Abstract
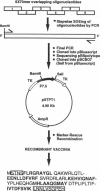
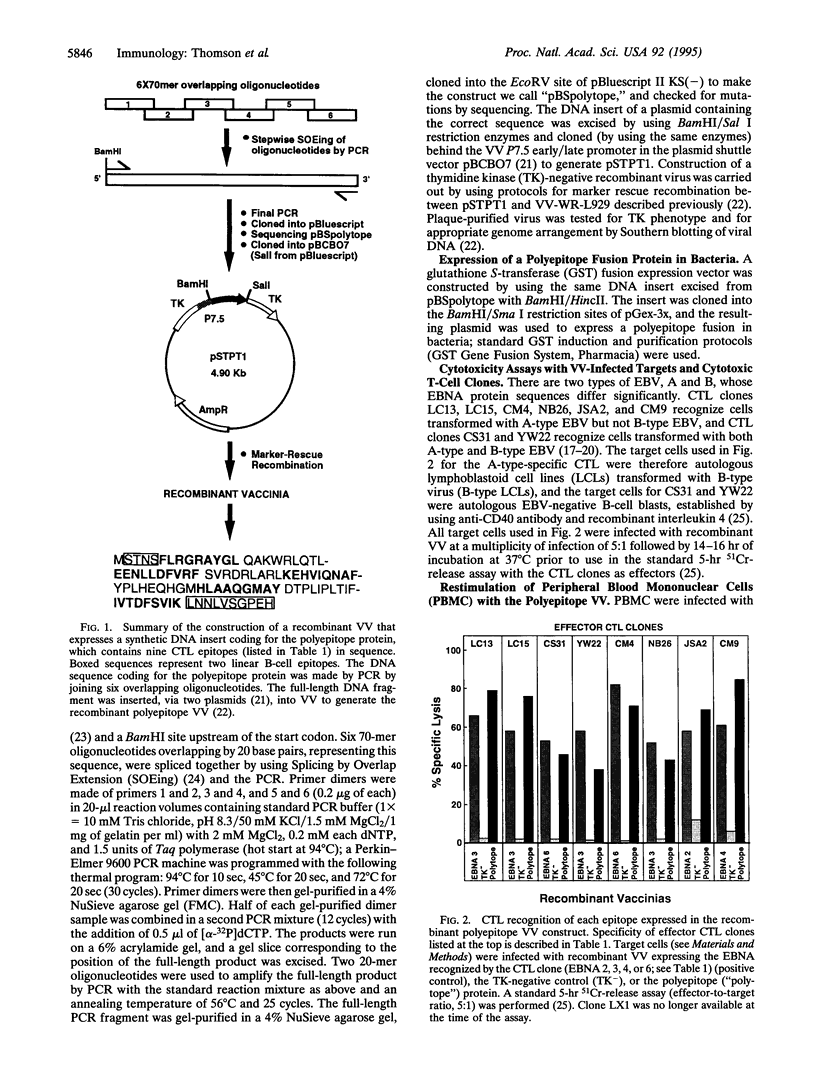
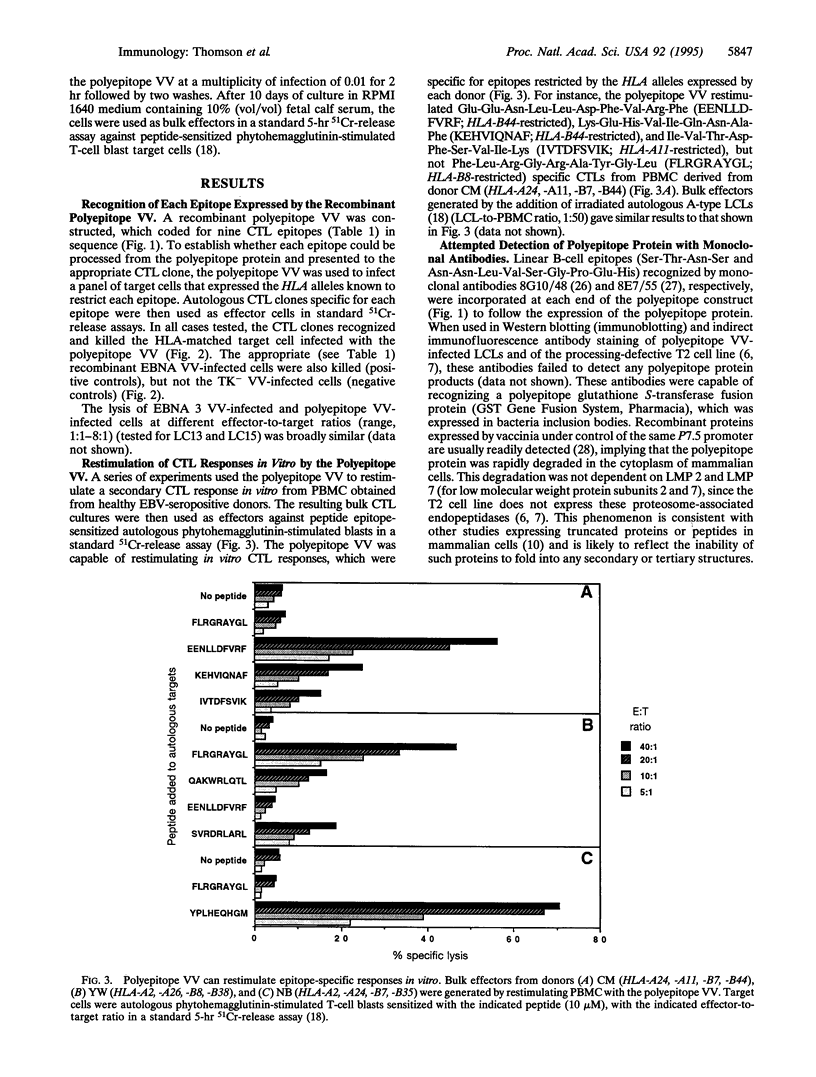
The epitopes recognized by CD8+ cytotoxic T lymphocytes (CTL) are generated from cytosolic proteins by proteolytic processing. The nature of the influences exerted by the sequences flanking CTL epitopes on these processing events remains controversial. Here we show that each epitope within an artificial polyepitope protein containing nine minimal CD8+ CTL epitopes in sequence was processed and presented to appropriate CTL clones. Natural flanking sequences were thus not required to direct class I proteolytic processing. In addition, unnatural flanking sequences containing other CTL epitopes did not interfere with processing. The ability of every CTL epitope to be effectively processed from a protein containing only CTL epitopes is likely to find application in the construction of recombinant polyepitope CTL vaccines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew M. E., Boyle D. B., Coupar B. E., Whitfeld P. L., Both G. W., Bellamy A. R. Vaccinia virus recombinants expressing the SA11 rotavirus VP7 glycoprotein gene induce serotype-specific neutralizing antibodies. J Virol. 1987 Apr;61(4):1054–1060. doi: 10.1128/jvi.61.4.1054-1060.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D., Driscoll J., Androlewicz M., Hughes E., Cresswell P., Spies T. Proteasome subunits encoded in the MHC are not generally required for the processing of peptides bound by MHC class I molecules. Nature. 1992 Nov 12;360(6400):171–174. doi: 10.1038/360171a0. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E., Both G. W. Multiple-cloning-site plasmids for the rapid construction of recombinant poxviruses. Gene. 1985;35(1-2):169–177. doi: 10.1016/0378-1119(85)90169-6. [DOI] [PubMed] [Google Scholar]
- Burrows S. R., Gardner J., Khanna R., Steward T., Moss D. J., Rodda S., Suhrbier A. Five new cytotoxic T cell epitopes identified within Epstein-Barr virus nuclear antigen 3. J Gen Virol. 1994 Sep;75(Pt 9):2489–2493. doi: 10.1099/0022-1317-75-9-2489. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Tse A. G., Salter R. D., Parham P., Townsend A. CD8 independence and specificity of cytotoxic T lymphocytes restricted by HLA-Aw68.1. Proc Biol Sci. 1991 May 22;244(1310):169–177. doi: 10.1098/rspb.1991.0066. [DOI] [PubMed] [Google Scholar]
- Chen W., Khilko S., Fecondo J., Margulies D. H., McCluskey J. Determinant selection of major histocompatibility complex class I-restricted antigenic peptides is explained by class I-peptide affinity and is strongly influenced by nondominant anchor residues. J Exp Med. 1994 Oct 1;180(4):1471–1483. doi: 10.1084/jem.180.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimini G., Pala P., Sire J., Jordan B. R., Maryanski J. L. Recognition of oligonucleotide-encoded T cell epitopes introduced into a gene unrelated to the original antigen. J Exp Med. 1989 Jan 1;169(1):297–302. doi: 10.1084/jem.169.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Val M., Schlicht H. J., Ruppert T., Reddehase M. J., Koszinowski U. H. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991 Sep 20;66(6):1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- Dick L. R., Aldrich C., Jameson S. C., Moomaw C. R., Pramanik B. C., Doyle C. K., DeMartino G. N., Bevan M. J., Forman J. M., Slaughter C. A. Proteolytic processing of ovalbumin and beta-galactosidase by the proteasome to a yield antigenic peptides. J Immunol. 1994 Apr 15;152(8):3884–3894. [PMC free article] [PubMed] [Google Scholar]
- Driscoll J., Brown M. G., Finley D., Monaco J. J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993 Sep 16;365(6443):262–264. doi: 10.1038/365262a0. [DOI] [PubMed] [Google Scholar]
- Eisenlohr L. C., Yewdell J. W., Bennink J. R. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992 Feb 1;175(2):481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Smith M., Driscoll P., McMichael A. Peptide selection by class I molecules of the major histocompatibility complex. Curr Biol. 1993 Dec 1;3(12):854–866. doi: 10.1016/0960-9822(93)90219-e. [DOI] [PubMed] [Google Scholar]
- Epping R. J., Goldstone S. D., Ingram L. T., Upcroft J. A., Ramasamy R., Cooper J. A., Bushell G. R., Geysen H. M. An epitope recognised by inhibitory monoclonal antibodies that react with a 51 kilodalton merozoite surface antigen in Plasmodium falciparum. Mol Biochem Parasitol. 1988 Feb;28(1):1–10. doi: 10.1016/0166-6851(88)90173-9. [DOI] [PubMed] [Google Scholar]
- Fehling H. J., Swat W., Laplace C., Kühn R., Rajewsky K., Müller U., von Boehmer H. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science. 1994 Aug 26;265(5176):1234–1237. doi: 10.1126/science.8066463. [DOI] [PubMed] [Google Scholar]
- Gaczynska M., Rock K. L., Goldberg A. L. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993 Sep 16;365(6443):264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. L. Proteolysis, proteasomes and antigen presentation. Nature. 1992 Jun 4;357(6377):375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Hahn Y. S., Braciale V. L., Braciale T. J. Presentation of viral antigen to class I major histocompatibility complex-restricted cytotoxic T lymphocyte. Recognition of an immunodominant influenza hemagglutinin site by cytotoxic T lymphocyte is independent of the position of the site in the hemagglutinin translation product. J Exp Med. 1991 Sep 1;174(3):733–736. doi: 10.1084/jem.174.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Hahn C. S., Braciale V. L., Braciale T. J., Rice C. M. CD8+ T cell recognition of an endogenously processed epitope is regulated primarily by residues within the epitope. J Exp Med. 1992 Nov 1;176(5):1335–1341. doi: 10.1084/jem.176.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Johnson R. P., Trocha A., Buchanan T. M., Walker B. D. Recognition of a highly conserved region of human immunodeficiency virus type 1 gp120 by an HLA-Cw4-restricted cytotoxic T-lymphocyte clone. J Virol. 1993 Jan;67(1):438–445. doi: 10.1128/jvi.67.1.438-445.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara U., Murray B., Pam C., Lahnstein J., Gould H., Kidson C., Saul A. Chemical characterization of the parasitophorous vacuole membrane antigen QF 116 from Plasmodium falciparum. Mol Biochem Parasitol. 1990 Jan 1;38(1):19–23. doi: 10.1016/0166-6851(90)90200-6. [DOI] [PubMed] [Google Scholar]
- Khanna R., Burrows S. R., Kurilla M. G., Jacob C. A., Misko I. S., Sculley T. B., Kieff E., Moss D. J. Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992 Jul 1;176(1):169–176. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R., Jacob C. A., Burrows S. R., Kurilla M. G., Kieff E., Misko I. S., Sculley T. B., Moss D. J. Expression of Epstein-Barr virus nuclear antigens in anti-IgM-stimulated B cells following recombinant vaccinia infection and their recognition by human cytotoxic T cells. Immunology. 1991 Nov;74(3):504–510. [PMC free article] [PubMed] [Google Scholar]
- Khanna R., Jacob C. A., Burrows S. R., Moss D. J. Presentation of endogenous viral peptide epitopes by anti-CD40 stimulated human B cells following recombinant vaccinia infection. J Immunol Methods. 1993 Aug 26;164(1):41–49. doi: 10.1016/0022-1759(93)90274-b. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Michalek M. T., Grant E. P., Gramm C., Goldberg A. L., Rock K. L. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature. 1993 Jun 10;363(6429):552–554. doi: 10.1038/363552a0. [DOI] [PubMed] [Google Scholar]
- Momburg F., Ortiz-Navarrete V., Neefjes J., Goulmy E., van de Wal Y., Spits H., Powis S. J., Butcher G. W., Howard J. C., Walden P. Proteasome subunits encoded by the major histocompatibility complex are not essential for antigen presentation. Nature. 1992 Nov 12;360(6400):174–177. doi: 10.1038/360174a0. [DOI] [PubMed] [Google Scholar]
- Momburg F., Roelse J., Howard J. C., Butcher G. W., Hämmerling G. J., Neefjes J. J. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature. 1994 Feb 17;367(6464):648–651. doi: 10.1038/367648a0. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Burrows S. R., Khanna R., Misko I. S., Sculley T. B. Immune surveillance against Epstein-Barr virus. Semin Immunol. 1992 Apr;4(2):97–104. [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Eddleston M., de la Torre J. C., McKee T., Whitton J. L. Vaccination to prevent persistent viral infection. J Virol. 1993 Jul;67(7):4372–4378. doi: 10.1128/jvi.67.7.4372-4378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich N. R., McInnes P., Klein D. L., Hall B. F. Vaccine technologies: view to the future. Science. 1994 Sep 2;265(5177):1401–1404. doi: 10.1126/science.7521064. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Donnelly J. J., Parker S. E., Rhodes G. H., Felgner P. L., Dwarki V. J., Gromkowski S. H., Deck R. R., DeWitt C. M., Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993 Mar 19;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- Whitton J. L., Sheng N., Oldstone M. B., McKee T. A. A "string-of-beads" vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993 Jan;67(1):348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos-Lima P. O., Levitsky V., Brooks J., Lee S. P., Hu L. F., Rickinson A. B., Masucci M. G. T cell responses and virus evolution: loss of HLA A11-restricted CTL epitopes in Epstein-Barr virus isolates from highly A11-positive populations by selective mutation of anchor residues. J Exp Med. 1994 Apr 1;179(4):1297–1305. doi: 10.1084/jem.179.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]