Abstract
Objective:
In the present study, a high performance liquid chromatography (HPLC) coupled with photodiode array detection was developed for simultaneous quantitation of esculetin, quercetin-3-O-β-D-glucuronide, quercetin-3-O-β-D- glucuronopyranoside methyl ester and quercetin in Polygonum perfoliatum L.
Materials and Methods:
The chromatographic separations were performed on a reversed-phase C18 column using a mobile phase composed of acetonitrile -0.5% aqueous acetic acid with gradient elution. The calibration curves for the analytes demonstrated good linearities within the investigated ranges. The satisfactory intra- and inter-day precision, repeatability and stability of the developed analytical method were shown in the method validation procedure. The recoveries of the established method ranged from 95.76 to 102.10% for all the analytes.
Results:
This proposed method was successfully applied for simultaneous quantification of the four compounds in effective part of Polygonum perfoliatum L. from different regions. Hierarchical clustering analysis (HCA) and principal components analysis (PCA) were performed to characterize and classify the samples based on the contents of the four compounds in Polygonum perfoliatum L.
Conclusion:
The established HPLC method combined with chemometric approaches was proven to be useful and efficient for quality control of Polygonum perfoliatum L.
Keywords: High performance liquid chromatography, diode array detector, hierarchical clustering analysis, principal components analysis, Polygonum perfoliatum L., quality control
INTRODUCTION
Polygonum perfoliatum L. (P. perfoliatum L.), listed in Chinese Pharmacopeia (version 2010),[1] is used and distributed widely in China, especially in Guizhou province. Historically, P. perfoliatum L. was used for the relief of fever and antidote and for promoting blood circulation.[2] Recent pharmacological studies indicated that extracts from P. perfoliatum L. showed anti-inflammatory,[3,4] anti-bacterium,[3,5] antitumor,[6,7] antioxidant and inhibitory α-glucosidase activities,[8] that could also be used for relieving cough, reducing sputum,[4] suppressing herpes simplex virus- I (HSV - I) disease,[9] and for protecting acute liver injury.[10]
So far, the investigations for P. perfoliatum L. have been focused mainly on phytochemistry, that revealed that a variety of compounds including flavonoids,[11,12,13] triterpenoids, steroids,[14,15,16] phenylpropanoids,[15,16,17,18,19,20] and benzoquinones[16,21] were found in this herb. However, until now, there have been few reports on the detection and quantification of quercetin,[22,23,24] caffeic acid[25] or quercetin-3-O-β-D-glucuronide.[26] Moreover, as one of the famous “Miao Medicines”, it has been widely used by Miao nationality in Guizhou for thousands of years. Nowadays, P. perfoliatum L. was used as one of the raw materials to treat gynecological diseases in many traditional medicine preparations. In light of its various bioactivities, pharmacological, phytochemical and analytical investigations of P. perfoliatum L. were carried out in our laboratory. First, effective part of P. perfoliatum L. has been screened out based on pharmacological studies by means of anti-inflammation test and then several chemical compounds were isolated from effective part using phytochemical approaches. Anti-inflammatory activity was tested by using different extracts from effective part then. The results demonstrated that efficacy of the extract obtained from ethyl acetate extracted was the best, in which four bioactive chemical compounds including esculetin, quercetin-3-O-β-D-glucuronide, quercetin-3-O-β-D-glucuronopyranoside methyl ester and quercetin were isolated and purified in our lab. It was reported that esculetin possessed a variety of pharmacological activities including antioxidant activity,[27] apoptosis-inducing activity,[28] and antihyperglycemic effect.[29] Quercetin-3-O-β-D-glucuronide was verified to show anti-inflammatory and antiviral activities,[26] and could inhibit lipid peroxidation,[30,31] form the reactive oxygen species (ROS).[32] Quercetin-3-O-β-D-glucuronopyranoside methyl ester was tested to have anti-inflammatory activity in our lab. Quercetin has been demonstrated to have antioxidant,[33] anti-inflammatory, analgesis,[34,35] and antitumor[36,37] activities that also inhibited poliovirus RNA replication[38] and showed ameliorative experimental autoimmune myocarditis activity.[39] Therefore, these four compounds were scientifically selected as biomarkers for control the quality of P. perfoliatum L.
MATERIALS AND METHODS
Chemicals
Methanol, acetonitrile (TEDIA, Company Inc. USA) and acetic acid (Damao, Tianjin, China) were for HPLC analysis. Water was obtained from water purification system (18.2 MΩ, Sartorius Germany), analytical-grade methyl acetate (Kelong, Chendu, China), ethanol (Guoyao, Shanghai, china) and methanol (Fuyu, Tianjin, China) were used for preparation of samples.
Esculetin, quercetin, quercetin-3-O-β-D-glucuronide and quercetin-3-O-β-D-glucuronopyranoside methyl ester were extracted, isolated and purified from P. perfoliatum L. in our laboratory. The compounds were identified by MS, 1H-NMR, 13C-NMR, IR and UV spectrometric techniques, of which the purities were more than 98% [Figure 1].
Figure 1.
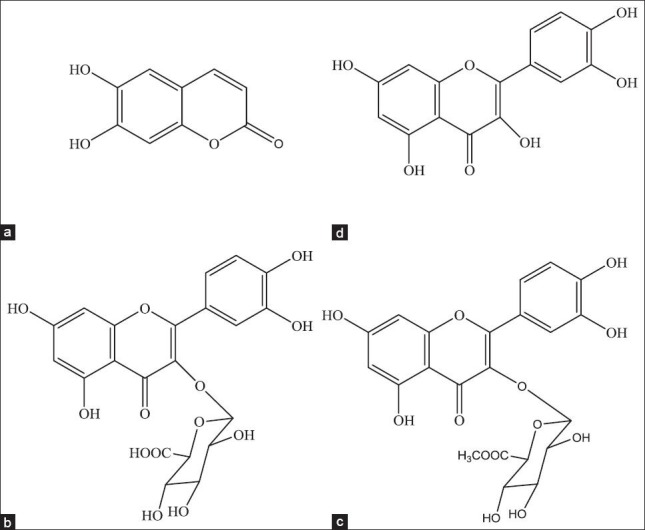
The structure of four compouds: (a) Esculetin; (b) Quercetin-3-O-β-D-glucuronide; (c) Quercetin-3-O-β-D-glucuronopyranoside methyl ester; (d) Quercetin
Thirty eight batches of P. perfoliatum L. were collected from various locations in China, including Guizhou, Henan, Hunan, Anhui provinces, et al. The plants were authenticated and met the standard of the Chinese Pharmacopoeia. The specimen was deposited at the Research Center for Quality Control of Nature Medicine, Guizhou Normal University.
Sample preparation
Dried samples of P. perfoliatum L. were grounded into powder, 0.3 g of each sample was weighed actually and refluxed by 40 mL of 80% ethanol for 2 h at 80°C, the extraction was repeated twice and the extraction solvents were combined and concentrated in a rotary evaporator under reduced pressure until the ethanol disappeared and then the residue was dissolved and extracted repeatedly with 10 mL ethyl acetate by for 5 min.
The extraction was dried under reduced pressure and was dissolved in 10 mL of methanol. Before the HPLC analysis, solution was filtered with 0.45 μm membrance.
Standard solution preparation
The appropriate amounts of reference compounds were accurately weighed and dissolved with methanol to make stock solutions, then, the working solutions were prepared by diluting the stock solutions with methanol. The solutions were stocked at 5°C and away from light.
HPLC analysis
An Agilent series 1100 liquid chromatography equipped with vacuum degasser, a quaternary pump, an autosampler and a diode array detector (DAD) system was used for HPLC analysis. The separation was performed on a reversed-phase column (Lichrosher C18 column, 250 × 4.6 mm, 5 μm, Jiangsu Hanbang Science and Technology Co. Ltd, China) together with C18 guard column (250 × 4.6 mm) by gradient elution. The mobile phase consisted of acetonitrile (A) and 0.5% aqueous acetic acid (B). The program was as follow: 0-10 min, 10-15% A; 10-13 min, 15-16% A; 13-25 min, 16-20% A; 25-30 min, 20-30% A; 30-47 min, 30-42% A. The column temperature was set at 25°C, the injection column was 20 μL, the detection wavelength was 346 nm and the flow rate was set at 1 mL/min.
RESULTS AND DISCUSSION
Optimization of extraction method
In term of pharmacological investigation, extraction solvent (80% ethanol) and extraction temperature (80°C) were selected. In order to extract the active compounds efficiently, the liquid-liquid extraction was performed to concentrate the extraction, solvent volume (20, 30, 40 and 50 mL), liquid-liquid extraction time (1, 2 and 3 h), extraction solvent volume (5, 10 and 15mL) and soaking time (5, 10 and 15 min) were optimized. Finally, the satisfactory conditions were optimize and were shown in section for Sample Preparation.
Optimization of the chromatographic conditions
Acetonitrile–water and methanol–water were tested with gradient elution in the present study. To obtain better resolution and peak shape, different concentrations of acetic acid were added to the mobile phase to reduce tailing. The column temperature (25, 30, 35°C) was optimized further. The results indicated that short analysis time, good resolutions and shapes of the peaks could be obtained by using the mobile phase consisted of acetonitrile (A) and 0.5% aqueous acetic acid with column temperature at 25°C. On overall consideration of the maximum of the four analytes, 346 nm was selected as the detection wavelength [Figure 2].
Figure 2.
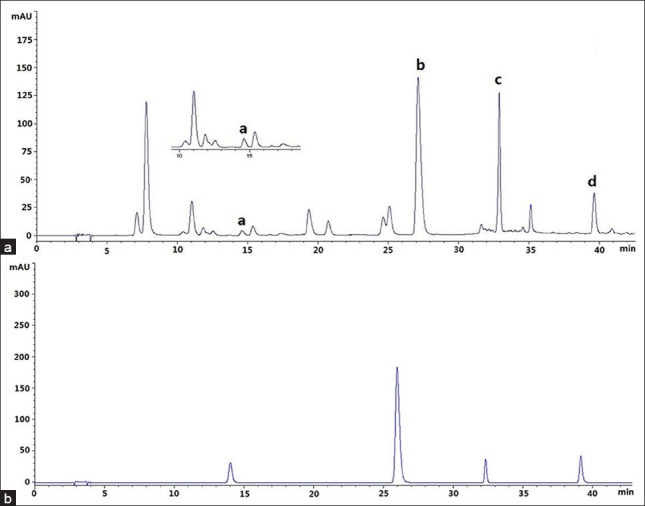
HPLC chromatograms of sample and standard of four compouds: (a) Esculetin; (b) Quercetin-3-O-β-D-glucuronide; (c) Quercetin-3-O-β-D-glucuronopyranoside methyl ester; (d) Quercetin
Validation of the HPLC method
Calibration curves, limits of detection and quantification
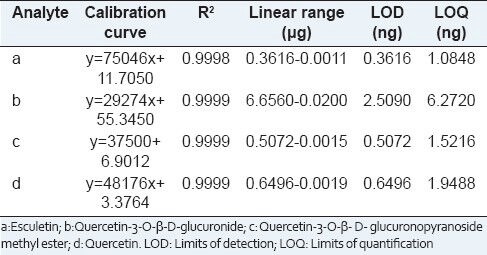
The mixed stock solution containing four compounds for linear regression analysis was prepared and was appropriately diluted with methanol to nine different working solutions. Each concentration was assayed in triplicate. Then, the calibration curves were plotted by the peak areas versus the concentrations using linear regressions. The limit of detections and quantifications were estimated at a signal-to-noise ratio (S/N) of about 3 and 10, respectively. The results are showed in Table 1.
Table 1.
Linearity, LOD and LOQ for analytes
Precision, stability and repeatability
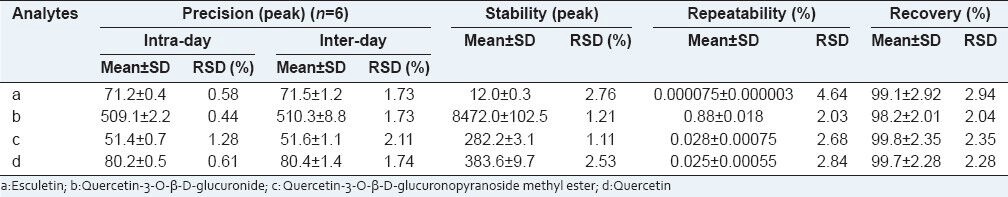
The intra-day and inter-day precisions were measured by sample solutions, both the precisions were carried out on six repeatedly analyses within one day and twice analyses on three consecutive days. The sample solution was assayed at 0, 4, 8, 12, 24, 48 h for stability test. The repeatability was evaluated by six different solutions from the same sample. The results were shown in Table 2.
Table 2.
Precision, stability, repeatability and recovery of analytical method for P. perfoliatum L.
Recovery
The recovery of method was tested by spiking three different concentrations (low, middle, high) into samples; each sample was prepared in triplicate. The samples were extracted and analyzed according to the section for Sample Preparation and HPLC Analysis. The results were shown in Table 2.
Sample analysis
The developed method was applied to determine the four compounds in P. perfoliatum L. from various locations. The results were listed in Table 1. The content of esculetin was greatly the lowest and was not determined in majority of P. perfoliatum L., the contents of quercetin-3-O-β-D-glucuronide, quercetin-3-O-β-D-glucuronopyranoside methyl ester and quercetin ranged from 0.1566 to 9.7644 mg/g, 0.0054 to 1.3871 mg/g, 0.0518 to 0.3745 mg/g, respectively. The content of quercetin-3-O-β-D-glucuronide was the highest in P. perfoliatum L.
Table 3.
The content of 4 compounds in P. perfoliatum L. from different habits in China (mg/g, n=3)
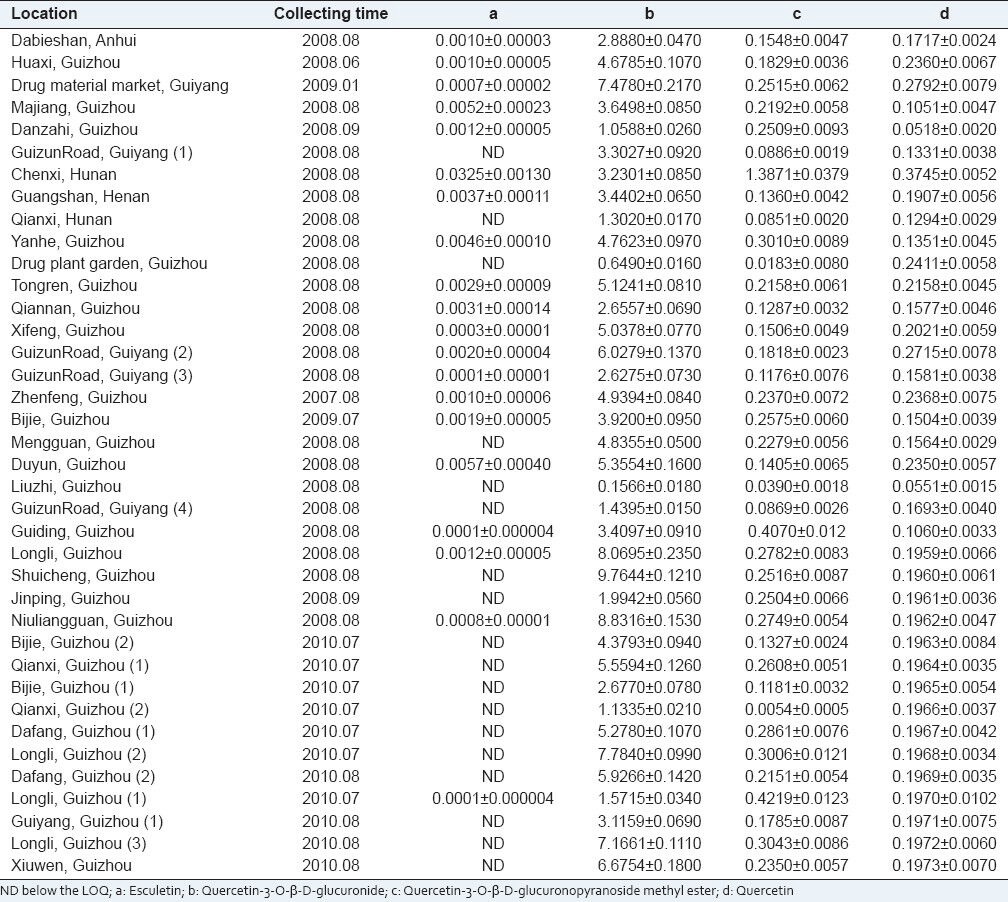
Based on the contents of the four compounds, HCA and PCA, which were the unsupervised clustering methods,[40] were applied to analyze and classify the 38 batches of samples from different locations. HCA and PCA were investigated by SPSS 16.0.
HCA of the Samples
Cluster Analysis was a classified method of individuals based on its similarities.[41] Between group linkage and squared Euclidean distance were selected as cluster method and measurement, respectively. According to the contents of the four investigated compounds in effective part of P. perfoliatum L., 38 batches of P. perfoliatum L. were divided into 2 groups by HCA, each group was divided into 2 subgrouds [Figure 3]. Group one (A1) contained sample nos. 13, 16, 30, 1, 4, 18, 6, 8, 23, 36 and 7, in which the contents of quercetin-3-O-β-D-glucuronide ranged from 2.6 to 4.0mg/g. Group two (A2) contained sample nos. 11, 21, 9, 22, 31, 5, 26 and 35 and the contents of quercetin-3-O-β-D-glucuronide ranged from 0.15 to 2.00 mg/g in this group. Group three (B1) contained sample nos. 15, 34, 38, 20, 32, 29, 12, 14, 17, 10, 19, 2 and 28, in which the contents of quercetin-3-O-β-D-glucuronide ranged from 4.6 to 6.0 mg/g. Group four (B2) contained sample no. 24, 33, 3, 37, 25 and 27 and the content of quercetin-3-O-β-D- glucuronide in this group ranged from 5.9 to 10.0 mg/g. Thus, the content of quercetin-3-O-β-D- glucuronide played an important role in HCA, which was also higher than that of other bioactive chemical compounds.
Figure 3.
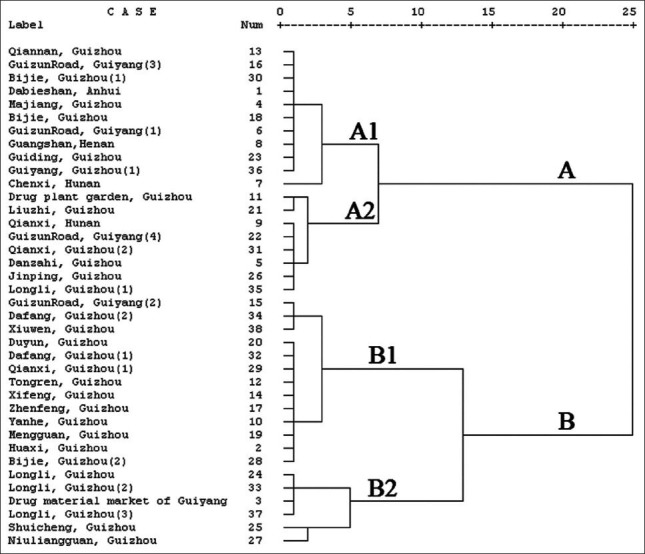
Dendrogram of the 38 tested samples from different locations
PCA of the samples
PCA which allows the reduction of data dimensionality for turning lots of variables into a few comprehensive irrelative variables was enable the problem to be simple and visualized.[42]
PCA loading plot which was effect on distribution of samples on score plot, were used to explain which markers contributed to the PCs.[43] The loading plot showed in Figure 4. Marker b and d contributed to higher PC2 score and c contributed mainly to PC1 score. Marker b and d represent peak of quercetin-3-O-β-D-glucuronide and quercetin, which resulted in three groups at the left of the PCA score plot. The marker a and c were peak of esculetin and quercetin-3-O-β-D-glucuronopyranoside methyl ester from samples, which cluster a group containing sample 7 at the right of the PCA score plot.
Figure 4.
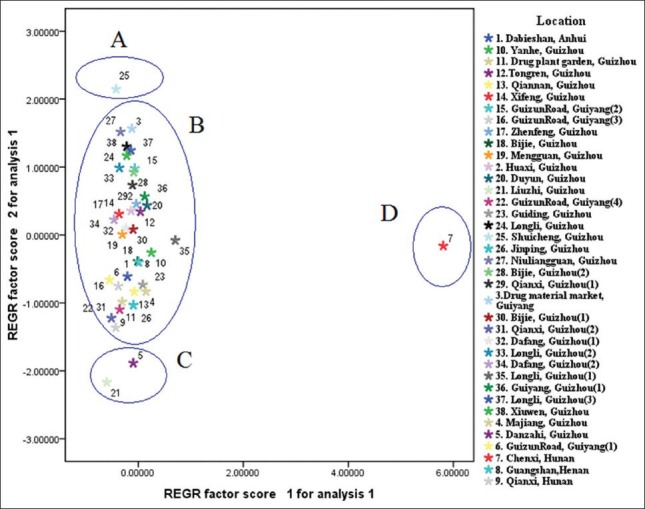
Scores Plot for the 38 tested samples from different locations based on quantitative data
Based on the contents of the four makers, 38 batches of P. perfoliatum L. samples were classified into four groups, which shown in the score plot [Figure 5]. Therefore, group A only consisted of sample no. 25, in which the content of quercetin-3-O-β-D-glucuronide was the highest (9.7644 mg/g). The contents of quercetin in those in group C were the lowest (about 0.05 mg/g). Group D only included sample no. 7, in which the contents of esculetin and quercetin-3-O-β-D-glucuronopyranoside methyl ester were the highest (0.0325 and 1.3871 mg/g, respectively). Other samples were in group B, in which the contents of the four bioactive chemical compounds varied differently. Herein, according to the content of four bioactive chemical compounds which contributed to classify, two-dimensional PCA score plot showed a tendency to discriminate the samples. This method would be more comprehensive evaluation the quality of samples.
Figure 5.
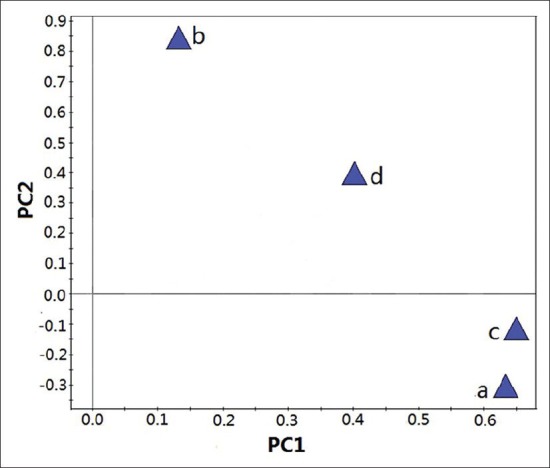
PCA loading plots for PC1 and PC2
CONCLUSION
In the present study, the optimized method was applied to determine the four compounds including three flavonoids and one coumarin in effective part of P. perfoliatum L. for the first time. Then the method was successfully applied for analyses of P. perfoliatum L. from different regions. The validation of the method showed good linearity, precision, repeatability, stability and recovery in analysis time within 45 min. The quantitative results demonstrated the contents of the four markers in samples from various sources were significantly different. Based on the contents of the four chemical constituents, HAC and PCA were successfully applied for quality control of P. perfoliatum L. and for discriminating and classifying P. perfoliatum L. from different locations. The results showed that the qualities of P. perfoliatum L. were obviously different, which possibly led to different clinical effects. Thus, it should establish GAP cultivation base to ensure stabile quality of Traditional Chinese Medicine. The proposed method could be useful and hopeful to control quality of P. perfoliatum L.
ACKNOWLEGEMENTS
The research work was supported by: (1) Natural Science Foundation of China (No. 81060340). (2) Science and Technology Innovation Talents Team Construction Project of Guizhou Province [No. (2011) 4008)]. (3) PK & PD Platform Construction Project of Guizhou Province (No. ZY [2011] 3013). (4) The Characteristic Key Laboratory of Guizhou Province Education Department. (No. KY [2012] 005). (5) Development and Reform Commission of Guizhou province for development and Reform of High Technology (No. [2013] 2068).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vol. 1. Beijing, China: China Medical Science and Technology Press; 2010. National Commission of Chinese Pharmacopoeia. Pharmacopoeia of Peoples Republic of China; p. 155. [Google Scholar]
- 2.Xinyixueyuan. Shanghai, China: Shanghai Scientific and Technologic Press; 2000. Dictionary of Traditional Chinese Medicine; p. 869. [Google Scholar]
- 3.Huang HF, Zhang CC, Yuan D, Zhou ZY. Anti-inflammatory and antibacterial effects of Polygonum perfoliatum L. Anhui Med Pharm J. 2008;12:595–6. [Google Scholar]
- 4.Long WY, Li YH. Experimental study on the effect of relieving cough and reducing sputum of Polygonum perfoliatum L. Chin J Clin Ration Drug Use. 2010;3:34–5. [Google Scholar]
- 5.Fu YX, He XR, Li JC, Liu XX. Study of chemical constitution and anti-bacterial effect of Herb Polygonum perfoliatum L. Prog Vet Med. 2008;29:45–9. [Google Scholar]
- 6.Chang MY. Changsha, China: Hunan Science and Technology Press; 1998. Anticancer medicine, revised ed; pp. 227–8. [Google Scholar]
- 7.Zhang YH. Nanjing, China: Jiangsu Science and Technology Press; 2000. Kang ai da quan; p. 217. [Google Scholar]
- 8.Xing YJ, Wang HY, Wang JX, Li YY, Kang WY. α-Glucosidase inhibitory and antioxidant activities of Polygonum perfoliatum. Chin J Exp Tradit Med Formul. 2011;17:189–91. [Google Scholar]
- 9.Zhang CC, Huang HF, Zhou ZY, Yuan D. Study on extraction of Polygonum perfoliatum L. suppress herpes simplex virus-I (HSV-I) disease. Lishizhen Med Mater Med Res. 2010;21:2835–6. [Google Scholar]
- 10.Cai XL, Zhang XL, Zhang KF. Protective effect of Polygonum perfoliatum on CCl4-induced acute liver injury in rats. Chin Pharm. 2010;21:4059–60. [Google Scholar]
- 11.Zhu GH, Wang DY, Meng JC. New compounds from Polygonum perfoliatum L. Indian J Heterocycl Chem. 2000;10:41–4. [Google Scholar]
- 12.Shen T, Jia ZJ, Zheng SZ. Studies on chemical constituents of Polygonum perforliatum L. J Asian Nat Prod Res. 2007;9:129–33. doi: 10.1080/1028602042000324844. [DOI] [PubMed] [Google Scholar]
- 13.Zhong RL, Sun XC, Li WX, Wu LJ, Huang J, Sun BH. Isolation and identification of chemical constituents of Polygonum perfoliatum L. J Shenyang Pharm Univ. 2008;25:105–7. [Google Scholar]
- 14.Liu JM, Wang DY, Zheng SZ. A new limonoid of Polygonum perfoliatum L. Indian J Heterocycl Chem. 1999;9:69–70. [Google Scholar]
- 15.Zhao C, Zhou X, Qin A, Yang ZN, Chen HG. Study on chemical constituents of Polygonum perfoliatum L. Chin Tradit Herb Drugs. 2009;31:1610–1. [Google Scholar]
- 16.Li HF, Ma QY, Liu YQ, Qian JF, Zhou J, Zhao YX. Constituents from Polygonum perfoliatum. Chin J Appl Environ Biol. 2009;15:615–20. [Google Scholar]
- 17.Sun X, Sneden AT. Neoflavoniods from Polygonum perfoliatum. Planta Med. 1999;65:671–3. doi: 10.1055/s-2006-960846. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, Chen HG, Gong XJ, Cao GH, Zhou CY, Zhou X. Study on chemical constituents of Polygonum perfoliatum L. (II) Chin Tradit Herb Drugs. 2010;41:365–7. [Google Scholar]
- 19.Sun X, Zimmermann ML, Campagne JM, Sneden AT. New sucrose phenypropanoid esters from Polygonum perfoliatum. J Nat Prod. 2000;63:1094–7. doi: 10.1021/np000055e. [DOI] [PubMed] [Google Scholar]
- 20.Dreyer DL. Citrus bitter principles III. Isolation of deacetylnomilin and deoxylinmonin. J Am Chem Soc. 1965;30:749. [Google Scholar]
- 21.Wang DY, Lu JH. Chemical constituents in roots of Polygonum perfoliatum. Subtrop Plant Sci. 2004;33:10–2. [Google Scholar]
- 22.Chen HG, Zhou X, Zhao C, Gong XJ, Qin A. RP-HPLC determination of quercetin in Polygonum perfoliatum L. Chin J Pharm Anal. 2009;29:1749–51. [Google Scholar]
- 23.Xiu QL, Bao JK, Mao XJ, Tong H, Zuo HD, Li JF, et al. Determination of quercetin in Polygonum perfoliatum L. by HPLC. Chin J Chin Mater Med. 2009;34:2105–7. [Google Scholar]
- 24.Liu Q, Li L, Huang JY, Liu T. Content determination of quercetin in Polygonum perfoliatum. Chin J Mod Drug Appl. 2009;3:27–8. [Google Scholar]
- 25.Bao JK, Xu QL, Mao XJ, Lin RC, Wang GL, Zhou N, et al. HPLC determination of caffeic acid in Polygonum perfoliatum L. from different source. Chin J Pharm Anal. 2010;30:903–5. [Google Scholar]
- 26.Fan DS, Zhou X, Zhao C, Chen HG, Zhao Y, Gong XJ. Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-D-glucuronide in Polygonum perfoliatum L. Fitoterapia. 2011;82:805–10. doi: 10.1016/j.fitote.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Lin WL, Wang CJ, Tsai YY, Liu CL, Hwang JM, Tseng TH. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch Toxicol. 2000;74:467–72. doi: 10.1007/s002040000148. [DOI] [PubMed] [Google Scholar]
- 28.Chu CY, Tsai YY, Wang CJ, Lin WL, Tseng TH. Induction of apoptosis by esculetin in human leukemia cells. Eur J Pharmacol. 2001;416:25–32. doi: 10.1016/s0014-2999(01)00859-7. [DOI] [PubMed] [Google Scholar]
- 29.Prabakaran D, Ashokkumar N. Antihyperglycemic effect of esculetin modulated carbohydrate metabolic enzymes activities in streptozotocin induced diabetic rats. J Funct Food. 2012;4:776–83. [Google Scholar]
- 30.Begum AN, Terao J. Protective effect of quercetin against cigarette tar extract-induced impairment of erythrocyte deformability. J Nutr Biochem. 2002;13:265–72. doi: 10.1016/s0955-2863(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 31.Shirai M, Moon JH, Tsushida T, Terao J. Inhibitory effect of a quercetin metabolite, quercetin 3-O-beta-D-glucuronide, on lipid peroxidation in liposomal membranes. J Agric Food Chem. 2001;49:5602–8. doi: 10.1021/jf010713g. [DOI] [PubMed] [Google Scholar]
- 32.Shirai M, Kawal Y, Yamanishi R, Kinoshita T, Chuman H, Terao J. Effect of a conjugated quercetin metabolite, quercetin 3-glucuronide, on lipid hydroperoxide-dependent formation of reactive oxygen speciesin differentiated PC-12 cells. Free Radic Res. 2006;40:1047–53. doi: 10.1080/10715760600794287. [DOI] [PubMed] [Google Scholar]
- 33.Haleagrahara N, Radhakrishnan A, Lee N, Kumar P. Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in the hypothalamus of rats. Eur J Pharmacol. 2009;621:46–52. doi: 10.1016/j.ejphar.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Meng DS, Wang FJ, Wang SL. Effects of quercetin on metabolism of cyclooxygenase in IEC26 cells stimulated by the serum of burned rats. Acta Acad Med Militaris Tertiae. 2003;25:1345–8. [Google Scholar]
- 35.Valério DA, Georgetti SR, Magro DA, Casagrande R, Cunha TM, Vicentini FT, et al. Quercetin reduces inflammatory pain: Inhibition of oxidative stress and cytokine production. J Nat Prod. 2009;72:1975–9. doi: 10.1021/np900259y. [DOI] [PubMed] [Google Scholar]
- 36.Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Zhong XG, Wu KN, He S, Ma SW, Kong LQ. Effects of quercetin on the proliferation and apoptos is in transplantation tumor of breast cancer in nude mice. Sichuan Da Xue Xue Bao (Yi Xue Ban) 2003;34:439–43. [PubMed] [Google Scholar]
- 38.Castrillo JL, Carrasco L. Action of 3-methylquercetin on poliovirus RNA replication. J Virol. 1987;61:3319–21. doi: 10.1128/jvi.61.10.3319-3321.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milenković M, Arsenović RN, Stojić VZ, Bufan B, Vučićević D, Jančić I. Quercetin ameliorates experimental autoimmune myocarditis in rats. J Pharm Pharm Sci. 2010;13:311–9. doi: 10.18433/j3vs3s. [DOI] [PubMed] [Google Scholar]
- 40.Romarís-Hortas V, García-Sartal C, Barciela-Alonso MC, Moreda-Piñeio A, Bermejo-Barrera P. Characterization of edible seaweed harvested on the Galician Coast (Northwestern Spain) using pattern recognition techniques and major and trace element data. J Agric Food Chem. 2010;58:1986–92. doi: 10.1021/jf903677y. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Chen LH, Zhang Q, Lai MX, Wang Q. Quality assessment of cortex Cinnamomi by HPLC chemical fingerprint, principle component analysis and cluster analysis. J Sep Sci. 2007;30:1276–83. doi: 10.1002/jssc.200600389. [DOI] [PubMed] [Google Scholar]
- 42.Han BX, Chen NF, Yao Y. Discrimination of Radix pseudostellariae according to geographical origin by FT-NIR spectroscopy and supervised pattern recognition. Pharmacogn Mag. 2009;5:279–86. [Google Scholar]
- 43.Zhao Y, Kao CP, Chang YS, Ho YL. Quality assessment on Polygoni Multiflori Caulis using HPLC/UV/MS combined with principle component analysis. Chem Cent J. 2013;7:106–18. doi: 10.1186/1752-153X-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]


