigy ODS3 150mm x 4.6mm 5um (this is a very standard analytical column)
Mobile phase: 98%/2% methanol water, 2 mL/min at 55C (standard methanol phase modified in this case with water to slow down elution and speed up by high temperature at high flow rate). NB Some authors just speed up by adding ethanol to methanol.
Detection: T 0 min 325 nm, T 2.5 min 292 nm, T 4.5 min 450 nm (programmed wavelength changes at peak maxima of each measured peak). Run with D2 lamp so not the best for baseline noise.
Quantitation: Retinol and β-carotene against retinyl acetate, tocopherol against tocopheryl acetate.
Key features:
Separation of all components without gradient in under 25 min.
This chromatogram to demonstrate the importance of resolving β-cryptoxanthin (a ubiquitous pro-retinol carotenoid) from tocopheryl acetate.
Carotenoids absorb strongly at around 275nm and will interfere with the tocopherols.
Carotenoids are not strong absorbers at 325nm and so resolution of the lutein/zeaxanthin at 1.7min from retinol is not essential.
Technically More Advanced Discussion Re Cis- Isomers
Furthermore another interesting chromatogram on a 16-month-old boy is shown below (same chromatographic conditions).
Key features:
Very high β-carotene with normal lycopene (from tomatoes) and β-cryptoxanthin (e.g. from orange juices).
Low normal retinol and tocopherol. Retinol levels are not related to β-carotene (different transport in serum).
Elution orders are predictable. Most polar dihydroxy < hydroxy < keto < naked β-carotene.
Carotenoids always elute in α-- and β- pairs. The α- carotenoid always elutes first on C18 columns.
You can easily see α-- and β-carotene. The α-- and β-canthaxanthin (keto-carotene) are visible at ∼9 min. A--cryptoxanthin (hydroxy-carotene) is concealed by tocopheryl acetate. Zeaxanthin and lutein (α-- β- pair despite different names, dihydroxy-carotene) coelute just next to retinol.
B-carotene has 4 major cis isomers 9-cis, 11-cis and 11-cis which elute in numeric order on C18 columns (different order on C30 columns).
9-cis has greatest concentration. Di-cis- are present in extremely low levels (unless is sample oxidised).
Look closely at the chromatogram to see a cis-isomer peak just visible at ∼22 min (resolved).
Now look closely at the contour plot of β-carotene below. You will see a small bulge rightwards at ∼340nm.
Now look closely at the spectrum of the β-carotene peak below showing a spectral overlay at the top of the peak and at 50% and 20% front and back (resp) points. You can see a peak with maximum at ∼340nm. This is the famous ‘cis peak’ which is absent in the all-trans isomer.
Hence the 9-cis peak is UNDER the all-trans peak and will be there for ALL C18 columns.
9-cis isomer has certain favourable nutritional properties but there is no need to resolve it for the purposes of our best practice document.
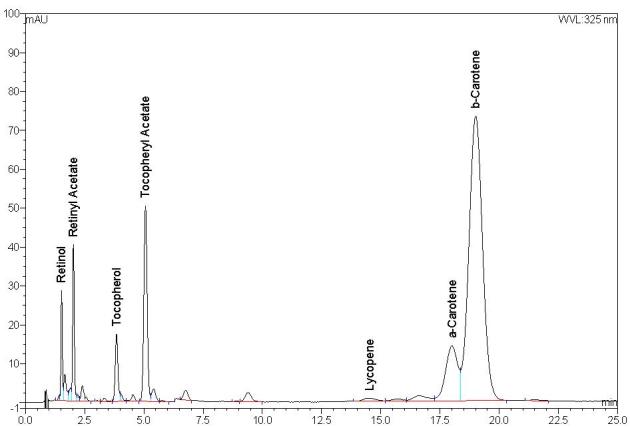
Appendix 3. External quality assurance programs for serum vitamin A, E and carotenoids. Adapted from Greaves, 2012.38
| EQA Program Organiser | Country | Survey name | Vitamins offered as part of the same program | Sample frequency | Website |
|---|---|---|---|---|---|
| RCPAQAP | Australia | Vitamin | A, E, B1, B2 B6 (whole blood and plasma), C, β-carotene and Total carotenoids | 2 samples per survey; 12 surveys per year. Total of 24 samples per annum. | www.rcpaqap.com.au/chempath |
| CDC | United States of America | VITAL | A | 9 samples to be run in duplicate over three days per survey, 2 surveys per year. Total 18 samples. | www.cdc.gov/labstandards/vitaleqa.html |
| DGKL | Germany | Vitamins and analgesics | A, B6, B12, D, E and folate | 2 samples per survey, 2 surveys per year. Total 4 samples per annum. | www.dgkl-rfb.de |
| NEQAS | United Kingdom | Vitamin Assays (Carotene and Vitamins A & E) | A, E, β carotene, Total carotenoids, Carotenoid fractions (Vitamin K offered as a separate program) | 5 samples per survey, 6 surveys per year. Total 30 samples per annum. | www.ukneqas.org.uk/ |
| NIST | United States of America | Micronutrients Quality Assurance Program | Total retinol, trans retinol, Vitamin E fractions, β-carotene, Carotenoid fractions | 5 samples per survey; 2 surveys per year. Total of 10 samples per annum. | www.nist.gov/mml/csd/organic/mmqap.cfm |
| SKML | The Netherlands | Vitamin A/E and β-carotene | A, E, β-carotene (Whole blood Vitamin B1 and B6 offered as a separate program) | 6 samples per survey, 2 surveys per year. Total 12 samples per annum. | www.skml.nl/en/rondzendingen/overzicht/rondzending?id=58 |
CAP, College of American Pathologists; CDC, Center for Disease Control and Prevention; DGKL, Deutsche Vereinte Gesellschaft fur Klinische Chemie und Laboratoriumsmedizin; RCPAQAP, RCPA Quality Assurance Programs; NEQAS, National External Quality Assurance Program; NIST, National Institute of Standards and Technology; SKML, Stichting Kwaliteitsbewaking Medische Laboratoriumdiagnostiek.
Footnotes
Competing Interests: None declared.
References
- 1.Schneider HG. Vitamins. In: Kaplan LA, Pesce AJ, editors. Clinical Chemistry: Theory, Analysis, Correlation. 5th ed. St. Louis, Missouri: Mosby; 2010. pp. 822–53. [Google Scholar]
- 2.World Health Organization . Global prevalence of vitamin A deficiency in populations at risk 1995–2005 WHO global database on vitamin A deficiency. Geneva: World Health Organization; 2009. http://whqlibdoc.who.int/publications/2009/9789241598019_eng.pdf (Accessed 24 June 2013) [Google Scholar]
- 3.Key TJ, Appleby PN, Allen NE, Travis RC, Roddam AW, Jenab M, et al. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr. 2007;86:672–81. doi: 10.1093/ajcn/86.3.672. [DOI] [PubMed] [Google Scholar]
- 4.Epplein M, Shvetsov YB, Wilkens LR, Franke AA, Cooney RV, Le Marchand L, et al. Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the Multiethnic Cohort Study: a nested case-control study. Breast Cancer Res. 2009;11:R49. doi: 10.1186/bcr2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donkena KV, Karnes RJ, Young CY. Vitamins and prostate cancer risk. Molecules. 2010;15:1762–83. doi: 10.3390/molecules15031762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RCPA Quality Assurance Programs- Chemical Pathology http://www.rcpaqap.com.au/chempath/ (Accessed 24 June 2013)
- 7.Armbruster D, Miller RR. The Joint Committee for Traceability in Laboratory Medicine (JCTLM): a global approach to promote the standardisation of clinical laboratory test results. Clin Biochem Rev. 2007;28:105–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Greaves RF. A guide to harmonisation and standardisation of measurands determined by liquid chromatography - tandem mass spectrometry in routine clinical biochemistry. Clin Biochem Rev. 2012;33:123–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Greaves R, Jolly L, Woollard G, Hoad K. Serum vitamin A and E analysis: comparison of methods between laboratories enrolled in an external quality assurance programme. Ann Clin Biochem. 2010;47:78–80. doi: 10.1258/acb.2009.009116. [DOI] [PubMed] [Google Scholar]
- 10.Greaves RF, Hoad KE, Woollard GA, Walmsley TA, Briscoe S, Johnson LA, et al. External quality assurance target setting with NIST SRM 968d material: performance in the 2010 Royal College of Pathologists of Australasia Quality Assurance Program with retinol, α-tocopherol and β-carotene. Ann Clin Biochem. 2011;48:480–2. doi: 10.1258/acb.2011.011054. [DOI] [PubMed] [Google Scholar]
- 11.International Union of Pure and Applied Chemistry (IUPAC) Commission on the Nomenclature of Biological Chemistry Definitive rules for the nomenclature of amino acids, steroids, vitamins, and carotenoids. J Am Chem Soc. 1960;82:5575–84. [Google Scholar]
- 12.Kurlandsky SB, Xiao JH, Duell EA, Voorhees JJ, Fisher GJ. Biological activity of all-trans retinol requires metabolic conversion to all-trans retinoic acid and is mediated through activation of nuclear retinoid receptors in human keratinocytes. J Biol Chem. 1994;269:32821–7. [PubMed] [Google Scholar]
- 13.IUPAC-IUB Joint Commission on Biological Nomenclature (JCBN) Nomenclature of retinoids – Recommendations 1981. Eur J Biochem. 1982;129:1–5. [Google Scholar]
- 14.Sommer A. Vitamin A deficiency and its consequences: A field guide to detection and Control. 3rd ed. Geneva: World Health Organization; 1995. [Google Scholar]
- 15.Beaton GH, Martorell R, Aronson KJ, Edmonston B, McCabe G, Ross AC, et al. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. 1993. Nutrition Policy Discussion Paper No. 13. http://www.unscn.org/layout/modules/resources/files/Policy_paper_No_13.pdf (Accessed 24 June 2013)
- 16.Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- 17.Goodman DS, Huang HS, Shiratori T. Mechanism of the biosynthesis of vitamin A from beta-carotene. J Biol Chem. 1966;241:1929–32. [PubMed] [Google Scholar]
- 18.Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the β-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. 2012;142:161S–5S. doi: 10.3945/jn.111.140756. [DOI] [PubMed] [Google Scholar]
- 19.Linus Pauling Institute at Oregon State University http://lpi.oregonstate.edu/infocenter/phytochemicals/carotenoids/#biological_activity (Accessed 24 June 2013)
- 20.Tang G. Techniques for measuring vitamin A activity from β-carotene. Am J Clin Nutr. 2012;96:1185S–8S. doi: 10.3945/ajcn.112.034603. [DOI] [PubMed] [Google Scholar]
- 21.Brigelius-Flohé R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 22.Traber MG, Burton GW, Hughes L, Ingold KU, Hidaka H, Malloy M, et al. Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism. J Lipid Res. 1992;33:1171–82. [PubMed] [Google Scholar]
- 23.Traber MG, Burton GW, Ingold KU, Kayden HJ. RRR- and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins. J Lipid Res. 1990;31:675–85. [PubMed] [Google Scholar]
- 24.Singh U, Devaraj S. Vitamin E: inflammation and atherosclerosis. Vitam Horm. 2007;76:519–49. doi: 10.1016/S0083-6729(07)76020-X. [DOI] [PubMed] [Google Scholar]
- 25.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–74. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 26.Zingg JM, Azzi A. Non-antioxidant activities of vitamin E. Curr Med Chem. 2004;11:1113–33. doi: 10.2174/0929867043365332. [DOI] [PubMed] [Google Scholar]
- 27.Vasudevan DM, Sreekumari S, Kannan V. Textbook of Biochemistry for Medical Students. 6th ed. Jaypee Brothers Medical Publishers; 2010. p. 388. [Google Scholar]
- 28.Oski FA, Barness LA. Vitamin E deficiency: a previously unrecognized cause of hemolytic anemia in the premature infant. J Pediatr. 1967;70:211–20. doi: 10.1016/s0022-3476(67)80416-5. [DOI] [PubMed] [Google Scholar]
- 29.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 30.Fraser CG. Quality Specifications In: Biological Variation: From Principles to Practice. Washington, DC, USA: AACC Press; 2001. pp. 29–66. [Google Scholar]
- 31.Jones GR, Sikaris K, Gill J. ‘Allowable limits of performance’ for external quality assurance programs -an approach to application of the Stockholm Criteria by the RCPA Quality Assurance Programs. Clin Biochem Rev. 2012;33:133–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Westgard JO. Westgard QC. 2009. http://www.westgard.com/right-quality-goal.htm (Accessed 24 June 2013)
- 33.Kenny D, Fraser CG, Hyltoft Petersen P, Kallner A. Strategies to set global analytical quality specifications in laboratory medicine – consensus agreement. Scand J Clin Lab Invest. 1999;59:585. [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention . Final Technical Report 2007: Phase I. Atlanta: US Department of Health and Human Services; 2008. Laboratory Medicine Best Practices: Developing an Evidence-Based Review and Evaluation Process. [Google Scholar]
- 35.Barreto-Lins MH, Campos FA, Azevedo MC, Flores H. A re-examination of the stability of retinol in blood and serum, and effects of a standardized meal. Clin Chem. 1988;34:2308–10. [PubMed] [Google Scholar]
- 36.Borel P, Dubois C, Mekki N, Grolier P, Partier A, Alexandre-Gouabau MC, et al. Dietary triglycerides, up to 40 g/meal, do not affect preformed vitamin A bioavailability in humans. Eur J Clin Nutr. 1997;51:717–22. doi: 10.1038/sj.ejcn.1600466. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-Campillo M, Larqué E, González-Silvera D, Martínez-Tomás R, García-Fernández M, Avilés F, et al. Changes in the carotenoid concentration in human postprandial chylomicron and antioxidant effect in HepG2 caused by differently processed fruit and vegetable soups. Food Chem. 2012;133:38–44. [Google Scholar]
- 38.Greaves RF. Vitamin A: Serum Vitamin A Analysis. In: Preedy VR, editor. Food and Nutritional Components in Focus: Chemistry, Analysis, Function and Effects. 1st Ed. Cambridge, UK: The Royal Society of Chemistry; 2012. pp. 162–83. [Google Scholar]
- 39.Young DS. Effects of Preanalytical Variables on Clinical Laboratory Tests. 3rd ed. Washington, DC: AACC Press; 2007. [Google Scholar]
- 40.Brouwer DA, Molin F, van Beusekom CM, van Doormaal JJ, Muskiet FA. Influence of fasting on circulating levels of α-tocopherol and β-carotene. Effect of short-term supplementation. Clin Chim Acta. 1998;277:127–39. doi: 10.1016/s0009-8981(98)00118-1. [DOI] [PubMed] [Google Scholar]
- 41.Traber MG, Jialal I. Measurement of lipid-soluble vitamins—further adjustment needed? Lancet. 2000;355:2013–4. doi: 10.1016/S0140-6736(00)02345-X. [DOI] [PubMed] [Google Scholar]
- 42.Talwar DK, Azharuddin MK, Williamson C, Teoh YP, McMillan DC, St J, O’Reilly D. Biological variation of vitamins in blood of healthy individuals. Clin Chem. 2005;51:2145–50. doi: 10.1373/clinchem.2005.056374. [DOI] [PubMed] [Google Scholar]
- 43.Mathews-Roth MM, Stampfer MJ. Some factors affecting determination of carotenoids in serum. Clin Chem. 1984;30:459–61. [PubMed] [Google Scholar]
- 44.Clark S, Youngman LD, Chukwurah B, Palmer A, Parish S, Peto R, et al. Effect of temperature and light on the stability of fat-soluble vitamins in whole blood over several days: implications for epidemiological studies. Int J Epidemiol. 2004;33:518–25. doi: 10.1093/ije/dyh064. [DOI] [PubMed] [Google Scholar]
- 45.Craft NE, Brown ED, Smith JC., Jr Effects of storage and handling conditions on concentrations of individual carotenoids, retinol, and tocopherol in plasma. Clin Chem. 1988;34:44–8. [PubMed] [Google Scholar]
- 46.Su Q, Rowley KG, O’Dea K. Stability of individual carotenoids, retinol and tocopherols in human plasma during exposure to light and after extraction. J Chromatogr B Biomed Sci Appl. 1999;729:191–8. doi: 10.1016/s0378-4347(99)00162-0. [DOI] [PubMed] [Google Scholar]
- 47.Hsing AW, Comstock GW, Polk BF. Effect of repeated freezing and thawing on vitamins and hormones in serum. Clin Chem. 1989;35:2145. [PubMed] [Google Scholar]
- 48.Comstock GW, Alberg AJ, Helzlsouer KJ. Reported effects of long-term freezer storage on concentrations of retinol, β-carotene, and α-tocopherol in serum or plasma summarized. Clin Chem. 1993;39:1075–8. [PubMed] [Google Scholar]
- 49.Rodriguez-Amaya DB. A guide to carotenoid analysis in foods. 2001. http://pdf.usaid.gov/pdf_docs/PNACQ929.pdf (Accessed 31 December 2013)
- 50.Lehrer M. Chromatographic Techniques. In: Kaplan LA, Pesce AJ, editors. Clinical Chemistry: Theory, Analysis, Correlation. 5th ed. St. Louis, Missouri: Mosby; 2010. pp. 81–117. [Google Scholar]
- 51.Honour JW. Development and validation of a quantitative assay based on tandem mass spectrometry. Ann Clin Biochem. 2011;48:97–111. doi: 10.1258/acb.2010.010176. [DOI] [PubMed] [Google Scholar]
- 52.Gueguen S, Herbeth B, Siest G, Leroy P. An isocratic liquid chromatographic method with diode-array detection for the simultaneous determination of α-tocopherol, retinol, and five carotenoids in human serum. J Chromatogr Sci. 2002;40:69–76. doi: 10.1093/chromsci/40.2.69. [DOI] [PubMed] [Google Scholar]
- 53.Talwar D, Ha TK, Cooney J, Brownlee C, O’Reilly DS. A routine method for the simultaneous measurement of retinol, α-tocopherol and five carotenoids in human plasma by reverse phase HPLC. Clin Chim Acta. 1998;270:85–100. doi: 10.1016/s0009-8981(97)00224-6. [DOI] [PubMed] [Google Scholar]
- 54.Lee BL, New AL, Ong CN. Simultaneous determination of tocotrienols, tocopherols, retinol, and major carotenoids in human plasma. Clin Chem. 2003;49:2056–66. doi: 10.1373/clinchem.2003.022681. [DOI] [PubMed] [Google Scholar]
- 55.Woollard GA, Hammer-Plecas A. Comparison of a complete commercial kitset Clinrep® with a validated extraction method for the determination of serum vitamins A & E. Clin Biochem Rev. 2008;29:S149. [Google Scholar]
- 56.Steghens JP, van Kappel AL, Riboli E, Collombel C. Simultaneous measurement of seven carotenoids, retinol and α-tocopherol in serum by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;694:71–81. doi: 10.1016/s0378-4347(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 57.Thibeault D, Su H, MacNamara E, Schipper HM. Isocratic rapid liquid chromatographic method for simultaneous determination of carotenoids, retinol, and tocopherols in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1077–83. doi: 10.1016/j.jchromb.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 58.Bortolotti A, Lucchini G, Barzago MM, Stellari F, Bonati M. Simultaneous determination of retinol, α-tocopherol and retinyl palmitate in plasma of premature newborns by reversed-phase high-performance liquid chromatography. J Chromatogr. 1993;617:313–7. doi: 10.1016/0378-4347(93)80505-x. [DOI] [PubMed] [Google Scholar]
- 59.Karppi J, Nurmi T, Olmedilla-Alonso B, Granado-Lorencio F, Nyyssönen K. Simultaneous measurement of retinol, α-tocopherol and six carotenoids in human plasma by using an isocratic reversed-phase HPLC method. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867:226–32. doi: 10.1016/j.jchromb.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Sharpless KE, Thomas JB, Sander LC, Wise SA. Liquid chromatographic determination of carotenoids in human serum using an engineered C30 and a C18 stationary phase. J Chromatogr B Biomed Appl. 1996;678:187–95. doi: 10.1016/0378-4347(95)00494-7. [DOI] [PubMed] [Google Scholar]
- 61.Nilsson LB, Eklund G. Direct quantification in bioanalytical LC-MS/MS using internal calibration via analyte/stable isotope ratio. J Pharm Biomed Anal. 2007;43:1094–9. doi: 10.1016/j.jpba.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 62.Albahrani AA, Rotarou V, Roche PJ, Greaves RF. Comparison of three commercial calibrators for α-tocopherol using liquid chromatography-tandem mass spectrometry. Clin Biochem. 2013;46:1884–8. doi: 10.1016/j.clinbiochem.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Albahrani AA, Rotarou V, Roche P, Greaves RF. Candidate reference method for quantitation of fat soluble vitamins using liquid chromatography -tandem mass spectrometry. Clin Biochem Rev. 2013;34:S20. doi: 10.1016/j.clinbiochem.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Chow FI, Omaye ST. Use of antioxidants in the analysis of vitamins A and E in mammalian plasma by high performance liquid chromatography. Lipids. 1983;18:837–41. [PubMed] [Google Scholar]
- 65.Mosca F, Fattorini D, Bompadre S, Littarru GP. Assay of coenzyme Q(10) in plasma by a single dilution step. Anal Biochem. 2002;305:49–54. doi: 10.1006/abio.2002.5653. [DOI] [PubMed] [Google Scholar]
- 66.Lucini L, Pellizzoni M, Baffi C, Molinari GP. Rapid determination of lycopene and β-carotene in tomato by liquid chromatography/electrospray tandem mass spectrometry. J Sci Food Agric. 2012;92:1297–303. doi: 10.1002/jsfa.4698. [DOI] [PubMed] [Google Scholar]
- 67.Finckh B, Kontush A, Commentz J, Hübner C, Burdelski M, Kohlschütter A. Monitoring of ubiquinol-10, ubiquinone-10, carotenoids, and tocopherols in neonatal plasma microsamples using high-performance liquid chromatography with coulometric electrochemical detection. Anal Biochem. 1995;232:210–6. doi: 10.1006/abio.1995.0009. [DOI] [PubMed] [Google Scholar]
- 68.Ferruzzi MG, Sander LC, Rock CL, Schwartz SJ. Carotenoid determination in biological microsamples using liquid chromatography with a coulometric electrochemical array detector. Anal Biochem. 1998;256:74–81. doi: 10.1006/abio.1997.2484. [DOI] [PubMed] [Google Scholar]
- 69.Cals MJ, Succari M, Pontezière C, Desmoulins D, Morgant G, Jardel A, et al. [Usual values of serum vitamin A using a modified fluorimetric method] Ann Biol Clin (Paris) 1982;40:685–9. [PubMed] [Google Scholar]
- 70.Thomas JB, Duewer DL, Mugenya IO, Phinney KW, Sander LC, Sharpless KE, et al. Preparation and value assignment of standard reference material 968e fat-soluble vitamins, carotenoids, and cholesterol in human serum. Anal Bioanal Chem. 2012;402:749–62. doi: 10.1007/s00216-011-5447-8. [DOI] [PubMed] [Google Scholar]
- 71.Joint Committee for Traceability in Laboratory Medicine Appendix III, The JCTLM Framework: A Framework for the international recognition of available higher-order reference materials, available higher-order reference measurement procedures and reference measurement laboratories for laboratory medicine. 2002. http://www.bipm.org/utils/en/pdf/jctlm_framework.pdf (Accessed 24 June 2013)
- 72.Joint Committee for Traceability in Laboratory Medicine Database of higher-order reference materials, measurement methods/procedures and services. 2011. http://www.bipm.org/jctlm/ (Accessed 24 June 2013)
- 73.Kock R, Seitz S, Delvoux B, Greiling H. Two high performance liquid chromatographic methods for the determination of α-tocopherol in serum compared to isotope dilution-gas chromatography-mass spectrometry. Eur J Clin Chem Clin Biochem. 1997;35:371–8. doi: 10.1515/cclm.1997.35.5.371. [DOI] [PubMed] [Google Scholar]
- 74.National Institute of Standards and Technology . Fat-Soluble Vitamins, Carotenoids, and Cholesterol in Human Serum. Gaithersburg, Marylands, USA: 1999. Certificate of Analysis, Standard Reference Material 968c. [Google Scholar]
- 75.National Institute of Standards and Technology . Fat-Soluble Vitamins, Carotenoids, and Cholesterol in Human Serum. Gaithersburg, Marylands, USA: 2008. Certificate of Analysis, Standard Reference Material 968d. [Google Scholar]
- 76.National Institute of Standards and Technology . Fat-Soluble Vitamins, Carotenoids, and Cholesterol in Human Serum. Gaithersburg, Marylands, USA: 2010. Certificate of Analysis, Standard Reference Material 968e. [Google Scholar]
- 77.Woollard GA, Hammer-Plecas A, Hoad KE, Walmsley TA, Briscoe S, Johnson LA, et al. Calibrators for human serum β carotene made from bovine serum. Clin Biochem Rev. 2013;34:S39. [Google Scholar]
- 78.Stöckl D, Sluss PM, Thienpont LM. Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chim Acta. 2009;408:8–13. doi: 10.1016/j.cca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 79.Bio-Rad Vitamin A and E control set. http://www.bio-rad.com/en-au/product/vitamin-ae-reversed-phase (Accessed 8 January 2014)
- 80.Vitamin Profiling - Chromsystems Instruments & Chemicals GmbH. Available at http://chromsystems.com/en/produkte/vitaminstatus (Accessed 8 January 2014)
- 81.RECIPE – HPLC Diagnostics http://www.recipe.de/en/products.html (Accessed 8 January 2014)
- 82.Jolly L, Woollard G, Hoad K, Freemantle M, Greaves R. Vitamin A and E methods: does a common calibrator improve the between laboratory agreement of results? Clin Biochem Rev. 2008;29:S128. [Google Scholar]
- 83.Miller WG, Myers GL, Rej R. Why commutability matters. Clin Chem. 2006;52:553–4. doi: 10.1373/clinchem.2005.063511. [DOI] [PubMed] [Google Scholar]
- 84.Westgard JO. Basic Method Validation. Madison, WI, USA: Westgard Quality Corporation; 1999. p. 250. [Google Scholar]
- 85.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(Suppl 1):S49–52. [PMC free article] [PubMed] [Google Scholar]
- 86.Ricos C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491–500. doi: 10.1080/00365519950185229. http://www.westgard.com/biodatabase1.htm (Accessed June 24th, 2013). [DOI] [PubMed] [Google Scholar]
- 87.Herbeth B, Didelot-Barthelemy L, Le Devehat C, Lemoine A. Rétinol, caroténoïdes et tocophérols plasmatiques: facteurs de variation biologique entre 18 et 45 ans. Ann Nutr Metab. 1988;32:297–304. doi: 10.1159/000177472. [DOI] [PubMed] [Google Scholar]
- 88.Koerbin G, Greaves RF, Robins H, Farquhar J, Hickman PE. Total intra-individual variation in sweat sodium and chloride concentrations for the diagnosis of cystic fibrosis. Clin Chim Acta. 2008;393:128–9. doi: 10.1016/j.cca.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 89.Joint Committee for Guides in Metrology International vocabulary of metrology – Basic and general concepts and associated terms (VIM) Third edition. 2012. http://www.bipm.org/utils/common/documents/jcgm/JCGM_200_2012.pdf (Accessed 24 June 2013)
- 90.International Standards Organization . Medical laboratories – Particular requirements for quality and competence. Geneva: ISO; 2012. ISO 15189:2012. [Google Scholar]
- 91.White GH, Farrance I, AACB Uncertainty of Measurement Working Group Uncertainty of measurement in quantitative medical testing: a laboratory implementation guide. Clin Biochem Rev. 2004;25:S1–24. [PMC free article] [PubMed] [Google Scholar]
- 92.Burns DT, Danzer K, Townshend A. Use of the terms “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002) Pure Appl Chem. 2002;74:2201–5. [Google Scholar]
- 93.Henry RJ, Segalove M. The running of standards in clinical chemistry and the use of the control chart. J Clin Pathol. 1952;5:305–11. doi: 10.1136/jcp.5.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Westgard JO, Barry PL, Hunt MR, Groth T. A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem. 1981;27:493–501. [PubMed] [Google Scholar]
- 95.Westgard J. Basic QC Practices. 3rd Edition. Madison, WI, USA: Westgard Quality Corporation; 2010. [Google Scholar]
- 96.Levey S, Jennings ER. The use of control charts in the clinical laboratory. Am J Clin Pathol. 1950;20:1059–66. doi: 10.1093/ajcp/20.11_ts.1059. [DOI] [PubMed] [Google Scholar]
- 97.Bermes EW, JR, Erviti V, Forman DT. Statistics, normal values, and quality control. In: Tietz NW, editor. Fundamentals of Clinical Chemistry. Philadelphia: WB Saunders; 1976. pp. 60–102. [Google Scholar]
- 98.Streitberg GS, Angel L, Sikaris KA, Bwititi PT. Laboratory information systems in clinical biochemistry in Australia. Aust J Med Sci. 2014 (in press) [Google Scholar]
- 99.The International System of Units (SI) http://www.bipm.org/en/si/si_brochure (Accessed 4 October 2013)
- 100.Jay Clinical Services Clinical Analyte Unit Conversion. http://dwjay.tripod.com/conversion.html (Accessed 30 September 2013) [Google Scholar]
- 101.Jones GR, Bryant S, Fullinfaw R, Ilett K, Miners JO, Morris RG, et al. Mass or molar? Recommendations for reporting concentrations of therapeutic drugs. Med J Aust. 2013;198:368–9. doi: 10.5694/mja12.10366. [DOI] [PubMed] [Google Scholar]
- 102.Hawkins RC, Johnson RN. The significance of significant figures. Clin Chem. 1990;36:824. [PubMed] [Google Scholar]
- 103.Badrick T, Wilson SR, Dimeski G, Hickman PE. Objective determination of appropriate reporting intervals. Ann Clin Biochem. 2004;41:385–90. doi: 10.1258/0004563041731583. [DOI] [PubMed] [Google Scholar]
- 104.World Health Organization Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. 2011. WHO/NHM/NHD/MNM/11.3. http://www.who.int/vmnis/indicators/retinol.pdf (Accessed 24 June 2013)
- 105.Lockitch G. Handbook of Diagnostic Biochemistry and Hematology in Normal Pregnancy. Boca Raton, FL: CRC Press; 1993. p. 537. [Google Scholar]
- 106.Lockitch G, Halstead AC, Wadsworth L, Quigley G, Reston L, Jacobson B. Age- and sex-specific pediatric reference intervals and correlations for zinc, copper, selenium, iron, vitamins A and E, and related proteins. Clin Chem. 1988;34:1625–8. [PubMed] [Google Scholar]
- 107.Soldin SJ, Brugnara C, Hicks JM. Pediatric Reference Ranges. 3rd Ed. Washington, DC, USA: AACC Press; 1999. [Google Scholar]
- 108.Karr M, Mira M, Causer J, Earl J, Alperstein G, Wood F, et al. Age-specific reference intervals for plasma vitamins A, E and β-carotene and for serum zinc, retinol-binding protein and prealbumin for Sydney children aged 9–62 months. Int J Vitam Nutr Res. 1997;67:432–6. [PubMed] [Google Scholar]
- 109.Lindblad BS, Patel M, Hamadeh M, Helmy N, Ahmad I, Dawodu A, et al. Age and sex are important factors in determining normal retinol levels. J Trop Pediatr. 1998;44:96–9. doi: 10.1093/tropej/44.2.96. [DOI] [PubMed] [Google Scholar]
- 110.Olmedilla B, Granado F, Blanco I, Rojas-Hidalgo E. Seasonal and sex-related variations in six serum carotenoids, retinol, and α-tocopherol. Am J Clin Nutr. 1994;60:106–10. doi: 10.1093/ajcn/60.1.106. [DOI] [PubMed] [Google Scholar]
- 111.Kieu NT, Yurie K, Hung NT, Yamamoto S, Chuyen NV. Simultaneous analysis of retinol, β-carotene and tocopherol levels in serum of Vietnamese populations with different incomes. Asia Pac J Clin Nutr. 2002;11:92–7. doi: 10.1046/j.1440-6047.2002.00283.x. [DOI] [PubMed] [Google Scholar]
- 112.Sklan D, Donoghue S. Vitamin E response to high dietary vitamin A in the chick. J Nutr. 1982;112:759–65. doi: 10.1093/jn/112.4.759. [DOI] [PubMed] [Google Scholar]
- 113.Hollander D. Intestinal absorption of vitamins A, E, D, and K. J Lab Clin Med. 1981;97:449–62. [PubMed] [Google Scholar]
- 114.Goodman DS. Vitamin A transport and retinol-binding protein metabolism. Vitam Horm. 1974;32:167–80. doi: 10.1016/s0083-6729(08)60011-4. [DOI] [PubMed] [Google Scholar]
- 115.Bjornson LK, Kayden HJ, Miller E, Moshell AN. The transport of α-tocopherol and β-carotene in human blood. J Lipid Res. 1976;17:343–52. [PubMed] [Google Scholar]
- 116.Baeten JM, Richardson BA, Bankson DD, Wener MH, Kreiss JK, Lavreys L, et al. Use of serum retinol-binding protein for prediction of vitamin A deficiency: effects of HIV-1 infection, protein malnutrition, and the acute phase response. Am J Clin Nutr. 2004;79:218–25. doi: 10.1093/ajcn/79.2.218. [DOI] [PubMed] [Google Scholar]
- 117.Weinman AR, Jorge SM, Martins AR, de Assis MD, Martinez FE, Camelo JS., Jr Assessment of vitamin A nutritional status in newborn preterm infants. Nutrition. 2007;23:454–60. doi: 10.1016/j.nut.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 118.Mishra A, Greaves R, Massie J. The limitations of sweat electrolyte reference intervals for the diagnosis of cystic fibrosis: a systematic review. Clin Biochem Rev. 2007;28:60–76. [PMC free article] [PubMed] [Google Scholar]
- 119.Clinical and Laboratory Standards Institute . CLSI document EP28-A3c. Third Edition. Wayne, PA, USA: CLSI; 2008. Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory. Approved Guideline. [Google Scholar]