Abstract
Background:
Bushen Huoxue Qubi (BHQ) granules, a traditional Chinese medicine preparation, has been clinically used for the treatment of the blood stasis syndrome.
Objective:
The main objective is to investigate whether the diseased condition would alter the pharmacokinetics and tissue distribution of tanshinone IIA in BHQ, which was given orally to the acute blood stasis rats.
Materials and Methods:
The main bioactive constituent in BHQ, tanshinone IIA, was measured in the plasma and tissues of animals by the high performance liquid chromatography with ultraviolet detection. The analysis was successfully performed on an Agilent TC-C18 column (250 × 4.6 mm I.D., 5 μm) protected with a Octadecylsilane (ODS) guard column (10 × 4.6 mm I.D., 5 μm). The mobile phase was aqueous solution (A) (containing 0.40% aqueous acetic acid) and acetonitrile (B). The conditions of the solvent gradient elution were 35-40% (B) in 0-15 min, 40-42% (B) in 15-18 min and 42-70% (B) in 18-30 min at a flow rate of 1.0 mL/min. Detection was conducted with wavelength of 270 nm at 30°C.
Results:
Good linearity relationships were found (r2> 0.9955) over the investigated concentration range for bio-samples. Blood stasis was associated with significantly higher area under the concentration-time curve (AUC), the maximum plasma concentration (Cmax) and biological half-life (t1/2), lower total body clearance (CL) and apparent volume of distribution (Vd) of tanshinone IIA in plasma and higher AUC0-t of tanshinone IIA in the analyzed tissues of rats treated with BHQ.
Conclusion:
Blood stasis could alter pharmacokinetics and tissue distribution of tanshinone IIA in BHQ.
Keywords: Blood stasis, Bushen Huoxue Qubi granules, high performance liquid chromatography, pharmacokinetics, tanshinone IIA, tissue distribution
Bushen Huoxue Qubi (BHQ) granules is a traditional Chinese medicine (TCM) preparation composed of 10 medicinal herbs, namely, Radix Salvia miltiorrhiza, Radix Celastri Orbiculati, Rhizoma Drynariae, Folium Epimedii, Fructus Aurantii, Radix Paeoniae Alba, Radix Astragali, Cortex Acanthopanacis, Radix Angelica Sinensis, Radix Glycyrrhizae. BHQ is frequently prescribed by Chinese medicine practitioners to treat the blood stasis syndrome.[1] Patients with the blood stasis syndrome manifested slow blood flow or poor blood circulation.[2] According to the theory of TCM, the blood stasis syndrome results from Qi deficiency, Qi stagnation, Phlegm-turbidity, blood deficiency, blood-cold or blood-heat and trauma. Clinically, blood stasis is characterized by pain, swelling, bleeding and cyanosis. Once blood stasis develops, hemorheology and microcirculation will be further affected. It will lead to the elevation of blood viscosity, deterioration of erythrocyte deformability, acceleration of erythrocyte aggregation and platelet aggregation as well as microcirculatory dysfunction.[3] Consequently, these changes have direct effect on the drug pharmacokinetics and the tissue distribution.[4] In BHQ, Radix Salvia miltiorrhiza is the major herb. Salvia miltiorrhiza, a traditional Chinese medicinal plant (known as “Danshen”), has been widely used to treat cardiovascular and cerebrovascular diseases such as angina pectoris, cerebral thrombosis, hyperlipidemia and acute ischemic stroke.[5] Tanshinone IIA is an important bioactive constituent in Danshen and has been reported possessing anticoagulant,[6] anti-inflammatory[7] and anti-oxidative activities,[8] also used as biomarker for in vivo study.[9]
Recently, many analytical methods have been developed to determine the concentration of tanshinone IIA in the plasma or tissues of animals treated with tanshinone IIA or extract of Salvia miltiorrhiza.[9,10] However, majority of the pharmacokinetics and tissue distribution studies focused on monomer or extract of Salvia miltiorrhiza in the normal animals and few investigation of Chinese herbal formula containing tanshinone IIA was carried out under the pathological conditions. As we know, Chinese herbal formula is the main form of treatment in TCM and has played an indispensable role in the prevention and treatment of diseases for more than 2500 years. Danshen-containing preparations are widely applied to treat blood stasis syndrome. Little is known about the pharmacokinetics and tissue distribution of tanshinone IIA in the context of blood stasis after administration of Danshen-containing Chinese herbal formula. The study of the pharmacokinetics and tissue distribution study of tanshinone IIA in Chinese herbal formula under pathological conditions are much more valuable for the clinical application. Therefore, it is very important to establish an analytical method that can be used to study the pharmacokinetics and tissue distribution of tanshinone IIA in blood stasis rats treated with BHQ (Chinese herbal formula). Until now, the pharmacokinetics and tissue distribution study of tanshinone IIA in Chinese herbal formula under pathological conditions were reported for the first time.
MATERIALS AND METHODS
Materials and reagents
Acetonitrile (high performance liquid chromatograph [HPLC] grade) was purchased from Tedia Co. (Fairfield, OH, USA). Wahaha purified water was provided by Hangzhou Wahaha Group Co. Ltd (Hangzhou, China). Methanol, acetic acid and diethyl ether (analytical grade) were obtained from Changsha Reagent Company (Changsha, China). Tanshinone IIA standard [Lot number 110766-200518, purity >98%, the chemical structure shown in Figure 1a] and loratadine [Lot number 100615-201103, internal standard (IS), purity >98%, the chemical structure shown in Figure 1b] were purchased from National Institute for Food and Drug Control (Beijing, China). Adrenaline hydrochloride injection was obtained from Wuhan Grand Pharmaceutical Group Co. Ltd. (Lot number 120305, Wuhan, China). BHQ granules (Lot number 20120720) were supplied by the Second Xiangya Hospital of Central South University. The BHQ solution administered in the experiments was prepared by dissolving the granules in 0.4% (w/v) carboxymethyl cellulose sodium salt (CMC-Na) and the concentration of tanshinone IIA in the BHQ solution was determined by HPLC to be 3.03 mg/mL.
Figure 1.
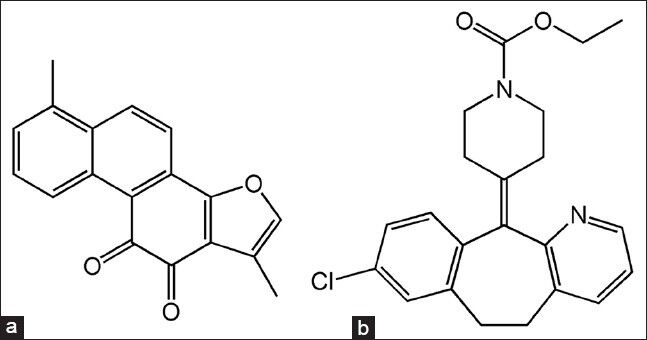
The chemical structures of tanshinone IIA (a) and internal standard loratadine (b)
Instrument and analytical conditions
Analyses were performed on an Agilent 1200 series HPLC system (Agilent Technologies, Waldbronn, Germany), equipped with a vacuum degasser, a quaternary pump, an auto sampler and a UV detector. Liquid chromatographic separation was achieved using an Agilent TC-C18 column (250 × 4.6 mm I.D., 5 μm) protected with a ODS guard column (10 × 4.6 mm I.D., 5 μm). The mobile phase was aqueous solution (A) (containing 0.40% aqueous acetic acid) and acetonitrile (B). The conditions of the solvent gradient elution were 35-40% (B) in 0-15 min, 40-42% (B) in 15-18 min and 42-70% (B) in 18-30 min at a flow rate of 1.0 mL/min. Detection was conducted with wavelength of 270 nm at 30°C.
Animals and induction of animal model
Male Sprague-Dawley (SD) rats, weighing 220 ± 20 g, were purchased from the Animal Center of Hunan University of Chinese Medicine (Certificate No. SCXK [Xiang] 2009-0004) and acclimatized in the laboratory for at least 5 days. Animals were individually housed in a plastic bottom cages with food and water provided ad libitum and maintained at temperature of 24 ± 2°C with 55 ± 5% relative humidity and a 12 h light/dark cycle. Prior to experiments animals were fasted for 12 h with free water supply.
The acute blood stasis was induced in SD rats as described previously.[4] Briefly, animals were subcutaneously injected at the intraperitoneal area with the adrenaline hydrochloride injection at the dose of 0.8 mg/kg. The injection was administered twice, each separated by 4 h. 2 h before the second injection, animals were soaked in ice water for 5 min with their heads above the water. Animals with the acute blood stasis were housed in the metabolic cages. All the experimental protocols involving animals and their care were approved by the Committee on Use of Human and Animal Subjects in Teaching and Research of the Hunan University of Chinese Medicine and were carried out according to the regulations of the National Institutes of Health of USA.
Drug administration and sample collection
Rats with acute blood stasis or normal rats were randomly divided into 13 groups with five rats per group. The dose of the BHQ solution (60 mg/kg of tanshinone IIA, equivalent dose in humans) was administered by gastric gavage to animals. After administration, blood samples (1.0 mL) were collected in heparinized eppendorf tubes through the caudal vein at the following time intervals: 0, 5, 10, 15, 30, 45, 60, 90, 120, 240, 360, 480 and 720 min. Blood samples were centrifuged at 4000 rpm for 10 min at room temperature and the resulting plasma samples were frozen at −20°C for further analysis. Tissue samples of heart, lung, liver and brain were taken at 30, 120, 360 and 720 min, respectively. These tissue samples were rinsed with phosphate buffered saline (PBS), weighed and then homogenized in PBS to prepare 0.5 g/mL homogenates. Tissue homogenates were frozen at −80°C for further analysis.
Sample extraction procedure
Tissue homogenates or plasma samples (470 μL) were mixed with 10 μL of loratadine (30.8 μg/mL) and 1.5 mL of acetic ether by vortexing for 60 s. The mixture was then centrifuged at 4000 rpm for 10 min. The acetic ether fraction was separated and transferred to a microcentrifuge tube. The water fraction was extracted with 1.0 mL of acetic ether again. The acetic ether fractions were combined and dried under a stream of nitrogen flow at 40°C. The residue was reconstituted in 100 μL of HPLC mobile phase and 20 μL of the solution was injected into the LC system.
Preparation of the standard solution, calibration standard and quality control sample
The stock solution of tanshinone IIA (123 μg/mL) and IS (loratadine) (308 μg/mL) were prepared in methanol. The working solutions of tanshinone IIA were obtained by diluting the stock solutions to different concentrations with methanol and stored at 4°C before analysis. A 30.8 μg/mL working solution of IS was similarly prepared by diluting a loratadine stock solution with methanol. Calibration standards of tanshinone IIA were prepared by spiking 20 μL of working solution into 450 μL of blank plasma or blank tissues. The final concentration of plasma, heart and brain was between 20.94 ng/mL and 523.40 ng/mL, between 20.94 ng/mL and 2617.0 ng/mL for liver and lung. The quality control (QC) samples were prepared in the same way as the calibration standards with three concentrations of 41.87, 83.74 and 418.72 ng/mL for plasma, heart, brain and 41.87, 167.48, 2093.6 ng/mL for liver and lung. The equations of the calibration curve and correlation coefficients were calculated by plotting the peak area ratio of tanshinone IIA/IS (Y) versus the concentration of tanshinone IIA (X) using weighted (1/χ2) least squares linear regression.
Method validation
Specificity
To investigate the specificity of the method, the chromatograms of blank plasma or blank tissues were compared with those of QC biosamples and biosamples after oral administration of BHQ spiked with IS loratadine.
Precision and accuracy
Precision and accuracy were assessed with the QC samples. Precision was determined by the relative standard deviations. The intra-day precision was evaluated by replicate analysis of six sets of QC samples at three concentrations (high, medium and low) on the same day. For the inter-day precision, QC samples at three concentrations were analyzed on three consecutive days. Accuracy was determined by comparing the observed concentration using calibration curves to the theoretical concentration.
Recovery
The recovery of tanshinone IIA was evaluated using the QC samples at three different concentrations by comparing the areas of spiked bio samples after liquid extraction with those at the same theoretical concentrations without liquid extraction.
Pharmacokinetic and statistical analyses
The maximum plasma concentration (Cmax) and the time to reach the maximum concentration (Tmax) were directly obtained from the plasma data. Other pharmacokinetic parameters, including area under the concentration-time curve (AUC), biological half-life (t1/2), apparent volume of distribution (Vd) and total body clearance (CL), were calculated using the non-compartmental method with the PKSolver program.[11] All the results were expressed as the mean ± S.D. Unpaired Student's t-test was used for data-comparison and P < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Method validation
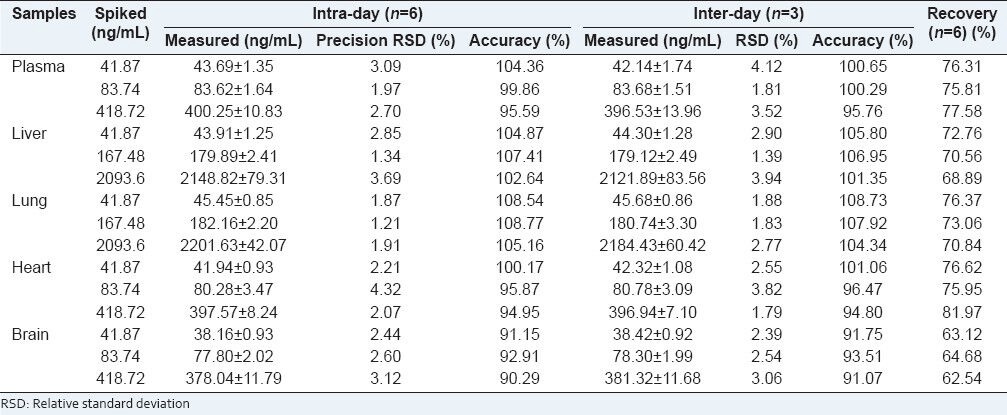
Under the conditions described above, we did not detect any interference peaks from endogenous constituents in blank plasma sample [Figure 2]. Both tanshinone IIA and loratadine (IS) were well separated with a retention time of 40.5 min and 17.1 min, respectively. The results of equations of calibration curve and correlation coefficients were as follows: y = 0.0125×−0.0213 (r2 = 0.9955) for plasma, y = 0.0112×+0.0321 (r2 = 0.9972) for lung, y = 0.0113×+0.0179 (r2 = 0.9985) for liver, y = 0.0128×−0.0158 (r2 = 0.9959) for heart, y = 0.0116×−0.0002 (r2 = 0.9970) for brain. They displayed excellent linearity over the concentration ranges (all correlation coefficients > 0.99). The intra-day precisions and inter-day precisions ranged from 1.21% to 4.32% and from 1.39% to 4.12%, respectively. The intra-day and inter-day accuracies were in the range of 90.29% to 108.77% and 91.07% to 108.73%, respectively. These results indicated that the present method has good precision and accuracy for all samples. The mean recovery of tanshinone IIA in plasma and tissues ranged from 62.54% to 81.97%, which suggests that tanshinone IIA could be extracted from the bio samples effectively. The results of precision, accuracy and recovery are shown in Table 1.
Figure 2.
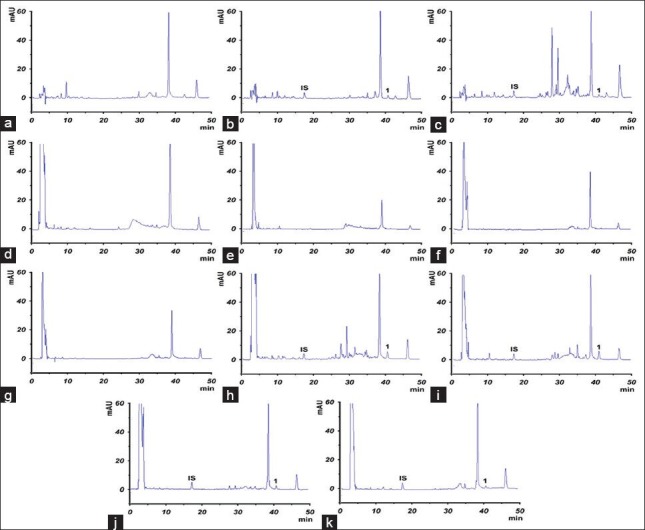
Representative chromatograms of tanshinone IIA in rat plasma and tissue homogenate. The samples from rat at 0.5 h following oral administration of Bushen Huoxue Qubi at a dose of 60 mg/kg tanshinone IIA: (a) Blank plasma; (b) blank plasma spiked with tanshinone IIA standard and internal standard loratadine; (c) plasma sample; (d) blank liver; (e) blank lung; (f) blank heart; (g) blank brain; (h) liver sample; (i) lung sample; (j) heart sample; (k) brain sample. (IS: loratadine; 1: tanshinone IIA)
Table 1.
Precision, accuracy, and recovery of tanshinone IIA in biosamples
Pharmacokinetic and tissue distribution studies
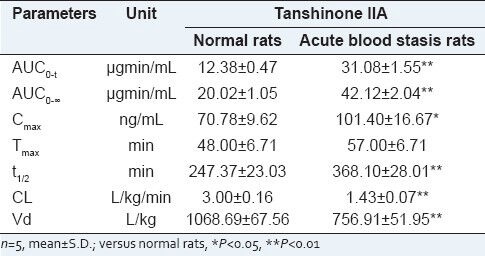
Plasma concentrations of tanshinone IIA in SD rats were determined successfully in normal rats and blood stasis rats after oral administration of BHQ. The mean plasma concentration-time profiles are presented in Figure 3. Notably, a multi-peak pattern was observed in the plasma concentration-time profiles of tanshinone IIA, which was consistent with the previous report.[12] It could be explained with relate to non-homogeneous gastrointestinal absorption, enterohepatic circulation, tissue drug redistribution and absorption[12] and metabolic transformation.[13] Cmax in the acute blood stasis rats was 1.5 times of that found in the normal rats and Tmax of tanshinone IIA lasted longer in the acute blood stasis rats than that in the normal rats. The pharmacokinetic parameters were calculated using the non-compartmental method with the PKSolver program[11] and are shown in Table 2. After comparing the pharmacokinetic parameters of tanshinone IIA in the normal rats with those in the acute blood stasis rats by t-test, significant differences were observed between the normal and acute blood stasis rats when orally administrated with BHQ, such as Cmax, AUC0-t, AUC0-∞, t1/2, Vd and CL. Particularly, remarkable increase of AUC, Cmax and t1/2 and decrease of CL, Vd of tanshinone IIA were observed in the acute blood stasis rats compared with the normal rats treated with BHQ.
Figure 3.
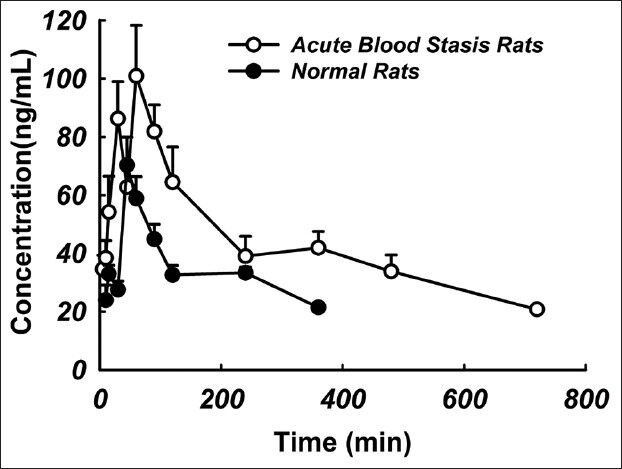
Mean concentration-time profiles of tanshinone IIA in normal and acute blood stasis rats treated with Bushen Huoxue Qubi (n= 5)
Table 2.
Pharmacokinetic parameters of tanshinone IIA in rats treated with BHQ
As listed in Figure 4, tanshinone IIA was detected in liver, lung, heart and brain at 0.5, 2, 6 and 12 h, after a single dose oral administration of BHQ (tanshinone IIA 60 mg/kg). Tanshinone IIA could be detected at 0.5 h post-dose in the analyzed tissues and the concentration reached peak level at the same time, which indicated that tanshinone IIA could be distributed rapidly into the tissues with abundant blood supply. High lipophilicity of tanshinone IIA brought about its rapid and wide distribution. Tanshinone IIA was still detectable in both lung and liver at 12 h post-dose, while it dropped below the limit of quantification in the heart and brain at 6 h post-dose in normal rats and at 12 h in acute blood stasis rats. AUC0-t of tanshinone IIA in tissues was also calculated using the non-compartmental method with the PKSolver program[11] and illustrated in Figure 5. Compared with the normal rats, AUC0-t of tanshinone IIA in liver, lung, heart and brain increased significantly in acute blood stasis rats [Figure 5]. Tanshinone IIA concentrations in the analyzed tissues were found to decrease in the order of lung >liver >brain >heart in the normal rats and lung >liver >heart >brain in the acute blood stasis rats. Lung displayed the highest tanshinone IIA concentration among all tissues examined in both normal and acute blood stasis rats.
Figure 4.
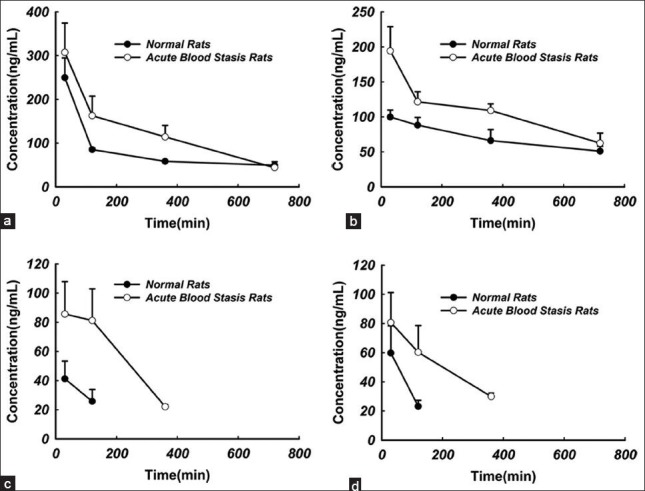
Tissue concentration-time profiles of tanshinone IIA in normal and acute blood stasis rats treated with Bushen Huoxue Qubi (n= 5): (a) lung; (b) liver; (c) heart; (d) brain
Figure 5.
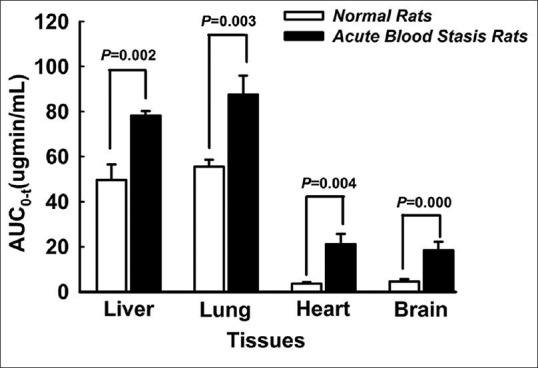
AUC0-t of tanshinone IIA in tissues in normal and acute blood stasis rats (n = 5)
Several pharmacokinetic and tissue distribution studies focus on normal animal,[14] regardless of pathological condition. Our study showed that blood stasis was associated with significantly higher AUC, Cmax, t1/2 and lower CL and Vd of tanshinone IIA in plasma and higher AUC0-t of tanshinone IIA in the analyzed tissues of rats treated with BHQ. According to the report,[15] blood stasis is associated with inflammation and the levels of inflammatory cytokines such as serum interleukin-6 and tumor necrosis factor are elevated in the animal models with blood stasis. Subsequently, the increase of these inflammatory cytokines can down regulate the expression and activity of drug transporters such as P-glycoprotein (P-gp),[16] a drug efflux transporter widely expressed in the intestinal wall, bile duct, tubule, blood-brain barrier and tumor tissue. Tanshinone IIA is a substrate and reversible agent for P-gp.[17] The decrease in P-gp-mediated efflux of tanshinone IIA into the gut lumen[17] and blood-brain barrier[18] may contribute to increasing absorption of tanshinone IIA in the animal model with blood stasis.
CONCLUSIONS
A simple, reliable and sensitive HPLC method for the analysis of tanshinone IIA in biosamples had been validated and successfully applied to pharmacokinetic and tissue distribution studies of tanshinone IIA in BHQ under blood stasis syndrome. The results indicated that tanshinone IIA could be distributed rapidly into the tissues and blood stasis could strongly influence the pharmacokinetic parameters and tissue distribution characteristics of tanshinone IIA in rats when administrated with BHQ. It might be a reference for the clinical application of BHQ on blood stasis syndrome.
Footnotes
Source of Support: This work was supported by the Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province for Innovation of Chinese Medicine and Resources sustained utilization (2010212), Hunan Provincial Science and Technology Department Foundation (2009TP4051-1), Changsha Scientific Research Foundation (k1003034-31).
REFERENCES
- 1.Gao G, Wu H, Tian J, Du J, Xie X, Gao J. Clinical efficacy of bushen huoxue qubi decoction on treatment of knee-osteoarthritis and its effect on hemarheology, anti-inflammation and antioxidation. Zhongguo Zhong Yao Za Zhi. 2012;37:390–6. [PubMed] [Google Scholar]
- 2.Zhu WF, Yuan ZK. 2nd ed. Beijing: China Medical Science Press; 2011. Chinese Diagnostics. [Google Scholar]
- 3.Wang T, Jia C, Chen Y, Li X, Cheng J. Analysis on establishment and affecting factors of qi stagnation and blood stasis rat model. Zhongguo Zhong Yao Za Zhi. 2012;37:1629–33. [PubMed] [Google Scholar]
- 4.Tian Y, Yang ZF, Li Y, Qiao Y, Yang J, Jia YY, et al. Pharmacokinetic comparisons of hydroxysafflower yellow A in normal and blood stasis syndrome rats. J Ethnopharmacol. 2010;129:1–4. doi: 10.1016/j.jep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L, Zuo Z, Chow MS. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–59. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 6.Wu LC, Lin X, Sun H. Tanshinone IIA protects rabbits against LPS-induced disseminated intravascular coagulation (DIC) Acta Pharmacol Sin. 2012;33:1254–9. doi: 10.1038/aps.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu LM, et al. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol. 2009;113:275–80. doi: 10.1016/j.jsbmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Cao EH, Liu XQ, Wang JJ, Xu NF. Effect of natural antioxidant tanshinone II-A on DNA damage by lipid peroxidation in liver cells. Free Radic Biol Med. 1996;20:801–6. doi: 10.1016/0891-5849(95)02211-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Zhang ZR, Fu H, Liu J, Chen Q, Nie Y, et al. Simultaneous determination and pharmacokinetic study of water-soluble and lipid-soluble components of danshen in rat plasma using HPLC-UV method. Biomed Chromatogr. 2007;21:1180–5. doi: 10.1002/bmc.872. [DOI] [PubMed] [Google Scholar]
- 10.Bi HC, Law FC, Zhong GP, Xu CS, Pan Y, Ding L, et al. Study of tanshinone IIA tissue distribution in rat by liquid chromatography-tandem mass spectrometry method. Biomed Chromatogr. 2007;21:473–9. doi: 10.1002/bmc.778. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99:306–14. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Bi HC, Zuo Z, Chen X, Xu CS, Wen YY, Sun HY, et al. Preclinical factors affecting the pharmacokinetic behaviour of tanshinone IIA, an investigational new drug isolated from Salvia miltiorrhiza for the treatment of ischaemic heart diseases. Xenobiotica. 2008;38:185–222. doi: 10.1080/00498250701767675. [DOI] [PubMed] [Google Scholar]
- 13.Song M, Hang TJ, Zhang ZhX, Du R, Chen J. Determination of cryptotanshinone and its metabolite in rat plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:205–9. doi: 10.1016/j.jchromb.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang Z, Zhao M, Tang J, Pan L. In vivo pharmacokinetic comparisons of ferulic acid and puerarin after oral administration of monomer, medicinal substance aqueous extract and nao-de-sheng to rats. Pharmacogn Mag. 2012;8:256–62. doi: 10.4103/0973-1296.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmith VD, Foss JF. Effects of inflammation on pharmacokinetics/pharmacodynamics: Increasing recognition of its contribution to variability in response. Clin Pharmacol Ther. 2008;83:809–11. doi: 10.1038/clpt.2008.62. [DOI] [PubMed] [Google Scholar]
- 16.Petrovic V, Teng S, Piquette-Miller M. Regulation of drug transporters during infection and inflammation. Mol Interv. 2007;7:99–111. doi: 10.1124/mi.7.2.10. [DOI] [PubMed] [Google Scholar]
- 17.Yu XY, Lin SG, Zhou ZW, Chen X, Liang J, Liu PQ, et al. Role of P-glycoprotein in the intestinal absorption of tanshinone IIA, a major active ingredient in the root of Salvia miltiorrhiza Bunge. Curr Drug Metab. 2007;8:325–40. doi: 10.2174/138920007780655450. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Zhou ZW, Xue CC, Li XX, Zhou SF. Role of P-glycoprotein in restricting the brain penetration of tanshinone IIA, a major active constituent from the root of Salvia miltiorrhiza Bunge, across the blood-brain barrier. Xenobiotica. 2007;37:635–78. doi: 10.1080/00498250701411258. [DOI] [PubMed] [Google Scholar]


