Abstract
Background:
Paraganglioma is a rare tumor arising from clusters of neuroendocrine cells in association with sympathetic and parasympathetic nervous system. It poses a diagnostic challenge because of its widespread anatomic distribution, subtle clinical manifestations, and a variety of morphologic patterns.
Aim:
The aim of this study is to have an insight into the diverse morphologic spectrum of extra-adrenal paraganglioma (EAP).
Materials and Methods:
A retrospective analysis of seven cytologically diagnosed cases of EAP over a period of 10 years was performed. There were five superficial swellings and two deep seated retroperitoneal masses. The superficial swellings were aspirated directly, and the retroperitoneal masses were aspirated under ultrasound guidance using 22-gauge lumbar puncture needle fitted to a 10 mL syringe. Smears were reviewed for cellularity, pattern, cell shape, cytoplasm, nuclear features, and background.
Results:
The age of patients ranged from 25 to 75 years; four patients were males and three were females. Sites involved were carotid body region (four cases), para-pharyngeal space (one case) and para-aortic region (two cases). All the cases yielded hemorrhagic material on fine-needle aspiration. Smears showed scattered and clusters of cells and loosely cohesive acini of tumor cells. Cells were round to polygonal with pleomorphic nuclei, granular chromatin, inconspicuous nucleoli, and moderate to abundant cytoplasm containing fine pink granules and vacuolations. The cases were confirmed on radiology and histopathology.
Conclusion:
The cytologic features in EAP along with pertinent clinicoradiologic findings help in making an accurate preoperative diagnosis of an otherwise rare tumor.
Keywords: Carotid body tumor, extra-adrenal, fine-needle aspiration, paraganglioma
Introduction
Paragangliomas (PG) are the neoplasms arising from clusters of neuroendocrine cells in association with sympathetic and parasympathetic nervous system. These are rare tumors, with incidence of 0.3/1,00,000.[1] These tumors include pheochromocytoma, carotid body tumor and other extra-adrenal paragangliomas (EAP), which have a widespread distribution. The occurrence of EAPs is still rarer with incidence of 2-8/million.[2] The use of fine-needle aspiration (FNA) in the diagnosis of paraganglioma has been controversial due to potential risk of hemorrhage within the lesion and precipitating a hypertensive crisis. Therefore, there are only a few studies highlighting the cytologic features of PG. However, when EAP is not suspected clinically due to lack of typical presentation, FNA is often used as a diagnostic modality for initial workup.
The clinical differential diagnoses of EAP in the head and neck region include lymphadenopathy, branchial cleft cyst, carotid artery aneurysm, carcinoma thyroid, and neural tumors.[3] EAP poses a diagnostic challenge because of its widespread anatomic distribution, subtle clinical manifestations and a variety of morphologic patterns. The aim of this study is to describe the cytological findings of EAP and discuss the differential diagnoses.
Materials and Methods
Seven cases cytologically diagnosed as EAP over a period of 10 years (2003-2012) were retrieved. There were five superficial swellings and two deep seated retroperitoneal masses. Only one case presenting as superficial swelling in right upper cervical region was clinically suspected to be carotid body tumor. The superficial swellings were aspirated directly, and the retroperitoneal masses were aspirated under ultrasound guidance using 22-gauge lumbar puncture needle fitted to a 10 mL syringe. No procedural complications were observed. Smears were reviewed for cellularity, architectural pattern, cell shape, cytoplasm, nuclear features, and background. Cell blocks were made in two cases, and chromogranin immunostaining was done. The diagnosis in all cases was subsequently confirmed on radioimaging and histopathology.
Results
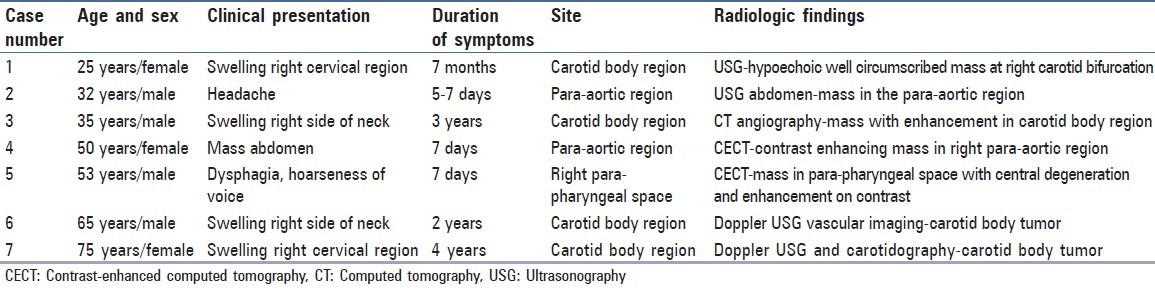
The clinical and radiologic features have been summarized in [Table 1]. The age range was 25-75 years (mean 48.76 years), four patients were males and three were females. The sites involved were carotid body region (four cases), para-aortic region (two cases) and para-pharyngeal space (one case). The presenting feature was a mass lesion in the superficial swellings. Headache, dysphagia and hoarseness of voice were the chief complaints in other cases.
Table 1.
Clinical details and radiologic findings in extra-adrenal paraganglioma (n = 7)
Cytomorphologic features
The smears were cellular in five cases, while two cases were paucicellular [Table 2]. The tumor cells were singly scattered and in clusters with focal acinar formations [Figure 1a]. The cells were mostly round to oval, polygonal with plasmacytoid appearance at places [Figure 1b]. One case with a para-aortic mass showed prominent spindling of tumor cells. The nuclei were single or multiple, pleomorphic with bland nuclear chromatin and prominent nucleoli in all cases. Irregular nuclear membranes and grooves were observed in three cases, with the presence of intranuclear inclusions in two and nuclear budding in one case [Figure 1c]. The cytoplasm was moderate to abundant with pink granularity in all cases. Immunohistochemisty for chromogranin was done on cell blocks in two cases. Intense granular cytoplasmic positivity was observed in the tumor cells [Figure 1d].
Table 2.
Spectrum of cellular details in extra-adrenal paraganglioma (n = 7)
Figure 1.
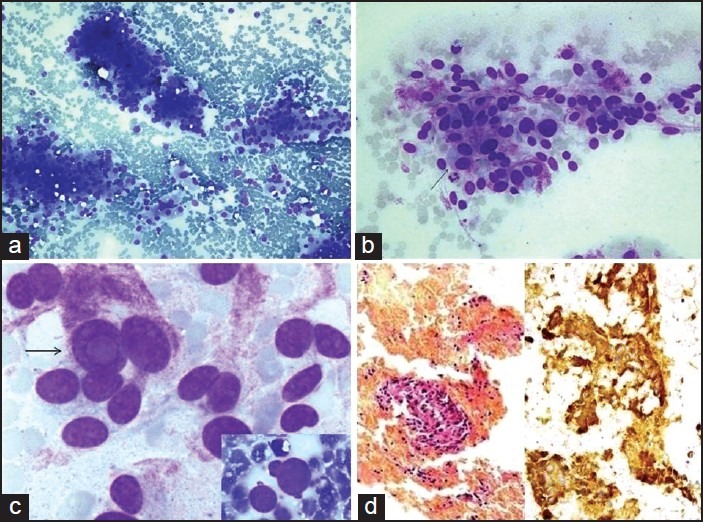
Smears from extra-adrenal paraganglioma showing: (a) Rich cellularity with the presence of cell clusters and dispersed cells (MGG, ×200), (b) Round to polygonal cells with nuclear pleomorphism, abundant granular cytoplasm and a few binucleated cells (arrow) (MGG, ×400), (c) Prominent intranuclear cytoplasmic inclusion (arrow) (MGG, ×600) and a cell with nuclear budding (inset) (MGG, ×600), (d) Cell block preparation with clusters of tumor cells on the left side (H and E, ×100) and immunohistochemistry for chromogranin with strong cytoplasmic positivity on the right side (IHC, ×400)
Follow-up of patients
Histopathologic examination was done in five cases. The tumor size varied between 3.5 cm and 12.8 cm. The cut section was dark brown with areas of hemorrhage. Sections showed solid tumor with typical organoid pattern and pseudo-rosettes were seen in one case. The tumor cells were large polygonal cells with cellular and nuclear pleomorphism and abundant granular cytoplasm. Bi- and multi-nucleation were observed in all cases. Focal areas of necrosis were seen in one case. Vascular or capsular invasion was not seen. All cases showed cytoplasmic immunopositivity for chromogranin and a final diagnosis of EAP was made.
Two cases were finally diagnosed as carotid body tumor on Doppler ultrasound vascular imaging and computed tomography (CT) angiography. One case was clinically suspected to be carotid body tumor based on ultrasound findings prior to the FNA procedure.
Discussion
A substantial number of EAPs are nonfunctional; hence, a correct clinical diagnosis is seldom made. FNA may be a first-line of investigation in such cases and help in arriving at a preliminary or final diagnosis. EAP are uncommon neoplasms, known to occur in a wide variety of anatomic sites. Among EAP, carotid body region is the most common site as in the present series, followed by the retroperitoneum and mediastinum.[1]
A wide spectrum of cytomorphologic features has been ascribed to EAP.[4,5,6,7,8,9] Majority have a dispersed cell population with acinar/rosette formations of round to oval, plasmacytoid or spindle shaped cells with considerable anisokaryosis. Fine and dusty chromatin with salt and pepper pattern is characteristic. Abundant cytoplasm with red granules, vacuolization and indistinct cytoplasmic borders cannot be overemphasized. The background is hemorrhagic in most of the cases. In our series, all cases showed clusters and singly scattered round to polygonal cells with pleomorphic nuclei and moderate to abundant cytoplasm with fine pink granularity. In addition, acinar formation, nuclear grooves, inclusions, membrane irregularity, budding, and nucleoli were also seen. Varma et al.[10] have reported nuclear budding in 8 out of 12 cytologically diagnosed cases of PG.
The cytologic differentiation of paraganglioma from other entities is a herculean task due to varied anatomic locations and tremendous cellular pleomorphism noted in these tumors. In the neck region, it can be difficult to differentiate paraganglioma from thyroid neoplasms, neurogenic tumors and metastatic carcinoma.[11] Acinar structures in paraganglioma can be mistaken for the follicles seen in follicular neoplasm of the thyroid. Identification of colloid is useful in such a setting. Occasional cases of paraganglioma may show intranuclear cytoplasmic inclusions as seen in papillary carcinoma thyroid. Presence of papillae and optically clear nuclei in papillary carcinoma thyroid are discriminating morphological features. Plasmacytoid appearance and intracytoplasmic reddish-pink granules in paraganglionic tumors may lead to a misdiagnosis of medullary carcinoma thyroid. However, significant discohesion, anisonucleosis and amyloid seen in medullary carcinoma thyroid helps. A misdiagnosis of neurogenic tumor is not unlikely when there is predominance of ovoid or spindle shaped nuclei in some PG. The uniform and benign looking chromatin pattern of the nucleus in paraganglioma helps in distinguishing it from metastatic carcinoma.
In the retroperitoneal location, differential diagnoses include epithelial tumors such as renal cell carcinoma, adrenal cortical tumor, carcinoid tumor, islet-cell tumor, chordoma, and metastatic adenocarcinoma.[12] In renal cell carcinoma, the cytoplasm is less fragile with more prominent vacuolization and cell borders are well-defined. Adrenal cortical tumors show a lipid background and are comparatively less pleomorphic with tiny lipid droplets in the cytoplasm.[13] The cytomorphologic features of chordoma are quite characteristic and show physaliferous cells in the background of chondroid matrix. Occasionally, hepatocellular carcinoma, neurogenic tumors, sarcomas and melanoma may also enter into the differential diagnosis.[12,14] Ancillary techniques are helpful in making a confirmatory diagnosis of EAP. It shows immunoreactivity for chromogranin, synaptophysin, neuron-specific enolase and S-100.[10,15] Radioimaging studies like ultrasound, CT scan with enhancement, magnetic resonance imaging and vascular imaging studies; like Doppler ultrasound and selective digital subtraction angiography are useful adjuncts to cytomorphology. These studies help in exact localization of EAP and also define their relation with major blood vessels.[4,16,17]
Conclusion
Cytomorphologic features like dispersed cell population with acinar pattern, plasmacytoid cells with salt and pepper nuclear chromatin and abundant granular vacuolated cytoplasm with indistinct cytoplasmic borders suggest a diagnosis of EAP. Ancillary techniques and clinical details help in reaching a definitive diagnosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Grufferman S, Gillman MW, Pasternak LR, Peterson CL, Young WG., Jr Familial carotid body tumors: Case report and epidemiologic review. Cancer. 1980;46:2116–22. doi: 10.1002/1097-0142(19801101)46:9<2116::aid-cncr2820460934>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Wen J, Li HZ, Ji ZG, Mao QZ, Shi BB, Yan WG. A decade of clinical experience with extra-adrenal paragangliomas of retroperitoneum: Report of 67 cases and a literature review. Urol Ann. 2010;2:12–6. doi: 10.4103/0974-7796.62919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapoor R, Saha MM, Das DK, Gupta AK, Tyagi S. Carotid body tumor initially diagnosed by fine needle aspiration cytology. Acta Cytol. 1989;33:682–3. [PubMed] [Google Scholar]
- 4.Zaharopoulos P. Diagnostic challenges in the fine-needle aspiration diagnosis of carotid body paragangliomas: Report of two cases. Diagn Cytopathol. 2000;23:202–7. doi: 10.1002/1097-0339(200009)23:3<202::aid-dc13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Chen LT, Hwang WS. Fine needle aspiration of carotid body paraganglioma. Acta Cytol. 1989;33:681–2. [PubMed] [Google Scholar]
- 6.Hood IC, Qizilbash AH, Young JE, Archibald SD. Fine needle aspiration biopsy cytology of paragangliomas. Cytologic, light microscopic and ultrastructural studies of three cases. Acta Cytol. 1983;27:651–7. [PubMed] [Google Scholar]
- 7.Fleming MV, Oertel YC, Rodríguez ER, Fidler WJ. Fine-needle aspiration of six carotid body paragangliomas. Diagn Cytopathol. 1993;9:510–5. doi: 10.1002/dc.2840090508. [DOI] [PubMed] [Google Scholar]
- 8.Mincione G, Urso C. Fine needle aspiration cytologic findings in a case of carotid body paraganglioma (chemodectoma) Acta Cytol. 1989;33:679–81. [PubMed] [Google Scholar]
- 9.Handa U, Bal A, Mohan H, Dass A. Parapharyngeal paraganglioma: Diagnosis on fine-needle aspiration. Am J Otolaryngol. 2005;26:360–1. doi: 10.1016/j.amjoto.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Varma K, Jain S, Mandal S. Cytomorphologic spectrum in paraganglioma. Acta Cytol. 2008;52:549–56. doi: 10.1159/000325596. [DOI] [PubMed] [Google Scholar]
- 11.Das DK, Gupta AK, Chowdhury V, Satsangi DK, Tyagi S, Mohan JC, et al. Fine-needle aspiration diagnosis of carotid body tumor: Report of a case and review of experience with cytologic features in four cases. Diagn Cytopathol. 1997;17:143–7. doi: 10.1002/(sici)1097-0339(199708)17:2<143::aid-dc11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RK, Cheung YK, Wakefield L, Wakefield SJ, Johnson P. Fine-needle aspiration cytology of malignant retroperitoneal paraganglioma. Diagn Cytopathol. 1998;18:287–90. doi: 10.1002/(sici)1097-0339(199804)18:4<287::aid-dc8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez-Heffernan JA, Vicandi B, López-Ferrer P, González-Peramato P, Pérez-Campos A, Viguer JM. Cytologic features of pheochromocytoma and retroperitoneal paraganglioma: A morphologic and immunohistochemical study of 13 cases. Acta Cytol. 2006;50:372–8. doi: 10.1159/000325975. [DOI] [PubMed] [Google Scholar]
- 14.Vera-Alvarez J, Marigil-Gómez M, Abascal-Agorreta M, Vázquez-Garcia J. Malignant retroperitoneal paraganglioma with intranuclear vacuoles in a fine needle aspirate. A case report. Acta Cytol. 1993;37:229–33. [PubMed] [Google Scholar]
- 15.McNicol AM. Update on tumours of the adrenal cortex, phaeochromocytoma and extra-adrenal paraganglioma. Histopathology. 2011;58:155–68. doi: 10.1111/j.1365-2559.2010.03613.x. [DOI] [PubMed] [Google Scholar]
- 16.Gong Y, DeFrias DV, Nayar R. Pitfalls in fine needle aspiration cytology of extraadrenal paraganglioma. A report of 2 cases. Acta Cytol. 2003;47:1082–6. doi: 10.1159/000326652. [DOI] [PubMed] [Google Scholar]
- 17.González-Cámpora R, Otal-Salaverri C, Panea-Flores P, Lerma-Puertas E, Galera-Davidson H. Fine needle aspiration cytology of paraganglionic tumors. Acta Cytol. 1988;32:386–90. [PubMed] [Google Scholar]


