Abstract
Background:
Sufu is a traditional Chinese fermented soybean food. Isoflavones are abundant in soybean and products incorporated with isoflavones exert many health benefits. The aim of this study was to investigate anti-fatigue effect of sufu fortified with isoflavones.
Materials and Methods:
In vivo anti-fatigue activity of sufu with fortification of isoflavones (IF) was investigated in this study via exhaustive swimming test using ICR mice and determination of biochemical parameters. Factors relating to fatigue, including hepatic glycogen, blood lactic acid (BLA), blood urea nitrogen (BUN) were determined. The isoflavone composition in the IF sufu was also determined to explore the anti-fatigue activity of isoflavones.
Results:
During fermentation, isoflavone glucosides were converted into aglycones and both sufu with and without fortification of IF prolonged the exhaustive swimming time of ICR mice. Intake of sufu also increased the hepatic glycogen content, while it decreased the levels of both the blood lactic acid (BLA) and blood urea nitrogen (BUN) content. A dose-response relationship was observed in both exhaustive swimming and BLA clearance test, with medium dose (1%) fortification of IF revealing the highest activity.
Conclusion:
IF sufu could possess high anti-fatigue activity.
Keywords: Anti-fatigue, exhaustive swimming test, isoflavone, sufu
INTRODUCTION
Fatigue is defined as difficulty in initiating or sustaining voluntary activities, which can be classified into mental and physical fatigue.[1] Among well-accepted mechanisms of exercise-induced fatigue is “clogging theory”,[2] which suggests that over accumulation of blood lactic acid (BLA) and blood urea nitrogen (BUN) will lead to metallic disorders, resulting in fatigue. Another fatigue mechanism, which is of particular interest to scientists, is “radical theory”. Harman's classical “radical theory” suggests that intense exercise can produce an imbalance between the body's oxidation and anti-oxidation system. “Oxygen paradox” is well documented as increasing O2 uptake and consumption can meet the skeletal muscle energy requirement during aerobic physical exercise, while further augmenting oxidative stress when scavenging capacity of both non-enzymatic and enzymatic defense mechanisms are overwhelmed.[3] Antioxidants, which protect cellular constituents from oxidation by neutralizing free radicals, can inhibit fatigue of skeletal muscle.[4] However, the mechanisms have not been elucidated.
Sufu is a traditional fermented soybean curd originating from China, and being part of Chinese diet for more than 1000 years. Through fermentation, the content of many nutrients including vitamins and soybean peptides increases. Sufu is considered to be not only nutritional, but also functional. Sufu has been reported to possess anti-oxidation activity, angiotensin I-converting enzyme inhibitory (ACE) and antimutagenicity activities in vitro.[5,6,7] However, most commercial sufu contains 6.2% -14.8% salt and diet high in salt increases health risk,[8] which limits the consumption of sufu. Some sufu manufacturers have launched low-salt sufu, the salt content of which is below 6%. The low-salt sufu we prepared in this study contained about 4% salt, which would not be decisive for dietary salt intake.
Soybean is abundant in isoflavones and products incorporated with isoflavones exert many health benefits. Isoflavones occur in the form of aglycones (daidzein, genistein, and glycitein) and corresponding glucosidic conjugates, which include glucosides (daidzin, genistin, and glycitin), malonyl-glucosides and acetyl-glucosides. Fermentation converts soybean isoflavones from the glycosides within tofu into the corresponding aglycones through hydrolysis by β-glycosidase,[9] which significantly improves the bioavailability and absorptivity of sufu compared to tofu.[10] A typical Chinese diet has an average daily intake of only about 20 mg isoflavones.[11] Considering sufu has an estimated annual production of over 300,000 metric tons in China,[12] fortifying isoflavones in sufu may be a possible way to improve intake of isoflavones, in particular the more beneficially healthy aglycones.
Up to now, there is scarce literature dealing with fortification of isoflavones in fermented soybean foods and anti-fatigue activity of sufu, as well as the mechanisms of in vivo anti-fatigue of isoflavones.
In this study, we prepared low-salt sufu of high isoflavone content and investigated in vivo anti-fatigue effect of sufu fortified with isoflavones by exhaustive swimming test of mice. Then several biochemical parameters related to fatigue, including hepatic glycogen, BLA, BUN were determined. The isoflavone composition in the IF sufu was also determined to relate to anti-fatigue activity of isoflavones.
MATERIALS AND METHODS
Materials
Commercial non-GMO soybeans (Zhonghuang 13, produced in 2009) were purchased from Chinese Academy of Agricultural Science (Beijing, China). Isoflavone extract from soybean was purchased from Guanghan Biochem Pharmaceutical Co., Ltd. (Sichuan, China). The extract is composed of 41.2% total isoflavones including 25% daizin, 9.7% glycitin, 5.6% genistin, 0.7% daidzen, 0.1% glycitein, and 0.1% genistein.
Animals
Male ICR mice (weighing from 18 to 20 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). They were housed in a SPF level room with a 12/12 h light-dark alternation cycle at constant room temperature of 23 ± 1°C and moderate humidity (55 ± 5%). Mice were allowed to adopt the surrounding environment for one week before experimental treatments were conducted. After adaptation, 50 mice were randomly divided into 5 groups each containing 10 mice. Mice were fed ad libitum continuously for 15 days on a commercial rodent diet and gavaged with distilled water (Group W), commercial Wang Zhihe red sufu (Group C), 0.5% IF sufu (Group L), 1% IF sufu (Group M) and 2% IF sufu (Group H). The administration dosage was 9.2 g/kg body mass per day.
Preparation of sufu with fortification of isoflavones (IF sufu)
IF sufu was prepared in the Wang Zhihe Corporation (Beijing, China). The preparation followed the method reported by Han, Rombouts and Nout[12] with some modifications:
-
(1)
Tofu preparation. Tofu was prepared by salt precipitation from boiled soymilk. The tofu was then sliced into cubes of 3.1 × 3.1 × 1.8 cm, weighing approximately 10 g per cube
-
(2)
Pre-fermentation. Actinomucor elegans was used as the fermentation starter. The mucor suspension was sprayed onto the surface of tofu and it was allowed to ferment for 72 h at the room temperature (28°C, RH >95%)
-
(3)
Salting. The cubes were salted for 5 days in a ceramic jar until the salt content of pehtze reached about 16%
-
(4)
Post-fermentation. Isoflavone extract was added into a commercial sufu red soup which mainly consists of red mold rice, Chinese distillate spirit, sugar, salt, wheat powder, spice. Each salted cube was transferred to one glass bottle (250 mL) and then fully filled with post-fermentation soup. Fermentation was carried out at room temperature of 25°C and RH above 60% with gentle ventilation for 75 days. The salt content of final product was within the range of 4.7-5.1 g/100 g. The sufu samples were dissolved in distilled water at the concentration of 20 mL/kg for further use.
Determination of the isoflavones contents in the IF sufu
The isoflavone content was determined based on the protocol as previously described by Klump et al.[13] Sufu cubes were vacuum freeze dried, then ground to a powder. For extraction, vacuum freeze dried sample powder (3.000 g) was weighed into an Erlenmeyer flask (250 mL) with aqueous methanol (80%, 40 mL) added. The flask was shaken in 65°C water bath for 2 h, and then was cooled to room temperature (25°C). NaOH (3 mL, 2 M) was added and the flask was shaken at room temperature on an orbital shaker for 10 min. The flask was removed from the shaker, then 1 mL glacial acetic acid was added. The suspension was poured into a graduated cylinder and diluted to 50 mL with aqueous methanol (80%). The solution was filtered through quantitative-grade filter paper, then 5 mL was pipeted into a 10-mL graduated cylinder, followed by 4.0 mL water and diluted to 10 mL with methanol. The cylinder was stoppered and inverted repeatedly. One milliliter extract was transferred into 1.5 mL centrifuge tube and centrifuged at 7000 × g for 5 min for further analysis. A LC-10ATvp liquid chromatograph (Shimadzu, Japan) equipped with a capcell pak C18 column (5 μm, 250 × 4.6 mm i.d., SHISEIDO Inc., Japan) and an ultraviolet spectrophotometer at a wavelength of 260 nm was used for isoflavone measurement. Isoflavone extracts were eluted at 40°C. The mobile phases for HPLC consisted of solvent (A) Water-methanol-acetic acid (88 + 10 + 2) and (B) methanol-acetic acid (98 + 2). The solvent gradient was as follows: the concentration of solvent (B) increased from 10 to 70% in 35 min. The flow rate was 1.2 mL/min. Quantitative data for each isoflavone was obtained by comparison with known standards.
Exhaustive swimming test
The mice were allowed to rest for 30 min after the last feeding. Then, tin wire weighing 5% of the body weight of a mouse was attached to the end of each mouse's tail. The mice were put into a swimming tank with water at a depth greater than 30 cm at 25 ± 1.0°C. The water was agitated to keep the mice swimming until the endpoint of the test, which was defined as the time point when the mice failed to rise to the surface for breathing within 7 s. Time period from the beginning of the swimming to the endpoint was recorded as the exhaustive swimming time.
Determination of hepatic glycogen
Mice were fasted for 8 h before the last feeding. Mice were sacrificed 30 min after the final oral administration, with the livers removed, immediately washed with saline and dried with filter papers. In accordance with instructions of the hepatic glycogen detection kit (Lot No. 20091215, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), liver samples were accurately weighted and the absorbance of the hepatic glycogen was measured under OD 620 nm using a 752 ultraviolet spectrophotometer (Shanghai Third Analytical Instrument Cooperation, Shanghai, China).
Determination of blood lactic acid (BLA)
The mice were put into the swimming tank with water temperature being 30°C to swim for 10 min without load. Blood samples of the mice were collected before, immediately and 20 mins after forced swimming. According to the instruction of the whole blood lactic acid detection kit (Lot No. 20091215, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), the BLA level of mice were measured at the OD 530 nm using KC – junior model microplate spectrophotometer (Bio Tek Instrument, Inc., USA). The area covered by blood lactic acid curve is defined as follows:
The area covered by blood lactic acid curve = 5 × (L1 + 3 × L2+ 2 × L3)
Where L1, L2 and L3 represent the blood lactic acid content tested before, immediately and 20 min after forced swimming.
Determination of blood urea nitrogen (BUN)
Mice 30 min after the last oral administration were individually forced to swim in a swimming tank containing water at temperature 30°C for 90 min without load. Mice were allowed to rest for 60 min, and then eyeballs of the mice were enucleated and 0.5 mL blood samples were collected following the retroorbital bleeding method reported by Taylor, Hayes and Toth.[14] After refrigeration for about 3 h at 4°C, the blood samples coagulated and were centrifuged at 2000 rpm/min for 15 min. The serum was collected for BUN measurement using a 7060 model automatic biochemical analyzer (Hitachi, Ltd., Japan).
Statistical analysis
The results were presented as the means ± standard deviations. Statistical analyses were performed with two-sided test conducted by SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). Probability values P < 0.05 (two-tailed) were considered as statistically significant and P < 0.01 were highly significant.
RESULTS
Isoflavone concentration of IF sufu
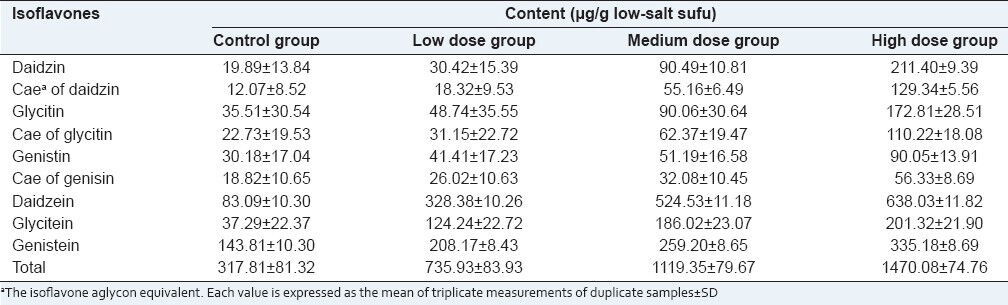
The content and composition of isoflavones may directly influence their bioactive activities. Isoflavone content in the IF sufu is summarized in Table 1. Yin et al., reported changes in the composition of isoflavones of sufu were detected during later fermentation as well as pre-fermentation, though with minor effects.[9] As shown in Table 1, the concentration of isoflavones increased as the fortification of isoflavones into the post-fermentation soup augmented. The accumulation of aglycones (daidzein, glycitein and genistein), in Group L, M, and H were 2.50, 3.67 and 4.45 fold compared to the control group.
Table 1.
The content of insoflavones in sufu of different groups
IF sufu prolonged the exhaustive swimming time
Exhaustive swimming model representative of muscular exercise endurance is a reliable model adopted in the study of the anti-fatigue test which gives a high reproducibility. Reduced susceptibility to fatigue is correlated with longer swimming time. As shown in Figure 1, all four sufu samples used on the diet could significantly prolong the swimming time of the mice (**P < 0.01) by 58.6%, 64.46%, 80.01%, 70.27%, respectively, indicating sufu possesses an anti-fatigue activity. Mice of Group L, M and H swam longer than Group C, and Group M is significantly more effective relative to Group C, suggesting isoflavone content might be critical in exerting anti-fatigue activity. To study the anti-fatigue mechanism of IF sufu, some biochemical parameters including hepatic glycogen, BLA, BUN were determined.
Figure 1.
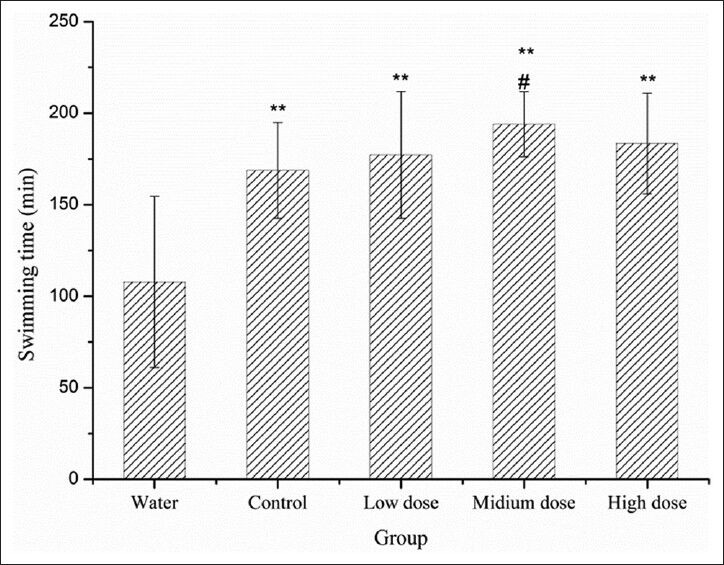
Effect of IF sufu on the exhaustive swimming time of mice. Results are presented as mean values ± standard deviation in triplicate. **P< 0.01 compared with water group. #P< 0.05 compared with control group
IF sufu increased the hepatic glycogen content
Energy for exercise is derived initially from the breakdown of glycogen and later, from circulating glucose released by the liver.[15] The role of hepatic glycogen is to complement the consumption of blood glucose and maintain the blood glucose in the physiological range. An effective way to improve endurance and retard fatigue is to increase the storage amount of glycogen before exercise starts.[16]
The effect of intake of IF sufu on the hepatic glycogen content is illustrated in Figure 2. Compared with Group W, the hepatic glycogen contents of Group C, Group M and Group H are significantly higher (*P < 0.05), which suggests sufu was capable of increasing the hepatic glycogen content, thus having a potential effect on retarding fatigue. In contrast to exhaustive swimming test, the sufu with fortification of isoflavones did not show any significant difference compared with the control group, indicating isoflavones are not the key factor for increased hepatic glycogen content.
Figure 2.
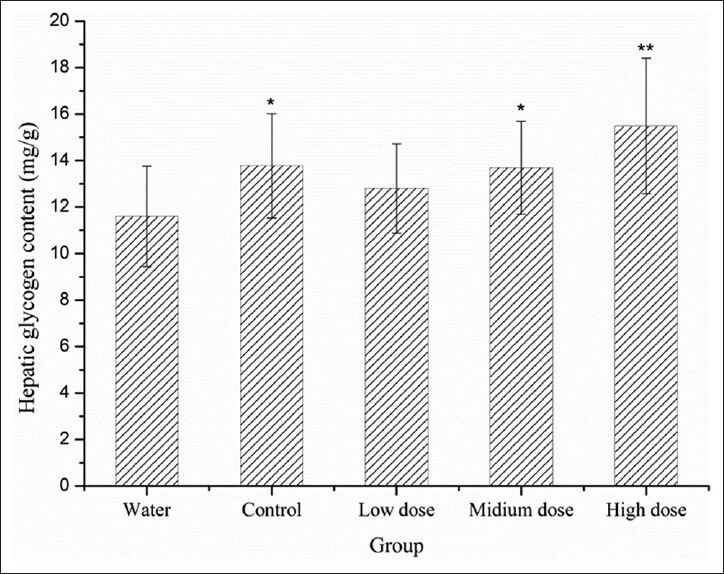
Effect of IF sufu on the hepatic glycogen content in the mice. Results are presented as mean values ± standard deviation in triplicate. **P< 0.01 and *P< 0.05 compared with water group
IF sufu decreased the BLA content during exercise
BLA is the glycolysis product of carbohydrate under an anaerobic condition and glycolysis is the main energy source for intense exercise in a short time. BLA accumulates during exercise, which decreases the pH value of the blood and muscle tissue, affecting both the cardio-circulating system and the skeletal muscle system function. The decrease in the contractive strength of the muscle eventually induces fatigue.[17] If the accumulation of lactic acid could be inhibited or the clearance of the lactic acid during exercise could be accelerated, anti-fatigue activity will be accomplished.
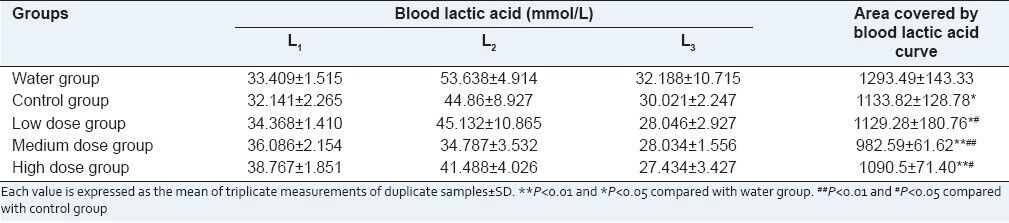
The BLA content before, immediately after and 20 min after exhaustive swimming test is shown in Table 2. The calculated area covered by blood lactic acid curve declaring the removing of blood lactic acid activity of tested samples is also illustrated in Table 2. Sufu significantly promoted scavenging of blood lactic acid which was produced during exercise. The area covered by blood lactic acid curve of IF sufu was significantly lower than the Group C (#P < 0.05), with Group M showing a decrease of 13.3% compared with the control. Additionally, the result shows a positive dose-dependent effect, that is, increasing dose of isoflavones within a certain range, can improve the effect of clearing the blood lactic acid.
Table 2.
The blood lactic acid content
IF sufu reduced the BUN content
Dynamophores in sports include sugar, fat, and protein. When movement time does not exceed 30 min, protein seldom participates in energizing and the BUN content is stable. Protein and amino acids have a stronger katabolic metabolism when the body cannot obtain enough energy by sugar and fat catabolic metabolism. After an extended time of movement, urea nitrogen increases.[2] It is reported the BUN content is significantly positive correlated with the exercise intense and endurance time.[18]
Sufu significantly reduced the BUN content compared with the water group (*P < 0.05) [Figure 3]. The difference between the control group and the water group is highly significant (**P < 0.01). However, BUN content in the treatment groups is higher compared with the control group with no significant difference. It is suggested that the fortification of isoflavones is not essential for reducing BUN content and can even act as a negative factor. Possibly, other functional components such as soybean peptides and red rice have a major effect on reducing BUN content.
Figure 3.
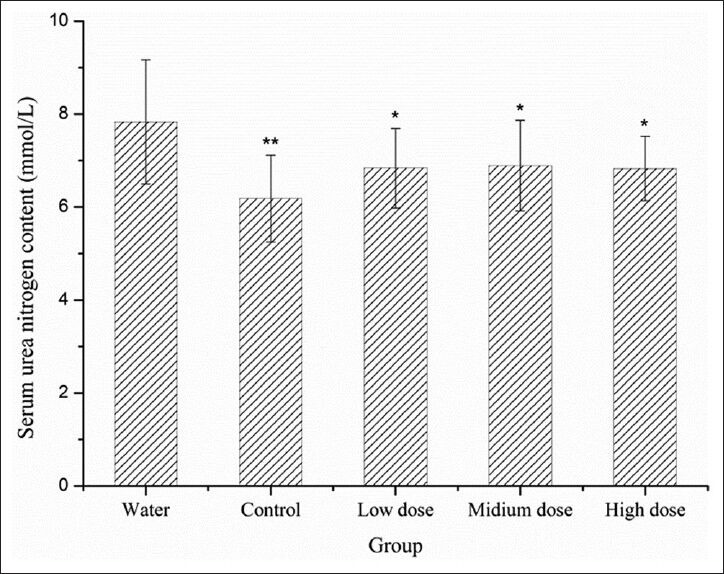
Effect of IF sufu on the BUN content in mice. Results are presented as mean values ± standard deviation in triplicate. **P< 0.01 and *P< 0.05 compared with water group
DISCUSSION
Numerous epidemiological studies suggest that dietary flavonoids are closely related to the prevention of degenerative diseases, but absorptivity of these compounds appears extremely low and much of what is absorbed appears to be rapidly converted to inactive conjugated metabolites.[19] Isoflavone aglycones, which show a different absorption pattern from that of glucosides, are absorbed in the rat stomach more efficiently.[20] Theoretically, fortification of isoflavones during sufu ripening can effectively improve absorptivity of IF via transforming the glucosides into aglycones.
In the sufu sample with fortification of isoflavones, the content of daidzein is the highest among the aglycones, while in the control sufu sample, genistein is the highest. Gardnet, Chatterjee and Franke observed possible saturation of bioavailability of genistein at doses of 288 vs. 144 mg total isoflavones/day.[21] However, no evidence of saturation of daidzein bioavailability has been previously reported, which indicates the potential to increase the bioavailability of isoflavones by increasing daidzein content of isoflavones. Therefore, adding an extract of isoflavones during the post-fermentation, would facilitate the transformation from glucosides to aglycones, increase higher aglycones content in IF sufu in comparison with control, and could overcome the bioavailability saturation of genistein as well.
Exhaustive swimming test indicated that IF sufu prolonged the exhaustive swimming time. Adding 5% body weight attached to mice in the duration of the swim-to-exhaustion could effectively simulate the fatigue stress, while not prohibiting mice swimming freely. Water temperature could significantly influence animal's behavior. Water temperature of 30°C prevents exchange between water and body temperatures, which also helps to maintain the body temperature. At the water temperature of 25°C, pilo-erection and increasing of paw muscle tone were observed. These behaviors are adopted to avoid heat loss thus maintaining the body temperature.[22] In our experiment, the water temperature is set at 25°C, and the cold water increased the mice's sympathetic nervous outflow,[23] which could be considered as another stress factor.
Soybean isoflavones have been reported to have antioxidant activities in vitro by ferric reducing-antioxidant power (FRAP) and anti-DPPH free radical assays.[24] Our data suggest that isoflavones could have beneficial effects on endurance capacity by decreasing contribution of exercise-induced oxidative stress. A previous study evaluated anti-fatigue activity of flavonoids from corn silk (FCS) and demonstrated that FCS is able to elevate anti-fatigue activity of mice.[25] It is estimated that isoflavones may share some biological attributes such as anti-fatigue activity with other flavonoids.
From results of determining hepatic glycogen, BLA, BUN, we suggest that anti-fatigue activity of sufu is a comprehensive and complex effect contributed to by various substances including isoflavones. The amount of amino acids, especially alpha-aminobutyric acid, alanine, glycine, isoleucine, serine, valine, threonine, and tyrosine in the plasma drops rapidly during successive exercise trials to exhaustion.[26] During fermentation, soybean protein is degraded into peptides and free amino acids, which are rich in these key 8 amino acids.[27] There is the possibility that replenishing the amino acids might help return to the normal level, which could not be accomplished by isoflavones alone. Red mold rice is an important ingredient for the post-fermentation soup which colors the surface of the sufu. Wang et al., found that it also exerts positive effect on anti-fatigue, which extends the swimming time for the rats, effectively delays the lowering of glucose in the blood, and prevents the increase in lactate and BUN concentrations.[28] Red mold rice in the sufu samples in our experiments may contribute to the increase of hepatic glycogen rather than isoflavones. In accordance with a previous conclusion made by Shen et al., isoflavones do play a crucial part in decreasing BLA level.[29] IF sufu is able to inhibit the accumulation of lactic acid and accelerate the clearance of lactic acid by increasing total isoflavone content, especially the bioavailable and absorptive aglycones.
CONCLUSIONS
Our study is the first to report on in vivo anti-fatigue activity of sufu and IF sufu and developed a new method to increase the content of isoflavone aglycones in sufu, which are more bioavailable and absorptive. It is suggested that sufu possesses high anti-fatigue activity. The swimming time was significantly prolonged, the storage of glycogen was significantly increased, and both the BLA and BUN content were significantly reduced. The effect of isoflavones on anti-fatigue is shown to be dose-dependent. IF sufu with medium dose (1%) fortification of isoflavones demonstrates the highest activity among three levels of isoflavone additions (0.5%, 1%, 2%), which significantly prolongs the swimming time of the mice and accelerates the clearance of BLA during exercise relative to commercial sufu. However, IF sufu is not highly effective in glycogen accumulation and BUN elimination. Exploration of the underlying mechanism in cellular or molecular levels requires further study to explain why these biochemical parameters are not in accordance in explaining anti-fatigue effect and how each form of isoflavone provides anti-fatigue benefits. Further study is needed to assess the anti-fatigue activity of sufu on humans.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Arnie W. Hydamaka (Department of Food Science, University of Manitoba) for language checking of this paper.
Footnotes
Source of Support: Grants from the National Natural Science Foundation of the People’s Republic of China (No. 31171739), Program for New Century Excellent Talents in University (NETC-10-0776) and Beijing Municipal Talent Training Program (2011D009007000001)
Conflict of Interest: None declared.
REFERENCES
- 1.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–88. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Zhang HL, Lu R, Zhou YJ, Ma R, Lv JQ, et al. The decapeptide CMS001 enhances swimming endurance in mice. Peptides. 2008;29:1176–82. doi: 10.1016/j.peptides.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Ikeuchi M, Koyama T, Takahashi J, Yazawa K. Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol Pharm Bull. 2006;29:2106–10. doi: 10.1248/bpb.29.2106. [DOI] [PubMed] [Google Scholar]
- 4.Yu F, Lu S, Yu F, Feng S, Mcguire PM, Li R, et al. Protective effects of polysaccharide from Euphorbiakansui (Euphorbiaceae) on the swimming exercise-induced oxidative stress in mice. Can J Physiol Pharmacol. 2006;84:1071–9. doi: 10.1139/y06-052. [DOI] [PubMed] [Google Scholar]
- 5.Wang LJ, Saito M, Tatsumi E, Li LT. Antioxidative and angiotensin I-converting enzyme inhibitory activities of sufu (Fermented Tofu) Extracts. Japan Agric Res. 2003;37:129–32. [Google Scholar]
- 6.Ma YL, Cheng YQ, Yin LJ, Wang JH, Li LT. Effects of processing and NaCl on angiotensin I-converting enzyme inhibitory activityand γ-aminobutyric acid content during sufu manufacturing. Food Bioprocess Technol. 2013;6:1782–9. [Google Scholar]
- 7.Moy YS, Lai YJ, Chou CC. Effects of ripening process on the Mutagenicity and Antimutagenicity of sufu, a Chinese traditional fermented product of soybean. Food Bioprocess Technol. 2012;5:2972–7. [Google Scholar]
- 8.Han BZ, Beumer RR, Rombouts FM, Nout MJ. Microbiological safety and quality of commercial sufu-a Chinese fermented soybean food. Food Control. 2001;12:541–7. [Google Scholar]
- 9.Yin LJ, Li LT, Li ZG, Tatsumi E, Saito M. Changes in isoflavone contents and composition of sufu (fermented tofu) during manufacturing. Food Chem. 2004;87:587–92. [Google Scholar]
- 10.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–9. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 11.Ho SC, Chan SG, Yi Q, Wong E, Leung PC. Soy intake and the maintenance of peak bone mass in Hong Kong Chinese women. J Bone Miner Res. 2001;16:1363–9. doi: 10.1359/jbmr.2001.16.7.1363. [DOI] [PubMed] [Google Scholar]
- 12.Han BZ, Beumer RR, Rombouts FM, Nout MJ. A Chinese fermented soybean food. Int J Food Microbiol. 2001;65:1–10. doi: 10.1016/s0168-1605(00)00523-7. [DOI] [PubMed] [Google Scholar]
- 13.Klump SP, Allred MC, MacDonald JL, Ballam JM. Determination of isoflavones in soy and selected foods containing soy by extraction, saponification, and liquid chromatography: Collaborative study. J AOAC Int. 2001;84:1865–83. [PubMed] [Google Scholar]
- 14.Taylor R, Hayes KE, Toth LA. Evaluation of an anesthetic regimen for retroorbital blood collection from mice. J Am Assoc Lab Anim Sci. 2000;39:14–7. [PubMed] [Google Scholar]
- 15.Hollman PC, Katan MB. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–42. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 16.Piskula MK, Yamakoshi J, Iwai Y. Daidzein and genistein but not their glucosides are absorbed from the rat stomach. FEBS Lett. 1999;447:287–91. doi: 10.1016/s0014-5793(99)00307-5. [DOI] [PubMed] [Google Scholar]
- 17.Jia JM, Wu CF. Antifatigue activity of tissue culture extracts of Saussurea involucrate. Pharm Biol. 2008;46:433–6. [Google Scholar]
- 18.You LJ, Zhao M, Regenstein JM, Ren JY. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011;124:188–94. doi: 10.1021/jf2016368. [DOI] [PubMed] [Google Scholar]
- 19.Suh SH, Paik IY, Jacobs K. Regulation of blood glucose homeostasis during prolonged exercise. Mol Cells. 2007;23:272–9. [PubMed] [Google Scholar]
- 20.Bo Y, Zhang XL, Xiao MB, Feng XL, Xian QH. Scanvenging and anti-fatigue activity of fermented defatted soybean pepetides. Eur Food Res Technol. 2008;226:415–21. [Google Scholar]
- 21.Gardnet CD, Chatterjee LM, Franke AA. Effects of isoflavone supplements vs. soy foods on blood concentrations of genistein and daidzein in adults. J Nutr Biochem. 2009;20:227–34. doi: 10.1016/j.jnutbio.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calil CM, Marcondes FK. The comparison of immobility time in experimental rat swimming models. Life Sci. 2006;79:1712–9. doi: 10.1016/j.lfs.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Kirov SA, Talan MI, Engel BT. Sympathetic outflow to interscapular brown adipose tissue in cold acclimated mice. Physiol Behav. 1996;59:231–5. doi: 10.1016/0031-9384(95)02129-9. [DOI] [PubMed] [Google Scholar]
- 24.Lee CH, Yang L, Xu JZ, Yeung SY, Huang Y, Chen ZY. Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem. 2005;90:735–41. [Google Scholar]
- 25.Hu QL, Zhang LJ, Li YN, Ding YJ, Li FL. Purification and anti-fatigue activity of flavonoids from corn silk. Int J Phys Sci. 2010;5:321–6. [Google Scholar]
- 26.Bazzarre TL, Murdoch SD, Wu SM, Herr DG, Snider IP. Plasma amino acid responses of trained athletes to two successive exhaustion trials with and without interim carbohydrate feeding. J Am Coll Nutr. 1992;11:501–11. doi: 10.1080/07315724.1992.10718254. [DOI] [PubMed] [Google Scholar]
- 27.Han BZ, Rombouts FM, Nout MJ. Amino acid profiles of sufu, a Chinese fermented soybean food. J Food Compost Anal. 2004;17:689–98. [Google Scholar]
- 28.Wang JJ, Shieh MJ, Kuo SL, Lee CL, Pan PM. Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl Microbiol Biotechnol. 2006;70:247–53. doi: 10.1007/s00253-005-0051-5. [DOI] [PubMed] [Google Scholar]
- 29.Shen XY, Wang JB, Long Z, Yan SF, Xiao Y, Li Y. The alleviative effect of soybean isoflavone compound on physical fatigue in mice. Chin J Food Hyg. 2004;3:215–7. [Google Scholar]


