Abstract
Objective:
Atractylodes macrocephala, a famous herbal medicine, is used extensively in the practice of Traditional Chinese Medicine (TCM). Processing procedure is a common approach that usually occurs before A. macrocephala is prescribed. This paper describes a sensitive and specific assay for the determination of principal volatile compounds in crude and processed A. macrocephala.
Materials and Methods:
The present study concentrated on the development of a static headspace gas chromatography-mass spectrometry (SHS-GC/MS) for separating and identifying of volatile compounds from crude and processed A. macrocephala samples.
Results:
The results showed that the volatile oil in crude and processed A. macrocephala was markedly quantitatively and qualitatively different. Processing resulted in the reduction of volatile oil contents and variation of chemical compositions in A. macrocephala.
Conclusion:
The proposed method proved that SHS-GC/MS is rapid and specific, and should also be useful for evaluating the quality of crude and processed medicinal herbs.
Keywords: Atractylodes macrocephala, processed, quality control, static headspace gas chromatography-mass spectrometry, volatile compounds
INTRODUCTION
Atractylodes macrocephala (Bai Zhu in Chinese), derived from the rhizome of A. macrocephala Koidz, as a valuable Traditional Chinese medicine (TCM), has been used for thousands of years all over the world because of its special pharmacological activities.[1,2,3] The rhizome of A. macrocephala is an important ingredient of several chinese herbal prescriptions, and has been used in drugs for diarrhea, abdominal pain, and insufficiency of the stomach, intestine, liver, kidney, or insufficiency of the spleen with abundance of dampness. Many components, such as volatile oils, sesquiterpenoids, polysaccharides, amino acids, vitamins, resins and other ingredients have been found in Atractylodes macrocephala up to now.[4,5,6,7,8] The essential oil as the major constituent has been isolated from A. macrocephala and has several pharmacological functions.[9] The essential oil of the herb has anti-inflammatory and anti-ulcer properties, scavenges the CCl3 radical, inhibits lipid peroxidation and xanthine oxidase inhibition, inhibits the tert-butyl hydroperoxide-induced cytotoxicity and lipid peroxidation in primary culture of rat hepatocytes, and inhibits the growth of esophageal carcinoma cells and tumor cells.[10] The crude A. macrocephala and its processed products are used clinically for thousands of years.[11,12] A proper pharmaceutical processing methodology may significantly alter the pharmacological properties of the original crude TCM, such as the reduction of toxicity and the enhancement of pharmaceutical efficacy.[13,14]
Steam distillation and solvent extraction methods combined with gas chromatography (GC) or gas chromatography-mass spectrometry (GC/MS) are used as the routine methods for the analysis of the volatile oils of TCMs. GC/MS after steam distillation (SD) has been used for the analysis of essential oils in A. macrocephala.[15] However, it requires a relatively large amount of samples and is a time-consuming procedure. However, none has reported the development of static headspace gas chromatography-mass spectrometry (SHS-GC-MS) as a rapid and efficient analytical tool for the solvent-free fractionation of crude and processed A. macrocephala volatiles. Here, we present the development of an automated SHS with GC-MS based analytical method to the separation and identification of the essential oils present in the crude A. macrocephala and its processed products by stir-frying with wheat bran (SFWB). SHS sampling has great advantage in the analysis of highly volatiles compounds in A. macrocephala. In SHS, the samples are enclosed in a gas tight sealed vial and are heated at a defined temperature.[16] The developed method has been demonstrated to be a powerful tool to study the principal volatile compounds in crude and processed A. macrocephala.
MATERIALS AND METHODS
Plant materials
The crude A. macrocephala samples were collected from Zhejiang provice. Processed A. macrocephala of SFWB was obtained according to the Chinese Pharmacopoeia edited in 2010. These herbal samples were authenticated by Professor Jianbao Zheng (Research Center of TCM Processing Technology, Zhejiang Chinese Medical University). Voucher specimens were stored at the Research Center of TCM Processing Technology.
Sample preparation
The powder of crude and processed A. macrocephala samples were precisely weighed (2.0 g) and then introduced into a 20 mL headspace vial. The headspace vial was immediately sealed with a silicone septum and stored at −10°C until use. SHS equilibration was performed at 65°C for 20 min., shaking at 250 rpm. 350 μL of headspace gas were injected using a heated (85°C) gastight syringe (1 mL) in split mode 10:1.
Gas chromatograph-mass spectrometer analysis
Volatile oil analysis was carried out on a Thermo Fisher ISQ single quadrupole gas chromatograph-mass spectrometer (GC/MS). An DB-5MS capillary column (30 m × 0.25 mm, 0.25 μm) was used for separation of the volatile compounds in the A. macrocephala samples. The injection temperature was set at 250°C and a splitless mode was used. The oven temperature program was as follows: Initial temperature 60°C for 1 min, increased to 160°C at 3°C/min for 0 min, then increased to 250°C at 5°C/min, 250°C was maintained for 5 min. Helium (99.999%) carrier gas had a flow-rate of 1.0 ml/min. Mass spectra were recorded in electron impact (EI) with full scan mode at 70 eV, scanning the 40-450 m/z range. Ion source temperature was 200°C. Filament emission current was 50 MA. The MS transferline temperature was set at 250°C. Compounds were identified using the NIST Mass Spectral Search Program (National Institute of Standards and Technology, Washington, DC, USA).
RESULTS AND DISCUSSION
SHS-GC/MS is a technique suitable for determining volatile compounds in herbal medicine samples. In present study, 49 volatile compounds were identified from crude and processed A. macrocephala samples, representing 88.59 and 87.57% of the oils, respectively, according to their elution order. The typical GC/MS chromatogram of volatile oil in crude and processed A. macrocephala under the same column systems are depicted in Figure 1. The chemical constituents identified by GC/MS in the essential oil and their contents are summarized in Table 1. Although the identified components were similar in the essential oils derived from the two groups, the quantity of some components in each essential oil was significantly different.
Figure 1.
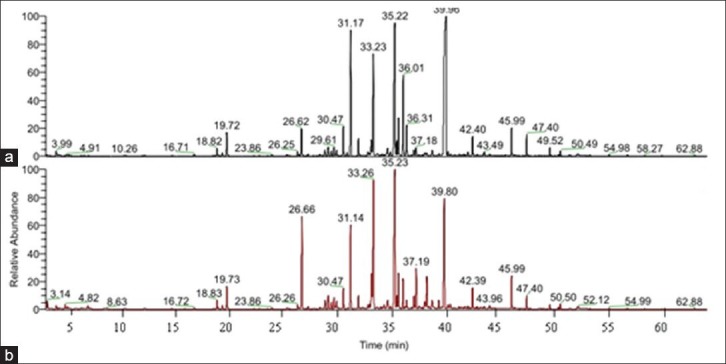
Typical GC/MS chromatogram of SHS analysis of volatile compounds from crude (a) and processed (b) Atractylodes macrocephala samples
Table 1.
Chemical composition of volatile oils in crude and processed Atractylodes macrocephala
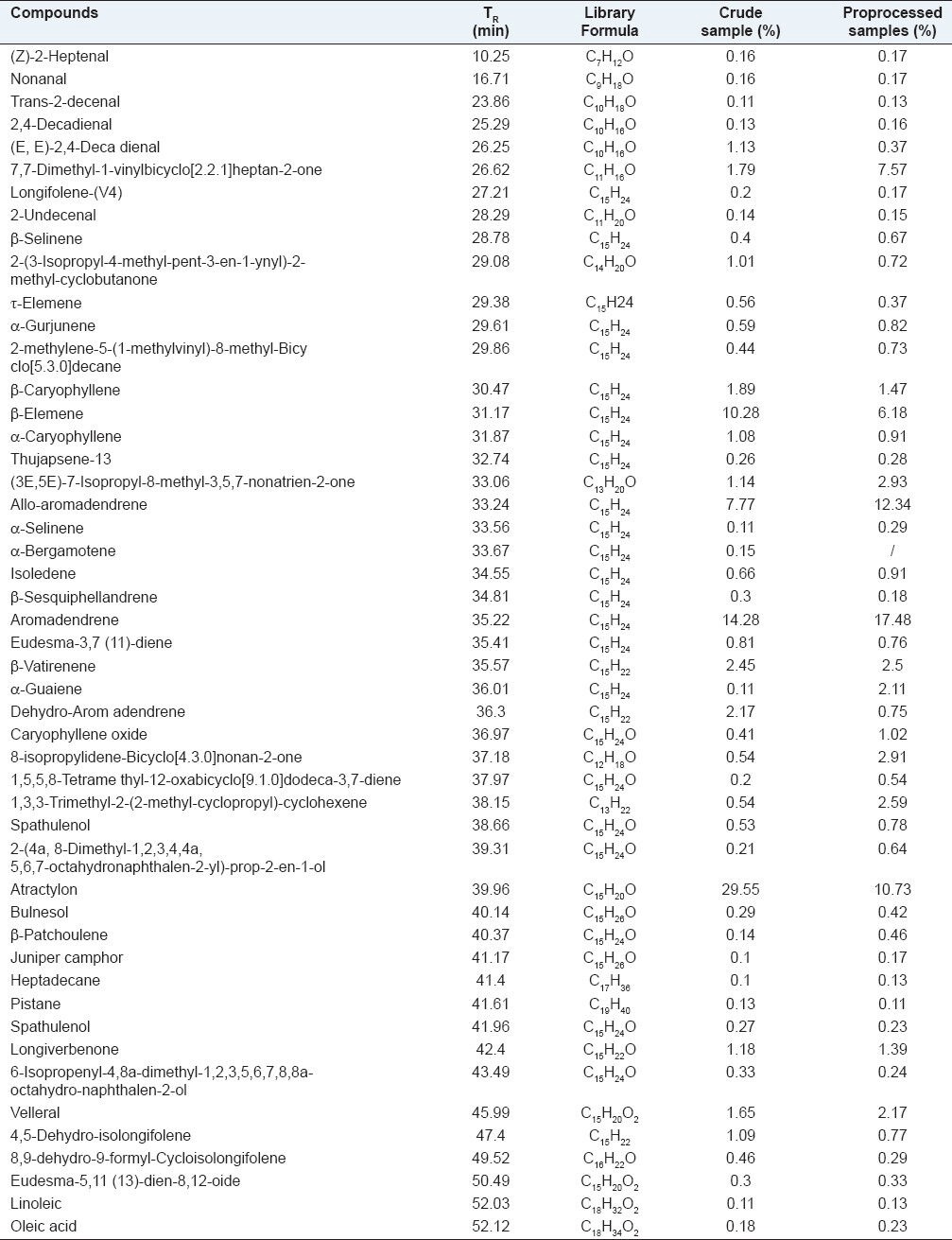
Processing procedure had a great effect on the volatile components and the content of them in A. macrocephala. There were more components in the oil from the processed samples than the crude ones. In this study, one compound (α-bergamotene), which was not identified in the oils of the processed samples, was lost during the processing in the oils of A. macrocephala. Moreover, there was a large difference in the contents of volatile composition between the crude and processed drugs. The main constituents in the essential oils of crude and processed A. macrocephala were atractylon, aromadendrene, τ-Elemene and allo-aromaden drene, accounting for more than 61.80% and 46.70% of the total oil from crude and processed samples, respectively. The structures and accurate mass spectra of the above four compounds were shown in Figure 2 and Figure 3. After processing, heat treatment increased relative amount of allo-aromaden drene and aromadendrene from 7.77-12.34%, 14.28-17.48%, respectively, however, the contents of τ-Elemene and atractylon in processed samples were lower than in crude ones. On the hole, except for 16 volatile compositions, other compounds from processed A. macrocephala were increased in various degrees. According to historical records, the processed Atractylodis rhizome of SFWB showed stronger effect of invigorating the function of the spleen than the crude ones.[17] It is presumed that the increased contents of volatile components induced by the processing procedure would be related to the effect of invigorating the function of the spleen.
Figure 2.
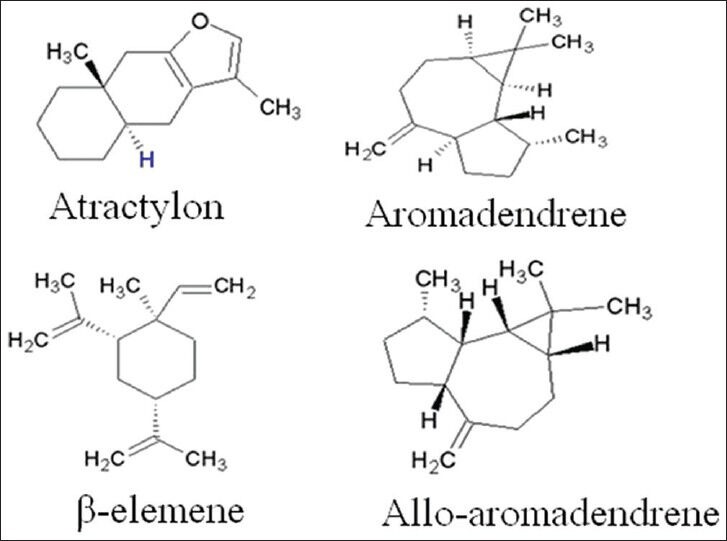
The structures of mian constituents in the volatile oils of crude and processed Atractylodes macrocephala
Figure 3.
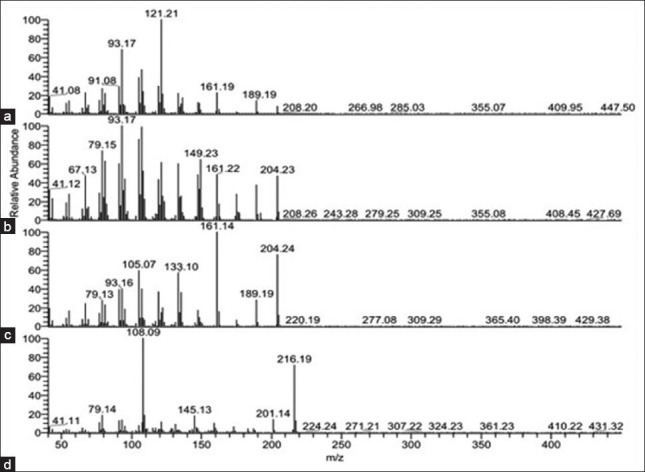
The accurate mass spectra of the four compounds in the volatile oils of crude and processed Atractylodes macrocephala. (a) β-elemene; (b) Allo-aromadendrene; (c) Aromadendrene; (d) Atractylon
CONCLUSIONS
In the present study, a fast, simple, and reliable method was developed to evaluate the quality of crude and processed A. macrocephala samples through determination of principal volatile compounds. The results demonstrate that processed procedure can reduce the volatile oil content and variation of chemical composition in A. macrocephala and the further pharmacological studies are necessary to determine the relationship between the variation in composition and curative effects. This study also contributes to the global quality investigation of decoctions derived from crude and processed herbal medicines, as well as safe and effective medicines for customers with great social and economic interest.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (No. 81202918), the Open Project of National First-Class Key Discipline for Science of Chinese Materia Medica, Nanjing University of Chinese Medicine (No. 2011ZYX2-006), the Project of Science and Technology for Chinese Medicine of Zhejiang Province, China (No. 2013KYB183), the Chinese Medicine Research Program of Zhejiang Province, China (No. 2014ZQ008), the Science and Technology Project of Hangzhou, China (No. 20130533B68, and No. 20131813A23), and the Science Foundation of Zhejiang Chinese Medical University (No. 2011ZY25, and No. 2013ZZ12).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chen Q, He H, Li P, Zhu J, Xiong M. Identification and quantification of atractylenolide I and atractylenolide III in Rhizoma Atractylodes macrocephala by liquid chromatography-ion trap mass spectrometry. Biomed Chromatogr. 2013;27:699–707. doi: 10.1002/bmc.2847. [DOI] [PubMed] [Google Scholar]
- 2.Shi YY, Guan SH, Tang RN, Tao SJ, Guo DA. Simultaneous determination of atractylenolide II and atractylenolide III by liquid chromatography-tandem mass spectrometry in rat plasma and its application in a pharmacokinetic study after oral administration of Atractylodes macrocephala Rhizoma extract. Biomed Chromatogr. 2012;26:1386–92. doi: 10.1002/bmc.2709. [DOI] [PubMed] [Google Scholar]
- 3.Rong S, Lin H, Gao N. Study on processing technology and processing principles of atractrylodis macrocephalae rhizoma. Zhongguo Zhong Yao Za Zhi. 2011;36:1001–3. [PubMed] [Google Scholar]
- 4.Li X, Lin J, Han W, Mai W, Wang L, Li Q, et al. Antioxidant ability and mechanism of rhizoma Atractylodes macrocephala. Molecules. 2012;17:13457–72. doi: 10.3390/molecules171113457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Chen J, Pan K. Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environ Sci Pollut Res Int. 2013;20:1441–9. doi: 10.1007/s11356-012-1048-0. [DOI] [PubMed] [Google Scholar]
- 6.Tsai CJ, Liang JW, Lin HR. Sesquiterpenoids from Atractylodes macrocephala act as farnesoid X receptor and progesterone receptor modulators. Bioorg Med Chem Lett. 2012;22:2326–9. doi: 10.1016/j.bmcl.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Shi YY, Guan SH, Tang RN, Tao SJ, Guo DA. Simultaneous determination of four sesquiterpenoids in Atractylodes macrocephala Rhizoma by GC-FID: Optimisation of an ultrasound-assisted extraction by central composite design. Phytochem Anal. 2012;23:408–14. doi: 10.1002/pca.1373. [DOI] [PubMed] [Google Scholar]
- 8.Jiang H, Shi J, Li Y. Screening for compounds with aromatase inhibiting activities from Atractylodes macrocephala Koidz. Molecules. 2011;16:3146–51. doi: 10.3390/molecules16043146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou RB, Wu J, Tong QZ, Liu YM, Liu XD. Studies on volatile oil from Atractylodes macrocephala with different distill methods. Zhong Yao Cai. 2008;31:229–32. [PubMed] [Google Scholar]
- 10.Yang C, Lao Y, Wu F, Su W. Advances in the study of Atractylodes macrocephala Koidz. Zhong Yao Cai. 2002;25:206–8. [PubMed] [Google Scholar]
- 11.Wang CS. The processing of Atractylodes macrocephala by stir-frying with wheat bran. Zhong Yao Tong Bao. 1983;8:18–9. [PubMed] [Google Scholar]
- 12.Hao C, Zhiwei X, Sucai L, Wenwen Z, Gang C, Xiao L, et al. Study on chemical fingerprinting of crude and processed Atractylodes macrocephala from different locations in Zhejiang province by reversed-phase high-performance liquid chromatography coupled with hierarchical cluster analysis. Pharmacogn Mag. 2012;32:300–7. doi: 10.4103/0973-1296.103659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun H, Ni B, Zhang A, Wang M, Dong H, Wang X. Metabolomics study on Fuzi and its processed products using ultra-performance liquid-chromatography/electrospray-ionization synapt high-definition mass spectrometry coupled with pattern recognition analysis. Analyst. 2012;137:170–85. doi: 10.1039/c1an15833c. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Wang Y, Su L, Li L, Zhang Y. Exploring potential chemical markers by metabolomics method for studying the processing mechanism of traditional Chinese medicine using RPLC-Q-TOF/MS: A case study of Radix Aconiti. Chem Cent J. 2013;7:36. doi: 10.1186/1752-153X-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Q, Cui ZJ, Liu TL, Zhang SD. Analysis of volatile compounds in Atractylodes macrocephala by GC-MS. Chin Traditional Herbal Drugs. 2002;33:998–1001. [Google Scholar]
- 16.Karthikeyan K, Arularasu GT, Devaraj P, Pillai KC. Determination of residual epichlorohydrin in sevelamer hydrochloride by static headspace gas chromatography with flame ionization detection. Sci Pharm. 2010;78:835–46. doi: 10.3797/scipharm.1007-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Fang L, Wang X, Zhang J, Guo LP, Huang LP. Comparison of th evolatile compounds of crude and processed Atractylodis rhizome analyzed by GC-MS. Afr J Pharm Pharmacol. 2012;6:2155–60. [Google Scholar]


