Abstract
Background:
The skin of Bufo bufo gargarizans Cantor, rich in bufadienolides, peptides, and alkaloids, has approved pharmacological activity for preliminary anti-liver and lung tumor treatment. However, few studies have systematically focused on the influence of the producing regions on the content and antitumor activity of the active constituents in toad skins.
Objective:
This study aims to compare toad skins obtained from six different regions in China (Jiangsu, Anhui, Henan, Hebei, Jiangxi, and Shandong province) for their bufadienolide and alkaloid content, and their cytotoxic activity on two lung carcinoma cell lines (SPC-A-1 cells and A549 cells).
Materials and Methods:
High performance liquid chromatography (HPLC) was used to quantificationally determine four bufadienolides, which included bufotalin, bufalin, cinobufagin, and resibufogenin in toad skins, from six different regions, respectively. In addition, an ultraviolet (UV) spectrophotometer was also employed to identify the content of the total alkaloids using 5-hydroxytryptamine (5-HT) as the reference substance. An MTT assay was performed to compare the antiproliferative effects of the toad skins’ ethanolic extracts from the different regions against SPC-A-1 and A549 cells.
Results:
In this study, the toad skins from Jiangsu province had the highest amount of bufadienolides (472.6 μg/g crude drug) and alkaloids (1.51 mg/g crude drug). Meanwhile, according to the extract, it exhibited the strongest cytotoxic effect against the lung carcinoma cell line (SPC-A-1 cells and A549 cells) with IC50 values of 24.82 ± 0.76 and 23.77 ± 0.63 μg crude drug/mL, respectively.
Conclusion:
The toad skins that originated from the Jiangsu province, have comparatively greater advantages over samples from other regions as far as active constituent content and potential anti-lung cancer activity is concerned, suggesting that it can be a promising chemotherapeutic agent in lung cancer therapy, in further studies.
Keywords: Active constituents, toad skins, Bufo bufo gargarizans cantor, producing regions, content determination, cytotoxicity, lung carcinoma cell
INTRODUCTION
Lung cancer is one of the most common malignancies worldwide, with more than a million deaths per year, and its incidence is still on the rise.[1] Characterized by its poor five-year survival, poor prognosis, and resistance to the apoptosis activity of antineoplastic drugs both in vivo and in vitro, new chemotherapeutic agents and more effective therapies for the treatment of lung cancer are mandatory.[2] In recent times, numerous traditional Chinese medicines and their active components, with potential antitumor activity, have attracted considerable attention, as candidates for the development of novel cancer therapeutics.[3]
The skins of the toad Bufo bufo gargarizans Cantor, a source of some Chinese medicines such as Chansu and cinobufacini (Huachansu), exhibit antipyretic, detoxicant, diuretic, stasis-eliminative, and pus-discharging properties.[4,5] Chansu and its preparations, such as Liu-Shen-Wan and She-Xiang-Bao-Xin-Wan, have been widely used as cardiotonic, diuretic, antimicrobial, local anesthetic, and anodyne and antineoplastic agents for thousands of years.[6] Cinobufacini, an aqueous extract of dried toad skin, is a traditional Chinese medicinal preparation, widely used in clinical cancer therapy, in China.[7] At present, cinobufacini has been developed into a variety of dosage forms such as tablets, oral solutions, and injections, which are approved by the Chinese State Food and Drug Administration (SFDA) (ISO9002) and displayed significant anti-cancer effects in a variety of cancers, including hepatic, pancreatic, gastric, and esophageal carcinomas.[8,9,10,11]
Previous studies have demonstrated that the major pharmacological constituents derived from toad skins are hydro-soluble indole alkaloids (bufotenine, bufotenidine, and cinobufotenine) and liposoluble steroidal cardiac glycosides mainly composed of bufadienolides.[12] Bufadienolides, such as, bufotalin, bufalin, cinobufagin, and resibufogenin, have been reported as Na+-K+-ATPase inhibitors and exhibit cytotoxic and growth-inhibitory activity against various human cancer cells in vitro.[13,14]
There is a direct relationship between the producing regions and the quality of the Chinese Materia Medica. The same Materia Medica in different regions may bring about a content difference in the effective ingredients, resulting in a significant difference in the curative effect. Therefore, the present study compared toad skins obtained from six different regions in China (Jiangsu, Anhui, Henan, Hebei, and the Jiangxi and Shandong province) for their bufadienolide and alkaloid contents, and cytotoxic activity on the lung carcinoma cell line (SPC-A-1 cells and A549 cells).
MATERIALS AND METHODS
Animal materials
The dried skins of Bufo bufo gargarizans Cantor from six different regions were purchased from individual farmers in the Jiangsu, Anhui, Henan, Hebei, Jiangxi, and Shandong provinces in China, and authenticated by Professor Dekang Wu of the School of Pharmacy, Nanjing University of Chinese Medicine, Jiangsu Province, P.R. China.
Reagents and materials
Bufotalin (10102631) and bufalin (11070631) standards were provided by Tauto Biotech Co., Ltd. (Shanghai, China). Cinobufagini (110803-200605), resibufogenin (110718-200507), and serotonin hydrochloride (5-HT, 111656-200401) standards were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Cisplatin were obtained from the Jiangsu Hengrui Medicine Co., Ltd. (Nanjing, Jiangsu). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St Louis, MO, USA). Acetonitrile was of HPLC grade and methanol was of an analytical grade. Deionized water was prepared with the help of the Millipore Mlli-Q water purification system.
Cell culture
The lung carcinoma cell line (SPC-A-1 cells and A549 cells) were maintained as exponentially growing cultures in an RPMI 1640 cell culture medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA). The cell lines were cultured at 37°C, in air/carbon dioxide (95:5) atmosphere.
High-performance liquid chromatography quantization of the bufadienolides in toad skins from six different regions
Apparatus and analytical conditions
High-performance liquid chromatography was performed using a Waters 600 system equipped with a vacuum degasser, quaternary solvent mixing autosampler, and a Waters 486 diode array detector. A Waters PC 800 chromatography workstation was utilized for instrument control, data collection, and data processing. The HPLC fingerprint was performed on a C18 column (Agilent, ZORBAX SB-C18, 4.6 × 250 mm, 5 μm) at 40°C, with a sample injection volume of 10 μL, and detection wavelength of 296 nm for the analysis. The mobile phase, consisting of methyl cyanides and 0.05% phosphoric acid (40:60, v/v), was at a flow rate of 1.0 mL/minute.
Preparation of sample solutions
The dry powder (1.0 g) of toad skins, obtained from different producing areas, was refluxed with 60 mL methanol for one hour. After filtrating, the residue was dissolved and made up to 5 mL with methanol. This solution was then filtered through a 0.45 μm microporous membrane and the filtrate was reserved for HPLC analysis.
Preparation of mixed standard solutions
Standard stock solutions of bufotalin (0.476 mg/mL), bufalin (0.392 mg/mL), cinobufagin (0.376 mg/mL), and resibufogenin (0.420 mg/mL) were prepared by dissolving accurately measured standards in methanol. The mixed standard working solutions containing 47.6 μg/mL bufotalin, 39.2 μg/mL bufalin, 37.6 μg/mL cinobufagin, and 42.0 μg/mL resibufogenin, were obtained by sequential dilution of the mixture of the four standard stock solutions using methanol.
Validation
The reliability of the HPLC method for analysis was validated through its linearity, precision, stability, and recovery.
Linearity
Stock solutions of bufotalin, bufalin, cinobufagin, and resibufogenin were diluted with methanol to five different concentrations to construct the calibration plots. The linearity of each standard curve was confirmed by plotting the peak area (y) against the corresponding concentration (x, μg/mL) of the analytes.
Precision
The precision of the method was evaluated by applying the method developed above, to analyze the same sample (mixed standard solutions) on six consecutive injections. The relative standard deviations (RSD) of the relative peak areas were calculated.
Stability
The sample solution of the toad skins from the Jiangsu Province was stored in a HPLC vial at 25°C and analyzed at 0, 2, 4, 8, 12, and 24 hours. Four chromatographic peaks were chosen to be inspected and the RSD of the relative peak areas were calculated.
Recovery
Six toad skin samples from the same source (Jiangsu Province) were extracted after a standard addition procedure with three levels (80, 100, and 120%) and analyzed using the method developed above. The recovery percentage and RSD were calculated.
Sample analysis
The newly established HPLC analytical method was subsequently applied for analyzing the four bufadienolides in the six samples from different areas in China.
Ultraviolet spectrophotometric quantization of the total alkaloids in toad skins from six different regions
Preparation of sample solutions
The dry powder (0.4 g) of toad skins obtained from the different producing areas was sonicated with 10 mL methanol for 30 minutes. After filtrating, 5 mL of 15% para-Dimethylaminobenzaldehyde solution was added to 1 mL of the filtrate and made up to 10 mL with deionized water.
Preparation of standard solutions
Standard stock solution of 5-HT was prepared by dissolving accurately measured standards (1.98 mg) in 25 mL deionized water.
Calibration curves
Various amounts of 5-HT stock solution, mixed with 5 mL 15% para-Dimethylaminobenzaldehyde solution, were added to 10 mL measuring flasks and made up to volume with deionized water. The absorbance at 555 nm was measured by an MV-2802 UV spectrophotometer (Unico, Shanghai, China). The calibration curves were performed by plotting the absorbance (y) against the corresponding concentration (x, μg/mL) of the analytes.
Sample analysis
The UV spectrophotometer method developed above was subsequently applied to assaying the total alkaloids in the toad skins from the six regions in China.
Comparison of the cytotoxic effects of the toad skin extracts from six different regions on SPC-A-1 and A549 cells
Preparation of the toad skins extract
The dry powder (5.0 g) of toad skins obtained from the different producing areas was refluxed twice with 50 mL of 95% ethanol for three hours. After filtrating, the filtrate was dried in a water bath and the residue was made up to 10 mL with ethanol, obtaining 0.5 g of crude drug/mL stock solution. Then, the 5 mg crude drug/mL testing solution was prepared by diluting 100 μL of the stock solution to 10 mL with phosphate buffered saline (PBS).
Cytotoxicity assay
The cytotoxicity assay was performed using the MTT method.[15] One hundred microliters of 13 × 104 cells/mL SPC-A-1 cells and 5 × 104 cells/mL A549 cells were seeded in 96-multiwell plates, each well separately. After 24 hours, the medium was replaced by 90 μL of fresh medium and 10 μL of toad skins extract at different concentrations. Thereafter, the final concentrations of the wells were 500, 100, and 10 μg crude drug/mL, respectively. Except for the wells with a concentration of 0 μM, cisplatin was added to the others, to serve as a positive control. After 36 hours of treatment, the cells were incubated with 10 μL of MTT (Sigma-Aldrich, USA) (5 mg/mL) in the dark, at 37°C, for four hours. Then the culture medium was removed and 100 μL of Dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystals. The optical density (OD) was measured at 550 nm, using a Multiskan MK3 microplate reader (Thermo, USA). All the samples were assayed in triplicate. The inhibitory rates (IR, %) were calculated according to the following formula:
IR (%) = [1− (OD of test group)/(OD of control group)] ×100%
The IC50 value (concentration of 50% cytotoxicity) was calculated using the SPSS 11.5 statistical software, to express the cytotoxicity.
Statistical analysis
Data were expressed as mean ± SD and were analyzed with the SPSS 11.5 statistical software. Analysis of variance and the student's t-test were used to evaluate the statistical significance. A value of P < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Content determination of bufadienolides
The chemical structures of bufotalin, bufalin, cinobufagin, and resibufogenin are shown in Figure 1. With the purpose of developing a better simultaneous quantification method for the four bufadienolides, various gradient elution systems, through adjusting the ratio of water to acetonitrile, were used as a suitable mobile phase in this study. In order to shorten the retention time and prevent potential ionization, phosphoric acid (0.05%) was added to the water to modulate the pH. Consequently, a mixture of acetonitrile: 0.05% phosphoric acid (40:60, v/v) gave a good resolution, short retention time, and less tailing. The HPLC chromatograms of mixed standard solutions and toad skin methanol extracts from the Jiangsu province are presented in Figure 2. The retention times of bufotalin, bufalin, cinobufagin, and resibufogenin were 9.5, 16.5, 24.6, and 28.0 minutes, respectively. No obvious interfering peak was observed in the matrix, suggesting an acceptable analytical technique.
Figure 1.
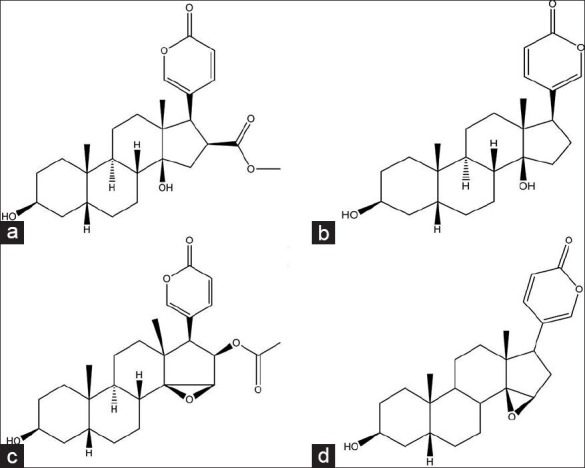
Chemical structures of four active bufadienolides components in toad skins. (a) Bufotalin, (b) Bufalin, (c) Cinobufagin, (d) Resibufogenin
Figure 2.
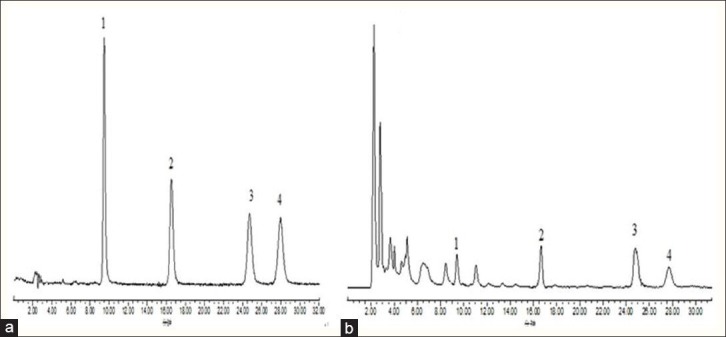
HPLC chromatograms of mixed standard solutions (a) and toad skin extracts from Jiangsu province (b). 1. Bufotalin; 2. Bufalin; 3. Cinobufagin; 4. Resibufogenin
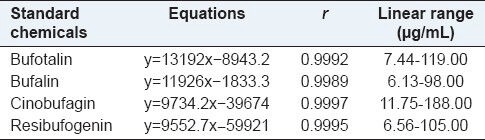
The calibration curves, according to the correlation coefficients of the four bufadienolides, are exhibited in Table 1. The precision of the HPLC method was evaluated by the repeatability test and the RSD of the relative peak area for bufotalin, bufalin, cinobufagin, and resibufogenin were 0.39, 0.27, 0.81, and 0.41%, respectively. The maximum RSD was lower than 2.0%, indicating a good reliability of this method. The results of the stability test showed that the RSD of the peak areas of bufotalin, bufalin, cinobufagin, and resibufogenin were 0.55, 0.75, 0.36, and 0.31%, respectively. It was suggested that these test solutions were stable within 24 hours, at room temperature. The average recoveries for bufotalin, bufalin, cinobufagin, and resibufogenin were 100.8, 99.9, 99.9, and 100.4%, with a small RSD, respectively, exhibiting good accuracy of the method. Therefore, the HPLC quantitation method was confirmed to meet the requirements of sample analysis (ICH, 2005).
Table 1.
Regression curves and correlation coefficients (r) of the four bufadienolides (n=5)
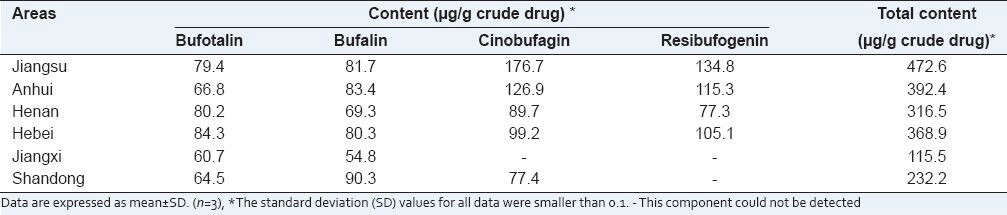
The average contents of the four bufadienolides in the various toad skins are listed in Table 2. The toad skins from the Hebei and Shandong provinces showed the maximum level of bufotalin (84.3 μg/g crude drug) and bufalin (90.3 μg/g crude drug), respectively. However, the toad skins from the Jiangsu province had a comparatively higher level of cinobufagin (176.7 μg/g crude drug) and resibufogenin (134.8 μg/g crude drug) than that from other regions. In a quantitative comparison with the total bufadienolide content of toad skins from other provinces (Anhui, Henan, Hebei, Jiangxi, and Shandong), the samples from Jiangsu had 472.6 μg/g of the crude drug, which was 20.4, 49.3, 28.2, 309.2, and 103.5% higher than that from the other provinces, respectively. In addition, no cinobufagin and resibufogenin were detected in toad skins from the Jiangxi province, and no resibufogenin was present in toad skins from the Shandong province, exhibiting a notable difference in content among the various toad skins.
Table 2.
Content determination of the four bufadienolides in toad skins from the six different regions
Content determination of total alkaloid
As indole alkaloids, primarily 5-HT, were the main alkaloids in toad skins, 5-HT was selected as the reference substance and determined by measuring the absorbance of the red compound formed from 5-HT and 15% para-Dimethylaminobenzaldehyde reagent in acidic conditions, at 555 nm, using spectrophotometry.[16,17] The standard curve for 5-HT was y = 0.028× +0.016, r = 0.9990. Good linearity was observed, within the range of 7.92 - 27.72 μg/mL, and the average recovery was 99.58%, with an RSD of 0.39%. The highest amount of total alkaloid was also found in toad skins from the Jiangsu province, with a content of 1.51 mg/g crude drug, which was 23.77, 21.77, 31.30, 38.53, and 29.06% higher than that in the toad skins from Anhui, Henan, Hebei, Jiangxi, and the Shandong provinces, respectively [Table 3].
Table 3.
Content determination of the total alkaloid in toad skins from the six different regions (mg/g crude drug)

Cytotoxicity against human lung carcinoma cell line
The ethanolic extract of toad skins from six regions (0.5, 0.1, 0.01 mg crude drug/mL) significantly decreased the cell viability to the lung carcinoma cell line (SPC-A-1 cells and A549 cells) in a dose-dependent manner [Figure 3]. The toad skin extracts from the Jiangsu province showed the strongest growth inhibitory effect on SPC-A-1 and A549 cells, at all predetermined concentrations. As shown in Table 4, with the highest amount of bufadienolides and alkaloids, the toad skins from Jiangsu province presented the lowest IC50 of 24.82 ± 0.76 and 23.77 ± 0.63 μg crude drug/mL for SPC-A-1 and A549 cells, respectively, producing the strongest cytotoxic effect. The differences were much more significant compared to the findings on toad skins that originated from Jiangxi and Shandong province, which did not contain resibufogenin and produced the least cytotoxic effect. It was indicated that the bufadienolides and alkaloids were the active constituents of toad skins, and their content might be related to the antitumor activity of toad skins. However, the toad skins from different regions exhibited a substantial difference in the cytotoxic effect, revealing the close correlation between the producing regions and the quality of the Chinese Materia Medica. Therefore, it is very important for the researchers to select a qualified Chinese Materia Medica, from the specified regions, for further study.
Figure 3.
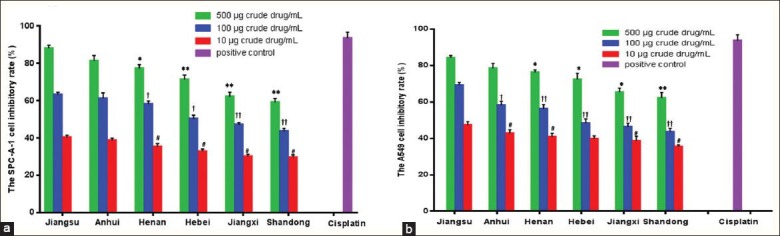
The inhibitory rate of the ethanolic extract of toad skins from six different regions on (a) SPC-A-1 cells and (b) A549 cells. Data expressed as mean ± SD (n = 3). *p < 0.05 and **p < 0.01, †p < 0.05 and ††p < 0.01, #p < 0.05 versus the ethanolic extract of toad skins from Jiangsu province at concentration of 500, 100 and 10 ?g crude drug / mL, respectively
Table 4.
IC50 of the ethanolic extract of toad skins from the six different regions on SPC-A-1 cells and A549 cells

CONCLUSIONS
In summary, we developed a simultaneous determination method for the four bufadienolides of toad skins Bufo bufo gargarizans Cantor from six different regions, by HPLC. Meanwhile, the quantitation of total alkaloid in toad skins from various sources was also done by using the UV spectrophotometer method. The established methods were confirmed to be rapid, accurate, and reliable for the content analysis of the bioactive compounds in toad skins, which were closely related to the producing areas. Our data suggested the toad skins from the Jiangsu province contained a significantly higher amount of bufadienolides and total alkaloids than the other toad skins, especially toad skins that originated from Jiangxi and Shandong. More importantly, the difference mentioned above led to an improved cytotoxic activity against lung cancer cell lines, indicating a promising chemotherapeutic agent in cancer treatment.
Footnotes
Source of Support: The financial grant of this work has been supported by the by the Jiangsu Provincial Chinese Medicine Leading Talent Project (LJ200913)
Conflict of Interest: None declared.
REFERENCES
- 1.Samarghandian S, Boskabady MH, Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–14. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bepler G. Lung cancer: Provoking new concepts, generating novel ideas, and rekinding enthusiasm. Cancer Control. 2003;10:275–6. doi: 10.1177/107327480301000401. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: How far have we come? Pharm Res. 2010;27:950–61. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 4.Wang SS, Zhai XF, Li B. Effects of cinobufacini injection on contents of serum thyroid-stimulating hormone and adrenaline in rats. Zhong Xi Yi Jie He Xue Bao. 2009;7:228–31. doi: 10.3736/jcim20090306. [DOI] [PubMed] [Google Scholar]
- 5.Zeng XM, Macritchie HB, Marriott C, Martin GP. Humidity-induced changes of the aerodynamic properties of dry powder aerosol formulations containing different carriers. Int J Pharm. 2007;333:45–55. doi: 10.1016/j.ijpharm.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Beijing: China Chemical Industry Press; The State Pharmacopoeia Commission of People's Republic of China. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- 7.Lu CX, Nan KJ, Lei Y. Agents from amphibians with anticancer properties. Anticancer Drugs. 2008;19:931–9. doi: 10.1097/CAD.0b013e3283139100. [DOI] [PubMed] [Google Scholar]
- 8.Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge Y, et al. Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer. 2009;115:5309–18. doi: 10.1002/cncr.24602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Zhu L, Wu Z, Wang Y. Chinese herbal medicines for induction of remission in advanced or late gastric cancer. Cochrane Database Syst Rev. 2013;4:CD005096. doi: 10.1002/14651858.CD005096.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin TJ, Zhao XH, Yun J, Zhang LX, Ruan ZP, Pan BR. Efficacy and safety of gemcitabine-oxaliplatin combined with huachansu in patients with advanced gallbladder carcinoma. World J Gastroenterol. 2008;14:5210–6. doi: 10.3748/wjg.14.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Jin Y, Xu Z, Zheng Z, Wan S. Involvement of caspase-3 activity and survivin downregulation in cinobufacini-induced apoptosis in A 549 cells. Exp Biol Med. 2009;234:566–72. doi: 10.3181/0811-RM-326. [DOI] [PubMed] [Google Scholar]
- 12.Qi FH, Li AY, Lv H, Zhao L, Li JJ, Gao B, et al. Apoptosis-inducing effect of cinobufacini, Bufo bufo gargarizans Cantor skin extract, on human hepatoma cell line BEL-7402. Drug Discov Ther. 2008;2:339–43. [PubMed] [Google Scholar]
- 13.Wang Z, Wen J, Zhang J, Ye M, Guo D. Simultaneous determination of four bufadienolides in human liver by high-performance liquid chromatography. Biomed Chromatogr. 2004;18:318–22. doi: 10.1002/bmc.322. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Sun Y, Liu JH, Yu BY, Xu Q. Microbial transformation of three bufadienolides by Nocardia sp. and some insight for the cytotoxic structure-activity relationship (SAR) Bioorg Med Chem Lett. 2007;17:6062–5. doi: 10.1016/j.bmcl.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 15.Samarghandian S, Boskabady MH, Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–14. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding XN, Zhang QL, Zhang Y. Determination of indole alkaoid in cinobufacin dispersible tablets by spectrophotometry. Anhui Med Pharm J. 2007;11:1093–4. [Google Scholar]
- 17.Wang L, Raju U, Milas L, Molkentine D, Zhang Z, Yang P, et al. Huachansu, containing cardiac glycosides, enhances radiosensitivity of human lung cancer cells. Anticancer Res. 2011;31:2141–8. [PubMed] [Google Scholar]


