Abstract
Prehypertension is associated with reduced conduit artery endothelial function and perturbation of oxidant/antioxidant status. It is unknown if endothelial dysfunction persists to resistance arteries and if exercise training effects oxidant/antioxidant balance in young prehypertensives. We examined resistance artery function using venous occlusion plethysmography measurement of forearm (FBF) and calf blood flow (CBF) at rest and during reactive hyperemia, as well as lipid peroxidation (8-iso-PGF2α) and antioxidant capacity (Trolox-equivalent antioxidant capacity; TEAC) before and after exercise intervention or time-control. Forty-three unmedicated prehypertensive and fifteen matched normotensive time-controls met screening requirements and participated in the study (age: 21.1±0.8 years). Prehypertensive subjects were randomly assigned to resistance exercise training (PHRT; n=15), endurance exercise training (PHET; n=13) or time-control groups (PHTC; n=15). Treatment groups exercised 3 days per week for 8 weeks. Peak and total FBF were lower in prehypertensives than normotensives (12.7±1.2 ml/min/100ml tissue and 89.1±7.7 ml/min/100ml tissue vs. 16.3±1.0 ml/min/100ml tissue and 123.3±6.4 ml/min/100ml tissue, respectively; p<0.05). Peak and total CBF were lower in prehypertensives than normotensives (15.3±1.2 ml/min/100ml tissue and 74±8.3 ml/min/100ml tissue vs. 20.9±1.4 ml/min/100ml tissue and 107±9.2 ml/min/100ml tissue, respectively; p<0.05). PHRT and PHET improved humoral measures of Trolox-equivalent antioxidant-capacity (TEAC) (+24% and +30%) and 8-iso-PGF2α (−43% and −40%, respectively; p<0.05). This study provides evidence that young prehypertensives exhibit reduced resistance artery endothelial function and that short term (8weeks) resistance or endurance training are effective in improving resistance artery endothelial function and oxidant/antioxidant balance in young prehypertensives.
Keywords: endothelial function, resistance arteries, oxidants, antioxidants, exercise, prehypertension
INTRODUCTION
Approximately one third of the U.S. population 20 years of age or older (~54 million Americans) are prehypertensive and this demographic is 11 times more likely to develop essential hypertension than normotensives.1–3 Prehypertension is associated with impaired conduit artery endothelial function, reduced nitric oxide (NO) bioavailability, and perturbation of oxidant/antioxidant status.4–6 However, it is unknown if reduced endothelial function persists to the resistance arteries in young prehypertensives. Prehypertension is not a disease category per se and individuals with prehypertension are not candidates for drug therapy.7 According to the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7), people with prehypertension should practice lifestyle modification to prevent the progressive rise in BP and increased risk of cardiovascular disease.7 The keystone of the JNC7 recommendations is participation in regular physical activity for the treatment of prehypertension.7 Resistance training is suggested as a complement to an endurance training routine to control blood pressure.8 The beneficial effects of endurance training for blood pressure control is well established, however, the effects of resistance training are less known.8
Therefore, in the present randomized and time-controlled study we examined resistance artery endothelial function using venous occlusion plethysmography to measure forearm and calf blood flow, at rest and during reactive hyperemia, and plasma markers of lipid peroxidation and antioxidant capacity in young prehypertensives and normotensives. Additionally, we tested the hypotheses that resistance and endurance exercise training would improve indices of oxidant/antioxidant status and resistance artery endothelial function in young prehypertensives.
METHODS
Baseline status of subjects
Forty-three consecutive young (aged 18–35 yrs) but otherwise healthy subjects determined to be prehypertensive through BP screening were enrolled from the University of Florida and surrounding Gainesville, FL area. Additionally, fifteen consecutive normotensive young healthy subjects were also recruited to serve as a normotensive healthy non-exercising control group (NMTC; n=15; 9M/6F). All subjects (n=58) were non-smokers, and considered to be novice exercisers who had not participated in a structured endurance and/or resistance training program in the past 6 months. Prehypertensive subjects were randomized to one of the following three groups; 1) resistance training (PHRT; n=15; 11M/4F), 2) endurance training (PHET; n=13; 9M/4F), and 3) non-exercising time control (PHTC; n=15; 10M/5F). All subjects were studied before training and after 8 weeks of exercise treatment or control time period. The study was approved by the Institutional Review Board of the University of Florida and written informed consent was obtained from all patients.
Resting brachial blood pressure screening
Prior to study enrollment, all subjects underwent BP screening to determine the presence, or absence, of prehypertension. Blood pressure measurements were performed according to the JNC7 guidelines.7 Briefly, subjects were screened on 3 separate visits during the same time of day and underwent at least 3 blood pressure measurements per visit. After 20–30 minutes of rest, BP measurements were spaced by 5 to 10 minutes intervals on the left arm in a seated position using an automated oscillometric blood pressure cuff (VSM MedTech, Ltd., BC, Canada). Prehypertensive subjects were included in the study when resting systolic blood pressure (SBP) was between 120 and 139, or diastolic blood pressure (DBP) was between 80 and 89 mmHg on all three visits. Normotensive subjects were included when resting SBP was below 120 and DBP was below 80 mmHg. After meeting screening criteria, subjects were asked to report on a separate day to the Cardiovascular Laboratory in the Center for Exercise Science at the University of Florida.
Exercise and time-control
At study entry, prehypertensive subjects were randomly assigned, using a random number generator, to either a group that performed resistance exercise training (RT), endurance training (ET), or to a nonexercising time-control group (TC). Over 8 weeks of training, PHRT and PHET groups performed exercise training on 3 non-consecutive days per week for 1 hour per visit. The RT regimen consisted of 2 sets of 8–12 repetitions to volitional fatigue on 7 variable resistance machines (MedX Corp., Ocala, FL, USA) chosen to exercise all major muscle groups: Leg Extension, Leg Curl, Leg Press, Lat Pull Down, Chest Press, Overhead Press, and Biceps Curl. When 12 repetitions were achieved on the second set, the training weight was increased 5% at the next training session. Recovery time between sets and exercises was 2–3 minutes. Subjects randomly assigned to the endurance training group were oriented to the Quinton endurance exercise treadmill (Cardiac Science Corp., Bothell, WA, USA) and underwent a symptom limited graded exercise test to determine peak oxygen consumption (Peak VO2) and maximum exercising heart rate (HRmax) before and after 8 weeks of exercise training. A 3 day familiarization period for RT and ET was instituted during the first week of training and set at 60% of each subjects predetermined one-repetition maximum (1-RM) or peak oxygen consumption (Peak VO2) and HRmax, respectively. The ET regimen consisted of interval treadmill walking/running to maintain a HR that was between 65–85% of their predetermined HRmax. Walking/running intervals were standardized for PHET participants and consisted of 3 minutes of walking at a speed and incline at 65% of HRmax and 2 minutes of running at a speed and incline at 85% of HRmax for a total of nine 5 minute intervals. All subjects performed a 10 minute warm-up at low speed (e.g. 2.5 mph) with no incline, then 45 minutes of aerobic or resistance exercise, followed by 5 minutes of cool-down, 3 days per week. Both PHTC and NMTC groups remained sedentary and refrained from initiating a structured exercise training program for duration of the study protocols. All subjects were instructed to maintain their current nutritional levels.
Forearm and calf blood flow
Following a 15 minute rest period in a supine position, HR and brachial artery blood pressure (BP) measurements were performed in triplicate in the left arm and an average of the three HR and BP measurements were used for resting values. Forearm (FBF) and calf blood flow (CBF) at rest and during reactive hyperemia (RH) was determined independently by venous occlusion plethysmography (EC-6, D.E. Hokanson, Inc., Bellevue, WA, USA) using calibrated mercury-in-silastic strain gauges, as previously described.9 Briefly, strain gauges were applied to the widest part of the non-dominant forearm or calf after limb circumference measurement. Subjects rested supine for 20 minutes with arms or legs elevated above heart level, which allows for venous emptying during the period of deflation without altering arterial inflow, to achieve stable baseline measurements of blood flow (BF). Due to the variations in vascular reactivity which occur throughout the phases of the menstrual cycle, female subjects were studied during the late luteal phase.10 To measure FBF and CBF, an upper arm or thigh cuff was inflated to 50 mmHg for 7 seconds each 15 second measurement cycle using a rapid cuff inflator (EC 20; DE Hokanson Inc.) to prevent venous outflow.11, 12 The venous collecting pressure was set at 50 mmHg based on evidence demonstrating that this pressure yields the most accurate blood flow measurements during RH.13 One minute before each measurement, a wrist or ankle cuff was inflated to 200 mmHg constant pressure to occlude hand or ankle circulation during FBF or CBF measurements. Eight plethysmography measurements were averaged for baseline BFvalues before and after exercise training or time-control. The plethysmography output signal was transmitted to the Non-Invasive Vascular Program (NIVP3) calibrated software program (DE Hokanson Inc.) on a computer and expressed as milliliters (ml) per minute per 100 ml of forearm or calf tissue (ml/min/100 ml). Absolute BF was determined by the rate of change of limb circumference (e.g. slope) during the seven-second venous occlusion, which correlates highly to arterial blood inflow into the limb.12, 14 FBF and CBF for one minute is the average of one plethysmographic measurement every 15 seconds for one minute. Forearm and calf vascular conductance was calculated as the ratio of FBF or CBF divided by mean arterial pressure expressed as mL/min/100 mL tissue/mmHg.
Forearm and calf blood flow during reactive hyperemia
Endothelium-dependent FBF and CBF were measured following 5 minutes of upper arm or thigh arterial occlusion during RH of the forearm or calf. Five minute occlusion of the conduit arteries results in the release of vasodilators (primarily nitric oxide) from the microvasculature thereby increasing velocity and blood flow resulting in vasodilation of the conduit artery and is a well established marker of microvascular function.15 Endothelium-dependent vasodilation (EDV) during RH in the forearm has been shown to correlate highly with acetylcholine-induced EDV in patients with essential hypertension and reactive hyperemia is a good non-invasive measurement of EDV of resistance vasculature.16–18 A blood pressure cuff was placed on the upper arm or thigh 5 cm above the anticubital or 10 cm above the popliteal fossa, respectively. After baseline FBF and CBF was confirmed to be stable for 2 minutes and recorded, the cuff was rapidly inflated to 200 mmHg for 5 minutes and then rapidly deflated. FBF and CBF was measured every 15 sec for 3 minutes. Peak FBF and CBF was recorded as the highest BF observed immediately following release of the cuff, and total FBF and CBF was recorded as the area under the time-curve after baseline BF was subtracted.16
Blood collection and biochemical assays
Venipuncture was performed before and after 8 weeks of exercise or time-control period and measurement of antioxidant-capacity and lipid peroxidation were used to determine redox balance. Briefly, venous blood samples were collected in Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) and centrifuged at 3,000 rpm for 15 minutes at 4°C. Plasma was aliquoted and immediately frozen and stored at −80°C. Plasma used for measurement of lipid peroxidation was stored with diethylenetriamine pentaacetic acid (DTPA) and butylated hydroxytoluene (BHT) for a final concentration of 0.01 mM to prevent auto-oxidation during freezing and thawing. Biochemical assays were performed after completion of the study. Universal precautions mandated by the Center for Disease Control and Prevention and the Occupational Safety Health Administration were followed in the handling of human blood.
Antioxidant capacity
Antioxidant capacity was measured in plasma using the Trolox-equivalent antioxidant-capacity (TEAC) technique. The TEAC assay is based on the scavenging of a radical by anitoxidants present in the sample.19, 20 Briefly, plasma was added to a free-radical-generating system whereby a radical cation of 2,2’-azinobis (3-ethylbenzothiazolone-6-sulfonate) (ABTS) reaction is quenched by existing antioxidant fortifications present in the plasma.21, 22 The TEAC assay technique produces a colorimetric solution that is quantified spectrophotometrically and inhibition of the free-radical reaction is proportional to the antioxidant capacity of the plasma. Intra and inter-assay coefficients of variation for the TEAC assay were 3.4% and 7.1, respectively.
Lipid peroxidation
Plasma levels of 8-isoprostane (8-iso-PGF2α) were assessed using a commercially available enzyme-linked immunosorbent assay kit (Assay Designs, Enzo Life Sciences, Inc., Farmingdale, New York, USA) as an index of total oxidative stress. Briefly, 8-iso-PGF2α in plasma competes for binding with 8-isoprostane covalently attached to alkaline phosphatase. The assay plate is then incubated with p-nitrophenyl phosphate and the reaction is stopped with the addition of an acid. The plate is quantified spectrophotometrically and the absorbance is inversely proportional to 8-iso-PGF2α in the plasma sample. Intra-assay and interassay coefficients of variation for the 8-iso-PGF2α assay were 1.3% and 2.2%, respectively.
Statistical analysis
Analysis of variance (ANOVA) was used to analyze baseline group differences between the PHRT, PHET, PHTC and NMTC groups. Continuous variable data are presented as mean ± standard error of the mean (SEM). All data were tested for normal distribution with the Shapiro-Wilk test for normality. An alpha level of p < 0.05 was required for statistical significance. Satterthwaite corrected two sample t-tests of baseline subject characteristics were performed to determine differences between groups at baseline. ANOVA with repeated measures were used to evaluate changes in continuous primary dependent variables; peripheral blood pressure and forearm and calf blood flow, and the secondary dependent variables; TEAC, 8-iso-PGF2α, subject characteristics, and all other data. When a statistically significant group-by-time interaction was observed, within-group comparisons between time point and between groups were analyzed with ANOVA and Tukey post hoc analysis. All statistical analyses were performed using SPSS version 18.0 for Windows (SPSS, Chicago, IL, USA).
RESULTS
All subjects completed the entire exercise treatment or matched time-control period without adverse events. Baseline descriptive and resting hemodynamic characteristics for all participants are presented in Table 1 The prehypertensive and the normotensive groups did not differ at baseline with respect to age, height, weight, body mass index, or resting HR. After randomization the prehypertensive groups did not differ at baseline with respect to resting brachial BP (Table 1). By design, the prehypertensive groups had significantly higher baseline SBP and DBP compared to normotensive time-controls at study entry (Table 1). After 8 weeks of either resistance or endurance training both resting SBP and DBP were significantly reduced in the prehypertensive groups but remained significantly elevated above those of the normotensive time-controls (Table 1). There was no demonstrable main effect of sex on the hemodynamic changes in response to exercise training.
Table 1.
Subject descriptive and resting hemodynamic characteristics before and after exercise training and time control
| PHRT (N=15; 11 male, 4 female) |
PHET (N=13; 9 male, 4 female) |
PHTC (N=15; 10 male, 5 female) |
NMTC (N=15; 9 male, 6 female) |
|||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Age, y | 21.1 ± 0.6 | − | 20.1 ± 0.9 | − | 21.6 ± 0.8 | − | 21.6 ± 0.7 | − |
| Height, cm | 174.8 ± 2.4 | − | 177.0 ± 2.0 | − | 180.1 ± 2.7 | − | 173.3 ± 2.4 | − |
| Weight, kg | 84.2 ± 4.7 | 85.0 ± 4.9 | 86.7 ± 3.6 | 84.1 ± 2.3 | 87.8 ± 4.3 | 88.5 ± 4.6 | 80.9 ± 3.4 | 81.7 ± 3.4 |
| BMI, kg/m2 | 27.4 ± 1.3 | 27.5 ± 1.4 | 28.7 ± 1.4 | 28.3 ± 1.6 | 27.0 ± 1.1 | 27.2 ± 1.2 | 24.5 ± 0.9 | 25.6 ± 0.8 |
| HR, bpm | 63 ± 3.4 | 61 ± 2.6 | 64 ± 4.4 | 56 ± 3.6 | 60 ± 2.8 | 58 ± 2.9 | 57 ± 2.1 | 58 ± 1.9 |
| SBP, mmHg | 130 ± 3* | 121 ± 2*† | 132 ± 3* | 120 ± 2*† | 130 ± 3* | 130 ± 4* | 111 ± 4 | 112 ± 4 |
| DBP, mmHg | 80 ± 2* | 72 ± 2*† | 81 ± 1* | 74 ± 2*† | 81 ± 2* | 81 ± 2* | 67 ± 2 | 68 ± 2 |
| MAP, mmHg | 94 ± 2* | 85 ± 2† | 94 ± 2* | 86 ± 2† | 95 ± 2* | 94 ± 2* | 80 ± 2 | 81 ± 2 |
| fVC, ml/min/100ml/mmHg | 0.029 ± 0.005* | 0.042 ± 0.004† | 0.032 ± 0.003* | 0.039 ± 0.005† | 0.030 ± 0.004* | 0.031 ± 0.003* | 0.039 ± 0.002 | 0.037 ± 0.003 |
| cVC, ml/min/100ml/mmHg | 0.024 ± 0.004* | 0.035 ± 0.003† | 0.026 ± 0.004* | 0.038 ± 0.004† | 0.028 ± 0.003* | 0.029 ± 0.004* | 0.036 ± 0.003 | 0.034 ± 0.004 |
Values are mean±SEM. PHRT indicates prehypertensive resistance trained; PHET, prehypertensive endurance trained; PHTC, prehypertensive time control; NMTC, normotensive time control; BMI, body mass index; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; fVC, forearm vascular conductance; cVC, calf vascular conductance. There were no significant differences between prehypertensive groups at baseline (P>0.05). Significance values are reported from between group and between timepoint repeated measures ANOVA and Tukey post hoc analysis.
P<0.05 versus normotensive control values at same timepoint;
P<0.05 versus pretreatment values.
Exercise improved limb blood flow
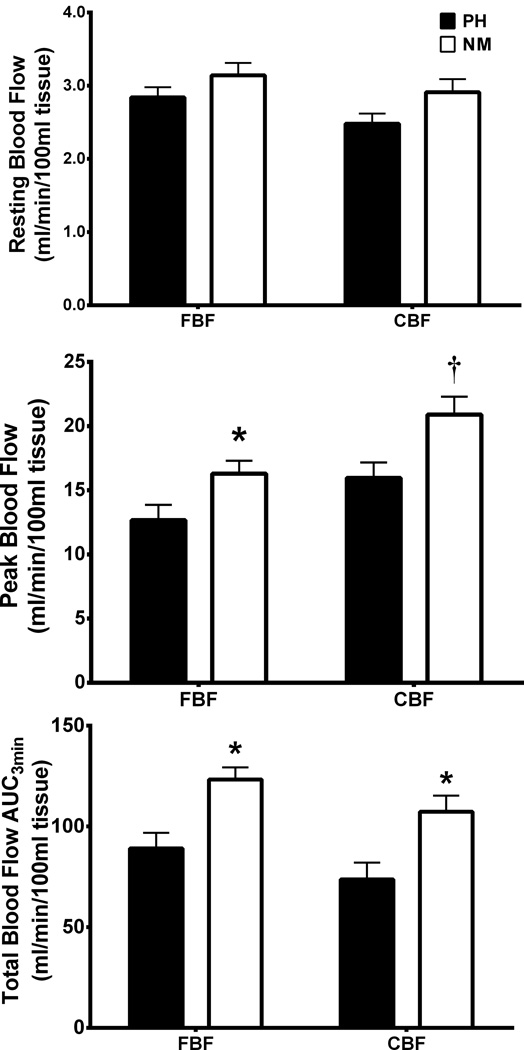
FBF and CBF results at rest and during RH are presented in Table 2 Resting FBF and CBF in the prehypertensive groups did not differ significantly from those of the normotensive groups at baseline (p=0.24). However, peak and total FBF and CBF during RH were significantly reduced in the prehypertensive groups when compared to normotensive time-controls at baseline (Figure 1), (p<0.05). Resistance and endurance exercise training improved resting, peak, and total FBF and CBF in both PHRT and PHET groups (p<0.05) (Figure 2). No significant changes in any FBF or CBF values at rest or during RH were exhibited by either time-control group. Forearm vascular conductance (fVC) and calf vascular conductance (cVC) were improved by resistance and endurance training (Table 1), (p<0.05). Male prehypertensives exhibited increased resting FBF when compared to female prehypertensives (3.65 ± 0.12 versus 2.77 ± 0.09; respectively, p = 0.038). There was no demonstrable main effect of sex on baseline hemodynamics between prehypertensives and normotensives.
Table 2.
Forearm and calf venous occlusion plethysmography before and after exercise training or time control
| PHRT (N=15; 11 male, 4 female) |
PHET (N=13; 9 male, 4 female) |
PHTC (N=15; 10 male, 5 female) |
NMTC (N=15; 9 male, 6 female) |
|||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Resting FBF | 2.70 ± 0.14 | 3.58 ± 0.14*† | 3.01 ± 0.17 | 3.32 ± 0.16† | 2.82 ± 0.12 | 2.94 ± 0.12 | 3.14 ± 0.17 | 3.02 ± 0.15 |
| Peak FBF | 12.7 ± 1.2* | 17.7 ± 1.0*† | 13.2 ± 1.4* | 16.1 ± 1.2† | 12.1 ± 1.0* | 12.5 ± 0.9* | 16.3 ± 1.0 | 16.2 ± 1.0 |
| Total FBF AUC3min | 251 ± 21* | 389 ± 38*† | 264 ± 19* | 346 ± 42† | 287 ± 29* | 269 ± 24* | 370 ± 18 | 362 ± 22 |
| Resting CBF | 2.30 ± 0.14 | 2.99 ± 0.18† | 2.49 ± 0.17 | 3.38 ± 0.23*† | 2.66 ± 0.12 | 2.76 ± 0.16 | 2.91 ± 0.18 | 2.79 ± 0.18 |
| Peak CBF | 14.3 ± 1.2* | 19.9 ± 1.4† | 18.5 ± 1.4 | 27.5 ± 1.7*† | 15.1 ± 1.0* | 14.8 ± 1.2* | 20.9 ± 1.4 | 21.0 ± 1.5 |
| Total CBF AUC3min | 231 ± 23* | 399 ± 58*† | 203 ± 25* | 437 ± 28*† | 229 ± 27* | 219 ± 18* | 322 ± 24 | 341 ± 27 |
Units are ml/min/100ml. Values are mean±SEM. FBF indicates forearm blood flow; AUC, area under the curve; CBF, calf blood flow. There were no significant differences between prehypertensive groups at baseline (P>0.05). Significance values are reported from between group and between timepoint repeated measures ANOVA and Tukey post hoc analysis.
P<0.05 versus normotensive control values at the same timepoint;
P<0.05 versus pretreatment values.
Figure 1.
Absolute values for forearm (FBF) and calf blood flow (CBF) at rest and during reactive hyperemia in prehypertensives vs. normotensives are presented. Between group comparisons at baseline were evaluated using a Satterthwaite correct t-test. *P<0.05, PH vs. NM; **P<0.01, PH vs. NM. Data are expressed as mean ± SEM.
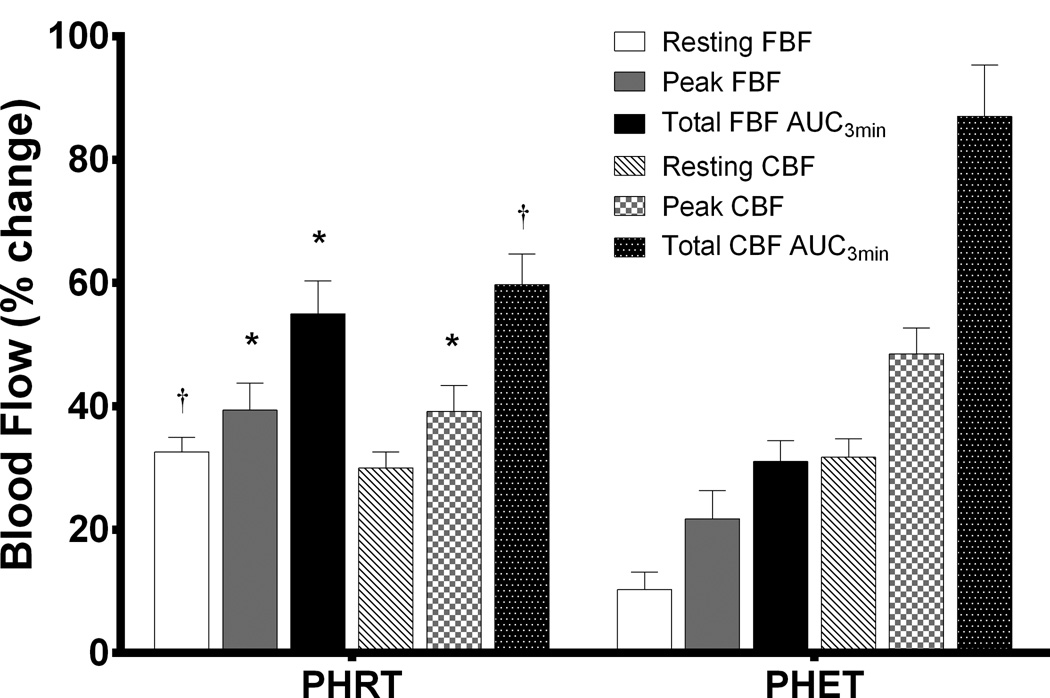
Figure 2.
Absolute values are presented as percent change from baseline for the exercise treated groups. P values are reported from within-group repeated measures ANOVA and Tukey post hoc analysis of between-group and between-timepoint differences in absolute values. Percent changes in FBF and CBF at rest and during reactive hyperemia are shown. *P<0.05, PHRT vs. PHET; †P < 0.01, PHRT vs. PHET, indicates significant differences between exercise trained groups after treatment. Data are expressed as mean ± SEM.
Exercise improved oxidant/antioxidant balance
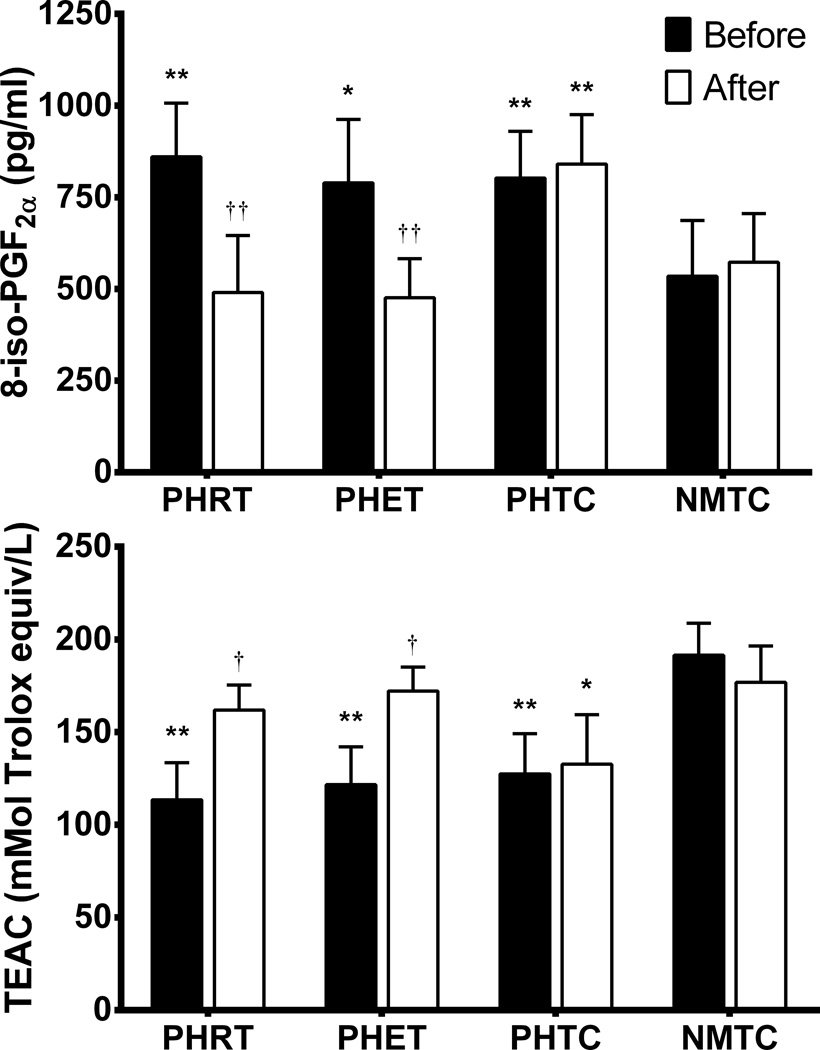
Plasma markers of antioxidant capacity (TEAC) and lipid peroxidation (8-iso-PGF2α) are presented in Figure 3. The PHRT, PHET, and PHTC groups exhibited significantly lower levels of TEAC (113.44±19.98 mMol Trolox equiv/L, 121.61±20.47 mMol Trolox equiv/L, 127.48±21.59 mMol Trolox equiv/L) and elevated levels of 8-iso-PGF2α (860.12±146.92 pg/ml, 789.24±172.8pg/ml, 802.76±127.11 pg/ml) when compared to normotensives at baseline (191.47±17.17 mMol Trolox equiv/L, 534.5±152.1 pg/ml); respectively, (p<0.01). Plasma levels of TEAC were not different from normotensive levels (176.83±19.67 mMol Trolox equiv/L) in the PHRT (161.91±10.55 mMol Trolox equiv/L) and PHET (172.14±10.91 mMol Trolox equiv/L) groups after exercise, respectively; (p = 0.53). Additionally, plasma concentrations of 8-iso-PGF2α were reduced by exercise training in the PHRT and PHET groups (490.31±155.42 pg/ml and 475.8±106.65 pg/ml), respectively, to similar levels exhibited by normotensives (572.9±132.3 pg/ml), (p=0.47).
Figure 3.
Absolute values for 8-isoprostane (8-iso-PGF2α) and Trolox-equivalent antioxidant-capacity (TEAC) are presented. P values are from within group repeated measures ANOVA and Tukey post hoc analysis of between group and between timepoint differences in absolute values. *P<0.05 vs. NMTC at same timepoint; **P<0.01 vs. NMTC at same timepoint; †P<0.05 vs pretreatment values; ††P<0.01 vs. pretreatment values. Data are expressed as mean ± SEM.
DISCUSSION
To the best of our knowledge, this is the first randomized and time-controlled study examining the independent effects of 8 weeks of resistance or endurance exercise training on resistance artery endothelial function and oxidant/antioxidant balance in young prehypertensives. Our findings suggest that microvascular function is impaired in sedentary young prehypertensives and that 8 weeks of moderate intensity resistance or endurance exercise training not only lowers blood pressure but also improves microvascular function and oxidant/antioxidant status.
Resting Hemodynamics
In the present study, prehypertensives did not exhibit differences in resting limb BF compared to those of matched normotensives (Table 1 and Figure 1). However, fVC and cVC were significantly lower at baseline when compared to normotensive values (Table 1). These reductions in vascular conductance exhibited by prehypertensives at baseline are presumably and primarily due to the elevated mean arterial pressures (MAP) in the prehypertensive groups. Interestingly, in a previous study of healthy young (23 ± 2 yrs) normotensive and prehypertensive men, prehypertensives exhibited increased resting FBF (3.4 ± 0.3 vs 2.5 ± 0.3 ml/min/100ml tissue).23 In the present study, male prehypertensives exhibited increased resting FBF when compared to female prehypertensives. The inclusion of both male and female prehypertensives in the current study may be responsible for our results differing from that in male prehypertensives of a similar age. Specific sex comparisons of resistance artery blood flow measurement at rest and during reactive hyperemia between young prehypertensive and normotensives is lacking and ‘normal’ limb blood flow values in these populations have not been defined. Unfortunately, the present study was not statistically powered to observe sex differences between prehypertensives and normotensives. Nevertheless, resting FBF and CBF were increased in both the PHRT and PHET groups after 8 weeks of exercise training. Further, fVC and cVC were increased in both PHRT and PHET groups after exercise due to the concomitant reductions in mean arterial pressure and increases in resting FBF and CBF (Table 1 and Figure 2). These findings are similar to those of a previous study that reported improvements in resting forearm blood flow and vascular conductance after resistance and endurance training in older pre- and stage-1 hypertensives.24
Microvascular Function and Exercise Training
FBF and CBF during reactive hyperemia are indicative of the vasodilatory capacity of resistance vessels and due to factors such as NO bioavailability produced by a healthy intact endothelial lining. Further, reactive hyperemic blood flow has been shown to be an independent predictor of cardiovascular events.25 In the present investigation, young prehypertensives exhibited reduced peak and total limb BF during RH compared to age matched normotensives indicating impaired microvascular function of the resistance arteries and/or microvascular rarefaction (Table 1 and Figure 1). A common characteristic of the hypertensive syndrome are the loss of terminal arterioles, small venules, and/or capillaries and endothelial microRNAs are potential therapeutic targets to correct capillary rarefaction and defective angiogenesis in hypertensives.26, 27 In fact, endurance training normalizes levels of endothelial micorRNAs, reduces blood pressure, improves vascularization, enhances angiogenic factors, and decreases apoptotic pathways.28 The beneficial effects of endurance training on BP reduction and endothelial function in hypertensives are well established; however, although recommended as a compliment to an endurance training regimen, the effects of resistance training are less defined. The results of a few studies targeting the effects of resistance and endurance exercise for BP control in prehypertensives have resulted in fairly promising findings.5, 24, 29, 30 To date, resistance and endurance training have been reported to be effective in reducing peripheral and central BP, improving conduit artery endothelial function, and increasing VC in pre- and stage-1 hypertensives.5, 24, 29, 30 Indeed, in the present study, peak and total blood FBF and CBF were improved in both the PHRT and PHET groups after exercise. The increased hyperemic BF suggests increased capillary or arteriolar proliferation and enhanced resistance vessel endothelial function. These findings are similar to reports by Collier and colleagues who investigated the effects of short term (4 weeks) resistance and endurance exercise training on FBF at rest and during RH in slightly older (age: 48.2 ± 1.3 yrs) pre- and stage-1 hypertensives.24
In the current study, we observed a more robust increase in resting, peak, and total forearm BF in PHRT groups (~33%, 39%, and 55%, respectively) when compared to PHET groups (~10%, 22%, and 31%, respectively) (Figure 2). Similarly, in the previous study, Collier and colleagues report that forearm resting, peak, and total blood flow were increased after 4 weeks of resistance exercise to a greater extent than those in the endurance trained (treadmill) group.24 Additionally, in the present study we report that endurance exercise training improved peak and total CBF (~49% and 87%) to a greater extent than resistance training (~39% and 60%), while resting CBF, although increased by both exercise regimens, resulted in similar improvements (~30 and 32%) (Figure 2).
It is reasonable to conclude that the increases in shear stress are in response and specific to the vasculature targeted by RT versus ET and may be responsible for these differences. For example, the PHRT group engaged the vasculature of the upper body and arms to a greater extent than the PHET group. Further, the PHET group engaged the lower limbs greater than the PHRT group during each training session. Indeed, Tinken and colleagues observed improvements in brachial artery endothelial function in normotensive subjects following 6 weeks of forearm resistance exercise but observed no change in the exercising forearm where acute flow during exercise was abolished. 31 They concluded that exercise-induced increases in shear stress were responsible for improved endothelial function and vascular remodeling.31 Additionally, Bank et al. report increased peak forearm reactive hyperemic blood flow and vasodilation in response to the endothelial dependent nitric oxide agonist acetylcholine in healthy subjects following forearm resistance exercise.32 These results are bolstered by the work of Heffernan and colleagues who reported significant increases in forearm blood flow and reductions in forearm vascular resistance following 6 weeks of resistance training in young normotensives.33 Resistance training not only improved microvascular function and limb BF, but the improvements were maintained following 4 weeks of detraining.33 Together, these findings suggest that the improvement in endothelial function due to exercise are limb specific, may be a local microvascular phenomenon, and the beneficial effects of exercise training may not be transient.
Oxidant/Antioxidant Balance and Exercise
The present study demonstrates that young prehypertensives appear to have a perturbation in oxidant/antioxidant status demonstrated by increased plasma levels of lipid peroxidation and reduced antioxidant capacity compared to matched normotensives.
F2-isoprostanes are a class of prostaglandin-like compounds that are produced by free radical-mediated lipid peroxidation of arachidonic acid, independent of cyclooxygenase, and their measurement is currently the best humoral assay to determine lipid peroxidation in biological samples. Importantly, limited amounts of isoprostanes can be absorbed and diet has a negligible effect on plasma levels of these compounds.34 One of these isoprostanes, 8-iso-PGF2α, is viewed as the most valid plasma marker to assess systemic oxidative stress and is a potent vasoconstrictor and an independent risk factor associated with coronary artery disease.35 In the present study we utilized plasma measures of 8-iso-PGF2α and TEAC as an index of total oxidative stress and total antioxidant status, respectively.36 Plasma levels of 8-iso-PGF2α in our groups of young sedentary prehypertensives were higher by ~21% when compared to our normotensive time-control groups (Figure 3). This finding is similar to previous reports by Sathiyapriya et al. who compared slightly older prehypertensives and normotensives (36±9 and 39±8 years), respectively.6 These authors report significant increases in oxidative stress in prehypertensives when measuring malondialdehyde (MDA), an end product of polyunsaturated fatty acid peroxidation, protein carbonyl, a biomarker of protein oxidation in whole blood, and erythrocyte glutathione peroxidase activity.6 Further, they determined that whole blood reduced glutathione (GSH) and erythrocyte catalase activities were significantly reduced among prehypertensives, suggesting that the antioxidant defenses in prehypertensives are compromised and prehypertensives may exhibit an imbalance in the oxidant/antioxidant ratio.6 Their results suggest that oxidative stress is present in prehypertensives and free radical damage is reflected systemically. Similarly, we observed significantly reduced levels of antioxidant capacity in young prehypertensives (~46%) when compared to normotensive age and activity matched controls. Our findings suggest that an oxidant/antioxidant imbalance may be present in prehypertensives at younger ages than previously reported.
In the present study, 8 weeks of resistance or endurance training effectively reduced levels of 8-iso-PGF2α the PHRT and PHET groups by 43% and 40%, respectively, to concentrations that were not different than those of the normotensives. The mechanisms responsible for decreased levels of 8-iso-PGF2α exhibited by the prehypertensive groups after exercise remains unclear. However, NO has been shown to have antioxidant properties and we previously observed a 19% and 23% increase in plasma nitrate/nitrite (NOx) levels after resistance and endurance exercise training in the same cohort of prehypertensives, respectively.5 Additionally, after both resistance and endurance training antioxidant capacity increased in the PHRT and PHET groups by ~43% and 42%, respectively (Figure 2). Oxidant/Antioxidant balance in plasma is sensitive to a host of redox sensitive apparatus of both the cellular and extra cellular compartments. Due to the lack of direct enzymatic analysis, we can only conclude that there appears to be a disparity in redox balance in young prehypertensives and this difference can be ameliorated by either resistance or endurance training.
Experimental Considerations and Future Directions
Indices of SNS activity were not measured in the present study and, consequently, the effects of SNS modulation in response to the exercise training cannot be ruled out. Previous evidence suggests that aerobic and resistance training similarly reduce resting blood pressure and improve resting forearm blood flow in pre- and stage-1 hypertensives but through differing mechanisms.24 That is, aerobic training improves the autonomic nervous system by increasing vagal tone and reducing sympathovagal balance whereas resistance training decreases sympathetic modulation of peripheral vessels while decreasing parasympathetic modulation of the heart.30 Schwartz and colleagues reported that prehypertension elicits a more dramatic pressor response to mental stress when compared to normotensive subjects and the augmented pressor response appears to be related to blunted forearm resistance artery endothelium dependent vasodilation and not augmented muscle sympathetic nerve activity (MSNA) in young prehypertensives.23 Moreover, Ray et al. demonstrated that isometric handgrip training improves endothelial function and decreases resting arterial blood pressure without altering MSNA or resting heart rate in normotensive subjects with ‘normal’ central sympathetic outflow.37 Important to the current study, resistance training in young subjects does not appear to alter MSNA.38 Additionally, the infusion of acetylcholine, serotonin, and/or bradykinin through brachial artery catheterization was not performed during the measurement of blood flow in the present study and the use hyperemic blood flow as an index of endothelial function was limited.
Conclusions
In conclusion, this study demonstrates that short term (8weeks) resistance or endurance training are independently efficacious in improving peak and total BF, fVC and cVC, and restoring oxidant/antioxidant balance in previously sedentary young prehypertensives. Given that forearm and calf BF measurements are indicative of the vasodilatory capacity of the resistance vessels, and are due to factors such as NO bioavailability, it is possible that both resistance and endurance training upregulated NO signaling. Moreover, concurrent improvements in oxidant/antioxidant balance likely contribute to improvements in NO bioavailability and resultant increases in limb BF at rest and during reactive hyperemia.
What is known about this topic
Prehypertensives are 11 times more likely to develop essential hypertension than normotensives.
Prehypertensives exhibit marked conduit artery endothelial dysfunction, a risk factor for future cardiovascular morbidity and mortality.
Prehypertension is associated with perturbation of oxidant/antioxidant status and reduced nitric oxide bioavailability.
Persistent prehypertension accelerates the progression of arterial stiffness and development of hypertension.
Prehypertension is not a disease category, prehypertensives are not candidates for pharmacotherapy, and exercise training is recommended as the primary lifestyle modification to treat prehypertension.
What this study adds
Conduit artery endothelial function persists to the resistance arteries in young prehypertensives.
Resistance and endurance exercise training improve resistance artery endothelial function in young prehypertensives.
Resistance and endurance exercise training are effective in improving oxidant/antioxidant status in young prehypertensives by reducing levels of oxidants and increasing antioxidant capacity in young prehypertensives.
Acknowledgements
All authors participated in the design and interpretation of the study and review of the manuscript. DTB and DPC conducted the experiments and analyzed the data. DTB, DPC, and JSM trained the subjects, supervised the trainers, and collected data for the study. DTB, RWB, DPC and JSM wrote and revised the manuscript. The authors also thank their subjects for their time and effort. This work was supported, in part, by a National Institutes of Health predoctoral training grant (NIH 5-T32-HL083810-04) awarded by the University of Florida Hypertension Center.
Footnotes
Disclosures/Conflicts of Interest: None
References
- 1.Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med. 2004;164:2113–2118. doi: 10.1001/archinte.164.19.2113. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV. Prehypertension revisited. Hypertension. 2006;48:812–814. doi: 10.1161/01.HYP.0000241684.29799.14. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 4.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvath T, et al. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 5.Beck DT, Casey DP, Martin JS, Emerson BD, Braith RW. Exercise training improves endothelial function in young prehypertensives. Exp Biol Med (Maywood) 2013;238:433–441. doi: 10.1177/1535370213477600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sathiyapriya V, Nandeesha H, Bobby Z, Selvaraj N, Pavithran P. Perturbation of oxidant-antioxidant status in non-obese prehypertensive male subjects. J Hum Hypertens. 2007;21:176–178. doi: 10.1038/sj.jhh.1002121. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. British journal of clinical pharmacology. 2001;52:631–646. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010;235:111–118. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson GC, Whelan RF. Reactive hyperaemia in the human forearm. Clinical science. 1955;14:197–211. [PubMed] [Google Scholar]
- 12.Hokanson DE, Sumner DS, Strandness DE., Jr An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans Biomed Eng. 1975;22:25–29. doi: 10.1109/tbme.1975.324535. [DOI] [PubMed] [Google Scholar]
- 13.Patterson GC, Whelan RF. The measurement of blood flow during reactive hyperaemia in man. The Journal of physiology. 1955;127:13–14P. [PubMed] [Google Scholar]
- 14.Greenfield AD, Whitney RJ, Mowbray JF. Methods for the investigation of peripheral blood flow. Br Med Bull. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- 15.Philpott A, Anderson TJ. Reactive hyperemia and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2007;27:2065–2067. doi: 10.1161/ATVBAHA.107.149740. [DOI] [PubMed] [Google Scholar]
- 16.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270:H1435–H1440. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 17.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Kajiyama G, Oshima T. A noninvasive measurement of reactive hyperemia that can be used to assess resistance artery endothelial function in humans. Am J Cardiol. 2001;87:121–125. doi: 10.1016/s0002-9149(00)01288-1. A129. [DOI] [PubMed] [Google Scholar]
- 18.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 19.Hudson MB, Hosick PA, McCaulley GO, Schrieber L, Wrieden J, McAnulty SR, et al. The effect of resistance exercise on humoral markers of oxidative stress. Med Sci Sports Exerc. 2008;40:542–548. doi: 10.1249/MSS.0b013e31815daf89. [DOI] [PubMed] [Google Scholar]
- 20.McAnulty SR, Hosick PA, McAnulty LS, Quindry JC, Still L, Hudson MB, et al. Effect of pharmacological lowering of plasma urate on exercise-induced oxidative stress. Appl Physiol Nutr Metab. 2007;32:1148–1155. doi: 10.1139/H07-131. [DOI] [PubMed] [Google Scholar]
- 21.Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998;68:1081–1087. doi: 10.1093/ajcn/68.5.1081. [DOI] [PubMed] [Google Scholar]
- 22.Cao G, Prior RL. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem. 1998;44:1309–1315. [PubMed] [Google Scholar]
- 23.Schwartz CE, Durocher JJ, Carter JR. Neurovascular responses to mental stress in prehypertensive humans. J Appl Physiol. 2011;110:76–82. doi: 10.1152/japplphysiol.00912.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collier SR, Kanaley JA, Carhart R, Jr, Frechette V, Tobin MM, Hall AK, et al. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens. 2008;22:678–686. doi: 10.1038/jhh.2008.36. [DOI] [PubMed] [Google Scholar]
- 25.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, et al. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 26.Murfee WL, Schmid-Schonbein GW. Chapter 12 Structure of microvascular networks in genetic hypertension. Methods Enzymol. 2008;444:271–284. doi: 10.1016/S0076-6879(08)02812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batkai S, Thum T. MicroRNAs in hypertension: mechanisms and therapeutic targets. Curr Hypertens Rep. 2012;14:79–87. doi: 10.1007/s11906-011-0235-6. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes T, Magalhaes FC, Roque FR, Phillips MI, Oliveira EM. Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: role of microRNAs-16, −21, and −126. Hypertension. 2012;59:513–520. doi: 10.1161/HYPERTENSIONAHA.111.185801. [DOI] [PubMed] [Google Scholar]
- 29.Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensives. American Journal of Hypertension. 2013;26 doi: 10.1093/ajh/hpt080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collier SR, Kanaley JA, Carhart R, Jr, Frechette V, Tobin MM, Bennett N, et al. Cardiac autonomic function and baroreflex changes following 4 weeks of resistance versus aerobic training in individuals with pre-hypertension. Acta Physiol (Oxf) 2009;195:339–348. doi: 10.1111/j.1748-1716.2008.01897.x. [DOI] [PubMed] [Google Scholar]
- 31.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 32.Bank AJ, Shammas RA, Mullen K, Chuang PP. Effects of short-term forearm exercise training on resistance vessel endothelial function in normal subjects and patients with heart failure. J Card Fail. 1998;4:193–201. doi: 10.1016/s1071-9164(98)80006-7. [DOI] [PubMed] [Google Scholar]
- 33.Heffernan KS, Fahs CA, Iwamoto GA, Jae SY, Wilund KR, Woods JA, et al. Resistance exercise training reduces central blood pressure and improves microvascular function in African American and white men. Atherosclerosis. 2009;207:220–226. doi: 10.1016/j.atherosclerosis.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 34.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, Pan J, Wang L, Zhu H, Yu R, Zou Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atheroscler Suppl. 2006;184:425–430. doi: 10.1016/j.atherosclerosis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Wang CC, Chu CY, Chu KO, Choy KW, Khaw KS, Rogers MS, et al. Trolox-equivalent antioxidant capacity assay versus oxygen radical absorbance capacity assay in plasma. Clin Chem. 2004;50:952–954. doi: 10.1373/clinchem.2004.031526. [DOI] [PubMed] [Google Scholar]
- 37.Ray CA, Carrasco DI. Isometric handgrip training reduces arterial pressure at rest without changes in sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2000;279:H245–H249. doi: 10.1152/ajpheart.2000.279.1.H245. [DOI] [PubMed] [Google Scholar]
- 38.Carter JR, Ray CA, Downs EM, Cooke WH. Strength training reduces arterial blood pressure but not sympathetic neural activity in young normotensive subjects. J Appl Physiol. 2003;94:2212–2216. doi: 10.1152/japplphysiol.01109.2002. [DOI] [PubMed] [Google Scholar]