Cerebral amyloid angiopathy (CAA) is characterized by beta-amyloid deposits in small- and medium-sized cerebral arteries and overlying leptomeninges, leading to impaired elasticity, subsequent ischaemia and haemorrhagic stroke. Although these lobar haemorrhages rarely occur before age 55 years [1], increased life expectancy and incidence of hypertension have made CAA haemorrhages increasingly common [1,2]. Autopsy studies demonstrate that the prevalence of CAA far exceeds observed lobar haemorrhages. CAA is found in routine post mortem studies in almost 50% of individuals over age 80 years [3].
Susceptibility-weighted imaging (SWI) is an magnetic resonance image (MRI) sequence that maximizes sensitivity to magnetic susceptibility effects [4] and is about four times more sensitive than standard gradient echo (GRE) techniques in detecting cerebral microhaemorrhages [5]. We describe an unusual patient with CAA who presented with rapid, progressive mental deterioration and symmetrically diffuse cerebral oedema, in which only SWI detected numerous microhaemorrhages confirmed by autopsy.
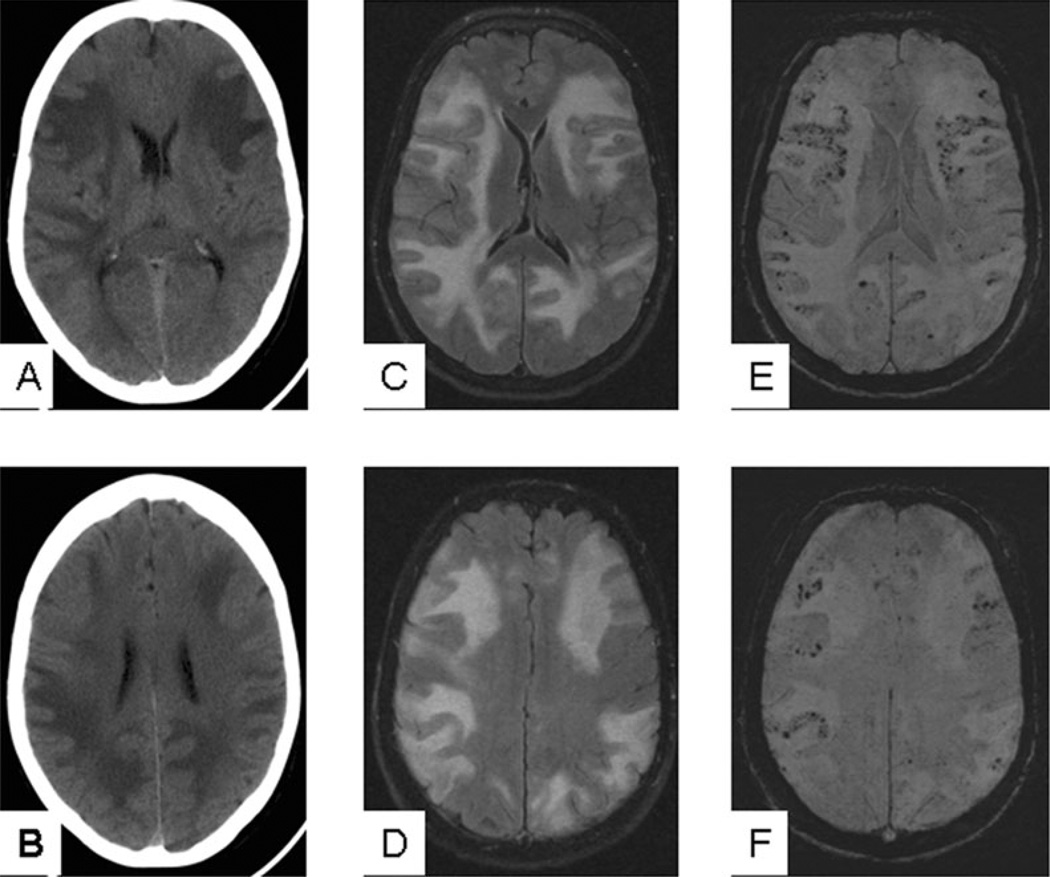
A 73-year-old woman presented to our emergency department with lethargy, confusion and inappropriate behaviour that progressed over 1 week. On admission, she was afebrile and stable. She was lethargic, disorientated to place and time, but followed simple commands. Cranial nerves were intact. She had no focal motor or sensory deficits. Head CT showed diffuse, bilateral hypodense areas in the cerebral white matter (Figure 1). MRI demonstrated bilateral increased signal intensity on fluid-attenuated inversion recovery and T2 sequences in the cortex and white matter of the hemispheres, without diffusion restriction or enhancement (Figure 1). SWI sequence (Figure 1) showed numerous, bilateral microhaemorrhages at the grey–white junction, congruent to regions of oedema. Imaging findings were suggestive of encephalitis from viral or granulomatous aetiology, or possibly of posterior reversible encephalopathy syndrome. A magnetic resonance angiography of the head and neck (not shown) revealed normal arterial vasculature.
Figure 1.
Head CT and MRI performed at presentation. (A,B) Head CT shows diffuse bilateral cerebral oedema mostly involving the white matter. (C,D) FLAIR sequence confirms the diffuse white matter oedema seen on head CT, and also shows some involvement of the cortical grey matter. (E,F) MR SWI sequence at the corresponding level shows diffuse microhaemorrhages at the grey–white matter junction that were not seen on either head CT or other MRI sequences. CT, computerized tomography; FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance image; SWI, susceptibility-weighted imageing.
Initial clinical diagnoses included cerebritis, metabolic encephalopathy, demyelinating disorder or vasculitis. Haematological evaluation was negative and included a complete blood count, basic metabolic panel, liver function tests, cardiac enzymes, sedimentation rate and coagulopathy panel. Lumbar puncture ruled out infectious, inflammatory, autoimmune and demyelinating processes. Cerebrospinal fluid cultures were negative.
The patient was placed on steroids, acyclovir and antibiotics prophylactically without significant clinical improvement, although her condition remained stable. However, 2 weeks after admission, she experienced sudden decompensation of mental status and developed decerebrate posturing with dilated pupils. Head CT demonstrated interval worsening of diffuse oedema and development of right frontal and fourth ventricular haemorrhage. Small haematomas were also seen in the right temporal lobe. According to the wishes of the family, further medical treatment was withdrawn.
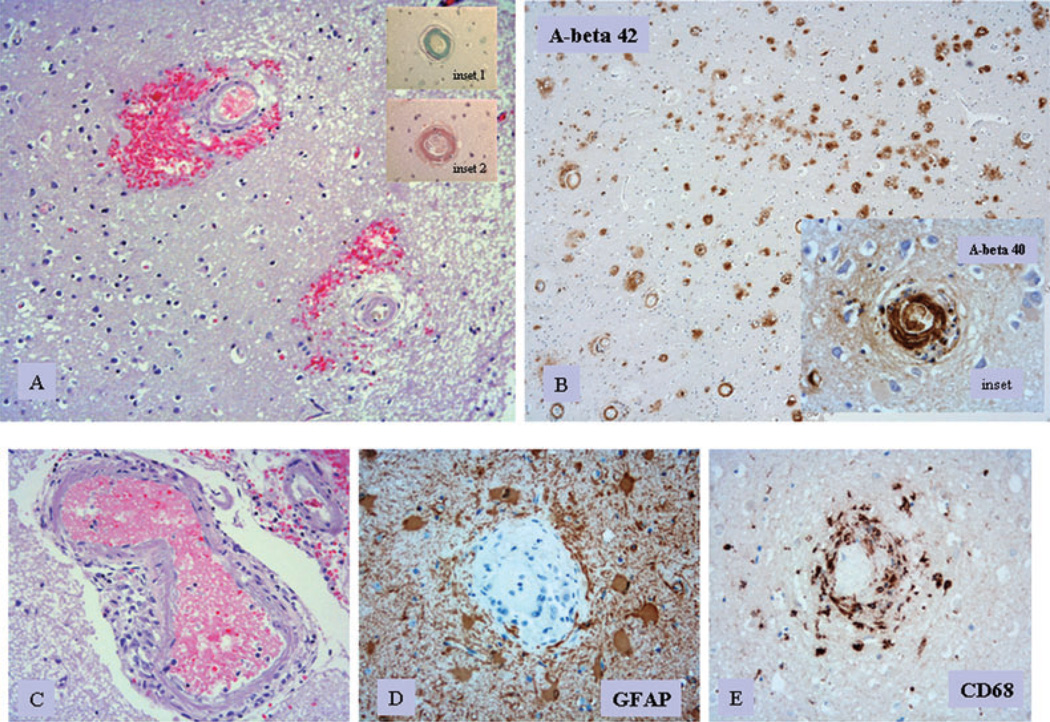
Autopsy revealed a large right frontal lobar haematoma (Figure 2) and diffuse bilateral perivascular petechial haemorrhages. Right cerebral oedema was associated with transtentorial herniation resulting in brainstem compression. Microscopic examination showed extensive CAA in the vessels of the cerebral cortex and leptomeninges, with microaneurysm formation and focal granulomatous inflammation (Figure 2A). Abundant A beta amyloid in capillaries, arterioles and small arteries was demonstrated on immunostaining using anti-A beta antibodies (Figure 2B). Sulphated mucin and Congo red were also positive for amyloid (Figure 2B).
Figure 2.
Post mortem pathology of cerebral tissue. (A) Perivascular acute haemorrhage and oedema surrounding cerebral vessels with amyloid deposits in their walls (H&E stain, ×10 objective; inset 1: sulphated alcian blue stain highlighting amyloid in vessel wall, ×20 objective; inset 2: Congo red stain highlighting amyloid in vessel wall, ×20 objective). (B) Numerous cerebral vessels showing amyloid angiopathy; immunostain for anti-A beta 1–42 (rabbit, Chemicon, Temecula, CA, USA; primary dilution 1:500, ×4 objective). Inset shows immunostaining by anti-A beta 1–40 (rabbit, primary dilution 1:500; ×10 objective). (C) Meningeal vessel with amyloid angiopathy surrounded by mononuclear inflammatory cell infiltrate (H&E stain; ×10 objective). (D) Cerebral vessel with amyloid angiopathy surrounded by reactive astrocytes (GFAP; Dako, Hamburg, Germany; primary dilution 1:500; ×20 objective). (E) Cerebral vessel with amyloid angiopathy; prominent macrophage infiltrates are noted within the vessel wall (CD68; Dako, primary dilution 1:500; ×20 objective).
Given the increasing prevalence of CAA, understanding the radiological variations may improve diagnosis. Although this may merely reflect an overall increase in life expectancy and an associated increased incidence of dementia, it may also reflect greater access to advanced neuroimaging tools. Greenberg et al. found a 38% rate of asymptomatic or minimally symptomatic microhaemorrhages on follow-up MRI GRE sequence in patients with history of lobar haemorrhage thought to be from CAA[6]. Ellis et al. reported that 40% of patients with CAA-related intracranial haemorrhage had concurrent diagnosis of dementia, and over 80% of patients with Alzheimer’s disease have CAA [7].
Wong et al. described a patient with word-finding difficulty and fatigue that had similar oedema patterns on CT and MRI [8]. The patient underwent biopsy that showed vasculitis and severe CAA. Radiological and clinical findings improved with intravenous methylprednisolone and methotrexate. The authors speculated that the radiological findings were probably due to a combination of ischaemia, microhaemorrhage (not evidenced by biopsy or radiological findings) and oedema. Unfortunately, these authors did not obtain an MR GRE or SWI sequence to assess for microhaemorrhages. The present case shares similarities with this group’s findings in that there was evidence of associated inflammatory response around affected vessels at brain autopsy. A primary vasculitis was not, however, seen in our patient.
Haacke et al. described a 70-year-old man with recurrent haemorrhages whose MRI showed multiple microhaemorrhages on both T2 GRE and SWI sequences [9]. The unusual feature of our case is the presentation with extensive diffuse white matter oedema with obvious ventricular compression and sulcal effacement, which their patient did not have. In addition, our patient presented with an acute rapidly progressive neurological deterioration, probably secondary to diffuse leukoencephalopathy.
CAA presenting with diffuse leukoencephalopathy has been previously described [10]. Nevertheless, the combination of leukoencephalopathy and microhaemorrhages make our case unique. Moreover, histopathology clearly demonstrated amyloid deposition as well as perivascular microhaemorrhages (Figure 2A).
There is speculation that CAA can cause an arteriopathy that leads to progressive white matter lesions and microhaemorrhages, contributing to altered mental status [11]. Recent studies associate deep intracerebral haemorrhages with increased expression of matrix metalloproteinase-9, a protein involved in the disruption of the blood brain barrier and development of perihaematomal oedema. Nevertheless, this protein fails to show the same expression in lobar haemorrhages [12]. The diffuse, massive bilateral hemispheric oedema we observed is somewhat unique to this case. A similar presentation of CAA was not found on review of the literature. We do not have a clear aetiology of the causal mechanism producing this cerebral oedema, but speculate that amyloid-associated loss of integrity of the vessel wall and secondary endothelial damage caused breakdown of the blood brain barrier and subsequent vasculitis.
This unique case of CAA presented with massive bilateral cerebral oedema and microhaemorrhages, which were only detected by SWI. There may be an expanding role for SWI in the detection and possible diagnosis of CAA.
Acknowledgements
HV Vinters supported in part by P50 AG16570, P01 AG 12435, and the Daljit S. & Elaine Sarkaria Chair in Diagnostic Medicine at UCLA.
References
- 1.Smith EE, Eichler F. Cerebral amyloid angiopathy and lobar intracerebral haemorrhage. Arch Neurol. 2006;63:148–151. doi: 10.1001/archneur.63.1.148. [DOI] [PubMed] [Google Scholar]
- 2.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 3.Vinters HV, Gilbert GG. CAA: incidence and complications in the ageing brain II. The distinction of amyloid vascular changes. Stroke. 1983;14:924–928. doi: 10.1161/01.str.14.6.924. [DOI] [PubMed] [Google Scholar]
- 4.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–277. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- 5.Tong KA, Ashwal S, Holshouser BA, Shutter LA, Herigault G, Haacke EM, Kido DK. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. 2003;227:332–339. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg SM, O’Donnell HC, Schaefer PW, Kraft E. MRI detection of new haemorrhages: potential marker of progression in cerebral amyloid angiopathy. Neurology. 1999;53:1135–1138. doi: 10.1212/wnl.53.5.1135. [DOI] [PubMed] [Google Scholar]
- 7.Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, Part XV. Neurology. 1996;46:1592–1596. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- 8.Wong SH, Robbins PD, Knuckey NW, Kermode AG. Cerebral amyloid angiopathy presenting with vasculitic pathology. J Clin Neurosci. 2006;13:291–294. doi: 10.1016/j.jocn.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Haacke EM, DelProposto ZS, Chaturvedi S, Sehgal V, Tenzer M, Neelavalli J, Kido D. Imaging cerebral amyloid angiopathy with Susceptibility-Weighted Imaging. AJNR Am J Neuroradiol. 2007;28:316–317. [PMC free article] [PubMed] [Google Scholar]
- 10.Loes DJ, Biller J, Yuh WT, Hart MN, Hart MN, Godersky JC, Adams HP, Jr, Keefauver SP, Tranel D. Leukoencephalopathy in cerebral amyloid angiopathy: MR imaging in four cases. AJNR Am J Neuroradiol. 1990;11:485–488. [PMC free article] [PubMed] [Google Scholar]
- 11.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, Greenberg SM. White matter lesions, cognition, and recurrent haemorrhage in lobar intracerebral haemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 12.Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, Alvarez-Sabin J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral haemorrhage. J Neurosurg. 2003;99:65–70. doi: 10.3171/jns.2003.99.1.0065. [DOI] [PubMed] [Google Scholar]