Abstract
Background:
Bronchial wash cytology of lung lesions is a non/minimally invasive procedure utilized for diagnosis of pulmonary lesions.
Aim:
The aim of this study was to evaluate the efficacy of bronchial wash cytology in the diagnosis of bronchopulmonary lesions and assess the role of morphometry in categorizing dysplastic/malignant lesions.
Materials and Methods:
All cases of bronchial wash cytology received from January 2006 to June 2010 were retrieved and reviewed. Cases with adequate clinical data or a subsequent biopsy were selected for the study and cytodiagnosis was correlated with available clinical details. Morphometry was done on alcohol fixed hematoxylin and eosin stained cytosmears using computer assisted Image Pro software.
Results:
One hundred and seventy-six cases of the 373 cases of bronchial cytology received were included for the study. Bronchial wash cytology technique showed high specificity. Cytohistopathology correlation showed 62.06% concordance rate. Cells from normal epithelium, reactive atypia, neoplastic atypia, squamous metaplasia, non-small cell and small cell carcinoma showed a mean nuclear diameter of 7.4 μm, 11.7 μm, 13.9 μm, 13.0 μm, 10.7 μm, and 17.7 μm, respectively, which was statistically significant with P < 0.05. Multiple comparisons between various groups using analysis of variance and Bonferroni tests also showed remarkable statistical significance.
Conclusions:
Bronchial wash cytology has low sensitivity in detecting pulmonary lesions. It can be of value in patients with contraindication for biopsy. Morphometry can be a useful adjunct to cytomorphology, especially in situations where biopsy is contraindicated.
Keywords: Bronchial wash cytology, efficacy, morphometry
Introduction
Cytology of pulmonary lesions provides valuable diagnostic information by non/minimally invasive procedures. It may be a valuable investigation in situations where biopsy procedure cannot be attempted due to high risk of hemorrhage.[1] The cytological methods presently available for studying the lung pathology are exfoliative (induced sputum), abrasive cytology (bronchioalveolar lavage [BAL], bronchial brushing [BB], bronchial washing and percutaneous/endobronchial fine-needle aspiration cytology).
Cell yield in a BB is better than aspirate and washing. However, wash technique samples out the areas beyond the reach of brush.[2] BAL was introduced as a therapeutic measure to clear alveolar spaces filled with secretion in alveolar proteinosis and bronchial asthma.[2] It was later utilized for diagnostic pulmonary cytology providing a high accuracy rate. Bronchial wash cytology is a widely accepted safe, simple, and minimally invasive technique to evaluate cell morphology. The aim of this study was to evaluate the efficacy of bronchial wash cytology in the diagnosis of bronchopulmonary lesions and assess the role of morphometry in categorizing dysplastic/malignant lesions.
Materials and Methods
All cases of bronchial wash cytology received from January 2006 to June 2010 were retrieved and only cases with adequate clinical data or a subsequent biopsy were selected for the study.
Cytohistopathological correlation was done on cases with biopsy. The cytological smears stained with May-Grünwald Giemsa and hematoxylin and eosin (H and E) were grouped into satisfactory/unsatisfactory and adequate/inadequate for interpretation. The satisfactory and adequate smears were further categorized as normal pattern, inflammatory, suspicious/atypical favoring neoplasm, and positive for malignant cells.
Adequate smears: Smears with bronchial epithelial cells/alveolar macrophage were considered adequate. Smears were considered satisfactory for reporting when there were no artifactual changes or excessive hemorrhage with blood elements obscuring cellular details.
Exclusion criteria: Unsatisfactory and inadequate smears.
Unsatisfactory smears: Smears were considered unsatisfactory based on the presence of degenerated/poorly preserved cell morphology, excessive hemorrhage with blood elements obscuring cellular details.
Inadequate smears: Smears that lacked alveolar macrophages or epithelial cells.
Significance of bronchial cytology in comparison with histopathology were assessed by deriving sensitivity, specificity, false positivity index, false negativity index, positive predictive value, negative predictive value, and accuracy rate. Histopathology diagnosis was used as the goal standard for assessing the above parameters.
Morphometry was done on alcohol fixed H and E stained cytological smears on all cases with subsequent histopathology, using Image Pro Plus software. In each case, minimum of 30 epithelial cells with preserved morphology and of diagnostic value were identified on cytosmears and their cell and nuclear diameter were measured. Parameters measured included nuclear diameter and cell diameter. Nucleo-cytoplasmic ratio (N:C) was derived from this data. Statistical analysis was performed to assess the significance of morphometric results.
Results
A total of 373 cases of bronchial cytology were received during the study period while only on 58 of these cases a lung biopsy was performed. Hence, cytohistopathology correlation could be done only in 58 cases of which 36 cases (62.06%) showed correlation [Table 1].
Table 1.
Cytohistopathology correlation
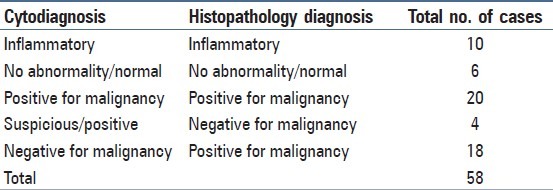
In 22 cases, cytology did not correlate with histopathology. Four of the suspicious cases on cytology on subsequent biopsy did not show any malignant cells. Patient did not have repeat biopsy at our center. However, on radiology mass lesions were present in all these cases. The quantitative measurements were within range of atypical cells favoring malignancy. In 18 cases, malignancy was missed on cytology. Morphometry in these 18 cases were within range of normal epithelial values.
Bronchial washing cytology showed a sensitivity of 52.63% and specificity of 80% with accuracy of 62.06%. The false positivity index and false negativity index was found to be 20% and 47.36%, respectively. A positive predictive value of 83.33% and negative predictive value of 47.05% was noted.
On cytological examination, malignant cases were categorized as small cell carcinomas (4 cases) and non-small carcinomas (20 cases). On histopathological examination, non-small carcinomas were further categorized as adenocarcinoma (8 cases), squamous cell carcinomas (5 cases) while 7 cases were found to be poorly differentiated carcinoma. Non-small cell and small cell carcinoma of lung showed distinct morphological features on cytosmears. Cytomorphology pattern of non-small cell carcinoma of lung included loose clusters/cell balls/acinar pattern with mild to moderate cytoplasm having ↑N:C, nuclear hyperchromasia and prominent nucleoli. Keratinized cells were seen occasionally in cases of squamous cell carcinoma. Cytomorphology pattern of small cell carcinoma of lung showed monomorphic cell clusters with small round cells having rounded nuclei with ↑N:C. Other nuclear features noted were nuclear molding with darkly stained nuclei or nuclei with fine chromatin clumping.
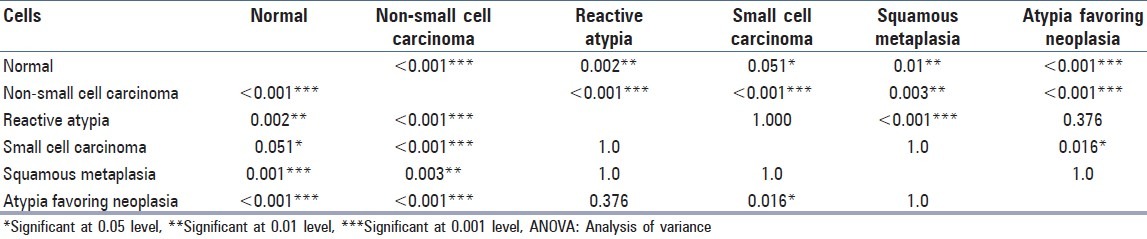
Morphometric analysis showed variable mean nuclear diameter (MND) in normal bronchioepithelial cells, metaplastic cells, reactive atypia, neoplastic atypia [Figure 1] and malignant cells [Table 2]. Cells from non-small cell [Figure 1] and small cell carcinoma showed distinct morphometric measurements [Table 2]. Analysis of variance with ANOVA test showed that the MND among the different groups were statistically significant with P < 0.05. Multiple comparisons between various groups showed variable statistical significance [Table 3].
Figure 1.
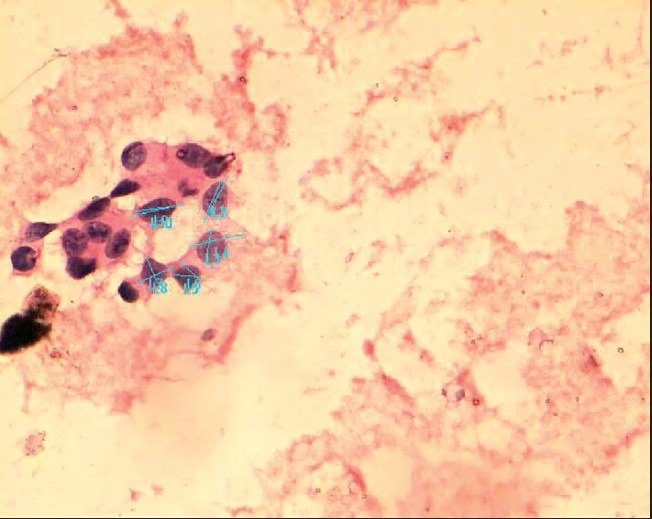
Computer assisted morphometry measuring mean nuclear and cell diameter in non-small cell carcinoma with neoplastic cells arranged in acinar pattern (H and E, ×200)
Table 2.
Morphometric parameters noted on cytosmears
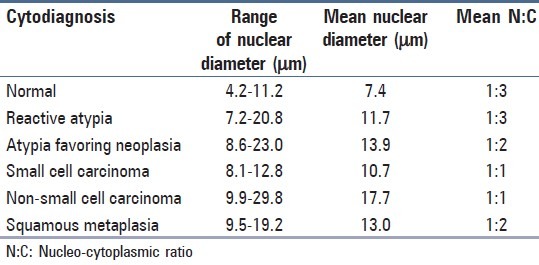
Table 3.
Comparison of mean nuclear diameter of multiple groups using ANOVA and bonferroni test
Discussion
The prevalence of lung malignancy has been on the rise and is found to be a major cause of death due to cancer in males. Bronchoscopic aided cytological sampling may provide useful information in the evaluation of neoplastic lesions of the lung. Hospitalization can be avoided in this specialized test as it can be done as a day care procedure contributing as an additional benefit.
Bronchoscopy provides direct visualization of the airways and permits more focused sampling of cells/tissue.[3] It is extremely helpful in diagnosing endobronchial or centrally located lesions such as squamous cell carcinoma and carcinoid tumor. On visualizing a mass, brushing can be done or wash or lavage could be performed for cytological diagnosis. However, a combined method of BB and washing has shown more sensitivity as compared to usage of individual methods.[3,4] Review of literature reveals variable findings with respect to sensitivity and specificity of lung cytology.[3,4,5,6] Overall the difference may possibly be because of the method adopted ranging from bronchial wash, BAL and transbronchial needle aspiration. The cell yield is much better in BAL, especially in peripheral lung lesions, resulting in a better accuracy. This is because lavage reaches the distal bronchial units and samples out more than one bronchi at the same time.[6,7] In our study, an average rate of sensitivity could be due to two reasons, one being the inherent sampling limitation that is related to location of the lesion and the other reason could be the usage of bronchial wash cytology technique. However, higher sensitivity and specificity was noted earlier in similar studies done to evaluate the efficacy of bronchial wash cytology.[1,5] A better sensitivity was noted in a study by Gaur et al.[4] as they adopted a utilized method of BB and thick-needle aspiration biopsy. A low accuracy rate and a high false negativity in our study as compared to results of similar studies in literature may be attributed to several factors such as low cell yield, peripheral lesion, necrotic tissue, crushing artifacts, and secondary inflammation.[3,8,9]
Eighteen of the 58 biopsied cases (31.03%) were false negative in this study resulting in an average cytohistopathological concordance rate (62.1%). The cytopathologist and clinician should be aware of the inherent limitations of this technique.[3] The reason for missing the lesion on cytology could be due several factors, which include secondary inflammation, nonrepresentative material or suboptimal cell yield. Four of the cases were false positive in our study. Reasons documented in literature for over-grading of lesions include reactive atypia secondary to inflammation, squamous metaplastic cells and basal cell hyperplasia.[1,3,6,8]
Radiotherapy and chemotherapeutic drugs may also induce changes in cell morphology producing cellular atypia, which may give a false positive diagnosis. Changes related to therapy begin within weeks of starting the treatment and resolve a month after stoppage of therapy. Cytopathologist needs to be aware of the possible pitfalls and simulators, while viewing the cytosmears of lung cytology to prevent morbidity in false positive diagnosis and delayed treatment with poor outcome in false negative cases.[9,10]
One of the most important information required by the clinician before designing treatment in lung cancers is categorization of tumors. In our study on cytosmears, the lesion could only broadly be categorized as a small cell carcinoma and non-small cell carcinoma based on cytomorphology. The difficulty in diagnosing specific subtype on cytology may be due to lack of acinar pattern, kertainization, overlapping of cells, scant cellularity and thick smears.[8] Typing of tumors is difficult especially in the absence of characteristic features, overlapping morphological features, indistinct pattern, and morphology. A precise diagnosis is important as treatment modalities and prognosis differs considerably for each of the subgroups. Even on histopathological examination, quite often it becomes difficult to subcategorize them and immunohistochemistry is performed to provide a more conclusive diagnosis. Though we did not encounter any case of bronchioalveolar carcinoma, one needs to be aware that it may easily be missed on cytology due to its morphological resemblance to normal bronchial epithelial cells with or without reactive atypia.[11]
In an era of evidence-based medicine, the significance of quantitative pathology cannot be ignored. The ability to reduce the element of interobserver variation by objectively measuring cells makes morphometry a reliable diagnostic aid to a visual microscopic evaluation. Numerical assessment reflects s objectivity, discreteness, and permanence. There are limited studies in literature with respect to utilization of morphometry in lung cytology and in this study we further objectively measured cells in cytological smears. Although, in this study MND of various cell types showed a wide range with overlapping values nevertheless, each category showed overall statistical significance with P < 0.05. Due to this finding of the wide range of MND with overlapping values, role of morphometry may still be limited. More studies on this aspect may probably help in specifying the precise range.
In a study by Fiorella et al.[11] morphometric measurement of 13.76 μm was noted for carcinoma group while reactive group showed 13.29 μm in contrast to our findings of 10.7 μm in nons-mall cell carcinoma and 11.7 μm in reactive atypia. However, MND of neoplastic atypia in our study was 13.9 μm, a value close to carcinoma group of Fiorella et al.[11] Influence of factors such as processing time interval, type of fixative and total fixation time[12] can also possibly result in cell morphology variability. A standardization of these factors may further helpful in providing more consistent results.
Fiorella et al.[11] performed morphometry in a limited number of cases and suggested morphometry may not be of any use in differentiating bronchioalveolar carcinoma and reactive atypical cells. In contrast to this, Zaman et al.[13] in a similar study as Fiorella et al.[11] found morphometery to be quite useful in distinguishing them. Although, we did not encounter bronchioalveolar carcinoma in our study, we studied the morphometry pattern in a spectrum of lesions ranging from normal to malignant cases with statistically significant results.
On comparing the MND between various groups, a good statistical significance was noted between them. However, no significance was noted between MND of reactive atypia with small cell carcinoma, squamous metaplastic cells and atypia favoring neoplasia. There was no statistical significance seen between MND of atypia favoring neoplasia with squamous metaplasia. However, N:C was found to be a useful parameter in reactive atypia to differentiate from malignant cells.
In 22 cases, cytology did not correlate with histopathology. Four of the suspicious cases on cytology were found to be negative on subsequent biopsy. The morphometry values were within range of atypical cells still favoring malignancy. However, we could not confirm whether the lesion was missed on biopsy because a repeat biopsy was not done at our center. In 18 cases, malignancy was missed on cytology. This could be due to the procedure limitation as at our institute they perform only bronchial wash and not BAL. Morphometry in these 18 cases were within range of normal epithelial values suggesting that the lesion was not sampled in cytology.
Small cell carcinoma showed a high N:C of 1:1, a feature that is well noted on cytosmears without the aid of morphometric evaluation. On morphometry, these cells showed distinctly higher MND than the normal cells, but lower than other reactive and neoplastic lesions with a high N:C of 1:1.
Squamous metaplasia showed an MND of 13 and mean N:C of 1:2, a feature also noted in non-small cell carcinoma. However, careful viewing of cytosmears may help in disregarding the higher measurement projected by morphometry in squamous metaplastic cells. Metaplastic cells have specific cytological features such as oval cells with abundant cytoplasm, which usually cannot be missed on microscopy. Nucleus of metaplastic cells appears vesicular unlike malignant cells, which are either hyperchromatic or show prominent nucleoli. This highlights the fact that morphometry should be used in combination with cytomorphological features and its usage as an independent method may lead to erroneous results. Morphometry can further be utilized to gain additional information in suspicious cases.
Conclusions
Bronchial wash cytology with a low sensitivity index plays a limited role in diagnosing pulmonary lesions. In spite of this limitation, due to its high specificity, it may still be a valuable method that provides significant information in the evaluation of lung pathology in centers not practicing BAL. Morphometry can be a useful adjunct to morphology in offering a conclusive diagnosis, especially in suspicious cases and where biopsy is contraindicated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Ahmad M, Afzal S, Saeed W, Mubarik A, Saleem N, Khan SA, et al. Efficacy of bronchial wash cytology and its correlation with biopsy in lung tumours. J Pak Med Assoc. 2004;54:13–6. [PubMed] [Google Scholar]
- 2.Koss LG, Melamed MR, editors. Koss’ diagnostic cytology and its histopathologic bases. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 573–5. [Google Scholar]
- 3.Atkins KA. The diagnosis of bronchioloalveolar carcinoma by cytologic means. Am J Clin Pathol. 2004;122:14–6. doi: 10.1309/BK8A-0XJT-BLW1-DFR9. [DOI] [PubMed] [Google Scholar]
- 4.Gaur DS, Thapliyal NC, Kishore S, Pathak VP. Efficacy of broncho-alveolar lavage and bronchial brush cytology in diagnosing lung cancers. J Cytol. 2007;24:73–7. [Google Scholar]
- 5.Truong LD, Underwood RD, Greenberg SD, McLarty JW. Diagnosis and typing of lung carcinomas by cytopathologic methods. A review of 108 cases. Acta Cytol. 1985;29:379–84. [PubMed] [Google Scholar]
- 6.Pirozynski M. Bronchoalveolar lavage in the diagnosis of peripheral, primary lung cancer. Chest. 1992;102:372–4. doi: 10.1378/chest.102.2.372. [DOI] [PubMed] [Google Scholar]
- 7.Henderson AJ. Bronchoalveolar lavage. Arch Dis Child. 1994;70:167–9. doi: 10.1136/adc.70.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sackett MK, Salomão DR, Donovan JL, Yi ES, Aubry MC. Diagnostic concordance of histologic lung cancer type between bronchial biopsy and cytology specimens taken during the same bronchoscopic procedure. Arch Pathol Lab Med. 2010;134:1504–12. doi: 10.5858/2009-0363-OA.1. [DOI] [PubMed] [Google Scholar]
- 9.Idowu MO, Powers CN. Lung cancer cytology: Potential pitfalls and mimics — a review. Int J Clin Exp Pathol. 2010;3:367–85. [PMC free article] [PubMed] [Google Scholar]
- 10.Saad RS, Silverman JF. Respiratory cytology: Differential diagnosis and pitfalls. Diagn Cytopathol. 2010;38:297–307. doi: 10.1002/dc.21205. [DOI] [PubMed] [Google Scholar]
- 11.Fiorella RM, Gurley SD, Dubey S. Cytologic distinction between bronchioalveolar carcinoma and reactive/reparative respiratory epithelium: A cytomorphometric analysis. Diagn Cytopathol. 1998;19:270–3. doi: 10.1002/(sici)1097-0339(199810)19:4<270::aid-dc8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Crothers BA, Tench WD, Schwartz MR, Bentz JS, Moriarty AT, Clayton AC, et al. Guidelines for the reporting of nongynecologic cytopathology specimens. Arch Pathol Lab Med. 2009;133:1743–56. doi: 10.5858/133.11.1743. [DOI] [PubMed] [Google Scholar]
- 13.Zaman SS, van Hoeven KH, Slott S, Gupta PK. Distinction between bronchioloalveolar carcinoma and hyperplastic pulmonary proliferations: A cytologic and morphometric analysis. Diagn Cytopathol. 1997;16:396–401. doi: 10.1002/(sici)1097-0339(199705)16:5<396::aid-dc4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]


