Abstract
Background:
Cordyceps militaris is widely used for various ethno medical conditions including cancer and inflammation complications in traditional Chinese medicine.
Objective:
To investigate the in vitro antitumor activity of Cordyceps militaris fraction (CMF) and the molecular mechanism underlying the apoptosis it induces in human chronic myeloid leukemia K562 cells.
Materials and Methods:
CMF was prepared according to our previous report. Cell viability was assessed by MTT assay. The rate of apoptosis, distribution of cell cycle and loss of mitochondrial membrane potential were measured by flow cytometry. Caspase activities were analyzed by Western blot and oxygen consumption rate was recorded using the Oxytherm system.
Results:
The results demonstrated that CMF triggered growth inhibition in K562 cells with only minor toxicity on a normal human cell line and inhibited the proliferation of K562 cells in a dose- and time-dependent manner with IC50 value of 34.1 ± 2.0 μg/ml after 48 h incubation. This most likely resulted from cell cycle arrest at the S phase and the induction of apoptosis. In addition, CMF induced activation of caspase-3 and subsequent cleavage of poly ADP-ribose polymerase (PARP). The caspase signals may originate from mitochondrial dysfunction, which was supported by the finding of decreased mitochondria transmembrance potential and the lower oxygen consumption rate.
Conclusion:
CMF possessed the in vitro antitumor effect on K562 cells and CMF-induced apoptosis might be involved by the mitochondrial dysfunction and valuable to research and develop as a potential antitumor agency.
Keywords: Apoptosis, Cordyceps militaris, K562 cells, mitochondria dysfunction
INTRODUCTION
New drugs, particularly those from herbal sources, are promising modalities for treatment of a variety of malignancies. Based on the ancient and modern Chinese herbal medicine literatures, there are many anti-cancer plants or herbal formulations, which could provide a guide for identification of new anticancer compounds or a source of alternative cancer therapy. It is widely accepted that those drugs have low toxicity, so they may be a better alternative choice in treatment of cancer.[1]
Cordyceps militaris is a caterpillar-shaped Chinese traditional medicine, which has been used for patients suffering from cancer in oriental medicine. It is an ascomycete that invades the pupae in the ground and that remains a worm during the winter and transforms into a mushroom in the summer. In recent years, a wide range of chemical compounds, constituents analysis and pharmacological activities has been reported for C. militaris, whose activities include immunomodulation,[2] antitumor,[3] anti-inflammation,[4] etc., Evidence from both in vivo and in vitro experiments demonstrated that extracts of C. militaris can inhibit the proliferation of human tumor cell lines, including colon 205, PC-3 and HepG2 cells.[5] These extracts exhibited antitumor effects mainly through induction of apoptosis in tumor cells, inhibition of angiogenesis and suppression of invasion and metastasis.
Although the extracts of C. militaris have been shown to be very effective in inhibiting a variety of solid tumors, few studies have reported their effects and the possible mechanisms associated with human chronic myeloid leukemia.
During the course of our extensive investigating program on cultured C. militaris, a novel, efficient and accurate fingerprint method using high-performance liquid chromatography-photodiode array detection (HPLC-DAD) was reported for the quality control of cultured C. militaris.[6] In the present study, we investigated the effects of C. militaris fraction (CMF) on K562 cells in vitro for the first time. We analyzed the cytotoxicity, cell-phase arrest and apoptosis, as well as mitochondrial function related to mitochondrial membrane potential and oxygen consumption. The results showed that CMF significantly inhibited the proliferation and induced caspase-dependent apoptosis associated with mitochondrial dysfunction in K562 cells, and indicated that CMF has the potential to be an effective chemotherapy agent.
MATERIALS AND METHODS
Plant material and fraction preparation
Cultured C. militaris was purchased from Honghao Biological Company of Jiangmen, Guangdong, China. The samples were prepared according to our previous report[6] with some improvements as follows: 1.0-g powder of dried materials was precisely weighed and put into a 50 ml volumetric flask. Approximately 20 ml of water was added to the flask, which was subsequently sonicated for 30 min for extraction at 25°C. The extraction step was repeated twice and the extracted solution was mixed and filtrated through analytical filter paper. The filtered solution was purified with macroporous adsorptive resins eluted with water-ethanol. The elution was vacuum-dried at 55°C and then CMF were obtained.
Reagents and antibodies
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, bicinchoninic acid (BCA) protein assay kit, Annexin V-fluorescein isothiocyanate (FITC)/PI staining kit, and RIPA buffer were all purchased from Beyotime (Shanghai, China). Positive drug Adriamycin (ADR) and dimethylsulfoxide (DMSO) were purchased from Sigma (USA). Rhodamine-123 was purchased from Invitrogen (California, USA). Antibodies against the following proteins were purchased: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), cleaved-caspase-3, cleaved-PARP protein (Cell Signaling Technology, Beverly, MA).
Cell lines and culture
Human chronic myeloid leukemia K562 cell line was used, along with L-02, a cell line representative of normal human liver. K562 cells and L-02 cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) plus 100 units/ml penicillin and 100 μg/ml streptomycin (all from Gibco/BRL USA). Both CML K562 and L-02 cells were purchased from the Cell Bank of China Science Academy (Shanghai, China) and cultured in a humidified atmosphere at 37°C and with 5% CO2.
Cell viability assay
Cell viability was assessed by MTT assay.[7] Briefly, 1.5 × 105 K562 cells/well was dispensed within 96-well plates in 100 μl volumes. Different concentrations of CMF (16, 63, 250, 1000 μg/ml) and positive drug ADR were then added into different wells, and then incubated for 24, 48 and 72 h. In addition, exponentially growing L-02 cells were seeded into 96-well culture plates (6 × 104 cells/well) and allowed to adhere overnight. Cells were then incubated with CMF at various concentrations (62.5, 125, 250, 500, 1000 μg/ml) for 48 h. Each of the concentrations above was regarded as one treated group, while the negative control group contained no CMF. Each CMF-treated or control group contained 3 parallel wells. After culture plates were incubated for indeed time, 20 μl of MTT working solution (5 mg/ml in PBS) was added to each well and incubated continuously for 4h at 37°C. MTT/medium was then removed and formazan was dissolved in DMSO, of which 200 μl/well was added. Finally, the absorbance (A value) of each well was measured at 570nm using a 96-well multi-scanner auto-reader (M450; Bio-rad, USA). The percentage of cell viability was calculated as A570(test)/A570(control)×100%. The IC50 values, defined as the concentration of drug that caused 50% inhibition of absorbance compared with the control cells treated with DMSO only, were calculated using the PrismPad computer program.
Cell cycle distribution analysis
Cell cycle distribution was determined by DNA staining with PI.[8] The DNA of cells was stained with PI and the population of non-apoptotic cells in different phases of the cell cycle was recorded. Briefly, 3 × 105 K562 cells were treated with various concentrations of CMF (100, 300 and 900 μg/ml) for 48 h. Then the cells were collected and washed twice with phosphate buffer solution (PBS) and fixed in ice cold 70% ethanol overnight. Cells were collected and re-suspended in PBS containing 50 μg/ml PI, 0.1 mg/ml RNase and 5% Triton X-100 and incubated at room temperature for 30 min. Cells were analyzed on a flow cytometer (Becton-Dickinson, CA, USA) and the percentage of cells in the different phases of the cell cycle was analyzed using Becton-Dickinson software. The data were determined by three independent experiments.
Detection of apoptosis
Apoptosis was measured by flow cytometry after staining with Annexin V-FITC (apoptotic cell marker) and PI (necrotic cell marker).[9] The staining method was used according to the Annexin V-FITC/PI staining kit. In brief, cells were grown in a 6-well plate at 6 × 105 cells/well in 2.5 ml volumes. The cells were treated with 100, 300 and 900 μg/ml of CMF for 48h. Treated and control cells were harvested, washed twice and re-suspended in 500 μl of PBS plus Annexin V-FITC and PI in dark for 15 min at room temperature. The degree of apoptosis was determined as the percentage of cells positive for Annexin V-FITC/PI. For each sample, at least 1 × 104 cells were analyzed by flow cytometry. The data were determined by three independent experiments.
Western blot analysis
Caspase activities were determined by Western blot.[10] Proteins of K562 cells incubated with CMF (100, 300 and 900 μg/ml) for 48 h were extracted in RIPA buffer. Total protein concentrations of whole cell lysates were determined using BCA protein assay kit. Equal amounts of protein samples were loaded onto 8-15% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels. After electrophoresis, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA), probed with primary antibodies, and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Specific protein bands were visualized using the chemiluminescence method and imaged by autoradiography. The amount of GAPDH was measured as an internal control.
Assessment of mitochondrial membrane potential transition
The mitochondrial membrane potential (ΔΨm) was determined in K562 cells after treatment with CMF. Briefly, K562 cells were cultured and treated in 6-well culture plates (6 × 105 cells/well) with or without CMF (300 μg/ml) for 12 h. Then, cells were stained with 1μM rhodamine-123 for 60 min to evaluate the mitochondrial transmembrane potential.[11] Analysis was performed using a FACS flow cytometer (Becton-Dickinson, CA, USA).
Determination of oxygen consumption
The oxygen consumption rate in intact cells was measured as an indication of the mitochondrial respiratory activity.[12] Five million control or CMF-treated cells were suspended in 1ml of culture medium pre-equilibrated with 21% oxygen, the oxygen content in the cell suspension medium was constantly monitored for 10 min and oxygen consumption rate was recorded by using the Oxytherm system (Hansatech Instrument, Cambridge, UK).
Statistical analysis
Statistical analysis of the data was performed using the one way ANOVA. Data are expressed as mean ± S.D. with significance at P < 0.05 or P < 0.01.
RESULTS
CMF inhibited K562 cell viability in a dose- and time-dependent manner
The inhibitory effect of CMF on K562 cells was investigated by varying incubation times in addition to concentrations. As shown in Figure 1b, CMF caused a decrease in the cell viability of the K562 cells in a dose- and time-dependent manner when compared with the control. After a 48h treatment, CMF caused a decrease in the cell viability of the K562 cells with IC50 value of 34.1 ± 2.0 μg/ml. ADR was used as a positive control with IC50 value of 197.3 ± 6.4 nM/ml.
Figure 1.
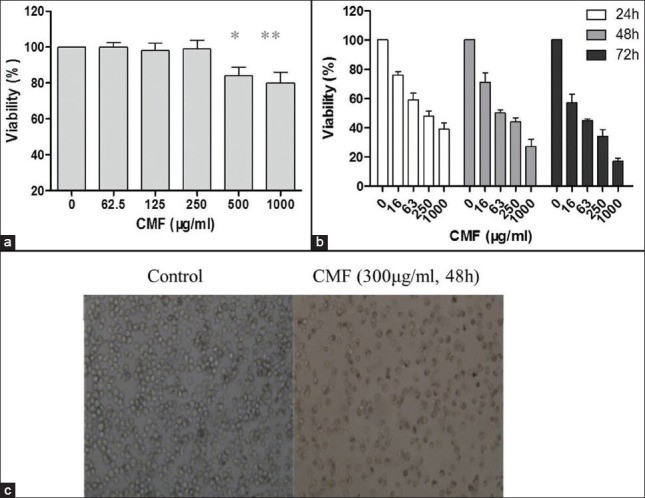
Effect of CMF on cytotoxicity in normal human liver cells and on cell viability in K562 cells. (a) Cytotoxic effects of CMF on normal human liver L-02 cells assessed by MTT assay. (b) Cell viability of K562 cells treated with various concentrations of CMF for 24, 48 and 72 h, evaluated by MTT assay. (c) Morphological study of K562 cells treated with CMF (300 μg/ml) for 48 h with inverted light microscope (×100). Data are means ± S.D. of three repeats. *P< 0.05 and **P< 0.01 vs. untreated control
Additionally, the morphological changes of K562 cells treated with CMF (300 μg/ml 48 h) were observed under inverted microscope. As shown in Figure 1c, in general, untreated K562 cells were found to be rotundity shape and the karyopyknosis phenomenon was seldom seen. The growth of cells treated with CMF was inhibited and irregular cells with different sizes were also observed, resulting in cell body enlargement.
CMF exerted low cytotoxicity on normal human cells
The L-02 cell line was used to assess the degree of cytotoxicity exerted by CMF on normal human cells.[13] According to the MTT assay results shown in Figure 1a, the growth inhibitory rate was less than 30% at the highest concentration (1000 μg/ml), which indicated that CMF exerted low cytotoxicity on normal human L-02 cells.
CMF induced cell cycle S arrest
On the basis of the above data, the effects of CMF on cell cycle progression were further investigated. The relative frequencies of cells in each cell cycle phase were estimated by propidium iodide (PI) mediated cellular DNA content measurement. After a 48 h treatment with CMF at different concentrations (100, 300 and 900 μg/ml), the K562 cells were harvested, PI stained and subjected to flow cytometric analysis. As summarized in Figure 2, as compared to the control cells, there was a significant reduction in population of cells in G0/G1 phase, while an accumulation of cells in S phase. Cells without CMF exposure demonstrated S population of 26.4 ± 3.2%, when treated with 100 μg/ml CMF, 29.3 ± 3.4% of the cells were arrested at the S phase of the cell cycle and when treated with 300 and 900 μg/ml CMF, the S fraction rose to 36.1 ± 4.2% and 46.5 ± 5.3% respectively. These results indicated that CMF arrested K562 cells in the S phase.
Figure 2.
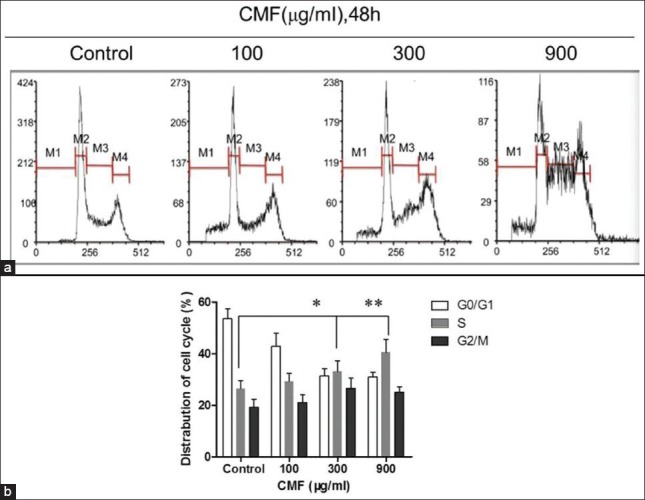
CMF-induced cell cycle S arrest. Evaluation of cell cycle by PI staining assay and flow cytometer analysis after CMF treatment (100, 300, 900 μg/ml) for 48 h. Percentages of cells in subG1 (M1), G1 (M2), S (M3), and G2/M (M4) phase are presented in histogram. The images are representative of three separate experiments. *P< 0.05 and **P< 0.01 vs. untreated control
CMF induced apoptosis through a caspase-mediated pathway
To determine whether CMF also decreased cell survival by the induction of apoptosis, K562 cells were cultured with CMF at different concentrations (100, 300 and 900 μg/ml) for 48 h and then assessed with Annexin V-FITC/PI dual staining assay. As shown in Figure 3a, cells in the lower left quadrant were negative for both Annexin V-FITC and in PI; in the lower right, positive for Annexin V-FITC, which indicated cells in the early stages of apoptosis; in the upper left, positive for PI only, which indicated cells that were dead; and in the upper right, positive for both Annexin V-FITC and PI, which indicated in cells in the later stage of apoptosis or necrosis. The values indicated in the quadrants show the percentage of cells positive for both Annexin V-FITC and PI or Annexin V-FITC alone. The results showed that the CMF treatment increased the percentage of late apoptotic cells (stained for both Annexin V-FITC and PI) from 2.9 ± 1.6% in the control group to 73.2 ± 5.9% in CMF-treated group (900 μg/ml).
Figure 3.
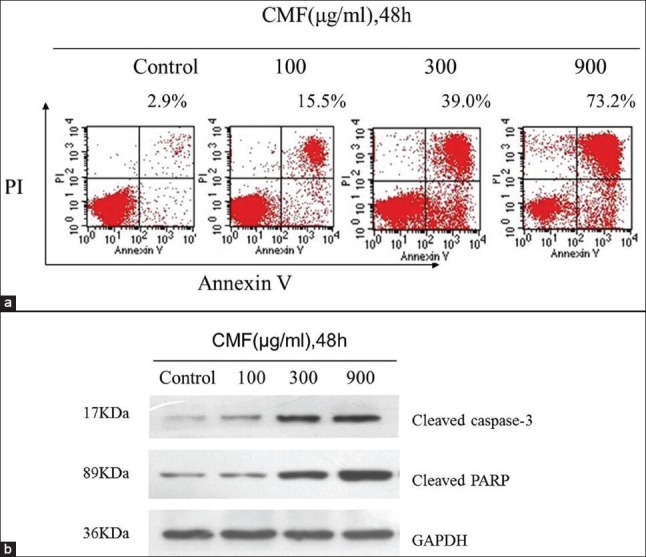
CMF induced apoptosis through a caspase-mediated pathway in K562 cells. (a) Evaluation of apoptosis by Annexin V-FITC/PI dual staining assay and flow cytometer analysis after CMF treatment (100, 300, 900 μg/ml) for 48 h. (b) Effects of CMF on the expression of caspase-3 and PARP proteins. Equal amounts of whole cell lysates (50 μg) were subjected to Western blot analysis. The amount of GAPDH was measured as an internal control
Next, the effects of CMF on the caspase family proteins were analyzed in K562 cells. The results demonstrated that CMF, at a concentration of 900 μg/ml, caused significantly activation of cleaved caspsed-3, which was accompanied by an evident cleavage of poly ADP-ribose polymerase (PARP, a major substrate of caspase-3), which denoted the involvement of the caspase in CMF-triggered irreversible apoptosis (Figure 3b). These results together suggested that CMF-driven apoptosis was mediated by caspase activation.
CMF-induced apoptosis is related to mitochondrial dysfunction
To determine the underlying mechanism of apoptosis induced by CMF, the mitochondrial transmembrance potential (ΔΨm) was first evaluated. As shown in Figure 4a, as compared to the control cells, the ΔΨm was rapidly decreased following treatment with CMF (300 μg/ml) only for 12 h.
Figure 4.
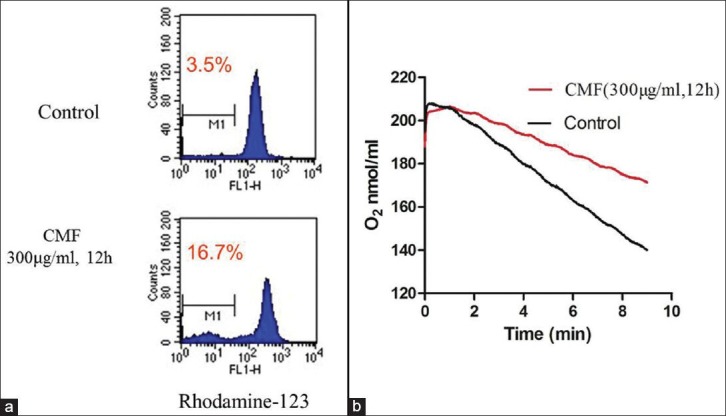
CMF-induced apoptosis related to mitochondrial dysfunction in K562 cells. (a) Cells treated with CMF (300μg/ml) for 12h were stained with rhodamine-123 to evaluate the mitochondrial transmembrane potential. (b) Alteration of oxygen consumption in K562 cells incubated with CMF (300ug/ml) for 12h compared with control. The images shown here are representative of three separate experiments
Another striking change in mitochondrial function observed in CMF-treated cells was the significant reduction in mitochondrial respiratory chain activity, as evidence by a substantial decrease in oxygen consumption rate. According to Figure 4b, the oxygen consumption rate of treated cells was much lower than that of the control cells for only 12 h CMF (300 μg/ml) treated. These results provided evidence that CMF-induced apoptosis in K562 cells related to mitochondrial dysfunction.
DISCUSSION
Recently, there has been a global trend toward the use of natural substances present in herbs as medicine. Current chemotherapeutic agents are known to destroy both cancerous and non-cancerous cells.[14] Thus, there is an urgent need to find new anticancer drugs that can kill cancerous cells with minimal toxicity to non-cancerous cells. There are roughly 13,000 medicinal methods in China and over 100,000 medicinal recipes recorded in the ancient literature. Undoubtedly, these traditional Chinese medicines are the abundant natural resources to search for new drugs. There is also some evidence that the cytostatic effect of multiple compounds in a whole plant extracts on cancer cells is often better than the effect of their particular biologically active compounds.[15] It has been suggested that there are multiple active ingredients in Chinese medicinal herbs which may inhibit the malignancies effectively through one or more of the following mechanisms of tumor metastasis: (i) induction of cells differentiation, (ii) interruption of DNA synthesis or blockage of cell division, and induction of cell apoptosis.[16,17]
Although many studies have demonstrated that the C. militaris is effective in treating cancers, few researches focus on its effect on human chronic myeloid leukemia in detail. In the present study, we investigated the effects of CMF on the proliferation and induction of apoptosis of K562 cells, and tried to determine the possible mechanisms of its antitumor effect.
The in vitro cytotoxicity analysis indicated that CMF showed a strong anti-proliferative effect on K562 cells with IC50 value of 34.1 ± 2.0 after 48 h incubation. In addition, CMF displayed less toxicity on normal human cell line L-02. The result of cell cycle analysis evaluated by flow cytometer showed that CMF could induce S phase arrest and apoptosis in K562 cells. Western blot analysis showed that CMF could cause significantly activation of cleaved caspsed-3, which was accompanied by an evident cleavage of PARP. These data proved the involvement of the caspase in CMF-triggered irreversible apoptosis in K562 cells.
It is known that mitochondria play an essential role in the regulation of apoptotic cell death, since both the intrinsic and extrinsic apoptosis pathways can converge at the mitochondrial level and trigger mitochondrial membrane permeabilization.[18] To determine the underlying mechanism of apoptosis induced by CMF, the mitochondrial transmembrance potential and the oxygen consumption rate of CMF-treated cells were then compared with those of the untreated cells. According to the results, the mitochondria function may be disrupted by decreased mitochondria transmembrance potential after CMF treated, indicating with the lower oxygen consumption rate. These data suggested that CMF-induced apoptosis might be involved by the mitochondrial dysfunction. Therefore, CMF may, in the future, have potential therapeutic applications for chronic myeloid leukemia.
ACKNOWLEDGMENTS
This research work was financially supported by National Scientific and Technological Key Projects of China (No. 2011ZX09102-001-33).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Schwartsmann G, Ratain MJ, Cragg GM, Wong JE, Saijo N, Parkinson DR, et al. Anticancer drug discovery and development throughout the world. J Clin Oncol. 2002;20:47–59s. [PubMed] [Google Scholar]
- 2.Wang ZM, Peng X, Lee KL, Tang JC, Cheung PC, Wu JY. Structural characterisation and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2011;125:637–43. [Google Scholar]
- 3.Lin YW, Chiang BH. Anti-tumor activity of the fermentation broth of Cordyceps militaris cultured in the medium of Radix astragali. Process Biochem. 2008;43:244–50. [Google Scholar]
- 4.Jeong JW, Jin CY, Kim GY, Lee JD, Park C, Kim GD, et al. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int Immunopharmacol. 2010;10:1580–6. doi: 10.1016/j.intimp.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Rao YK, Fang SH, Wu WS, Tzeng YM. Constituents isolated from Cordyceps militaris suppress enhanced inflammatory mediator's production and human cancer cell proliferation. J Ethnopharmacol. 2010;131:363–7. doi: 10.1016/j.jep.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Yu R, Ye B, Yan C, Song L, Zhang Z, Yang W, et al. Fingerprint analysis of fruiting bodies of cultured Cordyceps militaris by high-performance liquid chromatography-photodiode array detection. J Pharm Biomed Anal. 2007;44:818–23. doi: 10.1016/j.jpba.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 8.Yu DS, Ma CP, Chang SY. Establishment and characterization of renal cell carcinoma cell lines with multidrug resistance. Urol Res. 2000;28:86–92. doi: 10.1007/s002400050143. [DOI] [PubMed] [Google Scholar]
- 9.Jin L, Xiao CL, Lu CH, Xia M, Xing GW, Xiong S, et al. Transcriptomic and proteomic approach to studying SNX-2112-induced K562 cells apoptosis and anti-leukemia activity in K562-NOD/SCID mice. FEBS Lett. 2009;583:1859–66. doi: 10.1016/j.febslet.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 10.Jeong JW, Jin CY, Park C, Hong SH, Kim GY, Jeong YK, et al. Induction of apoptosis by cordycepin via reactive oxygen species generation in human leukemia cells. Toxicol in Vitro. 2011;25:817–24. doi: 10.1016/j.tiv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Hu YM, Lu WQ, Chen G, Wang P, Chen Z, Zhou Y, et al. K-rasG12V transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res. 2012;22:399–412. doi: 10.1038/cr.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emadi A, Anne L, Harwood CJ, Stagliano KW, Kamangar F, Ross AE, et al. Metabolic and electrochemical mechanisms of dimeric naphthoquinones cytotoxicity in breast cancer cells. Bioorg Med Chem. 2011;19:7057–62. doi: 10.1016/j.bmc.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khonkarn R, Mankhetkorn S, Hennink WE, Okonogi S. PEG-OCL micelles for quercetin solubilization and inhibition of cancer cell growth. Eur J Pharm Biopharm. 2011;79:268–75. doi: 10.1016/j.ejpb.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Tian Z, Chen S, Zhang YO, Huang MH, Shi L, Huang F, et al. The cytotoxicity of naturally occurring styryl lactones. Phytomedicine. 2006;13:181–6. doi: 10.1016/j.phymed.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Vickers A. Botanical medicines for the treatment of cancer: Rationale, overview of current data, and methodological considerations for phase I and II trials. Cancer Invest. 2002;20:1069–79. doi: 10.1081/cnv-120005926. [DOI] [PubMed] [Google Scholar]
- 16.Shin SY, Hyun J, Yu JR, Lim Y, Lee YH. 5-Methoxyflavanone induces cell cycle arrest at the G2/M phase, apoptosis and autophagy in HCT116 human colon cancer cells. Toxicol Appl Pharm. 2011;254:288–98. doi: 10.1016/j.taap.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Xie YZ, Li SZ, Yee A, Pierre DP, Deng ZQ, Lee DY, et al. Ganoderma lucidum inhibits tumour cell proliferation and induces tumour cell death. Enzyme Microb Technol. 2006;40:177–85. [Google Scholar]
- 18.Gurp MV, Festjens N, Loo GV, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487–97. doi: 10.1016/s0006-291x(03)00621-1. [DOI] [PubMed] [Google Scholar]


