Abstract
Object
Descriptions of temporal lobe arteriovenous malformations (AVMs) are inconsistent. To standardize reporting, the authors blended existing descriptions in the literature into an intuitive classification with 5 anatomical subtypes: lateral, medial, basal, sylvian, and ventricular. The authors’ surgical experience with temporal lobe AVMs was reviewed according to these subtypes.
Methods
Eighty-eight patients with temporal lobe AVMs were treated surgically.
Results
Lateral temporal lobe AVMs were the most common (58 AVMs, 66%). Thirteen AVMs (15%) were medial, 9 (10%) were basal, and 5 (6%) were sylvian. Ventricular AVMs were least common (3 AVMs, 3%). A temporal craniotomy based over the ear was used in 64%. Complete AVM resection was achieved in 82 patients (93%). Four patients (5%) died in the perioperative period (6 in all were lost to follow-up); 71 (87%) of the remaining 82 patients had good outcomes (modified Rankin Scale scores 0–2); and 68 (83%) were unchanged or improved after surgery.
Conclusions
Categorization of temporal AVMs into subtypes can assist with surgical planning and also standardize reporting. Lateral AVMs are the easiest to expose surgically, with circumferential access to feeding arteries and draining veins at the AVM margins. Basal AVMs require a subtemporal approach, often with some transcortical dissection through the inferior temporal gyrus. Medial AVMs are exposed tangentially with an orbitozygomatic craniotomy and transsylvian dissection of anterior choroidal artery and posterior cerebral artery feeders in the medial cisterns. Medial AVMs posterior to the cerebral peduncle require transcortical approaches through the temporooccipi tal gyrus. Sylvian AVMs require a wide sylvian fissure split and differentiation of normal arteries, terminal feeding arteries, and transit arteries. Ventricular AVMs require a transcortical approach through the inferior temporal gyrus that avoids the Meyer loop. Surgical results with temporal lobe AVMs are generally good, and classifying them does not offer any prediction of surgical risk.
Keywords: arteriovenous malformation, temporal lobe, anatomical subtype, microsurgical resection, vascular disorders
The temporal lobe houses important neurological functions.17 Memory and learning are located in the hippocampus and parahippocampus; language reception is located in the Wernicke center in the dominant superior temporal gyrus; auditory reception resides in the Heschl gyrus; and visual signals are transmitted in the optic radiations.8,17,19 The temporal lobe has an intimate relationship with the MCAs coursing through the sylvian fissure, with the PCAs coursing through the crural and ambient cisterns, and with the AChA supplying the choroid plexus of the temporal horn of the lateral ventricle.18 Venous anatomy of the temporal lobe is complex, with drainage anteriorly to temporopolar veins and the sphenoparietal sinus, posteriorly to the vein of Labbé and transverse sinus, medially to the BVR and the galenic system, and superiorly to superficial sylvian veins and the superior sagittal sinus.9 Anatomical and functional variability make temporal lobe AVMs highly complex.5,16.
Temporal lobe AVMs have been classified by neurosurgeons to better appreciate this variability in anatomy and surgical strategy. Yaşargil20 classified 70 temporal lobe AVMs as polar, dorsal, laterobasal, mediobasal, fistulous, and giant. Some authors have applied this classification (like Nagata et al.15 in a surgical series of 26 temporal patients with lobe AVMs), but it lacks intuitiveness and the distinctions between subtypes are poorly defined. Rhoton and colleagues3,7,18 described a more intuitive approach based on the 4 temporal lobe surfaces: lateral, medial, ba sal, and sylvian fissure. Rhoton, de Oliveira, and colleagues devoted much of their attention to AVMs in the medial temporal region rather than to temporal lobe AVMs as a whole. Other authors like Schramm (see Boström et al.1) applied this surface perspective, classifying 44 surgically resected AVMs as temporal lobe, temporomesial, ventricular, and sylvian fissure. Similarly, Malik et al.13 classified these AVM locations as lateral convexity, mesotemporal, and ventricular in their series of 24 patients. Rather than introducing a new classification, we were interested in blending existing ones into a single intuitive and practical system for these complex and variable AVMs. Therefore, we analyzed our surgical experience with temporal lobe AVMs according to 5 types: lateral, medial, basal, sylvian, and ventricular.
Methods
This study was approved by the UCSF Committee on Human Research and conducted in compliance with Health Insurance Portability and Accountability Act reg-ulations.
The 5 Types of Temporal Lobe AVMs
Lateral Temporal Lobe
These AVMs are based on or immediately beneath the lateral convexity surface (Fig. 1). The lateral temporal surface consists of the superior, middle, and inferior temporal gyri. These gyri lie below the sylvian fissure and are parallel to the sylvian line and to each other. The superior and inferior temporal sulci are landmarks on this surface above and below the middle temporal gyrus.
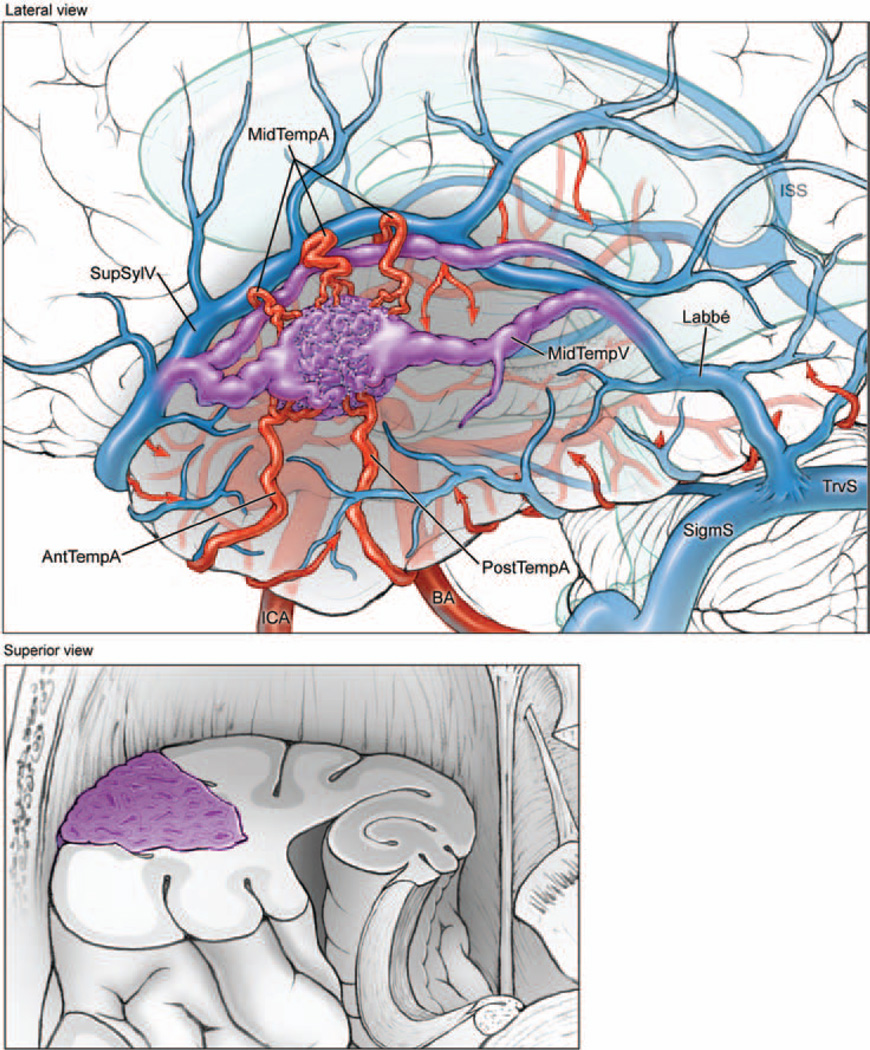
Fig. 1.
Lateral temporal AVMs are located on the lateral convexity (upper, lateral view of left hemisphere; lower, superior view of coronally transected left temporal lobe). The lesions are supplied by ATA (AntTempA) and MTA (MidTempA) branches from the inferior trunk of the MCA, as well as PTAs (PostTempA) from the PCA. These AVMs are drained anteriorly by superficial sylvian veins (SupSylV) and posteriorly by the vein of Labbé. ISS = inferior sagittal sinus; MidTempV = middle temporal vein; SigmS = sigmoid sinus; TrvS = transverse sinus.
Basal Temporal Lobe
These AVMs are based on or immediately beneath the basal surface (Fig. 2). The basal surface consists of the lower portion of the inferior temporal gyrus, the occipitotemporal (fusiform) gyrus, and the lower portion of the parahippocampal gyrus. The basal surface is traversed longitudinally by the collateral sulcus, which lies between the parahippocampal and occipitotemporal gyri. The occipitotemporal sulcus parallels the collateral sulcus and separates the occipitotemporal gyrus from the lower portion of the inferior temporal gyrus.
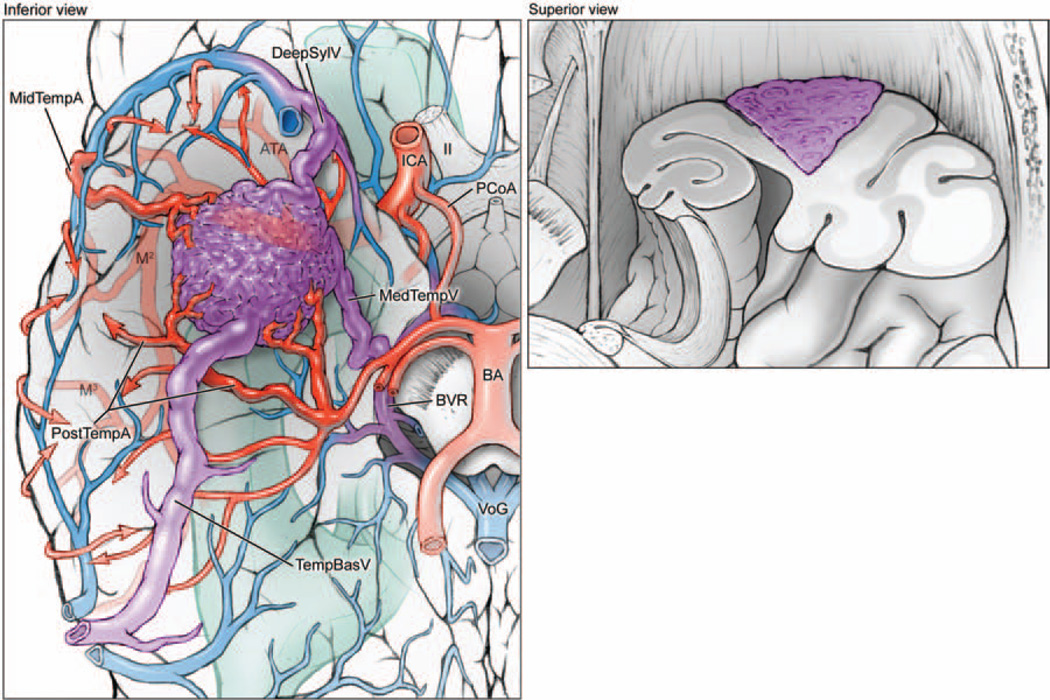
Fig. 2.
Basal temporal AVMs are located on the basal temporal surface (left, inferior view of right hemisphere; right, superior view of coronally transected right temporal lobe). The lesions are supplied by ATA and MTA branches from the inferior trunk of the MCA, as well as PTAs from the PCA. These AVMs drain anteriorly to deep sylvian veins (DeepSylV) and posteriorly to temporal basal veins (TempBasV). II = second cranial nerve; MedTempV = medial temporal vein; M2 = insular segment of MCA; M3 = opercular segment of MCA; VoG = Vein of Galen.
Medial Temporal Lobe
These AVMs are based on or immediately beneath the medial surface (Fig. 3). The medial temporal surface consists of the parahippocampal gyrus, uncus, and hippocampus (which is composed of the dentate gyrus, the Ammon horn, and the subiculum). The hippocampal sulcus separates the parahippocampal and dentate gyri. The fimbriodentate sulcus separates the dentate gyrus and the fimbria of the hippocampus, which collects hippocampal efferents as a bundle at the edge of the choroid plexus before leaving the hippocampus to form the crus of the fornix. The amygdala and hippocampal formation lie beneath the medial temporal surface.
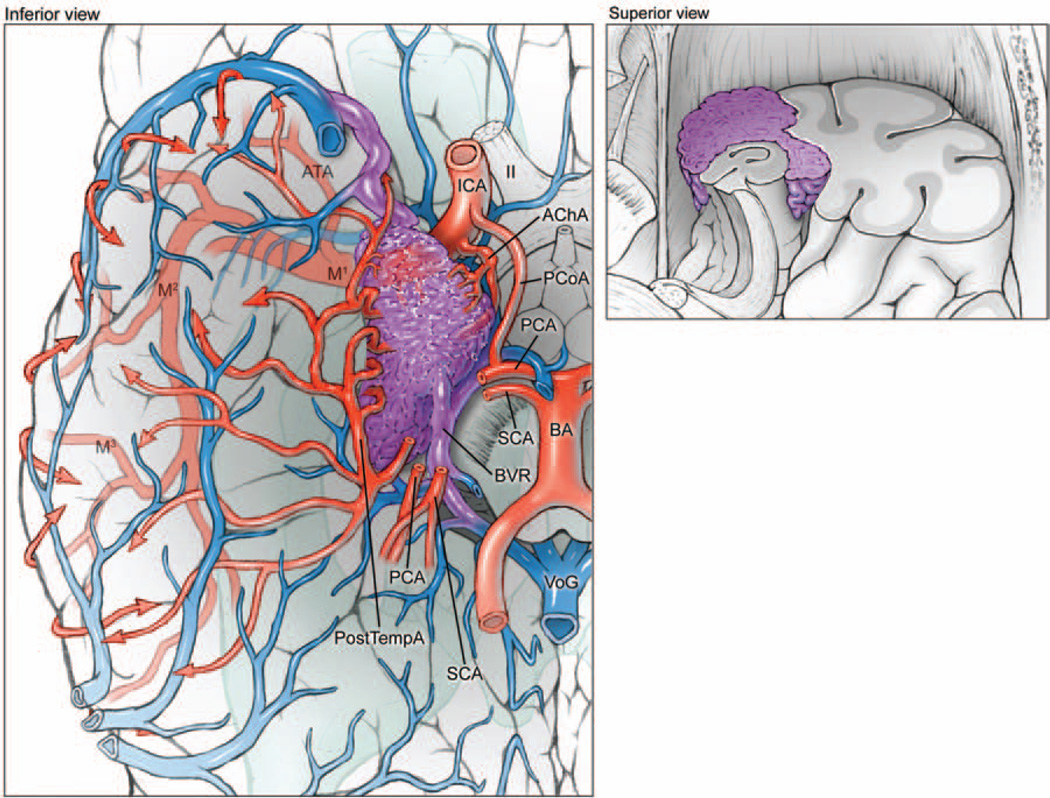
Fig. 3.
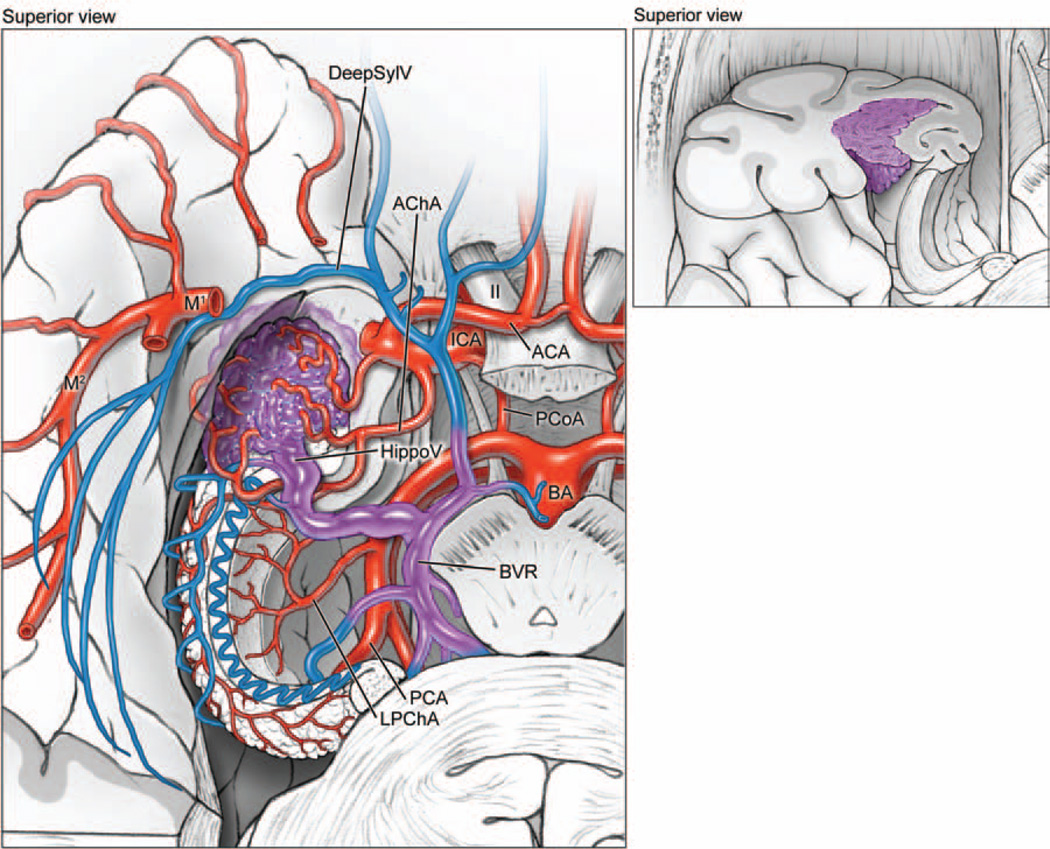
Medial temporal AVMs are based on the medial surface (left, inferior view of right hemisphere; right, superior view of coronally transected right temporal lobe). The lesions are supplied by the temporopolar artery, PCoA, AChA, and PTAs from the PCA. These AVMs drain to the BVR and vein of Galen. M1 = sphenoidal segment of MCA; SCA = superior cerebellar artery.
Sylvian Temporal Lobe
These AVMs are based on or immediately beneath the lateral surface of the sylvian fissure (Fig. 4). This surface forms the superior boundary of the temporal lobe and the lower lip of the opercular cleft. The anterior sylvian surface consists of the planum polare, formed by the upper edge of the superior temporal gyrus. The posterior sylvian surface consists of the planum temporale, formed by the Heschl gyrus and the transverse temporal gyri.
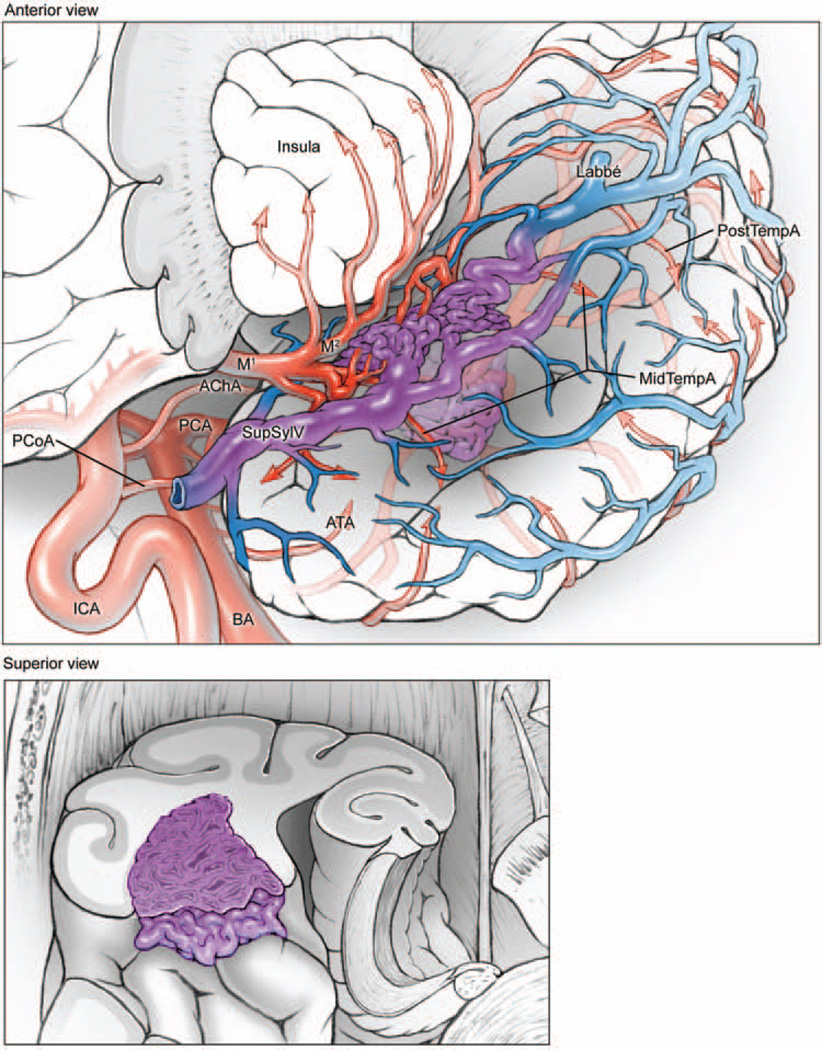
Fig. 4.
Sylvian temporal AVMs are based on the lateral surface of the sylvian fissure (upper, anterolateral view of the left temporal lobe, with lateral left frontal lobe transected parasagittally to expose insular cortex; lower, superior view of coronally transected left temporal lobe). The lesions are supplied by ATA, MTA, and PTA branches from the inferior trunk of the MCA. These AVMs drain to deep and superficial sylvian veins, as well as anterior, middle, and posterior temporal veins on the lateral convexity.
Ventricular
These AVMs in the temporal lobe are located in the temporal horn of the lateral ventricle, extending as far posteriorly as the atrium (Fig. 5). These AVMs are based on choroid plexus or on paraventricular white matter with extension into the ventricle, rather than a temporal lobe surface.
Fig. 5.
Temporal horn AVMs are located in the lateral ventricle, based on the choroidal fissure. The lesions are supplied by the AChA and the lateral posterior choroidal artery (LPChA). These AVMs drain to the hippocampal vein (HippoV) and the BVR. ACA = anterior cerebral artery.
Patient Population
During a 13-year period, a total of 500 patients with brain AVMs were treated surgically at the UCSF Medical Center by one neurosurgeon (M.T.L.) and recorded in the prospective registry of the UCSF Brain Arteriovenous Malformation Study Project. The registry was searched for patients with temporal lobe AVMs, and 88 patients were identified for inclusion in this study.
There were 42 men (48%) and 46 women (52%) with a mean age of 39 years (range 6–77 years) (Table 1). Of the 88 patients, 45 (51%) presented with hemorrhage, which was intraparenchymal in 27 (31%), subarachnoid in 9 (10%), and intraventricular in 9 (10%). The mean diameter of intraparenchymal hematomas was 4.7 cm (range 1–7 cm). Twenty-four patients (27%) presented with seizures, 15 (17%) with headaches, and 4 (5%) presented with other symptoms (steal symptoms, pulsatile tinnitus, or incidental).
TABLE 1.
Characteristics in 88 patients with temporal lobe AVMs*
| Total |
Lateral |
Basal |
Medial |
Sylvian |
Ventricular |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| patients | 88 | 100 | 58 | 66 | 9 | 10 | 13 | 15 | 5 | 6 | 3 | 3 |
| mean age (yrs) | 39.0 | 40.8 | 45.2 | 29.5 | 33.6 | 32.7 | ||||||
| sex | ||||||||||||
| male | 42 | 48 | 27 | 47 | 4 | 44 | 6 | 46 | 4 | 80 | 1 | 33 |
| female | 46 | 52 | 31 | 53 | 5 | 56 | 7 | 54 | 1 | 20 | 2 | 67 |
| presentation | ||||||||||||
| hemorrhage | 45 | 51 | 27 | 47 | 4 | 44 | 8 | 62 | 4 | 80 | 2 | 67 |
| seizures | 24 | 27 | 19 | 33 | 2 | 22 | 2 | 15 | 1 | 20 | 0 | 0 |
| headache | 15 | 17 | 9 | 16 | 2 | 22 | 3 | 23 | 0 | 0 | 1 | 33 |
| other | 4 | 5 | 3 | 5 | 1 | 11 | 0 | 0 | 0 | 0 | 0 | 0 |
| side | ||||||||||||
| lt | 59 | 67 | 42 | 72 | 5 | 56 | 7 | 54 | 3 | 60 | 2 | 67 |
| rt | 29 | 33 | 16 | 28 | 4 | 44 | 6 | 46 | 2 | 40 | 1 | 33 |
| S-M Grade | ||||||||||||
| I | 21 | 24 | 13 | 22 | 5 | 56 | 1 | 8 | 2 | 40 | 0 | 0 |
| II | 33 | 38 | 25 | 43 | 3 | 33 | 4 | 31 | 0 | 0 | 1 | 33 |
| III | 25 | 28 | 17 | 29 | 0 | 0 | 4 | 31 | 2 | 40 | 2 | 67 |
| IV | 8 | 9 | 3 | 5 | 1 | 11 | 4 | 31 | 0 | 0 | 0 | 0 |
| V | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 20 | 0 | 0 |
| supplementary grade | ||||||||||||
| I | 6 | 7 | 2 | 3 | 0 | 0 | 2 | 15 | 1 | 20 | 1 | 33 |
| II | 19 | 22 | 11 | 19 | 2 | 22 | 4 | 31 | 2 | 40 | 0 | 0 |
| III | 34 | 39 | 26 | 45 | 2 | 22 | 4 | 31 | 1 | 20 | 1 | 33 |
| IV | 25 | 28 | 17 | 29 | 5 | 56 | 1 | 8 | 1 | 20 | 1 | 33 |
| V | 4 | 5 | 2 | 3 | 0 | 0 | 2 | 15 | 0 | 0 | 0 | 0 |
| supplemented S-M Grade | ||||||||||||
| 1–3 (low risk) | 7 | 8 | 2 | 3 | 2 | 22 | 1 | 8 | 2 | 40 | 0 | 0 |
| 4–6 (moderate risk) | 66 | 75 | 48 | 83 | 6 | 67 | 8 | 62 | 2 | 40 | 2 | 67 |
| 7–10 (high risk) | 15 | 17 | 8 | 14 | 1 | 11 | 4 | 31 | 1 | 20 | 1 | 33 |
S-M = Spetzler-Martin.
Fifteen patients (17%) had undergone previous treatments. Three patients (3%) required hematoma evacuation before being transferred for AVM surgery; 5 (6%) had residual AVMs after incomplete resections at other centers; 1 pediatric patient (1%) had a recurrence 5 years after complete resection of his AVM, as confirmed by a negative postoperative angiogram; and 6 patients (7%) had incompletely obliterated AVMs after Gamma Knife surgery, of whom 2 patients (2%) were also treated with embolization before radiosurgery.
Characteristics of the AVMs
We systematically reviewed CT scans, MRI sequences, DSAs, operative reports, intraoperative photographs, and surgeon notes to grade AVMs according to the Spetzler-Martin and supplementary systems, and to classify temporal lobe AVMs according to the 5 types. Sylvian fissure AVMs based on the temporal surface were included, but pure sylvian fissure, medial/frontal sylvian, and deep/ insular sylvian AVMs, according to Sugita’s classification, were excluded. Large temporal lobe AVMs based on the surface and projecting deep to the ventricle were classified according to that surface.
A majority of AVMs were located on the left side (59 lesions, 67%). The mean AVM size was 2.7 cm (range 0.4–6.7 cm). The most common Spetzler-Martin grade was II (33 AVMs, 38%) and the most common supplementary grade was III (34 patients, 39%) (Table 1). Seventy-three patients (83%) had combined Spetzler-Martin and supplementary scores of 6 or less, indicating low to moderate surgical risk.
Intranidal aneurysms were found in 8 AVMs (9%), and 11 other aneurysms were found in 10 patients (11%) in the following locations: MCA (3), ACoA (2), and in the PCA, AChA, ATA, PCoA, ICA bifurcation, and ophthalmic artery (1 each).
Outcome Evaluation
Neurological outcome was assessed using the mRS. Neurological assessments were performed by a nurse clinician under the supervision of a neurologist, preoperatively, postoperatively, and up to 2 years postoperatively. Follow-up information was obtained during routine clinic visits or telephone interviews. Good outcomes were defined as a final mRS score of 0–2, and poor outcomes were defined as a final mRS score greater than 2. Improvement was defined as a decrease in mRS score, and deterioration was defined as an increase in mRS score.
Results
Anatomy of Temporal Lobe AVMs
A preponderance of temporal lobe AVMs were based on the lateral temporal surface (58 AVMs, 66%); 13 AVMs (15%) were based on the medial surface; 9 (10%) on the basal surface; and 5 (6%) on the sylvian surface. Ventricular lesions were least common (3 AVMs, 3%). Patient presentation and AVM grades were not significantly different between types (Table 1).
Feeding arteries from the MCA were present in 78 temporal lobe AVMs (89%) and from the PCA in 47 AVMs (53%). The MCA is the major supplier to lateral and sylvian AVM types, whereas the PCA is more frequently involved with medial, basal, and ventricular AVM types (Table 2). The AChA supplied all ventricular AVMs, and a majority of medial and sylvian AVMs. The MMAs and LSAs were infrequent suppliers to temporal lobe AVMs; the former were observed with lateral and basal types and the latter with medial and sylvian types.
TABLE 2.
Lesion anatomy in 88 patients with temporal lobe AVMs
| Total |
Lateral |
Basal |
Medial |
Sylvian |
Ventricular |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anatomy | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| patients | 88 | 58 | 9 | 13 | 5 | 3 | ||||||
| feeding arteries | ||||||||||||
| MCA | 78 | 89 | 55 | 95 | 6 | 67 | 10 | 77 | 5 | 100 | 2 | 67 |
| PCA | 47 | 53 | 28 | 48 | 5 | 56 | 9 | 69 | 2 | 40 | 3 | 100 |
| MCA + PCA | 37 | 42 | 25 | 43 | 2 | 22 | 6 | 46 | 2 | 40 | 2 | 67 |
| AChA | 18 | 20 | 5 | 9 | 0 | 0 | 7 | 54 | 3 | 60 | 3 | 100 |
| LSA | 3 | 3 | 0 | 0 | 0 | 0 | 2 | 15 | 1 | 20 | 0 | 0 |
| MMA | 12 | 14 | 9 | 16 | 2 | 22 | 0 | 0 | 1 | 20 | 0 | 0 |
| draining veins | ||||||||||||
| superficial | 77 | 88 | 56 | 97 | 6 | 67 | 8 | 62 | 4 | 80 | 3 | 100 |
| deep | 28 | 32 | 12 | 21 | 3 | 33 | 9 | 69 | 2 | 40 | 2 | 67 |
| superficial + deep | 14 | 16 | 10 | 17 | 0 | 0 | 1 | 8 | 1 | 20 | 2 | 67 |
Operative Strategies
Pterional, orbitozygomatic, or temporal craniotomies were used for temporal lobe AVMs. The pterional craniotomy was used for lateral and basal AVMs in the anterior temporal lobe (Table 3). The orbitozygomatic-pterional craniotomy was used for medial AVMs and for larger sylvian AVMs that required more exposure than the standard pterional craniotomy. The temporal craniotomy, based over the ear with more posterior exposure, was used in the majority of cases (56, 64%), including lateral, basal, medial, and ventricular AVM types.
TABLE 3.
Surgical approaches in 88 patients with temporal lobe AVMs
| Total |
Lateral |
Basal |
Medial |
Sylvian |
Ventricular |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approach | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| patients | 88 | 58 | 9 | 13 | 5 | 3 | ||||||
| craniotomy | ||||||||||||
| pterional | 19 | 22 | 14 | 24 | 3 | 33 | 0 | 0 | 2 | 40 | 0 | 0 |
| orbitozygomatic | 13 | 15 | 0 | 0 | 1 | 11 | 9 | 69 | 3 | 60 | 0 | 0 |
| temporal | 56 | 64 | 44 | 76 | 5 | 56 | 4 | 31 | 0 | 0 | 3 | 100 |
| approach | ||||||||||||
| transcortical | 61 | 69 | 58 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 100 |
| transsylvian | 14 | 16 | 0 | 0 | 0 | 0 | 9 | 69 | 5 | 100 | 0 | 0 |
| subtemporal | 13 | 15 | 0 | 0 | 9 | 100 | 4 | 31 | 0 | 0 | 0 | 0 |
The approach to sylvian and anterior medial types was transsylvian (Table 3). The approach to lateral and ventricular types was transcortical. The location of lateral AVMs determines the cortical entry sites, whereas ventricular AVMs were typically approached through the inferior temporal gyrus. The approach to basal and posterior medial types was subtemporal, working through the inferior temporal gyrus or occipitotemporal gyrus depending on AVM anatomy and the presence of an associated hematoma. Subtemporal approaches required drilling the squamosal portion of the temporal bone flat with the middle fossa floor to widen the exposure. Language mapping with awake anesthesia was performed for intraoperative language assessment in 5 patients and required a larger temporal-parietal craniotomy.
Surgical Results
Preoperative embolization was performed in 59 patients (67%). Transient neurological deficits after embolization occurred in 2 patients (2%). Complete AVM resection was achieved in 82 patients and confirmed angiographically (surgical obliteration rate 93%). Four of these patients had unexpected residual AVM on postoperative angiography and required a second stage to complete the resection; 2 of these patients had deliberate 2-stage resections. Of the 6 patients with incomplete AVM resections, 5 were treated with stereotactic radiosurgery and 1 refused further treatment. Ten aneurysms were clipped during the surgery for AVM resection, and one aneurysm that was not accessible during surgery was treated with coil insertion postoperatively. Major complications included 3 patients with postoperative hematomas that required evacuation.
Patient Outcomes
Four patients died in the perioperative period (surgical mortality 5%) (Table 4), of whom 2 presented in coma after severe intracerebral hemorrhages and failed to improve with aggressive management. One patient with a sylvian temporal AVM (Spetzler-Martin Grade IV) experienced a massive intraventricular hemorrhage on postoperative Day 1, an external ventricular drain was placed when intracranial pressure exceeded 90 mm Hg, and the brain rapidly herniated and the patient died. The final perioperative death was due to acute cholecystitis 3 weeks after an uncomplicated AVM resection that caused abdominal hemorrhage, renal failure, liver failure, and congestive heart failure. One additional patient died 3 months after surgery of unrelated causes, with a mRS score of 1 at last evaluation. Fifteen patients (17%) experienced transient neurological deterioration that resolved completely at late follow-up.
TABLE 4.
Outcomes in 88 patients after temporal lobe AVM resection
| Outcome | Total |
Lateral |
Basal |
Medial |
Sylvian |
Ventricular |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| patients | 88 | 58 | 9 | 13 | 5 | 3 | ||||||
| periop | ||||||||||||
| transient morbidity | 15 | 17 | 9 | 16 | 1 | 11 | 4 | 31 | 0 | 0 | 1 | 33 |
| surgical mortality | 4 | 5 | 3 | 5 | 0 | 0 | 0 | 0 | 1 | 20 | 0 | 0 |
| late outcome | ||||||||||||
| lost to follow-up | 6 | 7 | 3 | 5 | 1 | 11 | 2 | 15 | 0 | 0 | 0 | 0 |
| mRS Scores | ||||||||||||
| 0 | 27 | 33 | 15 | 27 | 4 | 50 | 6 | 55 | 2 | 40 | 0 | 0 |
| 1 | 32 | 39 | 24 | 44 | 2 | 25 | 2 | 18 | 2 | 40 | 2 | 67 |
| 2 | 12 | 15 | 7 | 13 | 2 | 25 | 2 | 18 | 0 | 0 | 1 | 33 |
| 3 | 3 | 4 | 2 | 4 | 0 | 0 | 1 | 9 | 0 | 0 | 0 | 0 |
| 4 | 3 | 4 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 5 | 6 | 4 | 7 | 0 | 0 | 0 | 0 | 1 | 20 | 0 | 0 |
| change in mRS | ||||||||||||
| improved/unchanged | 68 | 83 | 44 | 80 | 7 | 88 | 11 | 100 | 3 | 60 | 3 | 100 |
| worse/dead | 14 | 17 | 11 | 20 | 1 | 13 | 0 | 0 | 2 | 40 | 0 | 0 |
Six patients were lost to follow-up. Of the remaining 82 patients, 71 (87%) had good outcomes (mRS Scores 0–2) and 6 (7%) had poor outcomes (mRS Scores 3–4). Relative to their preoperative neurological condition, 68 patients (83%) were unchanged or improved, and 14 patients (17%) were worse or dead after surgery.
The rate of transient neurological morbidity was highest in the medial and ventricular AVM types, but these patients had some of the best outcomes at late follow-up. The rates of permanent neurological deterioration were highest in the lateral and sylvian types.
Discussion
This report describes a large surgical experience in 88 patients with temporal lobe AVMs. There is no definitive classification for reporting these diverse lesions.8,14 The Yaşargil classification20 is well known, but has weaknesses. He divided temporal lobe AVMs into dorsal and ventral, and further subdivided ventral AVMs into mediobasal and laterobasal, but the boundaries are unclear. He included categories for giant and fistulous AVMs, which are based on unclear size and shunt criteria rather than on temporal lobe anatomy. Polar AVMs, although observed in 14% of patients in Yaşargil’s experience, were uncommon in our (2%) and others’ experience (4% in Nagata et al.).15 After reviewing the various other anatomical descriptions and categorizations of these AVMs, we synthesized them into 5 intuitive types based on Roper and Rhoton’s17 temporal lobe surfaces: lateral, basal, medial, sylvian, and ventricular. Surgical results with temporal lobe AVMs are generally good, and classifying temporal AVMs did not offer any meaningful prediction of surgical risk.6 However, this classification does offer some practical assistance for planning surgical strategy.
Lateral Temporal Lobe AVMs
Lateral AVMs were by far the most common type and the easiest to expose surgically for a perpendicular approach, with the base of the AVM nidus exposed circum-ferentially, and with feeding arteries and draining veins accessible at the margins of the nidus (Fig. 6). A pterional craniotomy was used for AVMs in front of the external auditory canal (24% of lateral AVMs), and a temporal craniotomy was used for AVMs behind the external auditory canal (76% of lateral AVMs). Patients were positioned supine with 45° of lateral head rotation for anterolateral AVMs and 90° of lateral head rotation for posterolateral AVMs. Temporal craniotomies were centered over the external auditory canal and typically extended inferiorly to the middle fossa floor. The dural opening is based on the pterion with pterional craniotomies, and inferiorly with temporal craniotomies.
Fig. 6.
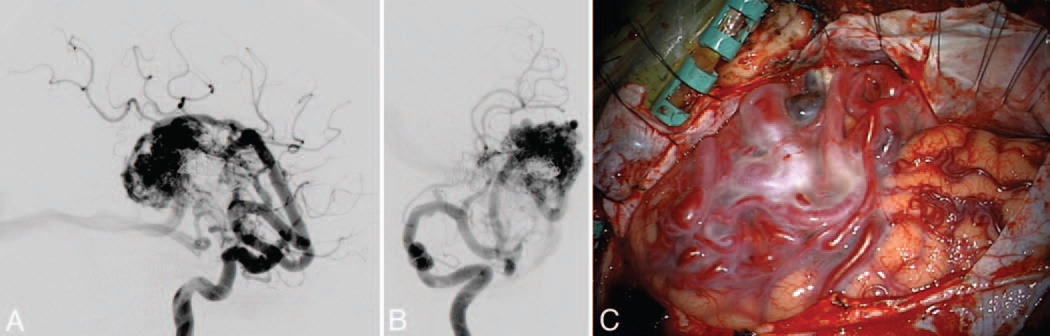
Lateral temporal AVM. This AVM (Spetzler-Martin Grade III+, supplementary Grade III) was supplied by large feeding arteries from the inferior trunk, as seen on DSAs (left ICA injection; lateral [A] and anteroposterior [B] views) after preoperative embolization with coils and glue. The lateral temporal lobe AVM (C) was exposed through an orbitozygomatic craniotomy for perpendicular access to its borders. The deep plane extended to the ependymal surface of the temporal horn of the lateral ventricle, where the AChA supplied the medial border.
Subarachnoid dissection defined the AVM borders, identified cortical feeding arteries as they joined the nidus, and isolated superficial draining veins as they left the nidus. Lateral AVMs in the superior temporal gyrus or large ones that extend to the sylvian surface required splitting the sylvian fissure. Lateral AVMs in the inferior temporal gyrus required opening the basal plane along the tentorium, cutting arachnoid adhesions and granulations. The Wernicke center is the only eloquent area on the lateral temporal surface, which is located in the superior temporal gyrus beyond 5 cm from the temporal pole, and in the middle temporal gyrus in some patients. Awake anesthesia and intraoperative stimulation mapping of speech function are indicated when speech function must be localized exactly.8
The STAs from the inferior trunk of the MCA are the main feeding arteries to these lateral AVMs. The dominant feeding arteries vary with nidus location in the lateral temporal lobe, and may be temporopolar, anterior temporal, middle temporal, posterior temporal, or tem-porooccipital arteries as the location shifts from anterior to posterior.7,18 The MCA feeding arteries emanate from the sylvian fissure and feed the nidus along its superior border. These are often large-caliber arteries that require aneurysm clips to occlude or temporary clips to stop flow during cauterization. The PTAs from the P2 segment of PCA become involved with lateral AVMs that are more inferiorly located on the lateral temporal surface. These feeders are identified subtemporally. The AChA is involved with larger AVMs that extend deep to the temporal horn of the lateral ventricle. Venous drainage is almost always superficial. The venous drainage can be ascending and anterior through superficial sylvian veins to the sphenoparietal sinus and cavernous sinus; drainage can be descending and posterior through anterior, middle, and posterior temporal veins to the vein of Labbé and the transverse sinus.
Basal Temporal Lobe AVMs
Like lateral AVMs, basal types are exposed with either a pterional craniotomy for anterior or a temporal craniotomy for posterior AVMs. However, the basal surface of the temporal lobe requires a subtemporal approach (Fig. 7). The head is positioned with more lateral neck flexion, with the vertex turned downward toward the floor, to allow the temporal lobe to fall away from the middle fossa floor. The inferior extent of the craniotomy is drilled flat with the middle fossa floor.
Fig. 7.
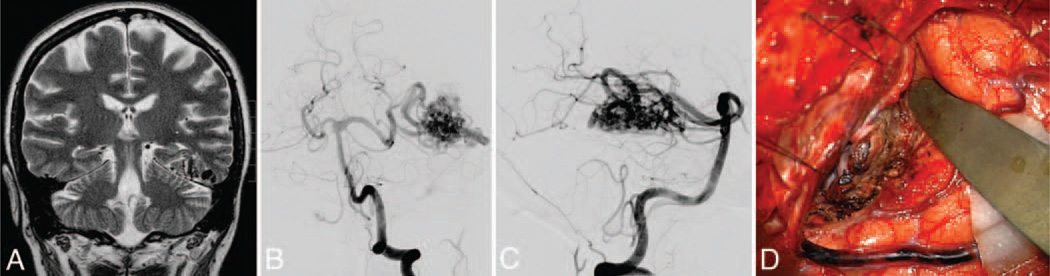
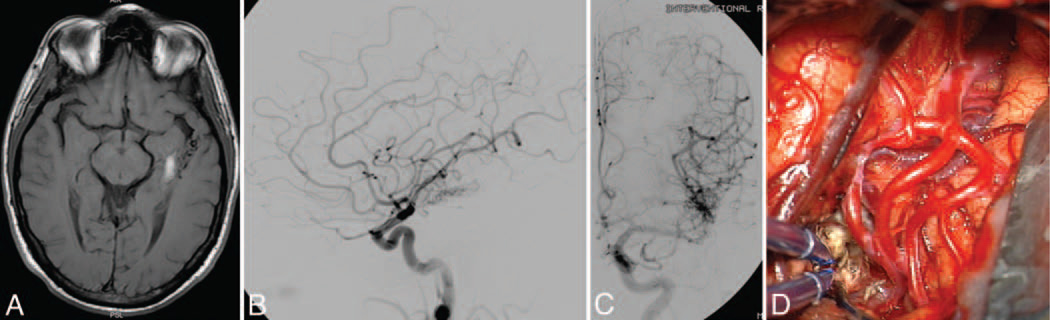
Basal temporal AVM. This AVM (Spetzler-Martin Grade III–, supplementary Grade III) was based on the basal surface of the temporal lobe (A), as seen on a coronal T2-weighted MRI study, and was supplied by posterior temporal branches of the PCA, as seen on DSAs (left VA injection; anteroposterior [B] and lateral [C] views). Basal temporal AVMs are not visible on the lateral temporal lobe, as exposed through a temporal craniotomy. Subtemporal dissection exposes the posterior temporal feeding arteries and draining temporal basal veins (D).
The important plane of dissection is the basal temporal surface, cutting adhesions to the tentorium and coagulating arachnoid granulations. Inferior temporal veins, including AVM draining veins, can bridge to the dura mater and are carefully protected. Release of these adhesions helps mobilize the temporal lobe, and release of CSF from the carotid and ambient cisterns slackens the brain. These maneuvers facilitate subtemporal retraction and bring the basal temporal surface into view, but in contrast to lateral temporal AVMs, it is a tangential rather than perpendicular view.5 Circumferential access is not available, and some feeding arteries and draining veins are outside the visible corridor of the approach.
There are no areas of eloquence on the basal temporal surface.17 When the nidus is not visible on the cortical surface, the draining vein can often be followed back to the nidus to localize the AVM. Feeding arteries are evenly split between MCA and PCA branches. The MCA branches are the ATA, MTA, and PTA that wrap around and under the lateral temporal surface, but are usually minor contributors. The PCA branches are the PTAs, which can arise as one large common temporal artery or as multiple branches that include hippocampal, anterior temporal, and middle temporal arteries, originating from the P2 segment. These inferior temporal arteries emanate from the crural and ambient cisterns and course over the tentorial incisura to supply the medial border of the nidus. These feeders are on the deep side of the nidus, and may not be accessible until after some circumferential dissection around the anterior and posterior sides of the nidus, later in the dissection. The basal surface is intimate with the dura of the middle fossa and had MMA feeders in nearly one-quarter of patients.
Basal temporal veins drain most AVMs on the basal surface. They are divided into anterior, middle, and posterior temporobasal veins, and course laterally to the vein of Labbé and the transverse sinus. One-third of AVMs had deep drainage via the BVR.
Medial Temporal Lobe AVMs
Medial AVMs are exposed tangentially with an orbitozygomatic craniotomy and transsylvian dissection,5 as with an approach to a BA bifurcation aneurysm. Removing the orbit opens a trajectory that is more anterior than with a pterional approach, creating a view along the medial temporal surface. Removing the zygoma facilitates temporal lobe retraction in a posterolateral direction that further opens this view along the medial temporal surface. Overall, the orbitozygomatic approach shortens the working distance to the AVM and widens the area of exposure. The medial temporal lobe is separated from its adhesions to the frontal lobe by opening the crural cistern and dissecting the AChA along its course around the uncus. This dissection leads to the AChA and PCA, the main feeders to these AVMs, running along the medial and inferior aspect of the nidus, respectively. Medial AVMs drain deep to the BVR, which enables the temporal pole to be retracted without sacrificing a critical draining vein. Arterial supply may also come from anterior thalamoper-forating vessels from the PCoA, temporopolar artery, and ATA from the MCA, and even LSAs in some anterior medial AVMs. The orbitozygomatic-transsylvian approach gives early access to AChA and P2 PCA along the medial border of the nidus. These vessels can be skeletonized to diminish AVM supply early in the dissection, while preserving normal branches from the cisternal segment of AChA that supply the optic tract and internal capsule, and normal branches from P2 PCA that include peduncular, circumflex, and thalamogeniculate perforators.
Involvement of the hippocampus in memory function makes these AVMs eloquent.2,4,17 Resection of some of the uncus and amygdala defines the lateral border of the AVM and deepens the resection. Larger AVMs may extend to the temporal horn of the lateral ventricle, and opening into the ventricle defines this lateral plane. The tangential trajectory of the orbitozygomatic-transsylvian approach obscures the superior and posterior margins of the AVM.5 These AVMs drain posteromedially through the BVR, which is often dark by the time it is visualized at the end of the dissection. Visual deficiencies from this tangential approach can be overcome with posterolateral temporal lobe retraction, which helps expose the medial temporal surface and make this more of a convexity approach.21
The posterior limit of safe exposure through an orbitozygomatic approach is the anterolateral edge of the cerebral peduncle. Medial AVMs that wrap around the posterolateral midbrain require approaches that are more subtemporal and transcortical (Fig. 8). They offer a more perpendicular approach to the AVM. When present, hematomas were used to open these corridors of exposure.10,11 This approach is like that described for the basal type AVMs, except that the approach is more medial, working through the fusiform or parahippocampal gyri rather than the inferior temporal gyrus. These AVMs are located medial to the temporal horn, and opening the ventricle widens access to the lateral border of the nidus. A transcortical corridor limits the operative view, and deep draining veins need to be traced medially to the ambient cistern to ensure that the medial extent of the nidus has been circumscribed. Transcortical approaches are also associated with increased risk to memory and language function.8,12 These approaches require some superior retraction on the temporal lobe, which can produce retraction injury or avulse the vein of Labbé. Tributaries to the vein of Labbé can cross the path of the cortical incision, necessitating separate incisions that straddle the veins.
Fig. 8.
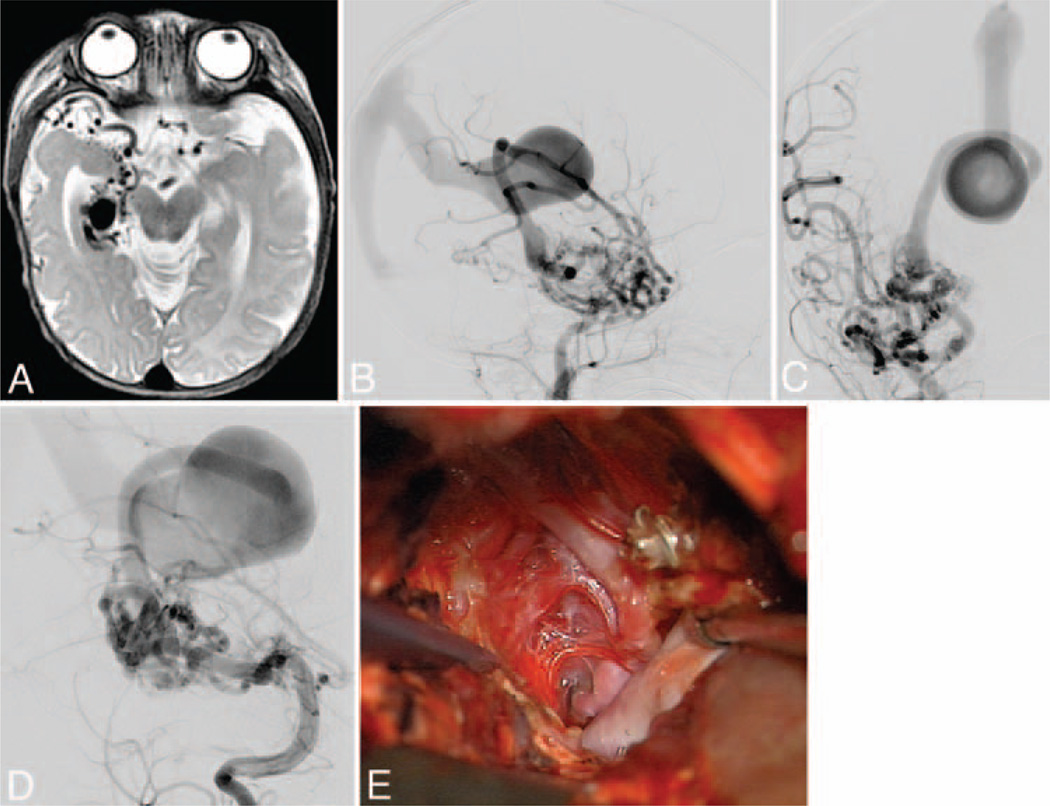
Medial temporal AVM. This AVM (Spetzler-Martin Grade III, supplementary Grade I) in a 3-year-old girl occupied the medial temporal lobe (A), as seen on an axial T2-weighted MRI study. It was supplied by AChA and PCoA branches as well as anterior and middle temporal branches of the MCA, as seen on DSAs (right ICA injection; lateral [B] and anteroposterior [C] views). Note the deep venous drainage to the BVR, which has a distal varix and drains into a persistent prosencephalic vein/falcine sinus. Posterior temporal branches from the PCA also fed the nidus (D), as seen on DSAs (right VA injection, anterior oblique view). The AVM was embolized extensively and exposed surgically through a temporal craniotomy, with resection of inferior temporal and occipitotemporal gyri to access the parahippocampus, lateral ventricle, and tentorial incisura. This transcortical dissection exposed the AVM’s lateral margin (E). A large posterior temporal feeding artery filled with coils was transected to access medial feeders from AChA, PCoA, and PCA. The arterialized BVR is seen under the sucker.
Sylvian Temporal Lobe AVMs
Sylvian AVMs are exposed with a pterional craniotomy and a wide sylvian fissure split. Superficial sylvian veins, the gatekeepers to the fissure, may be arterialized and are carefully preserved, including their connections to the sphenoparietal sinus and the vein of Labbé. Extensive subarachnoid dissection is performed to visualize the M1 segment, the inferior and superior trunks, and the course of the M2 insular segments. A wide overview of the sylvian vasculature identifies normal arteries that are uninvolved with the AVM, terminal feeding arteries, and transit arteries that supply the AVM but continue en passage to supply normal territories (Fig. 9). Wide splitting of the sylvian fissure also exposes the frontal opercula (pars orbitalis, triangularis, opercularis, precentral, and post-central gyri), temporal opercula (superior temporal and transverse temporal gyri), and insular cortex (long and short gyri). Sylvian AVMs in the temporal lobe are based on its superior surface, which is oriented vertically down the fissure. The nidus may be apparent on this cortical surface or it may lie just beneath, in which case an arterialized vein can offer a landmark. Temporal sylvian AVMs do not invade the insula or frontal cortex, or violate its pial layer. These AVMs are only eloquent when they are in the dominant hemisphere and posterior enough to involve the Wernicke center or the Heschl gyrus.
Fig. 9.
Sylvian temporal AVM. This AVM (Spetzler-Martin Grade III-, supplementary Grade III) is located on the sylvian surface of the temporal lobe, facing the sylvian fissure (A), as seen on an axial T1-weighted MRI study. The AVM is supplied by insular branches of the MCA, as seen on DSAs (left ICA injection; lateral [B] and anteroposterior [C] views). Left pterional cra-niotomy and wide splitting of the sylvian fissure exposed the MCA trifurcation and the arterialized vein beneath (D). By following the vein into the planum polare of the temporal lobe, the nidus was identified and circumdissected lateral to the inferior trunk, sparing the insula and frontal lobe.
These AVMs are supplied by the temporal arteries of the MCA (ATA, MTA, and PTA), either as large feeding trunks or insular branches. These arteries supply the anterior and superior margins of the AVM. The AChA supplies the medial margin when it extends to the temporal horn. The PCA feeding arteries are not typically major feeders to these AVMs. Venous drainage is superficial, via the sylvian veins (both superficial and deep).
Circumdissection of these AVMs is usually perpendicular, but the dissection is often conducted between branches of the MCA candelabra and between sylvian veins, which might also be draining veins. Terminal feeding arteries are typically high-flow vessels and are occluded at the AVM margins. Transit arteries are skeletonized, coagulating only the branches to the nidus, leaving the parent artery to continue past the AVM. Skeletonization in a distal-to-proximal direction ensures the preservation of the transit artery, which often continues to supply the sensorimotor strip, speech centers, and conduction pathways in the dominant hemisphere.
Ventricular Temporal Lobe AVMs
Ventricular AVMs in the temporal lobe are rare, and were the least common in our experience (3%). These AVMs do not present to 1 of the 4 surfaces of the temporal lobe, and therefore require a transcortical approach without any subarachnoid dissection. The temporal horn of the lateral ventricle parallels the middle temporal gyrus. However, optic radiations also parallel the middle temporal gyrus, exiting the lateral geniculate nucleus and wrapping around the lateral wall of the temporal horn in the Meyer loop. Therefore, the ventricle is approached through the inferior temporal gyrus to avoid injury to the Meyer loop and a superior visual quadrant deficit, staying as anteriorly as possible. A low entry avoids the Wernicke center. The trajectory to the ventricle requires a temporal craniotomy without extension to the middle fossa floor or subtemporal retraction.
A generous corticectomy in the inferior temporal gyrus and an ascending approach will lead to the temporal horn. Cortical draining veins on the lateral or basal surfaces can guide the approach to the ventricle. Once entered, the ventricle is opened widely along its axis and the choroidal arteries are identified medially as they enter the ventricle through the choroid fissure. The AChA is coagulated anterior to the nidus and the PChA is coagulated posterior to the nidus. The AVM is often within the ventricle and based on the choroid plexus, with CSF rather than the brain defining the planes of dissection. Venous drainage is often mixed, with superficial drainage to temporobasal or lateral temporal veins and deep drainage to BVR. The AVMs that drain medially can have a nidus medial to the ventricle, which should be dissected thoroughly. The superior border is the most difficult one to visualize, and medial feeders can be difficult to control along this plane. Transcortical exposures can be narrow and deep, and must be widened if exposure is inadequate.
Conclusions
Anatomical subtypes of temporal AVMs can assist with surgical planning and also standardize reporting. Lateral AVMs are common and easily exposed surgically, with perpendicular access to feeding arteries and draining veins. Basal, medial, and sylvian AVMs require more complex approaches with tangential exposures and more limited access to feeding arteries and draining veins. Ventricular AVMs are rare and require a transcortical approach through the inferior temporal gyrus that avoids visual tracts. Surgical results with temporal lobe AVMs are generally good, and classifying them does not offer any prediction of surgical risk.
Acknowledgments
This project is partially supported by NIH Grant Nos. R01 NS27713 and P01 NS44155 to Dr. Young. Dr. Rodríguez-Hernández is supported by a grant from La Caixa Foundation.
Abbreviations used in this paper
- AchA
anterior choroidal artery
- AcoA
anterior communicating artery
- ATA
anterior temporal artery
- AVM
arteriovenous malformation
- BA
basilar artery
- BVR
basal vein of Rosenthal
- DSA
digital subtraction angiogram
- ICA
internal carotid artery
- LSA
lenticulostriate artery
- MCA
middle cerebral artery
- MMA
middle meningeal artery
- mRS
modified Rankin Scale
- MTA
middle temporal artery
- PCA
posterior cerebral artery
- PcoA
posterior communicating artery
- PTA
posterior temporal artery
- STA
superior temporal artery
- UCSF
University of California, San Francisco
- VA
vertebral artery
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Lawton, Gabarrós Canals. Acquisition of data: Gabarrós Canals, Rodríguez-Hernández. Analysis and interpretation of data: Lawton, Gabarrós Canals, Rodríguez-Hernández. Drafting the article: Lawton, Gabarrós Canals, Rodríguez-Hernández. Critically revising the article: all authors. Re viewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Lawton. Statistical analysis: Rodríguez-Hernández. Administrative/technical/material support: Rodríguez-Hernández, Young. Study supervision: Lawton.
References
- 1.Boström A, Schaller K, Seifert J, Schramm J. The place for surgical treatment for AVM involving the temporal lobe. Acta Neurochir (Wien) 2011;153:271–278. doi: 10.1007/s00701-010-0885-1. [DOI] [PubMed] [Google Scholar]
- 2.Davies JM, Kim H, Young WL, Lawton MT. Classification schemes for arteriovenous malformations. Neurosurg Clin N Am. 2012;23:43–53. doi: 10.1016/j.nec.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 3.de Oliveira E, Tedeschi H, Siqueira MG, Ono M, Rhoton AL, Jr., Peace D. Anatomic principles of cerebrovascular surgery for arteriovenous malformations. Clin Neurosurg. 1994;41:364–380. [PubMed] [Google Scholar]
- 4.Du R, Dowd CF, Johnston SC, Young WL, Lawton MT. In-terobserver variability in grading of brain arteriovenous malformations using the Spetzler-Martin system. Neurosurgery. 2005;57:668–675. [PubMed] [Google Scholar]
- 5.Du R, Young WL, Lawton MT. “Tangential” resection of medial temporal lobe arteriovenous malformations with the orbi-tozygomatic approach. Neurosurgery. 2004;54:645–652. doi: 10.1227/01.neu.0000109043.56063.ba. [DOI] [PubMed] [Google Scholar]
- 6.Englot DJ, Young WL, Han SJ, McCulloch CE, Chang EF, Lawton MT. Seizure predictors and control after microsurgical resection of arteriovenous malformations in 440 patients. Neurosurgery. 2012;71:572–580. doi: 10.1227/NEU.0b013e31825ea3ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Miranda JC, de Oliveira E, Rubino PA, Wen HT, Rhoton AL., Jr. Microvascular anatomy of the medial temporal region: part 1: its application to arteriovenous malformation surgery. Neurosurgery. 2010;67(3 Suppl Operative):ons237–ons276. doi: 10.1227/01.NEU.0000381003.74951.35. [DOI] [PubMed] [Google Scholar]
- 8.Gabarrós A, Young WL, McDermott MW, Lawton MT. Language and motor mapping during resection of brain arteriovenous malformations: indications, feasibility, and utility. Neu rosurgery. 2011;68:744–752. doi: 10.1227/NEU.0b013e318207a9a7. [DOI] [PubMed] [Google Scholar]
- 9.Lawton MT. Seven Aneurysms: Tenets and Techniques for Clipping. New York: Thieme Medical Publishers; 2011. [DOI] [PubMed] [Google Scholar]
- 10.Lawton MT, Du R, Tran MN, Achrol AS, McCulloch CE, Johnston SC, et al. Effect of presenting hemorrhage on outcome after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2005;56:485–493. doi: 10.1227/01.neu.0000153924.67360.ea. [DOI] [PubMed] [Google Scholar]
- 11.Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010;66:702–713. doi: 10.1227/01.NEU.0000367555.16733.E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobato RD, Campollo J, Lagares A, Gómez PA, Ramos A, Al-day R, et al. [Arteriovenous malformation of the middle and posterior third section of the corpus callosum treated with embolization and surgery] Neurocirugia (Astur) 2002;13:209–215. doi: 10.1016/s1130-1473(02)70619-2. Span. [DOI] [PubMed] [Google Scholar]
- 13.Malik GM, Seyfried DM, Morgan JK. Temporal lobe arteriovenous malformations: surgical management and outcome. Surg Neurol. 1996;46:106–115. doi: 10.1016/0090-3019(96)00084-5. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz F, Clavel P, Molet J, Castaño C, de Teresa S, Solivera J, et al. [Current management of arteriovenous malformations. Retrospective study of 31 cases and literature review] Neurocirugia (Astur) 2007;18:394–405. Span. [PubMed] [Google Scholar]
- 15.Nagata S, Morioka T, Matsukado K, Natori Y, Sasaki T. Retrospective analysis of the surgically treated temporal lobe arteriovenous malformations with focus on the visual field defects and epilepsy. Surg Neurol. 2006;66:50–55. doi: 10.1016/j.surneu.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Potts MB, Chang EF, Young WL, Lawton MT. Transsylviantransinsular approaches to the insula and basal ganglia: operative techniques and results with vascular lesions. Neurosurgery. 2012;70:824–834. doi: 10.1227/NEU.0b013e318236760d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roper SN, Rhoton AL., Jr. Surgical anatomy of the temporal lobe. Neurosurg Clin N Am. 1993;4:223–231. [PubMed] [Google Scholar]
- 18.Wen HT, Rhoton AL, Jr., de Oliveira E, Castro LHM, Figueiredo EG, Teixeira MJ. Microsurgical anatomy of the temporal lobe: part 2—sylvian fssure region and its clinical application. Neurosurgery. 2009;65(6 Suppl):1–36. doi: 10.1227/01.NEU.0000336314.20759.85. [DOI] [PubMed] [Google Scholar]
- 19.Yamada S, Brauer F, Dayes L, Yamada S. Surgical techniques for arteriovenous malformations in functional areas: focus on the superior temporal gyrus. Neurol Med Chir (Tokyo) 1998;38(Suppl):222–226. doi: 10.2176/nmc.38.suppl_222. [DOI] [PubMed] [Google Scholar]
- 20.Yaşargil MG. Microneurosurgery: AVM of the Brain, Clin -ical Considerations, General and Special Operative Tech niques, Surgical Results, Nonoperated Cases, Cavernous and Venous Angiomas, Neuroanesthesia. Vol IIIB New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 21.Zador Z, Lu DC, Arnold CM, Lawton MT. Deep bypasses to the distal posterior circulation: anatomical and clinical comparison of pretemporal and subtemporal approaches. Neurosurgery. 2010;66:92–101. doi: 10.1227/01.NEU.0000362034.81037.FC. [DOI] [PubMed] [Google Scholar]