Abstract
Our previous study reported that inactivation of Shox2 led to dysplasia and ankylosis of the temporomandibular joint (TMJ), and that replacing Shox2 with human Shox partially rescued the phenotype with a prematurely worn out articular disc. However, the mechanisms of Shox2 activity in TMJ development remain to be elucidated. In this study, we investigated the molecular and cellular basis for the congenital dysplasia of TMJ in Wnt1-Cre; pMes-stop Shox2 mice. We found that condyle and glenoid fossa dysplasia occurs primarily in the second week after the birth. The dysplastic TMJ of Wnt1-Cre; pMes-stop Shox2 mice exhibits a loss of Collagen type I, Collagen type II, Ihh and Gli2. In situ zymography and immunohistochemistry further demonstrate an up-regulation of matrix metalloproteinases (MMPs), MMP9 and MMP13, accompanied by a significantly increased cell apoptosis. In addition, the cell proliferation and expressions of Sox9, Runx2 and Ihh are no different in the embryonic TMJ between the wild type and mutant mice. Our results show that overexpression of Shox2 leads to the loss of extracellular matrix and the increase of cell apoptosis in TMJ dysplasia by up-regulating MMPs and down-regulating the Ihh signaling pathway.
Keywords: Shox2, temporomandibular joint, articular cartilage, extracellular matrix, matrix metalloproteinase
1. Introduction
Temporomandibular joint (TMJ), a mammalian synovial joint essential for jaw function, consists of the fibrocartilaginous disc and the condyle, derived from a secondary cartilage by endochondral ossification, and the glenoid fossa arising from the otic capsule through intramembranous ossification [1,2]. During the processes of TMJ development, a large number of transcription factors and growth factors have been implicated in the development of primary cartilage and endochondral ossification, such as Sox9, Runx2, and Ihh [3,4,5]. The Hedgehog (Hh) signaling pathway plays a pivotal role in digit joint formation, which has been implicated in the initial formation and separation of the articular disc from the apex of condyle, and the proper structure and tissue homeostasis of the TMJ, as evidenced by the absence of the articular disc formation in Ihh−/− and Gli2−/− mutant mice, and the partial disc ankylosis and TMJ dysplasia in ablation of Ihh in the cartilage of neonatal mice [3,6,7].
Short stature homeobox 2 (Shox2) is expressed in the condylar chondrocytes and glenoid fossa of the developing TMJ, and there is an absence of Shox2 expression in the hypertrophic chondrocyte zone. Previous reports showed that targeted inactivation of Shox2 leads to severe defects in a number of developing organs including heart, palate, and limb, and that TMJ exhibits dysplasia and ankylosis in mice [8,9,10,11,12]. We also reported that although Shox2Shox–KI/KI mice did not exhibit TMJ dysplasia and ankylosis at birth, a phenotype observed in mice carrying inactivation of Shox2 in the cranial neural crest lineage, the mice did develop a different TMJ defect, a premature wearing out of the articular disc postnatally [2,13,14]. These results support a role proposed for the Shox2 genes in skeletogenesis and TMJ. However, the precise mechanisms of Shox2 on the development and function of the TMJ at the molecular and cellular level remain poorly understood. In this study, we sought to investigate the effect of overexpression of Shox2 on TMJ development to explore the mechanism of the TMJ dysplasia in the Wnt1-Cre; pMes-stop Shox2 mice.
2. Results
2.1. Wnt1-Cre; pMes-stop Shox2 Mice Show an Overexpression of Shox2 in the Temporomandibular Joint (TMJ)
Shox2, expressed in the developing temporomandibular joint (TMJ), plays an important role in the process of TMJ development. We aimed to determine if the expression of Shox2 significantly up-regulated in the developing TMJ of Wnt1-Cre; pMes-stop Shox2 mice. Our in situ assays show an overexpression of Shox2 in the condyle of Wnt1-Cre; pMes-stop Shox2 TMJ at embryonic day 16.5 (E16.5), compared to the wild type mice (Figure 1A,B).
Figure 1.
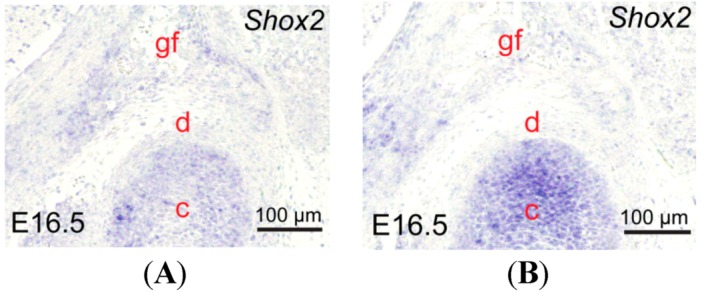
Expression of Shox2 in the developing temporomandibular joint (TMJ) of Wnt1-Cre; pMes-stop Shox2 mice. (A,B) In situ hybridization shows Shox2 expression in the developing TMJ of E16.5 wild type (A) and Wnt1-Cre; pMes-stop Shox2 embryo (B). c, condyle; d, disc; and gf, glenoid fossa.
2.2. Wnt1-Cre; pMes-stop Shox2 Mice Exhibit an Abnormal TMJ
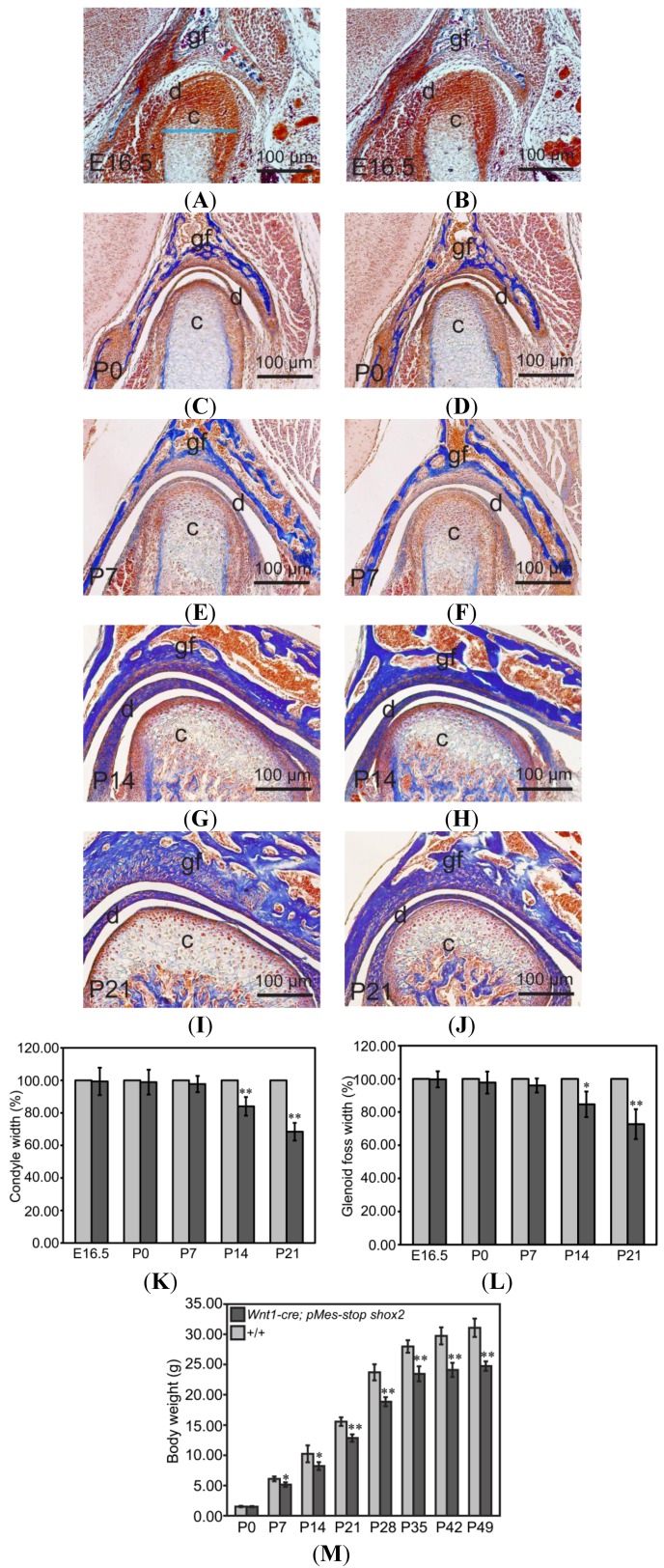
Histological analyses of TMJ showed no difference between wild-type mice and Wnt1-Cre; pMes-stop Shox2 mice at E16.5, postnatal day 0 (P0) and P7 stage, and a phenotype of TMJ dysplasia from the P14 stage. To investigate the cellular and molecular alternations that may contribute to this phenotype of TMJ, we started with a time course analysis of changes in the width of the condyle and glenoid fossa in the TMJ. We focused on the greatest width part where the defect appears most significant. The average width of the wild type condyle and glenoid fossa at each time point (n = 3 for each time point) is defined as 100%. We found that at E16.5 (Figure 2A,B), P0 (Figure 2C,D) and P7 (Figure 2E,F), the width of the condyle and glenoid fossa appeared comparable between wild type and mutant mice (n = 3). However, at P14 (Figure 2G,H), the width of the Wnt1-Cre; pMes-stop Shox2 condyle and glenoid fossa (n = 3) was reduced 15.97% ± 5.75% and 15.32% ± 7.69%, as compared to the wild type mice (Figure 1K,L). At P21 (Figure 2I,J), the Wnt1-Cre; pMes-stop Shox2 condyle and glenoid fossa (n = 3) was further reduced 31.58% ± 5.45% and 27.30% ± 9.01% compared to the wild type mice (Figure 2K,L). These results indicate that the condyle and glenoid fossa dysplasia occurs primarily in the second week after birth.
Figure 2.
Histological analyses of TMJ of Wnt1-Cre; pMes-stop Shox2 mice. (A–E) Coronal sections of the TMJ in wild type mice and Wnt1-Cre; pMes-stop Shox2 mice at E16.5 (A,B), P0 (C,D), P7 (E,F), P14 (G,H), and P21 (I,J); (K,L) Comparison of condyle and glenoid fossa width of the TMJ in wild type and Wnt1-Cre; pMes-stop Shox2 mice. Bright blue and red line segments in (A) point to the measured location of condyle and glenoid fossa on sections, respectively; and (M) Comparison of body weight at different time points in wild type and Wnt1-Cre; pMes-stop Shox2 mice. Standard deviation is shown as error bars. * p < 0.05, ** p < 0.01. c, condyle; d, disc; and gf, glenoid fossa.
To assess the effect of the condyle and glenoid fossa dysplasia on the body weight in Wnt1-Cre; pMes-stop Shox2 mice, we measured the changes of body weight at different time points (P0, P7, P14, P21, P28, P35, P42 and P49) (Figure 2M). The results show a comparable body weight for both wild type and Wnt1-Cre; pMes-stop Shox2 mice at birth, but this differs significantly at P7 and at other time points, indicating that the changes of condyle and glenoid fossa in Wnt1-Cre; pMes-stop Shox2 mice may affect the function of TMJ, resulting in the loss of body weight, and develop a wasting syndrome, clinically defined as TMJ disorders.
2.3. Wnt1-Cre; pMes-stop Shox2 Mice Display Normal Gene Expression in the Developing TMJ
Previous reports show that tissue-specific inactivation of Shox2 in the cranial neural crest cells leads to TMJ dysplasia and ankylosis, accompanied by significant down-regulation of Sox9, Runx2 and Ihh [2]. We sought to determine if the expression of these genes were altered in the developing TMJ of Wnt1-Cre; pMes-stop Shox2 mice. Our immunohistochemical assays display no alteration of Sox9 and Runx2 expression levels in the wild type and Wnt1-Cre; pMes-stop Shox2 TMJ at E14.5 and E16.5 (Figure 3A–H). On the other hand, in situ hybridization shows that comparable Ihh expression is also retained in the Wnt1-Cre; pMes-stop Shox2 TMJ at E15.5 (Figure 3I,J). These observations suggest that overexpression of Shox2 does not affect early TMJ development.
Figure 3.
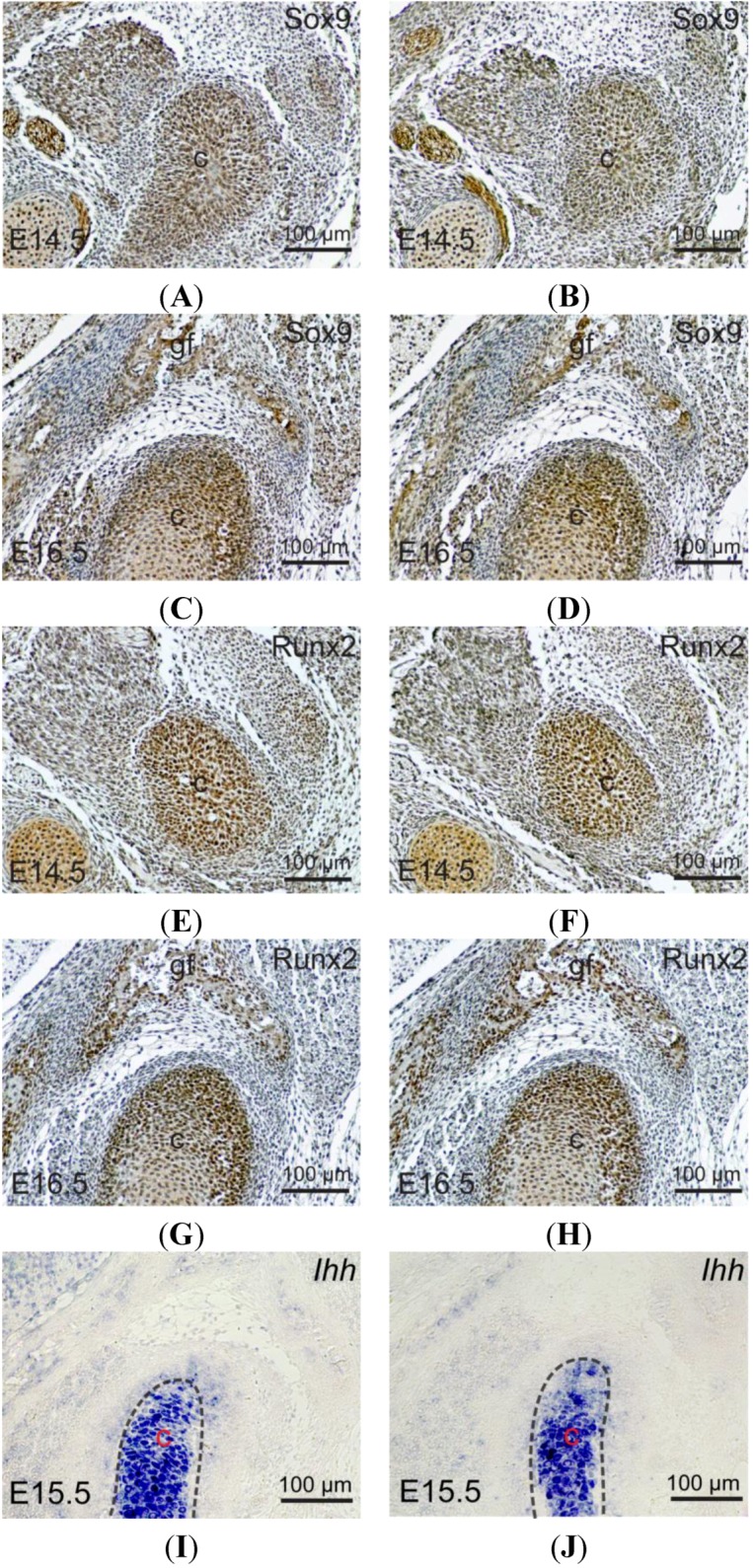
Expressions of Sox9, Runx2 and Ihh in the developing TMJ of Wnt1-Cre; pMes-stop Shox2 mice. (A–D) Immunohistochemical staining shows Sox9 expression in the developing condyle of E14.5 (A,B) and E16.5 (C,D) wild type and Wnt1-Cre; pMes-stop Shox2 embryo; (E–H) Immunohistochemical staining shows Runx2 expression in the developing TMJ of E14.5 (E,F) and E16.5 (G,H) wild type and Wnt1-Cre; pMes-stop Shox2 embryo; and (I,J) In situ hybridization shows Ihh expression in the developing condyle of E15.5 wild type and Wnt1-Cre; pMes-stop Shox2 embryo. c, condyle; and gf, glenoid fossa.
2.4. Reduction of Extracellular Matrix (ECM) Proteins in the Wnt1-Cre; pMes-stop Shox2 TMJ
The articular cartilage is composed of chondrocyte and extracellular matrix (ECM) components including collagens, proteoglycans (PGS) and glycosaminoglycans (GAGs) [15]. These ECM components play an important role in resistance to compressive forces and in maintaining the tensile properties of the tissue. Alterations of the ECM components are associated with cartilage degeneration [16], so we wondered if changes in the ECM components would account for the dysplasia of condyle and glenoid fossa in the Wnt1-Cre; pMes-stop Shox2 TMJ. We examined the expressions of several ECM components including Collagen type I (Col I), Collagen type II (Col II), and Aggrecan using P0 and P7 mice (Figure 4A–L). Immunohistochemcal analyses show that the expressions of Col I, Col II, and Aggrecan are comparable between wild type and Wnt1-Cre; pMes-stop Shox2 mice at P0 stage. However, at P7 stage, the expression of Col I is reduced in the glenoid fossa, and Col II expression is also decreased in the condyle in the TMJ of Wnt1-Cre; pMes-stop Shox2 mice. In addition, Aggrecan expression is not affected in the condyle of Wnt1-Cre; pMes-stop Shox2 TMJ as compared to the wild type mice. Thus, the reduction of Col I and Col II expressions could alter the ECM composition and contribute to the dysplasia of condyle and glenoid fossa in the Wnt1-Cre; pMes-stop Shox2 TMJ.
Figure 4.
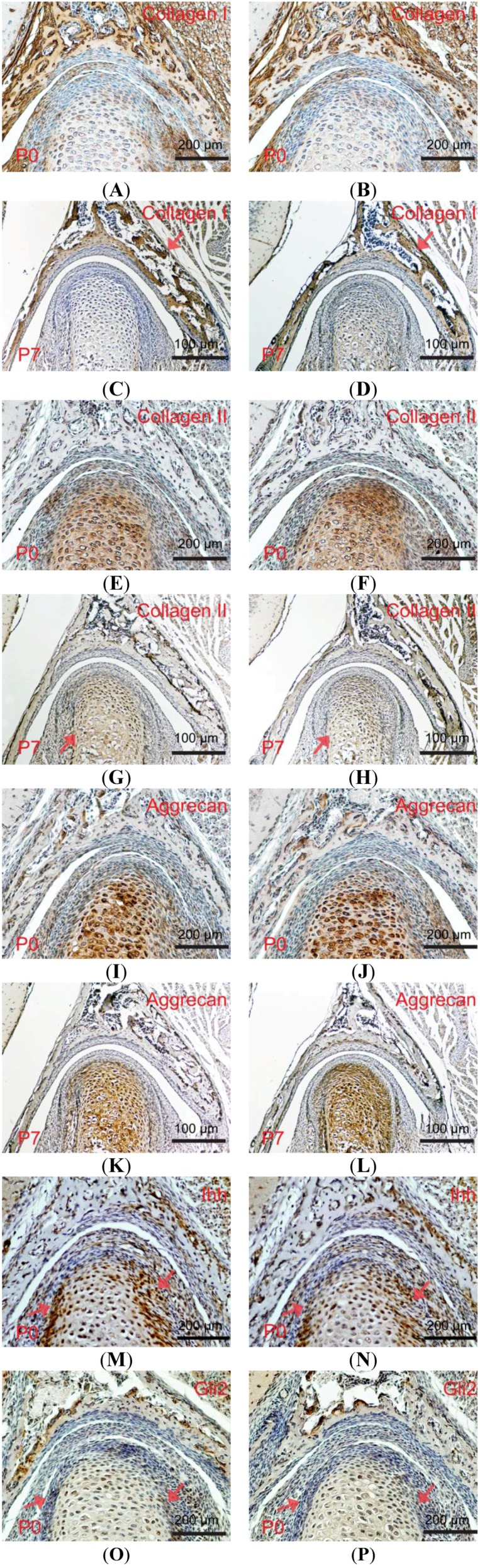
Expressions of extracellular matrix, Ihh and Gli2 in the postnatal TMJ of Wnt1-Cre; pMes-stop Shox2 mice. (A–L) Expressions of Col I (A–D), Col II (E–H), and Aggrecan (I–L) in the TMJ of P0 and P7 wild type (A,C,E,G,I,K) and Wnt1-Cre; pMes-stop Shox2 (B,D,F,H,J,L); (M–P) Immunohistochemical staining on coronal sections of P0 wild type (M,O) and Wnt1-Cre; pMes-stop Shox2 (N,P) TMJ shows Ihh (M,N) and Gli2 (O,P) protein expression and distribution. Arrow heads point to the condyle and glenoid fossa where expression is altered.
2.5. Down-Regulated Ihh Signaling Pathway in the Wnt1-Cre; pMes-stop Shox2 TMJ
Ihh is critical for the completion of postnatal TMJ growth and organization. To determine if it affected the dysplasia of the condyle and glenoid fossa in Wnt1-Cre; pMes-stop Shox2 mice, we investigated the protein levels of Ihh and Gli2 by immunohistochemistry at the P0 stage (Figure 4M–P). In the Wnt1-Cre; pMes-stop Shox2 mice, Ihh and Gli2 was significantly down-regulated compared to the wild type mice. Thus, the down-regulation of Ihh and Gli2 expressions can act as a promoter to the formation of condyle and glenoid fossa dysplasia in Wnt1-Cre; pMes-stop Shox2 mice.
2.6. Up-Regulated Matrix Metalloproteinases (MMPs) Activity in the Wnt1-Cre; pMes-stop Shox2 TMJ
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases capable of degrading ECM, including collagens and Aggrecan. We asked if the reduced levels of Col I and Col II in the Wnt1-Cre; pMes-stop Shox2 TMJ were a consequence of enhanced MMPs activities. To test this possibility, we first conducted in situ zymography to determine total MMPs activity in P0 wild type and Wnt1-Cre; pMes-stop Shox2 TMJ. As shown in Figure 5A,B, while strong MMPs activity is found in the glenoid fossa, and an above background level of MMPs activity is also seen in the condyle. Consistent with this enhanced total MMPs activity, we found that MMP9 and MMP13, which have been shown to be elevated in the TMJ osteoarthritis and are capable of degrading collagens and Aggrecan [17,18], exhibit enhanced expression in the Wnt1-Cre; pMes-stop Shox2 TMJ at P0 and/or P7 stage (Figure 5E–L). These results indicate that enhanced MMPs activities are responsible for the reduction of ECM components, which may ultimately lead to the dysplasia of condyle and glenoid fossa in Wnt1-Cre; pMes-stop Shox2 mice.
Figure 5.
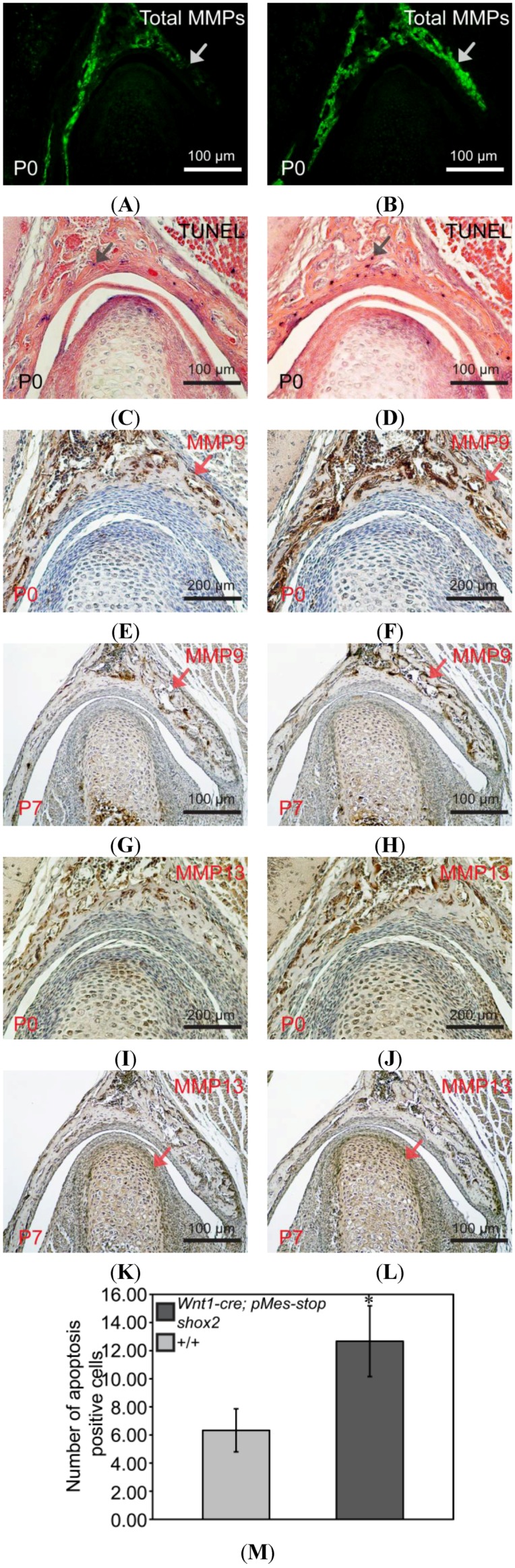
Altered expressions of MMPs and apoptosis in the postnatal TMJ of Wnt1-Cre; pMes-stop Shox2 mice. (A,B) In situ zymography on coronal sections of wild type (A) and Wnt1-Cre; pMes-stop Shox2 (B) TMJ shows MMPs activity. The ectopic MMPs activity in the glenoid fossa (arrow heads) of the Wnt1-Cre; pMes-stop Shox2 TMJ; TUNEL assay on coronal sections of P0 wild type (C) and Wnt1-Cre; pMes-stop Shox2 (D) TMJ shows an increase of apoptotic cells in the glenoid fossa of the Wnt1-Cre; pMes-stop Shox2 TMJ. Arrow heads in (C,D) point to the apoptotic cells in the glenoid fossa; (E–L) Immunohistochemical staining on coronal sections of wild type (E,G,I,K) and Wnt1-Cre; pMes-stop Shox2 (F,H,J,L) shows expressions of MMP9 (E–H) and MMP13 (I–L). Arrow heads in (E–H,K,L) point to the protein levels of MMP9 and MMP13in the condyle and glenoid fossa where expression is altered; (M) Comparison of numbers of apoptotic cells in the glenoid fossa between wild type and Wnt1-Cre; pMes-stop Shox2 mice. Standard deviation is shown as error bars. * p < 0.05.
2.7. Enhanced Apoptosis in the Condyle of the Wnt1-Cre; pMes-stop Shox2 TMJ
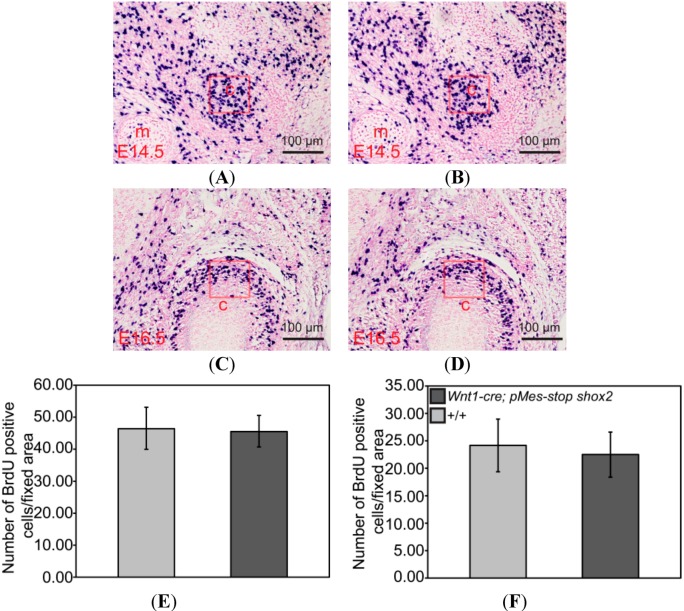
We further examined if there existed cellular defects that could potentially contribute to the phenotype of condyle and glenoid fossa in the Wnt1-Cre; pMes-stop Shox2 TMJ. Since apoptosis, or programmed cell death, is known to be responsible for tissue degeneration correlated with the severity of condyle and glenoid fossa pathologic processes [19,20], we determined if there was any alteration in cell apoptosis in the TMJ by TUNEL assay. Indeed, our results demonstrate a dramatically increased number of apoptotic cells in the glenoid fossa of Wnt1-Cre; pMes-stop Shox2 TMJ compared to that in the wild type at P0 (n = 3) (Figure 5C,D,M), indicating a contribution of apoptosis to the glenoid fossa dysplasia in Wnt1-Cre; pMes-stop Shox2 mice. In contrast, BrdU labeling assay revealed unaltered cell proliferation rate in the condyle (n = 3) of the Wnt1-Cre; pMes-stop Shox2 TMJ compared to the wild type at E14.5 and E16.5 (Figure 6A–F).
Figure 6.
Changes of proliferation in the TMJ of Wnt1-Cre; pMes-stop Shox2 mice. (A–D) BrdU labeling of cell proliferation in the condylar condensation of an E14.5 (A,B) and E16.5 (C,D) in wild type and Wnt1-Cre; pMes-stop Shox2 embryo; (E,F) Comparison of numbers of BrdUpositive cells in the fixed area (red frame) of the condylar primoridain between wild type (E) and Wnt1-Cre; pMes-stop Shox2 embryos (F). Standard deviation is shown as error bars.c, condyle; and m, Meckel’s cartilage.
3. Discussion
Shox2 and Shox, found only in vertebrates, are implicated to play a role in the development of the internal skeleton and its related structures [21]. In humans, mutations of Shox gene function have been associated with a series of short-stature conditions, such as Léri-Weill dyschondrosteosis, Turner syndrome, and Langer dysplasia, which exhibit abnormalities in the skeletal development [22,23]. Shox2 expression has also been found in the development of limbs in a complementary pattern to that of Shox [21]. However, any known syndrome that has been linked to Shox2 mutations remains unclear thus far. In mice, targeted inactivation of Shox2 leads to the virtual elimination of the stylopod in developing limbs [8,9]. A failure in growth, chondrogenesis, and endochondral ossification occurred in the mutant stylopodial cartilaginous element, accompanied by a down-regulation of several genes that are known to be essential for skeletogenesis, including Runx2, Runx3 and Ihh. Indeed, besides the limb phenotype, Shox2-deficient mice exhibit severe defects in a number of developing organs (heart and palate), as well as the TMJ that exhibits dysplasia and ankylosis [10,11,12]. These observations demonstrate a crucial role for the Shox2 genes in the development of the long bone that undergoes the endochondral ossification. Our previous studies support that inactivation of Shox2 leads to the abnormal development of TMJ [2,14], and the overexpression of Shox2 in the heart results in abnormal cardiac formation [24]. Therefore, we assume that the overexpression of Shox2 may affect the TMJ development.
The changes of the ECM composition are associated with pathological processes of cartilage degeneration in TMJ osteoarthritis, accompanied by the up-regulation of MMPs [17,25,26]. Cartilage degeneration is a characteristic feature that results from the loss of collagens and PGS. It is well established that elevated MMPs activities are responsible for degradation of the ECM in the processes of TMJ osteoarthritis. Our results show that the phenotype of condyle and glenoid fossa dysplasia, a congenital non-pathological degenerative defect, is associated with reduced amounts of Col I, Col II, Ihh and Gli2 in the Wnt1-Cre; pMes-stop Shox2 TMJ and an increase of apoptosis activity. The fact that enhanced MMPs activity was observed in the condyle and glenoid fossa of the Wnt1-Cre; pMes-stop Shox2 TMJ suggests a similar mechanism for this non-pathological degenerative process. Although it is currently unclear whether the reduction in Ihh and Gli2 expression is a causative factor in the elevated MMPs activity in postnatal Wnt1-Cre; pMes-stop Shox2 TMJ, it is consistent with an important role for Ihh signaling pathway in maintaining the proper structure and tissue homeostasis of postnatal TMJ [9], and warrants future investigation.
Congenital TMJ ankylosis has been reported in a patient with a short stature phenotype [27], however genetic alterations leading to congenital dysplasia and ankylosis of the TMJ are still unclear. Our results demonstrate a crucial role for Shox2 in TMJ function and development, and provide evidence for a genetic association with congenital non-pathological cartilage degeneration. Histological analysis and an investigation of the molecular mechanisms involved in these processes will contribute not only to basic science but to clinical research.
4. Experimental Section
4.1. Mice Embryonic and Postnatal Heads Collection
All animal procedures were performed according to guidelines approved by the animal care committee of Fujian University of Traditional Chinese Medicine, Fuzhou, China. The Wnt1-Cre; pMes-stop Shox2 mice from the cross of Wnt1-Cre mice [2] with pMes-stop Shox2 mice [24] were provided from the lab of Yiping Chen. The presence of a vaginal plug indicates E0.5. Embryonic heads were fixed in 4% paraformaldehyde (PFA)/phosphate buffer saline (PBS) at 4 °C overnight. The heads from P0, P7, P14, and P21 mice were fixed and decalcified in Surgipath Decalcifier I (Leica Biosystems Richmond Inc., Richmond, CA, USA) for various days according to the age of the mouse.
4.2. Histological Analyses
Paraffin-embedded heads were sectioned at 10 µm with a microtome. To analyses the histology of TMJ, the serial sections were stained with Alizarin red/Alcian blue (Sigma, Saint Louis, MO, USA) staining according to standard procedures.
4.3. Immunohistochemistry and in Situ Hybridization
Paraffin-embedded heads were sectioned at 8 μm for immunohistochemistry and 10 μm for in situ hybridization. Immunohistochemical staining was performed using polyclonal antibody against Sox9 (1:500, ab26414), Runx2 (1:1000, ab76956), Aggrecan (1:500, ab36861), Col I (1:500, ab34710), Col II (1:200, ab53047), MMP9 (1:300, ab38898), MMP13 (1:50, ab75606), Ihh (1:200, ab39634), Gli2 (1:100, ab7195) from Abcam (Cambridge, MA, USA) according to the manufacturer’s instruction. In situ hybridization was performed on 10 μm paraffin sections with Ihh and Shox2 digoxigenin-labeled probes as described [9,10].
4.4. In Situ Zymography
P0 mice heads were fixed in zinc-based fixative (ZBF) and embedded in optimum cutting temperature (OCT) compound. 10 μm Sections were incubated with dye-quenched (DQ)-gelatin (E12055, Molecular Probes Europe BV, Leiden, The Netherlands) according to the manufacturer’s instruction [28]. The gelatinolytic activity was observed as green fluorescence under a fluorescence microscopy (Fluorescence Microscope Model Axioskop 50, CarlZeiss, Oberkochen, Germany).
4.5. TUNEL Assay and Cell Proliferation Assay
Apoptosis of the TMJ was determined by in situ Cell Death Detection Kit and AP (alkaline phosphatase) from Roche (Mannheim, Germany). Paraffin-embedded heads were sectioned at 5 μm, and subjected to immunodetection for measurement of apoptosis according to the manufacturer’s protocol. Pregnant mice at E14.5 and E16.5 were injected with 1.5 mL labeling reagent/100 g body weight using the BrdU labeling and Detection Kit II from Roche (Mannheim, Germany) for 2 h. The Embryonic heads were fixed in Carnoy’s fixative, ethanol-dehydrated, paraffin-embedded, and sectioned at 5 μm. The sections were subjected to immunodetection for analysis of proliferation according to the manufacturer. Three independent experiments were conducted for TUNEL or cell proliferation assay.
4.6. Statistical Analysis
All experiments were repeated at least three times. Data were presented as mean ± Standard Deviation. Statistical analyses of the data were calculated using the Student’s t-test. p-values less than 0.05 were considered as statistically significant.
5. Conclusions
In summary, overexpression Shox2 leads to the loss of ECM and the increase of cell apoptosis in the TMJ dysplasia by up-regulation of MMPs and down-regulation of the Ihh signaling pathway. Our results suggest that Shox2 is essential for the process of TMJ development in mice, indicating that it may be further developed as a therapeutic target for the treatment of congenital TMJ dysplasia.
Acknowledgments
This study was supported by the Key Project of Fujian Provincial Department of Science and Technology (Grant no. 2012Y0046) and the Young Talent Scientific Research Project of Fujian Province Universities (Grant no. JA12165). The authors appreciate the help of Yiping Chen at the Department of Cell and Molecular Biology, Tulane University, New Orleans, LA, USA.
Supplementary Files
Author Contributions
X.Li and X.Liu designed and carried out the experiments, and wrote the manuscript; W.L., H.Y., X.W. and F.L. performed the experiment and analyzed the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wang Y., Liu C., Rohr J., Liu H., He F., Yu J., Sun C., Li L., Gu S., Chen Y. Tissue interaction is required for glenoid fossa development during temporomandibular joint formation. Dev. Dyn. 2011;240:2466–2473. doi: 10.1002/dvdy.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu S., Wei N., Yu L., Fei J., Chen Y. Shox2-deficiency leads to dysplasia and ankylosis of the temporomandibular joint in mice. Mech. Dev. 2008;125:729–742. doi: 10.1016/j.mod.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purcell P., Joo B.W., Hu J.K., Tran P.V., Calicchio M.L., O’Connell D.J., Maas R.L., Tabin C.J. Temporomandibular joint formation requires two distinct hedgehog-dependent steps. Proc. Natl. Acad. Sci. USA. 2009;106:18297–18302. doi: 10.1073/pnas.0908836106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori-Akiyama Y., Akiyama H., Rowitch D.H., de Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. USA. 2003;100:9360–9365. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata S., Yokohama-Tamaki T. An in situ hybridization study of Runx2, Osterix, and Sox9 in the anlagen of mouse mandibular condylar cartilage in the early stages of embryogenesis. J. Anat. 2008;213:274–283. doi: 10.1111/j.1469-7580.2008.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibukawa Y., Young B., Wu C., Yamada S., Long F., Pacifici M., Koyama E. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev. Dyn. 2007;236:426–434. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- 7.Ochiai T., Shibukawa Y., Nagayama M., Mundy C., Yasuda T., Okabe T., Shimono K., Kanyama M., Hasegawa H., Maeda Y., et al. Indian hedgehog roles in post-natal TMJ development and organization. J. Dent. Res. 2010;89:349–354. doi: 10.1177/0022034510363078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobb J., Dierich A., Huss-Garcia Y., Duboule D. A mouse model for human short-stature syndromes identifies Shox2 as an upstream regulator of Runx2 during long-bone development. Proc. Natl. Acad. Sci. USA. 2006;103:4511–4515. doi: 10.1073/pnas.0510544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu L., Liu H., Yan M., Yang J., Long F., Muneoka K., Chen Y. Shox2 is required for chondrocyte proliferation and maturation in proximal limb skeleton. Dev. Biol. 2007;306:549–559. doi: 10.1016/j.ydbio.2007.03.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L., Gu S., Alappat S., Song Y., Yan M., Zhang X., Zhang G., Jiang Y., Zhang Z., Zhang Y., et al. Shox2-deficient mice exhibit a rare type of incomplete clefting of the secondary palate. Development. 2005;132:4397–4406. doi: 10.1242/dev.02013. [DOI] [PubMed] [Google Scholar]
- 11.Gu S., Wei N., Yu X., Jiang Y., Fei J., Chen Y. Mice with an anterior cleft of the palate survive neonatal lethality. Dev. Dyn. 2008;237:1509–1516. doi: 10.1002/dvdy.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaschke R.J., Hahurij N.D., Kuijper S., Just S., Wisse L.J., Deissler K., Maxelon T., Anastassiadis K., Spitzer J., Hardt S.E., et al. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation. 2007;115:1830–1838. doi: 10.1161/CIRCULATIONAHA.106.637819. [DOI] [PubMed] [Google Scholar]
- 13.Liu H., Chen C.H., Espinoza-Lewis R.A., Jiao Z., Sheu I., Hu X., Lin M., Zhang Y., Chen Y. Functional redundancy between human SHOX and mouse Shox2 genes in the regulation of sinoatrial node formation and pacemaking function. J. Biol. Chem. 2011;286:17029–17038. doi: 10.1074/jbc.M111.234252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X., Liu H., Gu S., Liu C., Sun C., Zheng Y., Chen Y. Replacing Shox2 with human SHOX leads to congenital disc degeneration of the temporomandibular joint in mice. Cell Tissue Res. 2014;355:345–354. doi: 10.1007/s00441-013-1743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Z., Feng J., Shibata T., Hu J., Zhang Z. Type II collagen and aggrecan mRNA expression by in situ hybridization in rabbit temporomandibular joint posterior attachment following disc displacement. Arch. Oral Biol. 2003;48:55–62. doi: 10.1016/S0003-9969(02)00158-9. [DOI] [PubMed] [Google Scholar]
- 16.Garnero P., Rousseau J.C., Delmas P.D. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 2000;43:953–968. doi: 10.1002/1529-0131(200005)43:5<953::AID-ANR1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Planello A.C., Campos M.I., Meloto C.B., Secolin R., Rizatti-Barbosa C.M., Line S.R., de Souza A.P. Association of matrix metalloproteinase gene polymorphism with temporomandibular joint degeneration. Eur. J. Oral Sci. 2011;119:1–6. doi: 10.1111/j.1600-0722.2010.00803.x. [DOI] [PubMed] [Google Scholar]
- 18.Kapila S., Wang W., Uston K. Matrix metalloproteinase induction by relaxin causes cartilage matrix degradation in target synovial joints. Ann. N. Y. Acad. Sci. 2009;1160:322–328. doi: 10.1111/j.1749-6632.2009.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caltabiano R., Leonardi R., Musumeci G., Bartoloni G., Rusu M.C., Almeida L.E., Loreto C. Apoptosis in temporomandibular joint disc with internal derangement involves mitochondrial-dependent pathways. An in vivo study. Acta Odontol. Scand. 2013;71:577–583. doi: 10.3109/00016357.2012.700060. [DOI] [PubMed] [Google Scholar]
- 20.Spears R.1., Oakes R., Bellinger L.L., Hutchins B. Tumour necrosis factor-α and apoptosis in the rat temporomandibular joint. Arch. Oral Biol. 2003;48:825–834. doi: 10.1016/S0003-9969(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 21.Clement-Jones M., Schiller S., Rao E., Blaschke R.J., Zuniga A., Zeller R., Robson S.C., Binder G., Glass I., Strachan T., et al. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum. Mol. Genet. 2000;9:695–702. doi: 10.1093/hmg/9.5.695. [DOI] [PubMed] [Google Scholar]
- 22.Bobick B.E., Cobb J. Shox2 regulates progression through chondrogenesis in the mouse proximal limb. J. Cell Sci. 2012;125:6071–6083. doi: 10.1242/jcs.111997. [DOI] [PubMed] [Google Scholar]
- 23.Hirschfeldova K., Solc R., Baxova A., Zapletalova J., Kebrdlova V., Gaillyova R., Prasilova S., Soukalova J., Mihalova R., Lnenicka P., et al. SHOX gene defects and selected dysmorphic signs in patients of idiopathic short stature and Léri-Weill dyschondrosteosis. Gene. 2012;491:123–127. doi: 10.1016/j.gene.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Espinoza-Lewis R.A., Liu H., Sun C., Chen C., Jiao K., Chen Y. Ectopic expression of Nkx2. 5 suppresses the formation of the sinoatrial node in mice. Dev. Biol. 2011;356:359–369. doi: 10.1016/j.ydbio.2011.05.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y.D., Liao L.F., Zhang H.Y., Lu L., Jiao K., Zhang M., Zhang J., He J.J., Wu Y.P., Chen D., et al. Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis. Osteoarthr. Cartil. 2014;22:302–312. doi: 10.1016/j.joca.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gepstein A., Arbel G., Blumenfeld I., Peled M., Livne E. Association of metalloproteinases, tissue inhibitors of matrix metalloproteinases, and proteoglycans with development, aging, and osteoarthritis processes in mouse temporomandibular joint. Histochem. Cell Biol. 2003;120:23–32. doi: 10.1007/s00418-003-0544-1. [DOI] [PubMed] [Google Scholar]
- 27.Komorowska A. Congenital temporomandibular joint ankylosis—A case report. Eur. J. Orthod. 1997;19:243–248. doi: 10.1093/ejo/19.3.243. [DOI] [PubMed] [Google Scholar]
- 28.Sakakura Y., Hosokawa Y., Tsuruga E., Irie K., Yajima T. In situ localization of gelatinolytic activity during development and resorption of Meckel’s cartilage in mice. Eur. J. Oral Sci. 2007;115:212–223. doi: 10.1111/j.1600-0722.2007.00447.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.