Abstract
Background:
Rotator cuff pathology is a common source of shoulder pain with variable etiology and pathoanatomical characteristics. Pathological processes of fatty infiltration, muscle atrophy, and fibrosis have all been invoked as causes for poor outcomes after rotator cuff tear repair. The aims of this study were to measure the expression of key genes associated with adipogenesis, myogenesis, and fibrosis in human rotator cuff muscle after injury and to compare the expression among groups of patients with varied severities of rotator cuff pathology.
Methods:
Biopsies of the supraspinatus muscle were obtained arthroscopically from twenty-seven patients in the following operative groups: bursitis (n = 10), tendinopathy (n = 7), full-thickness rotator cuff tear (n = 8), and massive rotator cuff tear (n = 2). Quantitative polymerase chain reaction (qPCR) was performed to characterize gene expression pathways involved in myogenesis, adipogenesis, and fibrosis.
Results:
Patients with a massive tear demonstrated downregulation of the fibrogenic, adipogenic, and myogenic genes, indicating that the muscle was not in a state of active change and may have difficulty responding to stimuli. Patients with a full-thickness tear showed upregulation of fibrotic and adipogenic genes; at the tissue level, these correspond to the pathologies most detrimental to outcomes of surgical repair. Patients with bursitis or tendinopathy still expressed myogenic genes, indicating that the muscle may be attempting to accommodate the mechanical deficiencies induced by the tendon tear.
Conclusions:
Gene expression in human rotator cuff muscles varied according to tendon injury severity. Patients with bursitis and tendinopathy appeared to be expressing pro-myogenic genes, whereas patients with a full-thickness tear were expressing genes associated with fatty atrophy and fibrosis. In contrast, patients with a massive tear appeared to have downregulation of all gene programs except inhibition of myogenesis.
Clinical Relevance:
These data highlight the difficulty in treating massive tears and suggest that the timing of treatment may be important for muscle recovery. Specifically, earlier interventions to address tendon injury may allow muscles to respond more appropriately to mechanical stimuli.
Rotator cuff pathology is a common clinical problem. Approximately ten per 1000 patients seen by primary care physicians present with shoulder pain1; of these patients, 74% display signs of impingement, and 85% present with painful or torn rotator cuff muscle(s). In addition, a recent cadaveric study showed that the prevalence of full-thickness tears is approximately 30% in those over sixty years of age and is correlated with age2. Importantly, rotator cuff tears may lead to irreversible changes in the structure and physiological properties of the muscles3-5. Although repair of small tears may be successful in relieving pain and improving muscle biomechanics, repair of large and massive tears remains a challenge6,7. Several reasons likely underlie poor surgical outcomes, including the development of fatty infiltration of the muscle8,9, increased fibrosis7,10, and muscle atrophy8,11. Understanding the molecular basis of these muscular changes would help us to understand rotator cuff pathology, and it may subsequently lead to treatment strategies to prevent its initiation and/or progression.
There are many transcriptional pathways that control various aspects of the adipogenic, fibrogenic, and myogenic programs, and there is cross-talk between these pathways. In the adipogenesis program, CCAAT/enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptor γ (PPARG) coordinate the generation of mature adipocytes as well as triglyceride synthesis12-14. C/EBPs and PPARG can also regulate muscle differentiation15,16 and fibrosis17-19. In the myogenesis program, myogenic differentiation 1 (MYOD1) and myogenic factor 5 (MYF5) are important for myogenic differentiation of satellite cells, myoblast commitment, and induction of myogenin (MYOG, which helps control the formation of myotubes and normal muscle development)20. On the other hand, myostatin (MSTN), which is secreted by muscle, inhibits myogenesis21 and muscle hypertrophy22-24 (via regulation of mammalian target of rapamycin [MTOR]) but promotes fibrosis25,26 and adipogenesis21. An animal model of rotator cuff injury has also shown that MTOR modulates fatty infiltration27, and it regulates fibrosis28-30. Growth factors such as transforming growth factor β1 (TGFB1) and connective tissue growth factor (CTGF) are considered master regulators of fibrosis, which in simple terms represents an imbalance between the breakdown of extracellular matrix proteins by matrix metalloproteinases (MMPs) and the activity of tissue inhibitors of metalloproteinase (TIMPs), which inhibit MMPs31. Although there have been some studies of gene expression during muscle degeneration in animal models of the rotator cuff27,32-34, this process is poorly understood in humans; we are aware of only one study investigating the role of atrophy-related gene expression in human rotator cuff muscle35.
In the present study, the expression levels of five myogenic, six adipogenic, and nine fibrogenic genes were analyzed in the supraspinatus muscles of patients with bursitis, tendinopathy, a full-thickness tear, or a massive tear to understand the expression patterns reflecting the regulatory mechanisms of the factors that affect muscle mass and health in these patients. We broadly hypothesized that these families of genes are differentially expressed among patients who differ with respect to rotator cuff tendon tear severity. Specifically, we hypothesized that expression of myogenic genes would be lower in patients with a more severe rotator cuff tear than in patients with a less severe tear, whereas the reverse would be true for expression of adipogenic and fibrogenic genes. Overall, an understanding of these gene expression patterns and complex muscular adaptations to a tear may lead to the development of new human therapeutics that may alter current treatment.
Materials and Methods
Subjects
Under an institutional review board-approved protocol, biopsies of the supraspinatus were obtained arthroscopically from twenty-seven patients (fourteen male and thirteen female; mean age [and standard deviation], 55 ± 9 years) by one surgeon (J.G.L.). This muscle was selected because it is the mostly commonly injured rotator cuff muscle. Rotator cuff pathology was categorized on the basis of intraoperative observations and MRI (magnetic resonance imaging) findings36 as bursitis in ten patients, tendinopathy in seven, a full-thickness tear in eight, and a massive tear in two (Table I). Patients in the bursitis group were identified on the basis of the presence of inflamed tissue and gross synovitis. Patients in the tendinopathy group exhibited rotator cuff tendon fraying, thinning, or delamination, none of which required surgical repair. Patients in the full-thickness and massive tear groups were identified on the basis of a complete tendon detachment from the bone that required surgical repair, with a massive tear resulting in retraction of the musculotendinous unit to the level of the glenoid.
TABLE I.
Subject Demographics
| Age* (yr) | M/F | Body Mass Index* (kg/m2) | Diabetes, Y/N | |
| Bursitis, n = 10 | 50.00 ± 7.39 | 5/5 | 30.13 ± 5.10 | 2/8 |
| Tendinopathy, n = 7 | 54.14 ± 9.00 | 3/4 | 28.72 ± 5.00 | 2/5 |
| Full-thickness tear, n = 8 | 61.29 ± 6.24 | 4/4 | 28.37 ± 3.72 | 4/4 |
| Massive tear, n = 2 | 55.00 ± 3.54 | 2/0 | 33.02 ± 7.14 | 0/2 |
The values are given as the mean and the standard deviation.
Muscle Sampling
Muscle biopsy samples were obtained with an arthroscopic rongeur under direct observation, and post-biopsy muscle bleeding confirmed that the sample was from viable muscle. Muscle samples were immediately placed in RNAlater (QIAGEN, Valencia, California) on ice, then stored at −80°C until further processing.
qPCR (Quantitative Polymerase Chain Reaction)
Total RNA was isolated from muscle with use of an RNeasy kit (QIAGEN). Briefly, approximately 10 to 30 mg of tissue was pulverized in the presence of liquid nitrogen with use of a Cryopress (MicroTech-Nichion, Funabashi, Chiba, Japan). One milliliter of QIAzol lysis reagent and 200 μL of chloroform were added, and the sample was vortexed for five minutes and incubated on ice for thirty minutes. The sample was centrifuged for fifteen minutes at 10,000 rpm, the aqueous phase was removed, and an equal volume of 70% ethanol was added. The sample was immediately loaded onto an RNeasy column and centrifuged for thirty seconds at 9000 rpm, followed by two washing steps (with the buffers provided with the kit). RNA was eluted with 30 μL of RNase and DNase-free water. The RNA concentration was measured with use of a NanoDrop ND-1000 spectrophotometer (Wilmington, Delaware). Complementary DNA (cDNA) was prepared by reverse-transcribing 5 μg of total RNA to cDNA with use of a QuantiTect Reverse Transcription Kit (QIAGEN), and 2 μL of the cDNA was used for qPCR utilizing validated, gene-specific primers (QIAGEN) and the SYBR Green (QIAGEN) method. Standards were made by cloning the PCR products into the pDrive vector with use of a PCR cloning kit (QIAGEN). The results were expressed as molecules/mL and were normalized to the molecular concentration of 18S rRNA (ribosomal RNA).
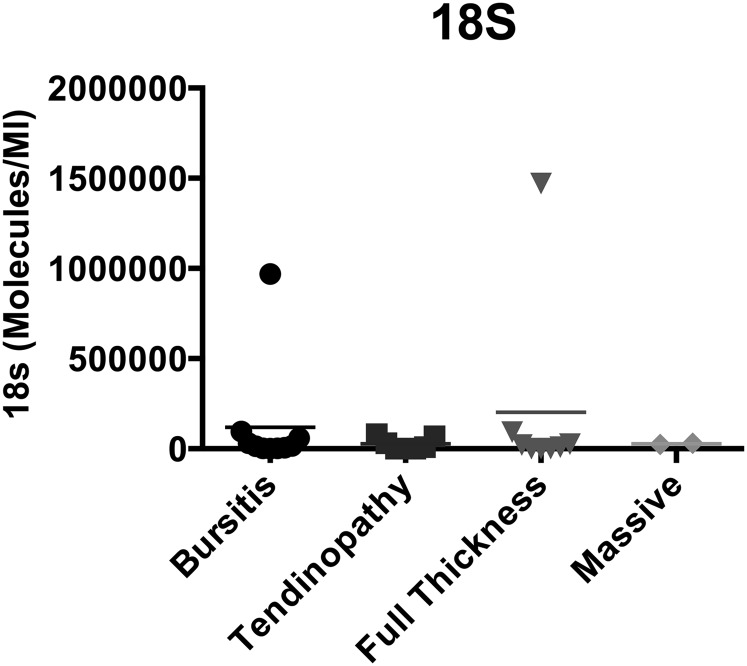
Selected genes (see Appendix) related to myogenesis (MYOD1, MYF5, MYOG, MTOR, and MSTN) were quantified in each sample and compared among the groups. Similarly, selected genes related to adipogenesis (PPARG, PPARD [PPAR delta], CEBPA [C/EBP alpha], ADIPOQ [adiponectin], LEP [leptin], and WNT10B) and to fibrosis (TGFB1, CTGF, COL1A1 [collagen type 1 A1], COL3A1 [collagen type 3 A1], TIMP1, TIMP3, MMP1, MMP3, and MMP9) were quantified and compared. The selection of these genes was based on previous reports of relevant gene expression in animal models of rotator cuff tears27,34,35,37 and a review of the literature related to muscle adipogenesis and muscle fibrosis31. Although measurement of expression of a larger number of genes would have been ideal, the size of the muscle sample collected, and therefore the amount of RNA that could be extracted, prohibited a broader assessment of the transcriptional profile. In preliminary experiments, expression of the 18S transcript was more stable than that of GAPDH (glyceraldehyde 3-phosphate dehydrogenase), so we chose to perform the normalization relative to this housekeeping gene. However, there was some variability in 18S expression among the sample groups (Fig. 1).
Fig. 1.
Expression of the 18S rRNA housekeeping gene in the biopsy sample from each patient, showing that two of the samples had values an order of magnitude greater than those in the other samples. The horizontal lines represent the means in the four rotator cuff pathology groups.
Data Analysis
Normalized gene expression was compared among groups with use of one-way ANOVA (analysis of variance) with post hoc Tukey testing. A p value of <0.05 was considered significant. All analyses were performed with SPSS software (version 21.0; IBM, Armonk, New York).
Source of Funding
Financial support for this work was provided by the Orthopaedic Research and Education Foundation and NIH (National Institutes of Health) grants R24 HD050837 and R01 HD073180.
Results
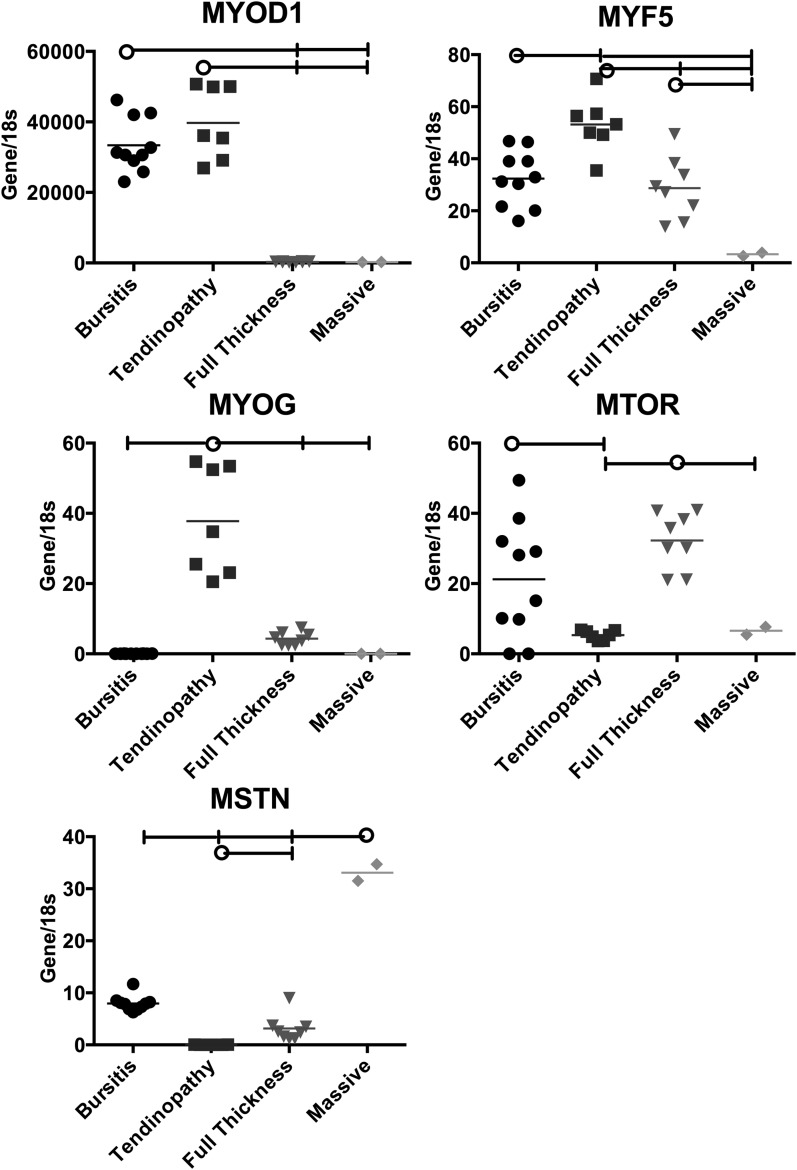
The myogenic gene expression patterns varied among the groups (Fig. 2). Expression of a gene involved with early determination (MYOD1) was greater in the bursitis group compared with the full-thickness and massive tear groups, and expression of a gene involved with differentiation (MYF5) was also greater in the bursitis group compared with the massive tear group. Interestingly, the bursitis group also exhibited greater expression of a gene related to muscle hypertrophy (MTOR) compared with the tendinopathy group. The tendinopathy group showed greater expression of genes related to myogenic determination (MYOD1) and myogenic differentiation (MYF5 and MYOG) compared with the full-thickness and massive tear groups. The full-thickness tear group showed greater expression of a gene related to muscle hypertrophy (MTOR) compared with the tendinopathy and massive tear groups, but the level was similar to that in the bursitis group. Perhaps most strikingly, the massive tear group demonstrated the lowest expression of all genes except MSTN (which was three to twentyfold greater than that in all other groups).
Fig. 2.
Expression of myogenesis genes relative to 18S rRNA in the samples from each rotator cuff pathology group. The horizontal lines at the tops of the panels indicate significant differences between the group identified with the circle and the groups identified with a vertical tick mark. The horizontal lines within the data points represent the means in the four rotator cuff pathology groups. MYOD1, MYF5, MYOG, and MTOR upregulate the myogenesis pathway. MSTN downregulates myogenesis.
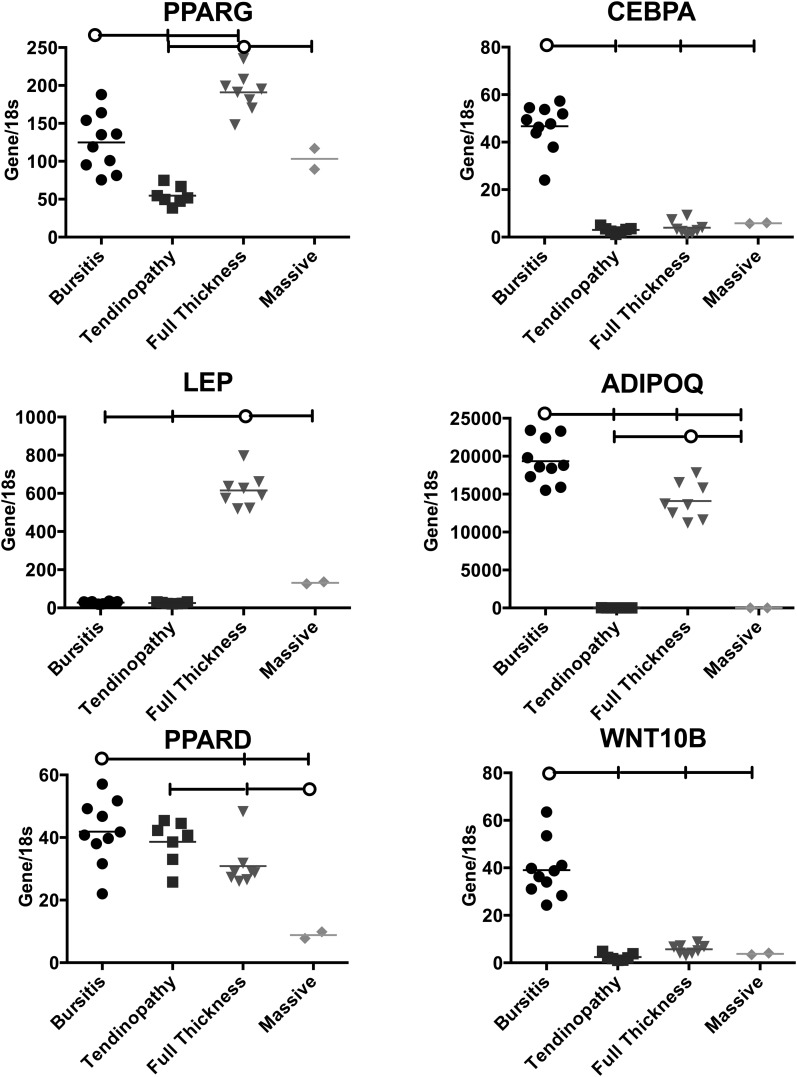
The bursitis group had greater expression of two adipogenic genes, CEBPA (a promoter of adipogenesis) and WNT10B (a potent inhibitor of adipogenesis), compared with the other groups (Fig. 3). The tendinopathy group expressed relatively low levels of all adipogenic genes except PPARD, which is associated with adipogenic inhibition. The full-thickness tear group showed slightly higher expression of PPARG and a large increase in LEP compared with other groups. Again, the massive tear group demonstrated low gene expression compared with the other groups except in the case of PPARG.
Fig. 3.
Expression of adipogenesis genes relative to 18S rRNA in the samples from each rotator cuff pathology group. The horizontal lines at the tops of the panels indicate significant differences between the group identified with the circle and the groups identified with a vertical tick mark. The horizontal lines within the data points represent the means in the four rotator cuff pathology groups. PPARG, CEBPA, ADIPOQ, and LEP upregulate the adipogenesis pathway. WNT10B and PPARD downregulate adipogenesis.
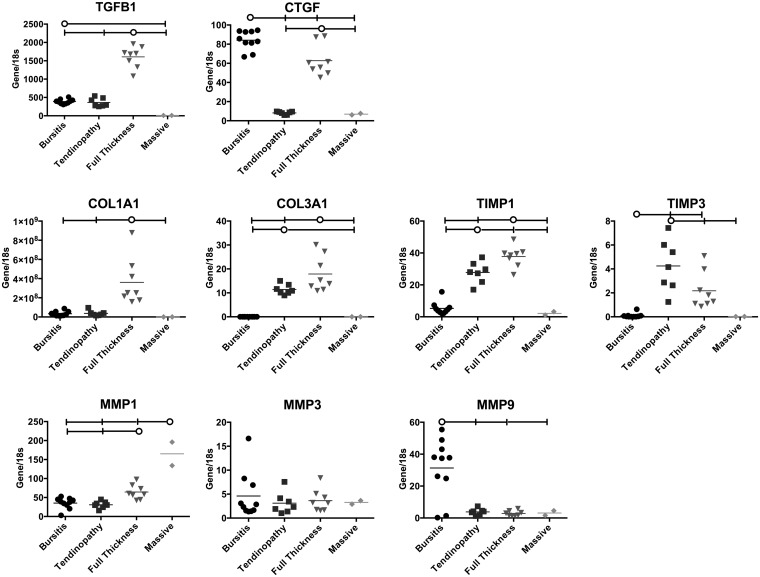
The gene expression patterns related to fibrosis were also interesting (Fig. 4). The bursitis group had greater expression of CTGF and MMP9 (involved in collagen breakdown) compared with all other groups. The tendinopathy and full-thickness tear groups demonstrated greater expression of COL3A1, TIMP1, and TIMP3 compared with the bursitis and massive tear groups. However, COL1A1, COL3A1, and TIMP1 were greater in the full-thickness tear group than in the tendinopathy group, and TIMP3 was greater in the tendinopathy group compared with the full-thickness tear group. The full-thickness tear group also showed greater expression of TGFB1 and CTGF, suggesting an ongoing inflammatory process in this group but not in the tendinopathy group. Finally, the massive tear group again showed lower expression of all genes except MMP1.
Fig. 4.
Expression of fibrosis genes relative to 18S rRNA in the samples from each rotator cuff pathology group. The horizontal lines at the tops of the panels indicate significant differences between the group identified with the circle and the groups identified with a vertical tic mark. The horizontal lines within the data points represent the means in the four rotator cuff pathology groups. TGFB1, CTGF, COL1A1, and COL3A1 upregulate the fibrosis pathway. MMP1, MMP3, and MMP9 break down fibrotic tissue (therefore downregulating fibrosis) and are inhibited by TIMP1 and TIMP3 (which therefore upregulate fibrosis).
Discussion
The purpose of this study was to understand the gene expression patterns in human supraspinatus muscles with various degrees of tendon injury. The data demonstrated that myogenic, adipogenic, and fibrogenic gene expression patterns are related to rotator cuff disease severity. First, the bursitis group demonstrated increased expression of genes involved in early myogenic determination (MYOD1), differentiation from satellite cells to myoblasts (MYF5), and muscle growth due to terminally differentiated myocytes (MTOR). Similarly, the tendinopathy group demonstrated increased expression of genes involved in early differentiation from satellite cells to myoblasts (MYOD1 and MYF5) and intermediate differentiation from myoblasts to terminally differentiated myocytes (MYOG). These data indicate that muscles with the milder types of rotator cuff injury are capable of, and in the process of, generating a repair or recovery process beginning from the earliest stages of muscle development, probably by recruitment of satellite cells. The full-thickness tear group showed increased expression of MTOR, which is related to muscle hypertrophy from terminally differentiated myocytes, and this may indicate a recovery response that is less robust than that in milder injuries. Perhaps most importantly, the massive tear group showed decreased expression of all genes related to myogenesis except MSTN, which is a potent inhibitor of myogenesis. These data indicate that severely (and perhaps more chronically) injured muscles are in an active state of muscle inhibition. This has been observed previously in human subjects with rotator cuff tears35, although the expression data presented here indicate a much greater ratio between muscles with a massive tear and less severe injury states as well as a more obvious effect when massive tears are compared with full-thickness tears. Similarly, upregulation of MSTN has been observed previously in animal models of rotator cuff injury, although the relative change compared with controls was extremely small38.
In the context of adipogenesis, the bursitis group had elevated gene expression of CEBPA and ADIPOQ (proadipogenic) but also a very large increase in WNT10B, which is a potent inhibitor of adipogenesis. These data indicate that there may be competing gene expression patterns that mitigate fatty deposition in these muscles. This is in contrast to the full-thickness tear group, which had increased expression of PPARG, LEP, and ADIPOQ compared with the other groups and no concomitant increase in WNT10B to suppress fat deposition. These data indicate that the full-thickness tear group is perhaps actively depositing fat in the muscle. Again, the massive tear group had relatively low levels of expression of this family of genes, indicating that fat deposition is no longer an active process in the muscle. Although we are aware of no human rotator cuff data regarding gene expression markers of adipogenesis, some data are available in animals. These data suggest similar trends to those observed in our study, although the relative differences between injured and control animals were much smaller and appear to be related to the addition of neurotomy38.
The family of genes related to fibrosis showed evidence of profibrotic activity in the tendinopathy group (COL3A1, TIMP1, and TIMP3). However, the full-thickness tear group showed increased gene expression of TGFB1 and CTGF, which are involved in inflammation, and COL1A1, COL3A1, TIMP1, and TIMP3, which are related to collagen synthesis and suppression of collagen breakdown. These data indicate that muscle fibrosis may begin as early as clinical tendinopathy and is likely fully underway in full-thickness tears. Again, the massive tear group demonstrated relatively low levels of expression of the genes in this family with the exception of MSTN, which has been shown to directly promote fibrosis and, as mentioned previously, inhibit myogenesis. This may indicate that the process of fibrosis has been completed in muscles with such an injury. We are aware of no previous data involving human rotator cuff muscles with which these values can be compared; however, the expression levels of TGFB1 and COL1A1 do parallel those observed in rodent models of complete tears34. Importantly, the values that we measured in massive tears are much lower than those observed in any of the animal models.
In order to relate gene expression to the clinical presentation of the individual patients, we examined all available MRI studies and quantified muscle and fat volumes and fractions in the suprascapular fossa with use of previously defined methods39. As all of our patients fell into the Goutallier stage-I and II categories despite the variability in tendon tear size, there were no significant correlations between gene expression and MRI-based measures of fatty infiltration. However, the failure to relate the gene expression and clinical results is most likely the result of an inadequate sample size relative to the small range in the amounts of muscle and fat. Many more subjects with a much wider range of structural adaptations will be needed to identify such correlations.
There are a number of limitations to this study. First, there was no control group. We explored the idea of obtaining muscle biopsy samples from patients without rotator cuff injury but ultimately decided against it because (1) it was considered unethical to explore the subacromial space without indication, and (2) we suspected that these patients would likely have pain that limited mobility and may therefore have altered gene expression patterns. We also chose to avoid using a rotator cuff muscle other than the supraspinatus as a control because we believe that there are inherent differences in gene expression among muscles40 and that the rotator cuff muscles have unique architectural features that influence their mechanosensitivity41. Second, there were relatively few patients in the massive tear group. We explored the idea of obtaining biopsies from patients scheduled for total shoulder arthroplasty but decided that such patients would not be representative of patients with a massive tear that was to undergo arthroscopic reconstruction. Third, we do not know the relative fractions of the various cell types in our biopsy samples. Although we know that each biopsy sample was obtained from viable muscle, it is unclear whether the patients with a more severe tear had greater fractions of fat or fibroblast cell types. This is a topic of much experimental work in our laboratory at this time. We are exploring the use of cell sorting and flow cytometry to address this question experimentally, but multinucleated muscle cells are excluded with each technique and there are no generally accepted fibroblast markers. Thus, although we obtained expression data in the present study, we were unable to ascertain the cellular source(s) of the individual transcripts. It is possible that an immunohistochemical approach will help answer this important question in the future. Fourth, similar to the situation in any gene expression study, the relative importance of each gene in a family is different. For example, a fivefold increase in one gene is not necessarily less important than a tenfold increase in another gene of the same program because the relative influence of each gene is unknown. Finally, the gene transcripts explored in this study represent an incomplete list of those known to be important in muscle plasticity, and information on their temporal regulation cannot be gleaned from patients with an unknown tear duration. Also, in future studies it will be important to measure protein levels and activation state (e.g., phosphorylation level) to support our gene expression data.
In conclusion, to our knowledge this is the first study to quantify myogenic, adipogenic, and fibrogenic gene expression in human rotator cuff muscles across a range of pathology severities, and it revealed varied gene expression patterns among these different groups. Specifically, patients with a massive tear demonstrated downregulation of adipogenic and myogenic genes, indicating that the muscle is not in a state of active change and may have difficulty responding to growth stimuli. Patients with a full-thickness tear showed upregulation of fibrotic and adipogenic genes, which, at the tissue level, are associated with the pathologies most detrimental to outcomes. Patients with bursitis or tendinopathy were still expressing myogenic genes, indicating that the muscle may be in a state of hypertrophy to accommodate the mechanical deficiencies induced by the tendon injury. Clinically, these data may shed some light on the difficulty in treating massive tears and suggest that the timing of treatment may be important for muscle recovery. Cases of severe or chronic injury may require a pharmacologic, biologic, or biomechanical treatment to the muscle to “jump-start” the hypertrophy or regeneration process.
Appendix
A table showing the genes and PCR primers used in the study is available with the online version of this article as a data supplement at jbjs.org.
Footnotes
Investigation performed at the Departments of Orthopaedic Surgery, Bioengineering, and Radiology, University of California San Diego, La Jolla, California
Alexander Choo, MD, and Meagan McCarthy, MD, contributed equally to the writing of this article.
A commentary by Theodore A. Blaine, MD, is linked to the online version of this article at jbjs.org.
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Ostör AJ, Richards CA, Prevost AT, Speed CA, Hazleman BL. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology (Oxford). 2005June;44(6):800-5 Epub 2005 Mar 15 [DOI] [PubMed] [Google Scholar]
- 2.Lehman C, Cuomo F, Kummer FJ, Zuckerman JD. The incidence of full thickness rotator cuff tears in a large cadaveric population. Bull Hosp Jt Dis. 1995;54(1):30-1 [PubMed] [Google Scholar]
- 3.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000April;82(4):505-15 [DOI] [PubMed] [Google Scholar]
- 4.Goutallier D, Postel JM, Lavau L, Bernageau J. [Impact of fatty degeneration of the supraspinatus and infraspinatus msucles on the prognosis of surgical repair of the rotator cuff] [French]. Rev Chir Orthop Reparatrice Appar Mot. 1999November;85(7):668-76 [PubMed] [Google Scholar]
- 5.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007May;35(5):719-28 Epub 2007 Mar 2 [DOI] [PubMed] [Google Scholar]
- 6.Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM. Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Joint Surg Am. 2001January;83(1):71-7 [DOI] [PubMed] [Google Scholar]
- 7.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004September;86(9):1973-82 [DOI] [PubMed] [Google Scholar]
- 8.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994July;304:78-83 [PubMed] [Google Scholar]
- 9.Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001May-Jun;10(3):199-203 [DOI] [PubMed] [Google Scholar]
- 10.Safran O, Derwin KA, Powell K, Iannotti JP. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 2005December;87(12):2662-70 [DOI] [PubMed] [Google Scholar]
- 11.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999Nov-Dec;8(6):599-605 [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Xie Y, Bucher NL, Farmer SR. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes Dev. 1995October1;9(19):2350-63 [DOI] [PubMed] [Google Scholar]
- 13.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol. 1997March;17(3):1552-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao D, Lazar MA. Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J Biol Chem. 1997August22;272(34):21473-8 [DOI] [PubMed] [Google Scholar]
- 15.Singh J, Verma NK, Kansagra SM, Kate BN, Dey CS. Altered PPARgamma expression inhibits myogenic differentiation in C2C12 skeletal muscle cells. Mol Cell Biochem. 2007January;294(1-2):163-71 Epub 2006 Jul 13 [DOI] [PubMed] [Google Scholar]
- 16.Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci U S A. 1995October10;92(21):9856-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005June;288(6):L1146-53 Epub 2005 Feb 25 [DOI] [PubMed] [Google Scholar]
- 18.Lakatos HF, Thatcher TH, Kottmann RM, Garcia TM, Phipps RP, Sime PJ. The role of PPARs in lung fibrosis. PPAR Res. 2007;2007:71323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sime PJ The antifibrogenic potential of PPARgamma ligands in pulmonary fibrosis. J Investig Med. 2008February;56(2):534-8 [DOI] [PubMed] [Google Scholar]
- 20.Venuti JM, Morris JH, Vivian JL, Olson EN, Klein WH. Myogenin is required for late but not early aspects of myogenesis during mouse development. J Cell Biol. 1995February;128(4):563-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artaza JN, Bhasin S, Magee TR, Reisz-Porszasz S, Shen R, Groome NP, Meerasahib MF, Gonzalez-Cadavid NF. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology. 2005August;146(8):3547-57 Epub 2005 May 5 [DOI] [PubMed] [Google Scholar]
- 22.Amirouche A, Durieux AC, Banzet S, Koulmann N, Bonnefoy R, Mouret C, Bigard X, Peinnequin A, Freyssenet D. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology. 2009January;150(1):286-94 Epub 2008 Sep 18 [DOI] [PubMed] [Google Scholar]
- 23.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009June;296(6):C1258-70 Epub 2009 Apr 8 [DOI] [PubMed] [Google Scholar]
- 24.Durieux AC, Amirouche A, Banzet S, Koulmann N, Bonnefoy R, Pasdeloup M, Mouret C, Bigard X, Peinnequin A, Freyssenet D. Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Endocrinology. 2007July;148(7):3140-7 Epub 2007 Mar 29 [DOI] [PubMed] [Google Scholar]
- 25.Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008July11;283(28):19371-8 Epub 2008 May 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bo Li Z, Zhang J, Wagner KR. Inhibition of myostatin reverses muscle fibrosis through apoptosis. J Cell Sci. 2012September1;125(Pt 17):3957-65 Epub 2012 Jun 8 [DOI] [PubMed] [Google Scholar]
- 27.Joshi SK, Liu X, Samagh SP, Lovett DH, Bodine SC, Kim HT, Feeley BT. mTOR regulates fatty infiltration through SREBP-1 and PPARγ after a combined massive rotator cuff tear and suprascapular nerve injury in rats. J Orthop Res. 2013May;31(5):724-30 Epub 2012 Dec 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Chen H, Wang C, Peng Y, Sun L, Liu H, Liu F. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One. 2012;7(3):e33626 Epub 2012 Mar 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patsenker E, Schneider V, Ledermann M, Saegesser H, Dorn C, Hellerbrand C, Stickel F. Potent antifibrotic activity of mTOR inhibitors sirolimus and everolimus but not of cyclosporine A and tacrolimus in experimental liver fibrosis. J Hepatol. 2011August;55(2):388-98 Epub 2010 Dec 17 [DOI] [PubMed] [Google Scholar]
- 30.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009December;20(12):2493-502 Epub 2009 Oct 29 [DOI] [PubMed] [Google Scholar]
- 31.Lieber RL, Ward SR. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol. 2013August1;305(3):C241-52 Epub 2013 Jun 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frey E, Regenfelder F, Sussmann P, Zumstein M, Gerber C, Born W, Fuchs B. Adipogenic and myogenic gene expression in rotator cuff muscle of the sheep after tendon tear. J Orthop Res. 2009April;27(4):504-9 [DOI] [PubMed] [Google Scholar]
- 33.Itoigawa Y, Kishimoto KN, Sano H, Kaneko K, Itoi E. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res. 2011June;29(6):861-6 Epub 2011 Jan 18 [DOI] [PubMed] [Google Scholar]
- 34.Killian ML, Lim CT, Thomopoulos S, Charlton N, Kim HM, Galatz LM. The effect of unloading on gene expression of healthy and injured rotator cuffs. J Orthop Res. 2013August;31(8):1240-8 Epub 2013 Mar 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmutz S, Fuchs T, Regenfelder F, Steinmann P, Zumstein M, Fuchs B. Expression of atrophy mRNA relates to tendon tear size in supraspinatus muscle. Clin Orthop Relat Res. 2009February;467(2):457-64 Epub 2008 Oct 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi LL, Boykin RE, Lin A, Warner JJP. Association of suprascapular neuropathy with rotator cuff tendon tears and fatty degeneration. J Shoulder Elbow Surg. 2014March;23(3):339-46 Epub 2013 Sep 20 [DOI] [PubMed] [Google Scholar]
- 37.Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012February;21(2):181-90 [DOI] [PubMed] [Google Scholar]
- 38.Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012July;21(7):847-58 Epub 2011 Aug 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samagh SP, Kramer EJ, Melkus G, Laron D, Bodendorfer BM, Natsuhara K, Kim HT, Liu X, Feeley BT. MRI quantification of fatty infiltration and muscle atrophy in a mouse model of rotator cuff tears. J Orthop Res. 2013March;31(3):421-6 Epub 2012 Sep 18 [DOI] [PubMed] [Google Scholar]
- 40.Smith LR, Meyer G, Lieber RL. Systems analysis of biological networks in skeletal muscle function. Wiley Interdiscip Rev Syst Biol Med. 2013Jan-Feb;5(1):55-71 Epub 2012 Nov 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward SR, Hentzen ER, Smallwood LH, Eastlack RK, Burns KA, Fithian DC, Friden J, Lieber RL. Rotator cuff muscle architecture: implications for glenohumeral stability. Clin Orthop Relat Res. 2006July;448(Jul):157-63 [DOI] [PubMed] [Google Scholar]