Abstract
Background
Adipose tissue represents a practical source of autologous mesenchymal stromal cells (MSC) and vascular-endothelial progenitor cells, available for regenerative therapy without in vitro expansion. One of the problems confronting the therapeutic application of such cells is how to immobilize them at the wound site. Here, we evaluated in vitro the growth and differentiation of human adipose stromal vascular fraction (SVF) cells after delivery using a fibrin spray system.
Methods
SVF cells were harvested from four human adult patients undergoing elective abdominoplasty using the LipiVage™ system. After collagenase digestion, mesenchymal and endothelial progenitor cells (pericytes, supra-adventitial stromal cells, endothelial progenitors) were quantified by flow cytometry before culture. SVF cells were applied to culture vessels using the Tisseel™ fibrin spray system. SVF cell growth and differentiation was documented by immunofluorescence staining and photomicrography.
Results
SVF cells remained viable following application and were expanded up to three weeks, when they reached confluence and adipogenic differentiation. Under angiogenic conditions, SVF cells formed endothelial (vWF+, CD31+ and CD34+) tubules surrounded by CD146+ and α-SMA+ perivascular/stromal cells.
Discussion
Human adipose tissue is a rich source of autologous stem cells, which are readily available for regenerative applications such as wound healing, without in vitro expansion. Our results indicate that mesenchymal and endothelial progenitor cells, prepared in a closed system from unpassaged lipoaspirate samples, retain their growth and differentiation capacity when applied and immobilized on a substrate using a clinically approved fibrin sealant spray system.
Keywords: Adipose-derived Stem Cells, Adipose Stromal Vascular Cells, Regenerative Therapy, Wound Healing, Fibrin sealant, Angiogenesis, Perivascular cells, Pericytes, Supra Adventitial-Adipose Stromal Cells, Multipotent mesenchymal stromal cells
Introduction
Wound healing is often hampered by physiologic and mechanical factors such as local infection, hypoxia, and trauma, or systemic problems such as diabetes mellitus. Fibrin plays a major role in the earliest phase of wound healing, while the proliferative is particularly dependent on the cells which mediate granulation and angiogenesis. Cultured autologous bone marrow derived multipotent mesenchymal stromal cells (MSC) suspended in fibrin glue and applied as an aerosol are currently undergoing phase I clinical trial for patients with acute wounds from skin cancer and non-healing lower extremity wounds (1), with encouraging results. The stromal vascular fraction (SVF) of human adipose tissue is heterogeneous and a ready source of autologous stem cells, including pericytes, and MSC-like supra-adventitial adipose stromal cells (SA-ASC) (2-4). We have shown that a small proportion of SVF pericytes are CD34+ (2), and recently demonstrated that they are highly proliferative (9.4 ± 1.9% DNA >2N, mean ± SEM), suggesting a transit amplifying population intermediate between pericytes and the CD34+ SA-ASC (4). We demonstrate here that adipose SVF cells, isolated from human lipoaspirate using a closed system device can give rise to mature adipocytes and small-vessel-like endothelial tubules when deposited onto the surface of culture vessels in a fibrin spray.
Materials And Methods
Adipose tissue collection and SVF preparation
Subcutaneous adipose tissue was harvested from human adult female patients undergoing abdominoplasty at Magee Womens Hospital, Pittsburgh, PA. A clinical collection using the LipiVage® Fat Harvest, Wash and Transfer system (Genesis Biosystems, Lewisville, TX) was simulated by aspirating 60 mL of adipose tissue in the sterile field immediately following excision. A schematic of the LipiVage transfer system is shown in Figure 1. All samples were waste materials collected as a byproduct of surgery. De-identified samples were collected under an IRB approved exemption (number 0511186, University of Pittsburgh IRB).
Figure 1.
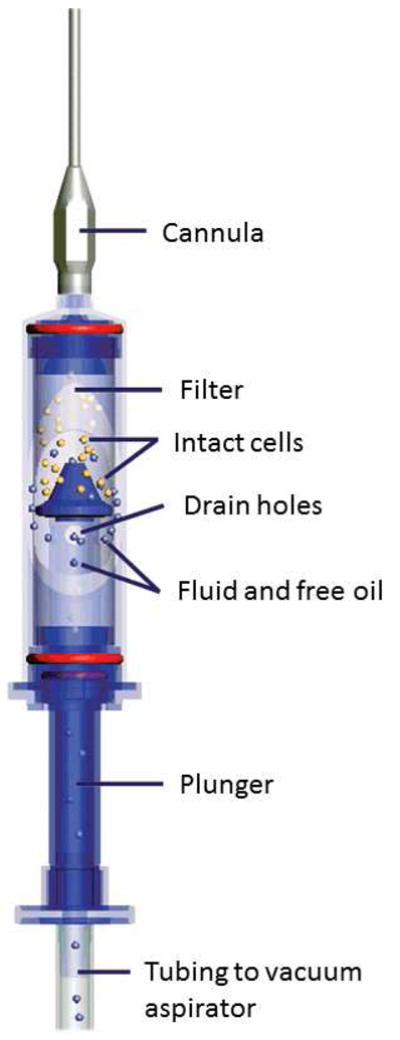
Schematic of the LipiVage Device. The LipiVage device was designed to harvest small volumes of autologous adipose tissue for cosmetic surgical applications. The device aspirates fat at low vacuum into a sterile chamber. Once inside, the fat cells are concentrated by filtration. Fluids and free oil are drawn into a waste canister. Diagram courtesy of Genesis Biosystems
Cell isolation was performed according to the manufacturer's recommendations, using the Tissue Genesis Isolation System (TGI, Tissue Genesis, Honolulu HI), a closed system automated device designed for the clinical setting. Lipoaspirate suspension was delivered to the UPMC HSCLab, a GMP-compliant facility, in 60 cc Luer-lock syringes at room temperature. After performing the automated self-test, the supplied tubing cartridge and an empty 60 cc transfer syringe were loaded and the waste bag hung. The medium bag was hung, spiked, and one liter of separation medium, consisting of 0.5% human serum albumin in 0.9% sterile saline was transferred to the medium bag. The Adipase™ solution (Tissue Genesis), a proprietary enzyme mixture containing collagenase and dispase, was rehydrated with sterile water, aspirated into a 60 cc syringe and loaded, after which the disposable centrifuge bowl and associated tubing was installed. When the set-up was complete, the 60 cc syringe containing the lipoaspirate was injected into the centrifuge bowl port and the processing commenced. The stromal vascular fraction (approximately 35 mL) was recovered in the transfer syringe. Processing took one hour and 25 minutes.
Cell recovery was measured using an AcT10 hematology analyzer (Beckman-Coulter, Miami FL) and confirmed with a manual hemocytometer. Viability was determined by Trypan blue exclusion and all preparations were documented with a Giemsa-Wright stained cytocentrifuge preparation.
Flow cytometry analysis
Freshly harvested fat SVF cells were kept on ice during subsequent steps and stained for analytical flow cytometry as previously described (2). After centrifugation (200 g, 7 min), all supernatant was discarded. The cell pellet was preincubated with 5 μL neat decomplemented mouse serum (Sigma) to minimize non-specific antibody binding. Cells were simultaneously stained with monoclonal mouse anti-human fluorochrome-conjugated antibodies (2μL each, CD105-FITC (Fitzgerald Industries 61R-CD105dHUFT), CD73-PE (BD Pharmingen 550257), CD146-biotin (Miltenyi Biotec 130-092-852), ECD-steptavidin (Beckman Coulter IM3326), Lineage PE-Cy5 (CD14 (Beckman Coulter IM2640U), Glycophorin-A (BD Biosciences 559944), CD33 (Beckman Coulter IM2647U), CD90-APC (BD Pharmingen 559869) and CD31-PE-Cy7 (Biolegend 303117), and CD45-APC-Cy7 (BD Biosciences, San Jose, CA, 348805). Following fixation (2% methanol-free formaldehyde (Polysciences, Inc., Warrington, PA)), cells were permeabilized in PBS, 0.1% saponin (Coulter), 0.5% BSA for 10 min at ambient temperature. 7.7 μg/mL 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) was added to sample tubes 10 minutes prior to sample acquisition. Samples were acquired on a three-laser Gallios cytometer (Beckman-Coulter) without exceeding an acquisition rate of 10,000 events/second. DAPI fluorescence was captured by 2 individual photomultiplier tubes (PMT) which were optimized for logarithmic (elimination of hypodiploid events) and linear (cell cycle analysis) scales, respectively. Offline compensation and analysis were performed using the high throughput parallel processing VenturiOne software (Applied Cytometry, Sheffield, UK). The compensation matrix was created based on the acquisition of BD Calibrite™ beads (FITC, PE and APC, BD Biosciences) and single antibody-stained mouse IgG capture beads (552843, BD Biosciences) for tandem-dyes (ECD, PE-Cy5, PE-Cy7 and APC-Cy7). Four independent samples were analyzed. Flow cytometric results were summarized as arithmetic means ± SEM, to convey the precision of the mean value estimates.
Delivery of aerosolized SVF cells suspended in fibrin sealant
The spray-on fat delivery was performed using the Tisseel and Easyspray system (Baxter Healthcare Corp.). Tisseel is a fibrin sealant which includes human fibrinogen and thrombin supplied in a two-part system. It is approved as an adjunct to surgical sealing procedures. Reagents were reconstituted to concentrations for fibrinogen (67 -106 mg/mL) and thrombin (10 IU/mL) optimized for reconstructive surgery by one of the authors (JPR). The fibrinogen was diluted into 1mL of fibrinolysis inhibitor solution as recommended by the manufacturer. The thrombin reagent was dissolved in 5mL sterile water, diluted 10-fold in sterile water and supplemented with the calcium chloride solution to a final CaCl2 concentration of 27 μmol/mL and 3.3 IU/mL thrombin. All solutions were kept at 37°C until use using the Fibrinotherm reagent warmer. The Duploject easy Prep System was prepared according to manufacturer instructions. 1x107 cells were resuspended in 400μL sterile PBS and added to the fibrinogen/fibrinolysis inhibitor solution. Both solutions were loaded into the syringe units of the Duplojet sprayer and delivered at a pressure of 20 lbs/in2 nitrogen gas using the EasySpray System onto the culture surfaces of 8-well Labtek™ chamber slides (Nunc™, Thermo-Fisher Scientific) or 35 mm Petri dishes. Cells were incubated for 10 min at 37°C prior to adding culture medium.
Culture of spray-on SVF cells
For SVF cell expansion, sprayed cells were cultured using “ASC culture medium” (DMEM and DMEM/F12, 1:2, 10% fetal bovine serum (FBS), 0.1μM dexamethasone (Sigma), 100 U/mL penicillin (Sigma), 50 μg/mL gentamycin sulfate (Sigma) for adipose differentiation, or in Clonetics® EGM®-2 endothelial growth medium (CC-3156 supplemented with CC-4176, Lonza) for endothelial tubule formation. After 21 days, cells were fixed in 2% formaldehyde (Polysciences, Inc.) in PBS for 15 min at room temperature. For spontaneous lipid vesicle formation assessment, fixed cells were washed in 60% isopropanol and stained for 10 min with Oil Red O (Sigma) at RT. After 5 consecutive washes in deionized water, the presence of red stained lipid was documented using bright field microscopy.
Immunofluorescence
Cell permeabilization and all washes were performed by two 5-min incubations in Dako Wash Buffer (Dako, Carpinteria, CA) at ambient temperature. Labtek chambers were removed to minimize incubation volumes during subsequent steps. Cells were incubated for 1 hour in blocking solution (PBS, 5% goat serum, 0.05% Tween 20) to reduce nonspecific antibody binding, and blocking solution was used for all subsequent antibody dilution. Cells were incubated with primary unconjugated antibodies (CD31 Dako (ready-to-use, N1596), CD34 (1:50, BD Biosciences, Cat No. 347660), CD146 (1:100, BD Biosciences, 550314,) overnight at 4°C. Washed slides were sequentially incubated for 1 hr with biotinylated secondary goat anti-mouse antibody (1:500, Dako) and for 30 min with streptavidin-Cy3 (1:500, Sigma) at ambient temperature. After 2 washes, cells were incubated with the second primary antibody (FITC-conjugated vWF (1:100, US Biological, V2700-01C) and FITC conjugated anti-alpha smooth muscle actin (aSMA, 1:200, Sigma, Cat No. F3777) for 1 hour at room temperature. Nuclear staining was performed with a 5-min incubation with (7.15 μM) DAPI (Invitrogen). Slides were mounted using Prolong® Gold Anti-fade Reagent (P36934, Invitrogen) and observed under an epi-fluorescence microscope (Nikon Eclipse TE 2000-U). Universal Negative Control for Mouse Primary Antibodies (Ready to use, N1698, Dako) substituted primary antibodies for negative controls.
Results
Stem/Progenitor content of the adipose stromal vascular fraction (SVF). Flow cytometric assessment of disaggregated SVF cells prepared from LipiVage samples on the Tissue Genesis Cell Isolation System revealed substantial populations of endothelial progenitors (CD14-/CD33-/CD45-/glycophoran-A-/CD34+/CD31+, 17.4 ± 5.7% of non-hematopoietic cells, mean SEM), pericytes (CD14-/CD33-/CD45-/glycophoran-A-/CD31-/CD146+, 14.3 ± 6.5%), and supra-adventitial adipose stromal cells (SA-ASC) (CD14-/CD33-/CD45-/glycophoran-A-/CD31-/CD146-/CD34+, 83.9 ± 7.6%) (Figure 2). Despite expression of CD34, SA-ASC uniformly expressed CD73, CD90 and CD105, markers associated with multipotent mesenchymal stromal cells.
Figure 2.
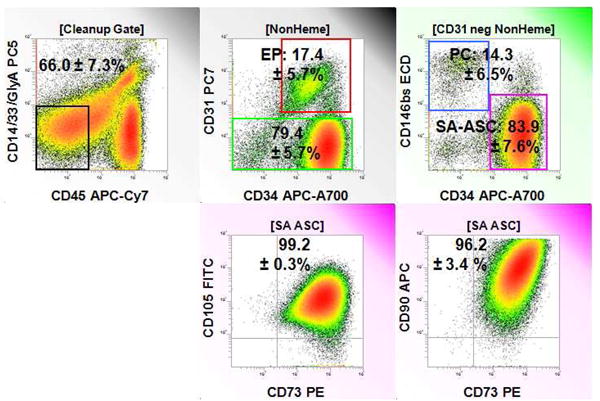
Stem/Progenitor content of freshly isolated adipose stromal vascular cells. A representative sample is shown. Statistics in analytical regions represent the percent positive (mean ± SEM) for four independent samples. The gating population for each histogram is shown in square brackets and comprises the denominator for percent calculations. From left to right, top to bottom: Nonhematopoietic (Non-Heme) cells are identified on events gated to remove autofluorescent cells, cell clusters, and events with <2N DNA [Cleanup Gate]. Endothelial progenitor cells (EP) are CD31+/CD34+. Pericytes (PC, CD146+/CD34-) and supra-adventitial adipose stromal cells (SA-ASC, CD146-/CD34+) are identified among CD31 negative, nonhematopoietic cells. SA-ASC are CD34+, but express MSC-associated markers CD73, CD105 and CD90.
SVF cells were suspended in the fibrin/fibrinolysis inhibitor solution of the 2-part Tisseel reagent set (Figure 3) and sprayed onto chamber slides or Petri dishes under nitrogen pressure. The cell-fibrin mixture formed a fibrous layer on the culture surface within minutes of delivery. Tissue culture medium was added 10 minutes after cell delivery. After 24 hours, cells could be observed associated with three dimensional fibrin structures.
Figure 3.
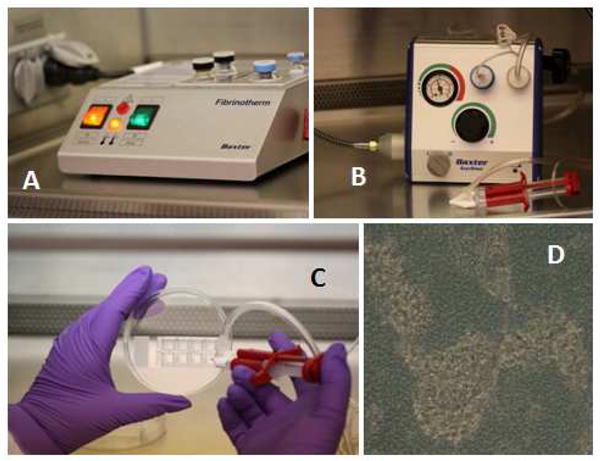
Composite schematic representation of Adipose Stromal Vascular Progenitor spray deposition. Panel A: Fibrinotherm reagent warmer and mixer. Cells and reagents are warmed to 37° C in this device. Panel B: The Tissomat spray module regulates nitrogen gas used to spray the cells suspended in reconstituted fibrinogen. The Duploject syringe system is in the foreground. Panel C: Cells mixed with fibrinogen sealer protein solution are sprayed onto 8-well chamber slides using the Duploject syringe system. Fibrinogen containing the cell suspension is mixed with thrombin and calcium as they enter the Duploject spray nozzle. Panel D: Light photomicrograph (40 x objective) taken 24 hours after cell/fibrin deposition. Darker areas are the fibrin substrate. Lighter areas represent areas where cells have coalesced within the fibrin, forming refractile clusters.
Cells grew in association with fibrin strands, spreading to semi-confluence in the culture vessel by 3 weeks. In the presence of medium containing dexamethasone, approximately 50% of cells differentiated into adipocytes, as evidenced by cytoplasmic fat vesicles as demonstrated by oil red-O staining (Figure 4). In the presence of endothelial growth medium, branched tubules reminiscent of microvasculature formed spontaneously. Tubules expressed CD31, vWF and CD34, and were associated with aSMA+ cells (Figure 5).
Figure 4.
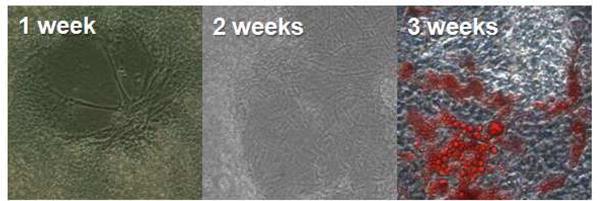
Growth and differentiation of spray-deposited adipose stromal vascular cells. After one week in culture in the presence of dexamethasone, cell colonies can be seen associated with fibrin strands. Cells continue to grow and at 3 weeks are semi-confluent and a proportion has spontaneously differentiated into adipocytes as determined by oil red-O staining.
Figure 5.
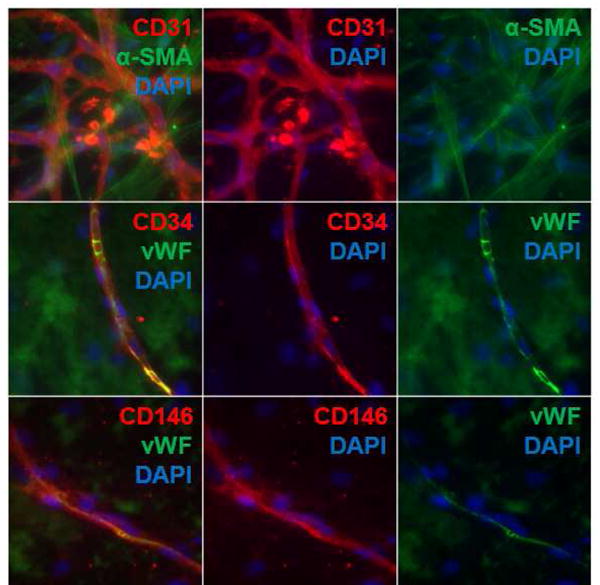
Endothelial progenitor cell survival and differentiation after spray deposition. Replicate cultures of spray-on cells cultured for 3 weeks in the presence of EGM2 spontaneously form endothelial tubules. The tubules are microvascular-like in structure with CD31+, CD34+ vWF+ cells in intimate association with alpha smooth muscle actin+ cells. Endothelial tubules grew on a matrix of fibrin-associated stromal cells (isolated blue nuclei).
Discussion
Delivery of single cell suspensions for regenerative therapy has been problematic. Direct injection of cells into soft tissue or muscle, or topical application of cells results in cell loss due to mechanical stress, shear forces, and the absence of a substrate conducive to cell adhesion. The ability to immobilize cells in a matrix of biocompatible material would greatly facilitate cell delivery in a variety of clinical applications including wound healing and reconstructive surgery. The fibrin tissue sealant Tisseel is an excellent candidate for a liquid cell delivery vehicle that rapidly polymerizes upon addition of thrombin and calcium, forming an adherent web of cells embedded in a fibrin meshwork.
The suitability of fibrin as a scaffold for a variety of regenerative therapeutic applications has been reviewed (5). As early as 1993, Tisseel fibrin sealant was used to promote the engraftment of cultured keratinocytes in a nude mouse model (6). Since that time, Tisseel has been tested in a variety of in vitro and in vivo model systems including the application of keratinocytes to treat burn wounds (7) and mesenchymal stromal cells to promote bone regeneration (8). More recently, Falanga et al. used Tisseel as a vehicle to spray cultured autologous mesenchymal stromal cells to treat non-healing lower extremity wounds (8 subjects) and skin cancer (5 subjects) (1). Tisseel has also been used to adhere human adipose SVF cells to a hydroxyapatite substrate for use in a murine model of osteogenesis (9). Despite numerous successful applications of fibrin as a support for cell growth, too high a fibrin density can result in poor cell survival (10). Here, we used a low thrombin concentration (3.3 IU/mL) and a moderate fibrinogen concentration (approximately 80 mg/mL) that we had previously optimized for use in reconstructive surgery. This resulted in a well-organized mesh that supported growth and differentiation of SVF cells.
In the present report we demonstrated that unpassaged SVF cells isolated from human subjects by LipiVage, a minimally invasive clinically approved procedure that routinely provides a high yield of viable adipocytes (11), and processed in a closed system (Tissue Genesis Cell Isolation System) could be suspended in Tisseel fibrin sealant and delivered using a commercially available aerosol spray system (12) while retaining the ability to differentiate into vessel-like endothelial tubules and adipocytes.
SVF cells prepared from LipiVage using the Tissue Genesis system were enriched for pericytes (14.3 ± 6.5% of nonhematopoietic cells), a multipotenital stem cell hypothesized to be the progenitor of MSC-like SA-ASC in adipose tissue (13), compared to our previously published results using whole fat and manual cell disaggregation, in which pericytes comprised 2.7 ± 1.8% of nonhematopoietic cells (2).
SA-ASC, the most prevalent progenitor subpopulation, comprising 83.9 ± 7.6% of non-hematopoietic/non-endothelial cells, expressed CD73, CD105 and CD90, a signature that would mark them as MSC, were it not for coexpression of CD34.
MSCs derived from bone marrow, synovium, umbilical vein, and human embryonic stem cells are all reportedly CD34 negative (reviewed in (14)). Further, the minimal criteria proposed by the International Society for Cellular Therapy for defining MSC are CD34-/CD45-/CD14-/CD19-/HLADR- and CD73+/CD90+/CD105+ (15), although it should be noted that this definition applies to plastic adherent cultured and not freshly isolated cells. The consensus group stressed that CD34 should be used to exclude hematopoietic and endothelial cells which may be co-isolated with plastic adherent MSC.
Despite the expression of CD34, adipose stem cells (ASC) derived by culture of SVF cells, secrete cytokines (VEGF, TGF-β, and IL-6) similar to those produced by MSC (16) and may be expected to exert anti-inflammatory as well as regenerative effects.
Finally, the system described here was developed to solve the problem of cell delivery and immobilization in wound healing applications. Although such systems have been proposed and tested by others (1, 6-8), our goal was to determine whether adipose tissue that has just been subjected to evulsion and collagenase digestion can withstand the mechanical stress of the spraying system, and will grow and differentiate when enmeshed in a fibrin scaffold. In particular, the visualization of endothelial tubules in culture demonstrates the survival of endothelial progenitors that may have an unprecedented impact on wound healing by directly mediating revascularization.
Given the challenge of obtaining FDA approval for combination therapies (a cellular product plus a device), we took care to design this procedure around devices and reagents that are either FDA approved (LipiVage, Tisseel, EasySpray) or undergoing approval and in clinical use under IND (Tissue Genesis). LipiVage can be performed on most individuals to obtain a volume of lipoaspirate (60 mL) sufficient for separation using the closed system Tissue Genesis apparatus. The Tisseel fibrin delivery system is in routine clinical use to promote healing of surgical wounds. The results presented here support the conclusion that adipose SVF cells can be administered and immobilized in a fibrin matrix through an aerosol delivery system while preserving the ability to grow and differentiate. The heterogeneity and rich stem/progenitor cell content of freshly isolated SVF cells, and their ability to differentiate along adipogenic and endothelial lineages after Tisseel delivery, suggest that they may participate in granulation, regeneration, angiogenesis and anti-inflammatory response when applied in a wound healing environment.
Acknowledgments
This project was supported by Production Assistance for Cellular Therapy (PACT) under contract #N01-HB-37165 and #R01-HL-085819 from the National Heart, Lung, and Blood Institute, and R01 CA 114246-7 from the National Cancer Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007 Jun;13(6):1299–312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010 Oct 22;77A:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic Potential of Adipose Stem Cell Subpopulations. Plast Reconstr Surg. 2011 May 12; doi: 10.1097/PRS.0b013e318221db33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerlin L, Donnenberg VS, Donnenberg AD. Pericytes: A universal adult tissue stem cell? Cytometry Part A. 2012;81A(1):12–4. doi: 10.1002/cyto.a.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed TA, Dare EV, Hincke M. Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Eng Part B Rev. 2008 May 1; doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 6.Auger FA, Guignard R, Lo'pez Valle CA, Germain L. Role and innocuity of Tisseel®, a tissue glue, in the grafting process andin vivo evolution of human cultured epidermis. British Journal of Plastic Surgery. 1993;46(2):136–42. doi: 10.1016/0007-1226(93)90145-2. [DOI] [PubMed] [Google Scholar]
- 7.Currie LJ, Martin R, Sharpe JR, James SE. A comparison of keratinocyte cell sprays with and without fibrin glue. Burns. 2003 Nov;29(7):677–85. doi: 10.1016/s0305-4179(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Boo JS, Ozawa R, Nagasaka T, Okazaki Y, Hata K, et al. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Craniomaxillofac Surg. 2003 Feb;31(1):27–33. doi: 10.1016/s1010-5182(02)00143-9. [DOI] [PubMed] [Google Scholar]
- 9.Muller AM, Mehrkens A, Schafer DJ, Jaquiery C, Guven S, Lehmicke M, et al. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cell Mater. 2010;19:127–35. doi: 10.22203/ecm.v019a13. [DOI] [PubMed] [Google Scholar]
- 10.Zurita M, Otero L, Aguayo C, Bonilla C, Ferreira E, Parajon A, et al. Cell therapy for spinal cord repair: optimization of biologic scaffolds for survival and neural differentiation of human bone marrow stromal cells. Cytotherapy. 2010 Jul;12(4):522–37. doi: 10.3109/14653241003615164. Comparative Study Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson REH, Cui X, Fink BF, Vasconez HC, Pu LLQ. The Viability of Autologous Fat Grafts Harvested With the LipiVage System: A Comparative Study. Annals of Plastic Surgery. 2008;60(5):594–7. doi: 10.1097/SAP.0b013e31817433c5. [DOI] [PubMed] [Google Scholar]
- 12.Chaurasia SS, Champakalakshmi R, Angunawela RI, Tan DT, Mehta JS. Optimization of Fibrin Glue Spray Systems for Ophthalmic Surgery. Translational Vision Science & Technology. 2012 doi: 10.1167/tvst.1.2.2. 2012/06/01:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerlin L, Donnenberg VS, Donnenberg AD. Pericytes: A universal adult tissue stem cell? Cytometry Part A. 2011:n/a–n/a. doi: 10.1002/cyto.a.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurer MH. Proteomic Definitions of Mesenchymal Stem Cells Stem Cells. International. 2011;2011 doi: 10.4061/2011/704256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011 Jan;17(1-2):93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]


