Abstract
Background:
Fine-needle aspiration cytology (FNAC) in the diagnosis of thyroid nodules is an easy and cost-effective method. The increase in malignancy rates of the excised nodules due to the high sensitivity and specificity rates of the FNAC is remarkable.
Aim:
The aim of this study was to assess the effectiveness of FNAC in the evaluation of thyroid nodules by comparing the results with histopathologic evaluation and comparing the consistency of the results with the literature.
Materials and Methods:
In this study, 1607 FNACs of 1333 patients which were classified according to the Bethesda system and 126 histopathological evaluations obtained from this group were evaluated. The mean age of the patients was 51.24 (range: 17-89, 17% male and 83% female). The sensitivity, specificity, positive and negative predictive values, and accuracy rates were evaluated.
Results:
The sensitivity was 87.1% and specificity was 64.6%. The positive and negative predictive value and accuracy rates were 76.1%, 79.5%, and 77.3%, respectively.
Conclusions:
In our study, the evaluation of thyroid FNAC samples with Bethesda system highly correlated with the results of histopathological diagnosis. However, combination of additional and advanced diagnostic methods such as immunocytochemical studies and molecular pathology techniques enhance the prognostic value of FNAC in patients with atypia of undetermined significance or follicular lesion of undetermined significance, lesions suspicious for malignancy, and suspected follicular neoplasm.
Keywords: Efficacy, fine-needle aspiration cytology, predictive value, sensitivity, specificity, thyroid nodule
Introduction
Epidemiological studies have shown that thyroid nodules can be detected by palpation in 5% of cases and detection with high-resolution ultrasound (US) ranged between 19 and 67%.[1] In North America, epidemiological studies showed that thyroid nodules can be detected with US in 67% of population.[2] Palpable thyroid nodules are more common in women, and male/female ratio ranged from 1.2 to 4.3.[1,3] Thyroid nodules may cause hypothyroidism, hyperthyroidism, cosmetic issues, and problems in other organs such as compression, and they also have the potential for malignancy.[4] Therefore, the accurate evaluation of thyroid nodules is crucial. In recent years, the role of fine-needle aspiration cytology (FNAC) is increasing regarding the management methods as well as its role in detection of malignancy potentials of thyroid nodules. No single diagnostic methods used for the definitive diagnosis of thyroid cancers, such as radiographs, US, scintigraphy and suppression therapy, is effective enough to make a benign/malignant differentiation alone. FNAC has been used since the 1950s, and is one of the effective methods in the diagnosis of thyroid nodules.[5]
In this study, the effectiveness of FNAC was evaluated through the identification of the correlation between the cytologic diagnoses of thyroid FNAC and the postoperative histopathologic diagnoses.
Materials and Methods
Between October 2008 and March 2013, results of 1607 cytologic aspirates (obtained by FNAC) of 1333 patients were evaluated retrospectively. Cytological and histopathologic diagnoses of 126 nodules of 123 patients (who had undergone surgical excision after FNAC) were compared.
Cytological evaluation was based on Bethesda classification.[6] Cytological results were evaluated in two main groups: Neoplasia negative group was consisted of nodular goiter and thyroiditis, whereas the other group was consisted of follicular neoplasm (FN)/(suspicious for follicular neoplasm) SFN, suspicious for malignancy and patients diagnosed with malignant cytology.
The specificity and sensitivity rates of cytological diagnoses were evaluated on the basis of histopathological diagnoses. After exclusion of the nondiagnostic results, cytological evaluation results were classified as positive and negative. According to the Bethesda 2007 classification, benign results are considered as a negative test results and results with atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS), FN/SFN, suspicious for malignancy, and malignant ones were considered as a positive test results. Patients with negative cytologic examination and diagnosed as carcinoma, follicular adenoma or Hurthle cell adenoma on histopathological examination were considered as false-negative. Patients with positive cytological examination and diagnosed as nodular goiter or thyroiditis on histopathological examination were considered as false positive.
Comparing the results of cytologic and histopathologic examinations, the sensitivity, specificity, positive and negative predictive value, and accuracy were calculated. These values were calculated by the following formulas. Patients with nondiagnostic FNAC were excluded from the calculations.
All statistical calculations were performed using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 19.0, Company ©1989-2010, SPSS Inc. an IBM Company) program. Chi-square test was used to assess the effect of gender independent variable on the results of histopathological and cytological tests, t-test was performed to compare the mean age between genders. Significance of the statistical tests was based on 95% confidence interval.
Results
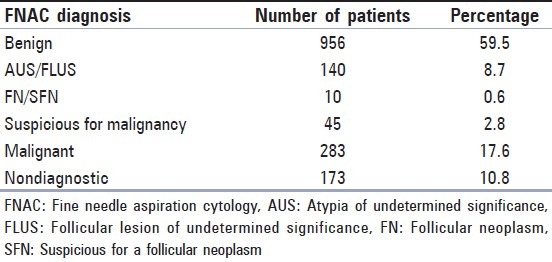
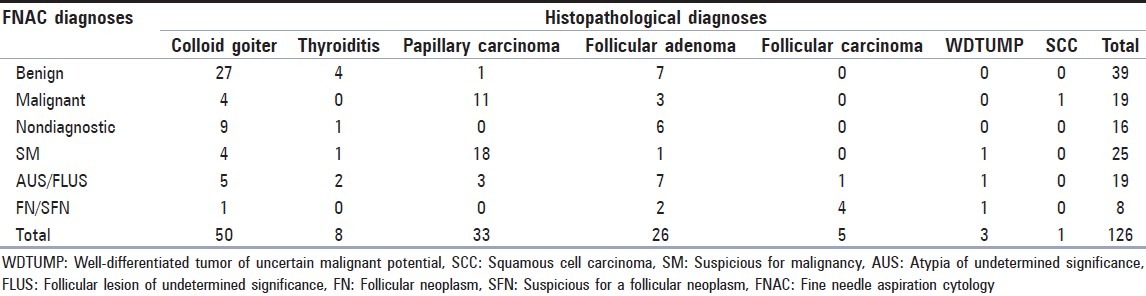
Of 1333 patients, 1107 were female (83%) and 226 of them were male (17%). The mean age was 51.24 (range: 17-89). The mean age of male patients was greater than the mean age of female patients (57.61 and 49.95 respectively, P = 0.000). The cytological diagnosis distribution is shown in Table 1. The percentage of cases with the diagnoses of suspicious for malignancy, AUS/FLUS and FN/SFN were approximately 12% in total.
Table 1.
Distribution of FNAC diagnoses of 1607 cases
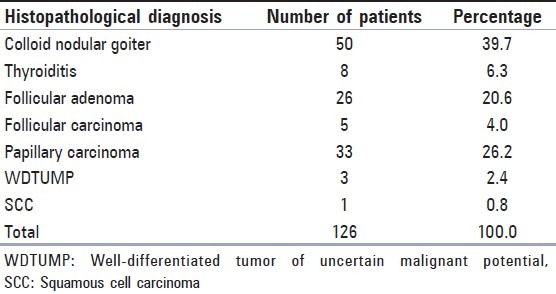
126 nodules of 123 patients who had cytologic diagnosis were operated and histopathological diagnoses were made by examination of excised materials [Table 2]. Patients with the histopathological diagnoses of follicular adenoma and carcinoma were categorized as malignant, while diagnoses of nodular goiter and thyroiditis were categorized as benign when comparing with cytological examinations [Table 2].
Table 2.
Distribution of histopathologic diagnoses of 126 cases
When compared with the previous cytologic examinations, the histopathologic examinations revealed malignancy in 20.5% of previous benign cytology, 63.2% of previous AUS/FLUS cytology [Figure 1], 80% of previous suspicious for malignancy cytology, 87.5% of previous FN/SFN cytology [Figure 2] and 79% of previous malignant cytology [Figure 3]. Comparisons of cytologic and histopathologic evaluations are summarized in Table 3.
Figure 1.
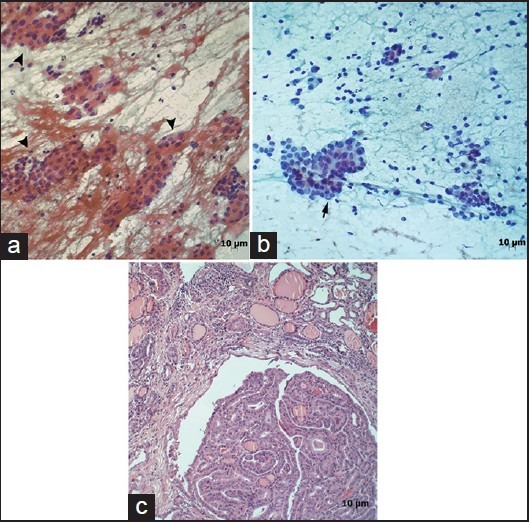
(a) Case with the diagnosis of atypia of undetermined significance due to thyrocytes with mild nuclear pleomorphism and tendency for overlapping (arrowhead) (Pap, ×200). (b) Second cytology of same case shows malignant thyrocytes with intranuclear inclusion (arrow) (Pap, ×200). (c) Histopathology section shows characteristics of papillary carcinoma (H and E, ×100)
Figure 2.
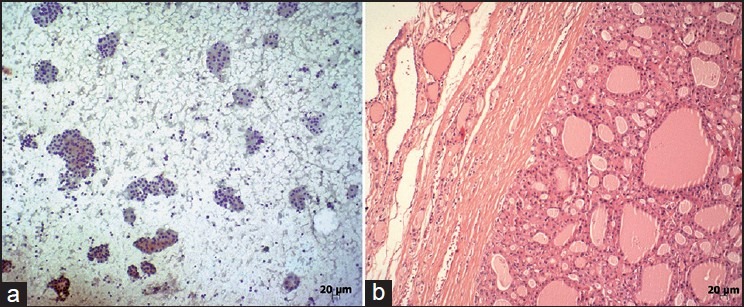
Case with the cytologic diagnosis of “suspicious for follicular neoplasia” (a) (Papanicolaou [PAP], ×100). Histopathologic diagnosis of the same case was Hurthle cell adenoma (b) (H and E, ×100)
Figure 3.
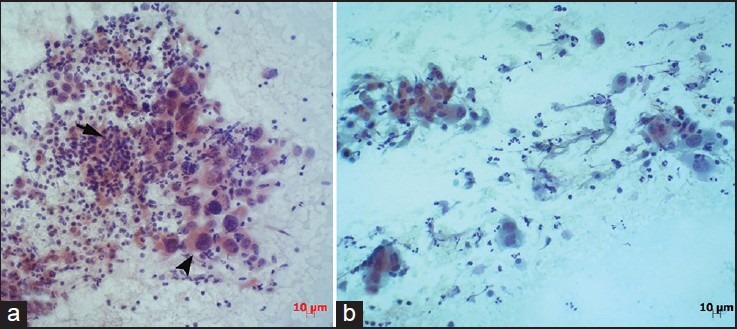
Two patients diagnosed with malignant cytology. The histopathological examinations confirmed these diagnoses. (a) squamous cell carcinoma (arrow: normal thyrocytes, arrow head: Squamous carcinoma cells, (Pap, ×200). (b) Anaplastic carcinoma with areas of papillary carcinoma (Pap, ×200)
Table 3.
FNAC and histopathologic correlations
Histopathological examinations revealed follicular adenoma in seven patients and papillary carcinoma in one patient with previous benign cytology. These cases were considered as false-negatives. Histopathological examinations showed nodular goiter in four patients with previous malignant cytology, AUS/FLUS in four patients with previous suspicious for malignancy cytology and nodular goiter in one patient with previous FN/SFN cytology. These cases were considered as false positives. In this study, the false positive rate of 15.5% and false-negative rate of 7.3% were found when nondiagnostic cases were excluded.
When classified as positive, negative, and nondiagnostic; 59.5% of all cases were negative, 29.7% were positive and 10.8% were nondiagnostic in cytologic examinations [Table 1]. Examination of the cytologic test results by gender revealed positive malignant rates of 41.7% for male patients and 31.7% for females, (P = 0.03). When classified as benign and malignant; 58 (46.0%) of 126 nodules were benign and 68 (54.0%) were malignant in histopathologic examinations. We did not found any difference-related to gender parameters between benign and malignant results. However, the patients with the histopathologic diagnosis (123) were 9.2% of all patients (1333). The number of patients who were diagnosed as intermediate-category and undergone surgical excision was 52 (41.3%). 39 (75.0%) of these patients were diagnosed as malignant in histopathological examination.
Statistical analysis of FNAC showed sensitivity, specificity, positive negative predictive value and accuracy to be 87.1%, 64.6%, 76.1%, 79.5%, and 77.3%, respectively.
Discussion
Cancer of the thyroid gland accounts for 1% of all cancers and is responsible for 0.5% of cancer-related deaths.[4] Early diagnosis still maintains its importance for higher life expectancy due to the low malignant potential of thyroid nodules, and slow progressing characteristics of thyroid gland cancers.
First cytological diagnosis with FNAC was made by Martin and Ellis in 1930s.[7] Many studies have been carried out in the following years; however; the method has been widely used after 1952.[5]
FNAC is easy to apply, has a low complication rates and high diagnostic value and is a cost-effective test used in the diagnosis of thyroid nodules.[6,8,9] In addition to clinical information for diagnosis and treatment of thyroid lesions, US, scintigraphy, radiographic evaluation of the soft tissues of the neck and FNAC studies are essential. The use of FNAC resulted in a decrease in the number of patients who underwent surgical treatment by 25-50%, while increasing the percentage of malignant results in the operated group of patients.[10] Currently, FNAC is the preferred diagnostic method for the initial stage of evaluation of thyroid nodules.[9]
Solitary thyroid nodules are unlikely to be malignant, which corresponds for 5% of these patients.[11,12] The Bethesda System for Reporting Thyroid Cytopathology group has identified six diagnostic categories in which the risk of malignancy increases respectively. These categories are reported as; benign: <1%, AUS/FLUS: 5-10%, FN: 20-30%, suspicious for malignancy: 50-75%, malignant: 100%.[13]
The sensitivity and specificity ratios for FNAC in published series range between 65% and 98% for sensitivity and 73-100% for specificity.[8,9,14,15,16] In this study, we found the sensitivity and specificity ratios of 64.6%, and 87.1%, respectively. The major reason for the wide range of sensitivity and specificity ratios is the differences in the categorization of “FN/SFN,” “suspicious for malignancy” and “AUS/FLUS” diagnoses. In addition, some authors categorize follicular lesions as histopathologically benign, while others categorize these lesions as malignant.[8,9,17,18] In our study, we evaluated the follicular lesions in the same category.
Among the factors that reduce the efficiency of FNAC include, inadequate sampling, inexperience of the cytopathologist and natural difficulties of differentiation of benign and malignant follicular lesions. The reported nondiagnostic test rates are ranging between 1.6% and 20% in the literature.[8,9,17,19] In our study, we found the rate of nondiagnostic tests as 10.8%. Ali et al.[19] suggested that the rate of nondiagnostic tests should be kept below 10%.
Inadequate sampling often results from sclerotic, calcified nodules or nodules with cystic degeneration in larger areas. US guided sampling reduces the nondiagnostic test rates in such conditions.[19,20]
Introduced by the Bethesda classification, AUS or FLUS is a category that covers a heterogeneous group of lesions that contain architectural abnormalities of follicular cells and/or nuclear atypia. These abnormalities and atypia must not be enough to be placed in other categories. The cytomorphologic interpretation of AUS is subjective; therefore the rate of AUS diagnosis is variable among institutions and pathologists. In some studies, the diagnoses of “AUS/FLUS,” “FN/SFN” and “suspicious for malignancy” are defined as intermediate-category.[8,18] We interpreted this as positive category. These diagnoses accounted for 12.1% in our cases.
We found the rate of false-negative as 7.3% and the rate of false-positive as 15.5%. In previous studies, false-negative rates were reported between 1% and 7% and false-positive rates between 1% and 11.60%.[8,9,14,21] Cytological evaluation errors can lead to increased rates of false negativity and false positivity. Bagga and Mahajan et al.[9] have reported that the decreased frequency of benign findings in the cytologic evaluations of patients who underwent surgery, complicates the identification of false-negative ratios. In our study we found this ratio as 46%. Most common lesions that contribute to false-positive results, as in our study, are the nodular hyperplasias with dense macropapillary structures. In general, it may be difficult to establish a cytologic differentiation between follicular hyperplastic nodules which are diagnosed as suspicious for malignancy or some of the follicular adenomas and well differentiated follicular carcinomas. It is reported that such conditions may lead to false-positive results.[17,22] Furthermore in our study, two patients with a cytological diagnosis of AUS/FLUS were then diagnosed as benign in histopathological examination. However micropapillary thyroid carcinoma was detected in close proximity to these nodules. We considered that the false-positive results were due to contamination from these zones. In the rest of the cases the false-positive tests, as in the literature, were resulted from a very suspicious single zone in the cytologic materials.[8,23] Multiple aspirations from different areas of the lesion may help cytologic evaluation to be more accurate.
We considered the significantly higher numbers (P = 0.03) of malignant cytology results in male patients were due to the higher mean age of these patients. In previous studies, it is reported that the malignancy rates increase in older patients.[1,2,24]
Conclusion
Comparison of results of this study with various previous studies is shown in Table 4. In conclusion, our results are consistent with the literature. In our study, as well as other publications, patients with “suspicious for malignancy,” “AUS/FLUS” and “FNS” constitute a significant proportion of cases. As stated in previous studies, we believe that addition of molecular techniques to the routine cytologic evaluation process should be considered.[18,19,24]
Table 4.
Comparison of results of present study with previous studies
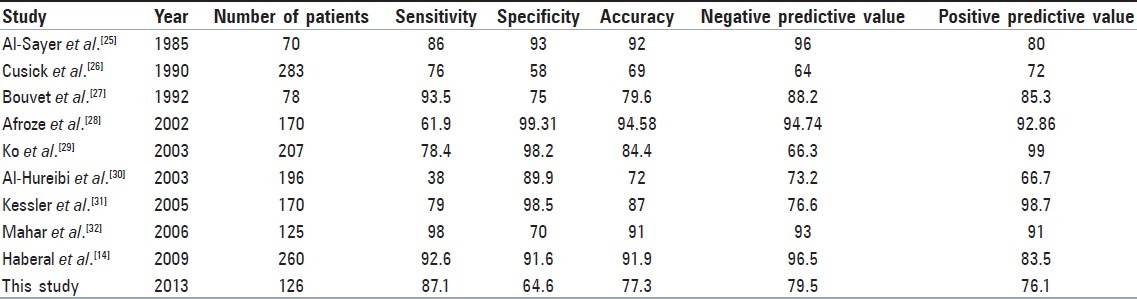
With the emerging data on AUS/FLUS subcategories and increased values of sensitivity and specificity together with the judicious use of molecular techniques may possibly help future management guidelines to avoid unnecessary surgeries.[32]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Gharib H, Papini E, Paschke R. Thyroid nodules: A review of current guidelines, practices, and prospects. Eur J Endocrinol. 2008;159:493–505. doi: 10.1530/EJE-08-0135. [DOI] [PubMed] [Google Scholar]
- 3.Tan GH, Gharib H. Thyroid incidentalomas: Management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–31. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Roman SA. Endocrine tumors: Evaluation of the thyroid nodule. Curr Opin Oncol. 2003;15:66–70. doi: 10.1097/00001622-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Soderstrom N. Puncture of goiters for aspiration biopsy. Acta Med Scand. 1952;144:237–44. [PubMed] [Google Scholar]
- 6.Cibas ES, Ali SZ. NCI Thyroid FNA State of the Science Conference. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132:658–65. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 7.Martin HE, Ellis EB. Biopsy by needle puncture and aspiration. Ann Surg. 1930;92:169–81. doi: 10.1097/00000658-193008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey P, Dixit A, Mahajan NC. Fine-needle aspiration of the thyroid: A cytohistologic correlation with critical evaluation of discordant cases. Thyroid Res Pract. 2012;9:32–9. [Google Scholar]
- 9.Bagga PK, Mahajan NC. Fine needle aspiration cytology of thyroid swellings: How useful and accurate is it? Indian J Cancer. 2010;47:437–42. doi: 10.4103/0019-509X.73564. [DOI] [PubMed] [Google Scholar]
- 10.Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111:508–16. doi: 10.1002/cncr.23116. [DOI] [PubMed] [Google Scholar]
- 11.Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–71. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 12.Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Oncologist. 2008;13:105–12. doi: 10.1634/theoncologist.2007-0212. [DOI] [PubMed] [Google Scholar]
- 13.Baloch ZW, Alexander EK, Gharib H, Raab SS. Overview of diagnostic terminology and reporting. In: Ali SZ, Cibbas ES, editors. The Bethesda System for Reporting Thyroid Cytopathology. Definitions, Criteria and Explanatory Notes. New York: Springer; 2010. pp. 1–3. [Google Scholar]
- 14.Haberal AN, Toru S, Ozen O, Arat Z, Bilezikçi B. Diagnostic pitfalls in the evaluation of fine needle aspiration cytology of the thyroid: Correlation with histopathology in 260 cases. Cytopathology. 2009;20:103–8. doi: 10.1111/j.1365-2303.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 15.Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine-needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125:484–8. doi: 10.5858/2001-125-0484-AOFNAO. [DOI] [PubMed] [Google Scholar]
- 16.Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118:282–9. doi: 10.7326/0003-4819-118-4-199302150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Esmaili HA, Taghipour H. Fine-needle aspiration in the diagnosis of thyroid disease: An appraisal in our institution. ISRN Pathology [Internet] 2012. Jun, [cited 2012 Aug 2]. 912728:[about 4 p.]. Available from: http://downloads.hindawi.com/journals/isrn.pathology/2012/912728.pdf .
- 18.Wang CC, Friedman L, Kennedy GC, Wang H, Kebebew E, Steward DL, et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011;21:243–51. doi: 10.1089/thy.2010.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali SZ. Thyroid cytopathology: Bethesda and beyond. Acta Cytol. 2011;55:4–12. doi: 10.1159/000322365. [DOI] [PubMed] [Google Scholar]
- 20.Borget I, Vielh P, Leboulleux S, Allyn M, Iacobelli S, Schlumberger M, et al. Assessment of the cost of fine-needle aspiration cytology as a diagnostic tool in patients with thyroid nodules. Am J Clin Pathol. 2008;129:763–71. doi: 10.1309/H86KM785Q9KBWPW5. [DOI] [PubMed] [Google Scholar]
- 21.Layfield LJ, Reichman A, Bottles K, Giuliano A. Clinical determinants for the management of thyroid nodules by fine-needle aspiration cytology. Arch Otolaryngol Head Neck Surg. 1992;118:717–21. doi: 10.1001/archotol.1992.01880070047009. [DOI] [PubMed] [Google Scholar]
- 22.Dündar E, Paşaoğlu Ö, Kebapçý M, Bildirici K. Retrospective evaluation of fine needle aspiration biopsy of the thyroid. Turkiye Klinikleri J Med Sci. 2002;22:14–7. [Google Scholar]
- 23.Kini SR. Thyroid. In: Kini SR, editor. Guides to Clinical Aspiration Biopsy Series. 2nd ed. New York: Igaku-Shoin; 1996. [Google Scholar]
- 24.Melillo RM, Santoro M, Vecchio G. Differential diagnosis of thyroid nodules using fine-needle aspiration cytology and oncogene mutation screening: Are we ready? F1000 Med Rep. 2010;2:62. doi: 10.3410/M2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Sayer HM, Krukowski ZH, Williams VM, Matheson NA. Fine needle aspiration cytology in isolated thyroid swellings: A prospective two year evaluation. Br Med J (Clin Res Ed) 1985;290:1490–2. doi: 10.1136/bmj.290.6480.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cusick EL, MacIntosh CA, Krukowski ZH, Williams VM, Ewen SW, Matheson NA. Management of isolated thyroid swellings: A prospective six year study of fine needle aspiration cytology in diagnosis. BMJ. 1990;301:318–21. doi: 10.1136/bmj.301.6747.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouvet M, Feldman JI, Gill GN, Dillmann WH, Nahum AM, Russack V, et al. Surgical management of the thyroid nodule: Patient selection based on the results of fine-needle aspiration cytology. Laryngoscope. 1992;102:1353–6. doi: 10.1288/00005537-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Afroze N, Kayani N, Hasan SH. Role of fine needle aspiration cytology in the diagnosis of palpable thyroid lesions. Indian J Pathol Microbiol. 2002;45:241–6. [PubMed] [Google Scholar]
- 29.Ko HM, Jhu IK, Yang SH, Lee JH, Nam JH, Juhng SW, et al. Clinicopathologic analysis of fine needle aspiration cytology of the thyroid. A review of 1,613 cases and correlation with histopathologic diagnoses. Acta Cytol. 2003;47:727–32. doi: 10.1159/000326596. [DOI] [PubMed] [Google Scholar]
- 30.Al-Hureibi KA, Al-Hureibi AA, Abdulmughni YA, Aulaqi SM, Salman MS, Al-Zooba EM. The diagnostic value of fine needle aspiration cytology in thyroid swellings in a university hospital, Yemen. Saudi Med J. 2003;24:499–503. [PubMed] [Google Scholar]
- 31.Kessler A, Gavriel H, Zahav S, Vaiman M, Shlamkovitch N, Segal S, et al. Accuracy and consistency of fine-needle aspiration biopsy in the diagnosis and management of solitary thyroid nodules. Isr Med Assoc J. 2005;7:371–3. [PubMed] [Google Scholar]
- 32.Mahar SA, Husain A, Islam N. Fine needle aspiration cytology of thyroid nodule: Diagnostic accuracy and pitfalls. J Ayub Med Coll Abbottabad. 2006;18:26–9. [PubMed] [Google Scholar]


