Abstract
Metformin is well-known as an anti-diabetic drug, but it seems to possess anti-cancerous properties as well. Adenosine monophosphate-activated protein kinase (AMPK) is a highly conserved regulator of the cellular response to the presence of low energy in all eukaryotic cells. It is considered a key sensor of the balance of cellular ATP and AMP concentrations. LKB1 serine/threonine kinase is a divergent yet evolutionarily well-conserved kinase, biochemically sufficient to activate AMPK in vitro and genetically required for AMPK activation. Because of this potent connection to AMPK, LKB1 may act as a central regulator of metabolism in vivo. Once activated, AMP kinase phosphorylates the transcriptional activator TorC2, thereby blocking its nuclear translocation and inhibiting the expression of genes involved in gluconeogenesis. Data suggest that LKB1/AMPK signaling plays a role in protection from apoptosis, specifically in response to agents that increase the cellular AMP/ATP ratio. Active AMPK signaling offers a protective effect by providing the cell with time to reverse the aberrantly high ratio of AMP/ATP. If unable to reverse this ratio, the cell will eventually undergo cell death. These observations offer the provocative suggestion of a potential therapeutic window in which LKB1-deficient tumor cells may be acutely sensitive to AMP analogues or sensitized to cell death by other stimuli when treated in combination with agents that increase the AMP/ATP ratio. LKB1 therefore is a classical tumor suppressor. AMPK is a direct LKB1 substrate. A consequence of AMPK activation by LKB1 is the inhibition of the mammalian target of rapamycin (mTOR) C1 pathway. Metformin's anti-cancerous properties have been demonstrated in various cancer cells in vitro, such as lung, pancreatic, colon, ovarian, breast, prostate, renal cancer cells, melanoma, and even in acute lymphoblastic leukemia cells. To test metformin's action in vivo, mice were implanted with transformed mammary epithelial cells and treated with three cycles of metformin and with the anthracycline doxorubicin. When combined with doxorubicin, metformin wiped out tumors and prevented recurrence. Metformin alone had no effect, and doxorubicin as a single agent initially shrank tumors, but they regrew later. Virtually no cancer stem cells were recovered immediately after treatment and the complete response was sustained for nearly two months. Further studies are needed to assess the anti-cancerous potentials of metformin in vivo. This article reviews the current knowledge on the actions of LKB1/AMPK and the effectiveness of metformin in cancer, specifically in diabetes patients.
Keywords: AMPK, LKB1, metformin, doxorubicin
Abbreviations: AMP - adenosine monophosphate; AMPK - adenosine-monophosphate-activated protein kinase; ATP - adenosine triphosphate; CAMKK - calmodulin-dependent protein kinase kinase; CBS - crystathionine-β synthase; IGF-IR - insulin-like growth factor insulin receptor; IR - insulin receptor; LKB1 - liver kinase B1 (a threonine/serine kinase); MAPK - mitogen-activated protein kinase; mTOR - mammalian target of rapamycin; Thr172 - threonine 172; TORC2 - target of rapamycin complex 2
Introduction
It is well-established that colorectal cancer incidence is increased among patients with type 2 diabetes mellitus [1-4]. Certain types of cancers are more common in people with diabetes than in those without. Diabetes is also associated with reduced survival after cancer [5-8]. Therefore, it is important to address the topic of cancer and in diabetes in future research.
Adenosine-monophosphate-activated protein kinase (AMPK)
Adenosine monophosphate-activated protein kinase (AMPK) is a highly conserved regulator of the cellular response to low energy expressed in all eukaryotic cells. AMPK is activated when intracellular adenosine triphosphate (ATP) concentrations decrease and AMP concentrations increase [9]. It is considered a key sensor for the balance of cellular ATP and AMP concentrations. AMPK is activated by:
1. Stimuli that induce stress including oxidative damage, osmotic shock, hypoxia, and deprivation of glucose or other nutrients.
2. Mitochondrial poisons.
3. Physiological stimuli including exercise, muscle contraction, and hormones such as leptin and adiponectin [10].
In mammals, AMPK has a critical role in metabolic processes, including glucose uptake and fatty acid oxidation in muscle, fatty acid synthesis and gluconeogenesis in the liver, and the regulation of food intake centrally at the hypothalamus level [11-13].
AMPK exists as a heterotrimer complex, composed of the catalytic kinase α subunit and two associated regulatory subunits, β and γ [11]. Upon energy stress, AMP directly binds to tandem repeats of crystathionine-β synthase (CBS) domains in the AMPK γ subunit, causing a conformation change that exposes the activation loop in the α subunit, allowing it to be phosphorylated by an upstream kinase [10]. The sequence flanking the activation loop threonine (Thr172 in human AMPKα1) is conserved across species and its phosphorylation is absolutely required for AMPK activation. Phosphorylation of a single invariant threonine residue in the activation loop of the catalytic subunit (Thr172 in human AMPKα1) has been shown to be required to activate all known AMPK homologues [12]. A number of laboratories have reported biochemical purification of a kinase activity, AMPK kinase (AMPKK), which is capable of phosphorylating Thr172 [14-17]. Calcium calmodulin-dependent protein kinase kinase (CAMKK) has been demonstrated to serve as a surrogate AMPKK in vitro, but not in vivo [18].
The LKB1 serine/threonine kinase is a divergent yet evolutionarily well conserved kinase that most closely resembles CAMKK in its catalytic domain. Threonine kinase LKB1 is biochemically sufficient to activate AMPK in vitro and is genetically required for AMPK activation by energy stress in a number of mammalian cell lines [19-20]. Because of this potent connection to AMPK, LKB1 may act as a central regulator of metabolism in vivo [21-26]. Once activated, AMP kinase phosphorylates the transcriptional activator TorC2, thereby blocking its nuclear translocation and inhibiting the expression of genes involved in gluconeogenesis (Figure 1) [23].
Figure 1. Action of metformin in diabetes.
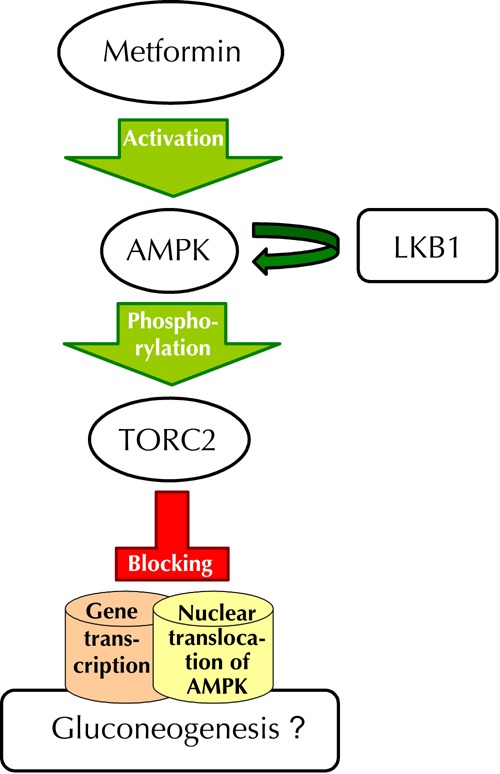
Metformin activates adenosine-monophosphate-activated protein kinase (AMPK), which phosphorylates the target of rapamycin complex 2 (TORC2), thereby blocking its nuclear translocation and transcription of the genes involved in gluconeogenesis. Liver kinase B1 (LKB1) is essential for the activation of AMPK. Positive AMPK signals prevent the mitogenic activity of mammalian target of rapamycin (mTOR) C1 pathway.
LKB1/AMPK and diabetes
The ability of metformin to lower glucose and insulin levels by inhibiting the expression of genes involved in gluconeogenesis is a satisfactory explanation of its therapeutic effect in diabetes [9]. Mice deficient in hepatic LKB1 develop hyperglycemia and are resistant to the glucose-lowering effects of metformin [21].
There is genetic and biochemical evidence that LKB1 is a critical regulator of AMPK in vivo. As such, LKB1 may play an unexpected role in multiple organ systems that mediate the diverse effects of AMPK on mammalian physiology. Importantly, AMPK has been shown to be a critical mediator of glucose uptake in skeletal muscle in mice. The kinase activity of AMPK is stimulated by two major anti-diabetic drugs, metformin and rosiglitazone [27-28]. Therefore, the identification of LKB1 as a major activator of AMPK in vivo may offer potential avenues to boost AMPK activity for the treatment of diabetes.
LKB1/AMPK and apoptosis
Data suggest that LKB1/AMPK signaling plays a role in protection from apoptosis, specifically in response to agents that increase the cellular AMP/ATP ratio. Active AMPK signaling induces a protective effect by providing the cell with time to reverse the aberrantly high ratio of AMP/ATP. If unable to reverse this ratio, the cell will eventually undergo cell death. These results offer the provocative suggestion of a potential therapeutic window in which LKB1-deficient tumor cells may be acutely sensitive to AMP analogues or sensitized to cell death by other stimuli when treated in combination with agents that increase the AMP/ATP ratio.
AMP kinase is activated by the product of the Peutz-Jegher tumor suppressor gene LKB1 [29]. Peutz-Jeghers syndrome patients develop numerous benign tumors in the gastrointestinal tract and have a 20-fold increased risk of developing malignant tumors at other sites. Mutations in the LKB1 gene are also seen in some sporadic cancers, especially lung adenocarcinoma [30-31]. Therefore, LKB1 is a classical tumor suppressor [32]. AMPK is a direct LKB1 substrate. A consequence of AMPK activation by LKB1 is the inhibition of the mammalian target of rapamycin (mTOR) C1 pathway through phosphorylation of tuberous sclerosis 2 or hamartin and raptor [33-34].
As mentioned above, loss of LKB1 function is a frequent finding in lung adenocarcinoma and squamous cell carcinomas [20]. Interestingly, the anti-diabetic drug rosiglitazone is known to stimulate AMPK signaling through alterations in the intracellular AMP/ATP ratio, suggesting that rosiglitazone may be useful in the treatment of LKB1-deficient tumors as well [28]. The observation that altered AMP/ATP ratios result in cell death in the absence of AMPK signaling indicates that other cellular proteins that are regulated by AMP may contribute to the cell death observed.
LKB1 tumor suppression seems to be the major activating kinase for AMPK in the liver. As Shaw et al. suggested, minor roles for other kinases cannot be ruled out, but under all the conditions they have examined, the loss of LKB1 was mirrored by the loss of AMPK phosphorylation [26].
mTOR, insulin, and LKB1 pathways represent a fundamental eukaryotic network governing cell growth in response to environmental nutrients; dysregulation of one of these pathways contributes to both diabetes and cancer [29-32].
Metformin and various cancer cells
The involvement of a tumor suppressor pathway as a target for metformin’s action in glucose homeostasis prompted studies of possible effects in tumor cells and animal cancer models. In vitro studies have shown that metformin inhibits the proliferation of colorectal cancer cells [33]. In vivo studies have demonstrated that metformin delays tumor onset in mouse models for p53 mutant colon cancer [34]. Another animal model of colon cancer has indicated that metformin inhibits colon carcinoma growth stimulated by a high-energy diet [35]. Two animal models of colorectal aberrant crypt foci showed that metformin significantly suppresses colonic epithelial proliferation by inhibiting the mTOR pathway [36-39]. This pathway has recently been found to be involved in T cell acute lymphoblastic leukemia [40]. Indeed, metformin has been shown to have therapeutic effects in T cells of acute lymphoblastic leukemia in vitro [41].
Metformin exerts in vitro inhibition of the proliferation of prostate, ovarian, and breast cancer cells [39]. This inhibitory effect is seen, however, at concentrations that are at least 10-fold higher than the peak plasma concentration attained with typical dosing in diabetics [42]. Even though most laboratory studies have been using doses that are much lower than the typical anti-diabetic dose of metformin used in vivo, there are emerging studies which show that even lower doses of metformin could have substantial anti-cancerous effects. For example, the proliferation of CD133+, but not CD24+CD44+ESA+ cells, which are considered pancreatic cancer stem cells, was inhibited by low doses of metformin [43]. Recently, it has been shown that the conventional anti-diabetic concentrations of metformin caused death in cancer cells and were preferentially cytotoxic to cancer stem cells related to non-cancer stem cells [44]. Also, to demonstrate the action of potential anti-cancerous properties of metformin in vivo, mice with transformed mammary epithelial cells were given three cycles of metformin and one cycle of doxorubicin, resulting in shrinking tumor cells and the prevention of recurrence. Mouse xenograft models demonstrate in vivo anti-tumor effects of metformin against pancreatic, prostate, and p53 mutant colon cancers [33, 45-47].
AMPK is critically linked to the phosphatidyl-inositol-3 kinase/AKT/mTOR signaling pathway, a vital cellular signaling cascade that is essential for cell growth in response to mitogenic stimuli or pathways activated by growth factor receptors [48]. AMPK activation directly inhibits phosphorylation and subsequent activation of the mTORC1 complex and is controlled partly by the upstream kinase AKT, whose activation decreases the AMP:ATP ratio [35, 49-51]. AKT also directly inhibits the activation of AMPK by phosphorylation of AMPK at Ser 485/491 [52-53]. Renal cell carcinoma is a highly aggressive genitourinary cancer for which the treatment options are limited [54]. This malignancy is characterized by over-activation of this AKT/mTOR signaling pathway [55]. Extensive work over the last few years has demonstrated the effectiveness of targeting the mTOR pathway for the treatment of renal cell carcinoma [56]. Temsirolimus, a known mTOR pathway inhibitor, has clinically significant activity in the treatment of renal cell carcinoma and is now an FDA-approved agent in the treatment of patients with renal cell carcinoma [57]. Studies have demonstrated that addition of metformin exerted suppressive effects on the tumorogenicity of renal cancer cells in vitro.
It has been suggested that inhibitory effects on the LKB1/AMPK axis in melanoma may constitute an important mechanism of tumorigenesis [58]. Studies using both melanoma cell lines harboring this BRAF mutation and melanoma cell lines without this mutation suggest that AMPL plays a role in the control of malignant melanoma cell growth. Taken together, the above-mentioned studies provide evidence for potent inhibitory effects of AMPK on malignant melanoma cell growth and survival and raise the potential of AMPK manipulation as a novel future approach for the treatment of malignant melanoma [59].
Metformin and breast cancer
The discovery that metformin selectively kills cancer stem cells adds further interest and may explain its antineoplastic properties. Hirsch et al. genetically manipulated human breast epithelial cells to enrich for stem cells and tested these together with three distinct breast tumor cell lines [60]. Using flow cytometry to track the effects of metformin, researchers found that the drug is selectively toxic to cancer stem cells. To test metformin's action in vivo, mice were implanted with transformed mammary epithelial cells and treated with three cycles of metformin and with the anthracycline doxorubicin. When combined with doxorubicin, metformin wiped out tumors and prevented recurrence. Metformin alone had no effect and doxorubicin as a single agent initially shrank tumors but they regrew later. Virtually no cancer stem cells were recovered immediately after treatment and the complete response was sustained for nearly two months [61]. Among patients with breast cancer, the metformin-treated subgroup has been related with better outcomes than patients not treated with metformin [62].
Further studies will delineate whether the AMP kinase pathway is important in cancer stem cells, and whether the synergistic effect of metformin and anthracyclines is generalized to other types of cancer and to its combination with other drugs [63].
Metformin and colorectal cancer
Type 2 diabetes has been associated with increased incidence of colorectal cancer [64]. Recently, a study has revealed a significant association between highly intensive use of metformin and lower mortality from colorectal cancer in diabetics with stage I-III colorectal cancer compared with non-diabetics with stage I-III colorectal cancer [65]. Also, metformin use has been related with decreased incidence of colorectal adenomas in diabetic patients with previous colorectal cancer [66]. Overall survival has been found to be better among patients with colorectal cancer and type 2 diabetes taking metformin as part of their anti-diabetic medication compared with diabetic patients with colorectal cancer not taking metformin as part of their anti-diabetic regimen [67]. In Korean patients with colorectal cancer, the use of metformin has been associated with reduced risk of overall mortality, especially in patients with stage III colorectal cancer [68].
Metformin, cancer prevention, and mortality
As shown by Curie et al., diabetic patients with type 2 diabetes taking metformin had a decreased risk of cancer compared with patients taking metformin plus sulfonylurea and patients on insulin treatment [69]. Metformin has been related with lower incidence of cancer among type 2 diabetes patients [70]. Many studies support the notion that the use of metformin results in lower incidence of cancer [71-75]. Lung, pancreatic, prostate, breast, ovarian, and hepatocellular cancer incidence has been found to be lower among patients receiving metformin [76-82]. Moreover, the mortality from cancer was lower in diabetes patients taking metformin as part of their anti-diabetic medication compared with those not taking metformin [83-86]. However, studies showing favorable effects of metformin on cancer are not always corroborated by large clinical trials. Larger studies are expected to investigate the possible antineoplastic effects of metformin more thoroughly [87-89].
Conclusions
Metformin exerts its anti-tumor effects mainly through the AMPK/LKB1/TORC1 signaling pathway, thereby causing apoptosis in cancerous cells [14, 21-23]. Another possible mechanism is amelioration of endogenous hyperinsulinemia by use of metformin therapy [90]. Insulin stimulates cellular proliferation, and multiple signaling pathways are activated after insulin receptors or insulin-like growth factor (IGF-I) receptors interact with their ligands [91-92].
Most cancer cells express insulin and IGF-I receptors. The A isoform of the insulin receptor is also commonly expressed, which may stimulate mitogenesis, even in cells deficient in IGF-I [93-94]. Metformin therapy decreases the levels of circulating insulin-like growth factors and insulin which, in turn, may reduce the risk of cancer (Figure 2).
Figure 2. Insulin-binding and metabolic action of insulin.
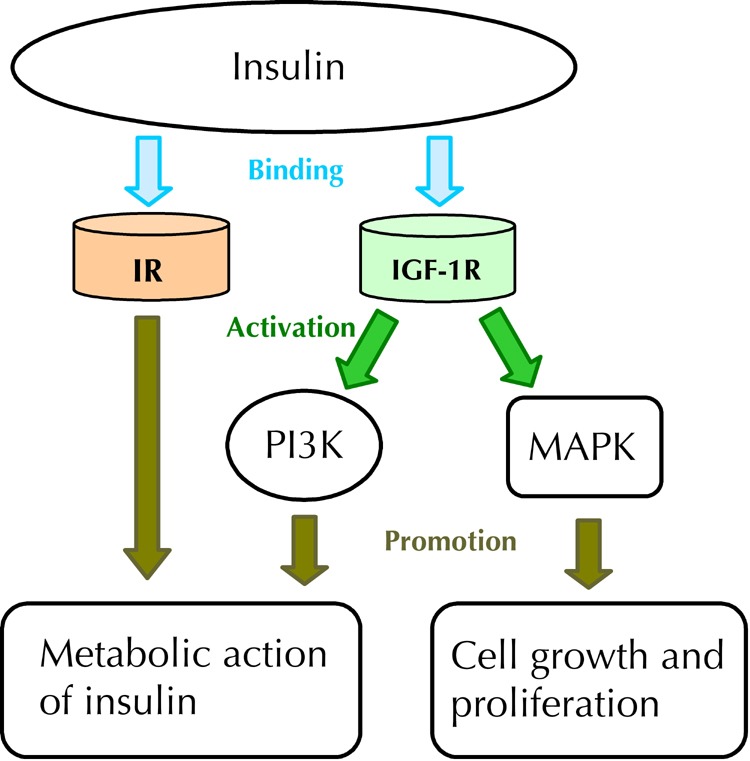
Insulin binds both to the insulin receptor (IR) and to insulin-like growth factor receptor 1 (IGF-1R). The binding of insulin to IGF-1R activates both phosphoinositol-3-kinase pathway (PI3K), which activates the metabolic pathway, and mitogen-activated-protein-kinase (MAPK), which promotes cell growth and cell proliferation. The binding of insulin to IR is responsible for the metabolic actions of insulin.
Other possible mechanisms underlying the potential anti-tumor effect of metformin could be the antagonization of obesity and anti-inflammatory effects. Interleukin-6, plasminogen activator inhibitor-1, tumor necrosis factor-α, and monocyte chemoattractant are produced by adipose tissue and can enhance cancer cell proliferation, p-53 activation, downregulation of cyclin D1, and killing of cancer stem cells [95-98]. Further studies investigating potential mechanisms of the anti-cancerous properties of metformin are needed.
If metformin effectively helps cancer patients, it will finally join drugs such as thalidomide, retinoic acid, and arsenic, which have unique mechanisms of action and were first used elsewhere in medicine, but have also found their way into the field of anticancer drugs.
Disclosure: The authors declare no conflict of interests.
References
- 1.Seow A, Yuan JM, Koh WP, Lee HP, Yu MC. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J Natl Cancer Inst. 2006;98:135–138. doi: 10.1093/jnci/djj015. [DOI] [PubMed] [Google Scholar]
- 2.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–1050. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Balluz LS, Ford ES, Okoro CA, Tsai J, Zhao G. Association between diagnosed diabetes and self-reported cancer among U.S. adults: findings from the 2009 behavioral risk factor surveillance system. Diabetes Care. 2011;34:1365–1368. doi: 10.2337/dc11-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 6.Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010;47:87–95. doi: 10.1007/s00592-010-0187-3. [DOI] [PubMed] [Google Scholar]
- 7.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. The impact of diabetes on survival following breast cancer. Breast Cancer Res Treat. 2008;109:389–395. doi: 10.1007/s10549-007-9654-0. [DOI] [PubMed] [Google Scholar]
- 8.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardie DG, Scott JW, Pan DA, Huson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:1113–1120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 11.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferree P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 13.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carlinq D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 14.Kim EK, Song MJ, Yoo EJ, Choe SS, Park SD, Kim JB. Regulatory role of glycogen synthase kinase 3 for transcriptional activity of ADD1/SREBP1c. J Biol Chem. 2004;279:19970–19975. doi: 10.1074/jbc.M405522200. [DOI] [PubMed] [Google Scholar]
- 15.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton SR, O'Donnell JB, Hammet A, Stapleton D, Habinowski SA, Means AR, Kemp BE, Witters LA. AMP-activated protein kinase kinase: detection with recombinant AMPK alpha1 subunit. Biochem Biophys Res Commun. 2002;293:892–898. doi: 10.1016/S0006-291X(02)00312-1. [DOI] [PubMed] [Google Scholar]
- 17.Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, Wallimann T, Carling D, Rider MH. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem. 2003;278:28434–28442. doi: 10.1074/jbc.M303946200. [DOI] [PubMed] [Google Scholar]
- 18.Hawley SA, Boudau J, Reid JL, Mustard JK, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila) 2008;1:369–375. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 20.Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5'-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270(45):27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 21.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, De Pinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 24.Pollak M. Insulin-like growth factor-related signaling and cancer development. Recent Results Cancer Res. 2007;174:49–53. doi: 10.1007/978-3-540-37696-5_4. [DOI] [PubMed] [Google Scholar]
- 25.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA. et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 26.Shaw RJ, Katja A, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The Kinase LKB1 Mediates Glucose Homeostasis in Liver and Therapeutic Effects of Metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 28.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 29.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27:6908–6919. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 30.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, Fan Z, Edgerton SM, Denq XS, Alimova IN, Lind SE, Thor AD. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–2040. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 32.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 33.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 35.Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010;17:351–360. doi: 10.1677/ERC-09-0252. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, Koide T, Tokoro C, Abe Y, Inamori M, Nakagama H, Nakajima A. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010;49:662–671. doi: 10.1002/mc.20637. [DOI] [PubMed] [Google Scholar]
- 38.Tomimoto A, Endo H, Sugiyama M, Fujisawa T, Hosono K, Takahashi H, Nakajima N, Nagashima Y, Wada K, Nakagama H, Nakajima A. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008;99:2136–2141. doi: 10.1111/j.1349-7006.2008.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010;17:351–360. doi: 10.1677/ERC-09-0252. [DOI] [PubMed] [Google Scholar]
- 40.Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C Boyer O, Bardet V, Park S, Foretz M. et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116:4262–4273. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- 41.Grimaldi C, Chiarini F, Tabellini G, Ricci F, Tazzari PL, Battistelli M, Falcieri E, Bortu R, Melchionda F, Iacobucci I. et al. AMP-dependent kinase/mammalian target of rapamycin complex 1 signaling in T-cell acute lymphoblastic leukemia: therapeutic implications. Leukemia. 2012;26:91–100. doi: 10.1038/leu.2011.269. [DOI] [PubMed] [Google Scholar]
- 42.Ben Sahra BI, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 43.Gou S, Cui P, Li X, Shi P, Liu T, Wang C. Low concentrations of metformin selectively inhibited CD133+ cell proliferation in pancreatic cancer and have anti-cancer action. Plos One. 2013;8(5):e63969. doi: 10.1371/journal.pone.0063969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song CW, Lee H, Dings RPM, Williams B, Powers J, Santos TD, Choi BH, Park HJ. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Scientific Reports. 2012 doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN, Bruchim I. In vitro metformin antineoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–250. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 47.Zhuang Y, Miskimins WK. Cell cycle arrest in metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3:18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider MB, Matsuzaki H, Haorah J, Shiomori K, Ogawa M. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120:1263–1270. doi: 10.1053/gast.2001.23258. [DOI] [PubMed] [Google Scholar]
- 49.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 51.Hardie DG. AMPK and Raptor: matching cell growth to energy supply. Mol Cell. 2008;30:263–265. doi: 10.1016/j.molcel.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 53.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 54.Longo R, D'Andrea MR, Sarmiento R, Salerno F, Gasparini G. Integrated therapy of kidney cancer. Ann Oncol. 2007;18:141–148. doi: 10.1093/annonc/mdm244. [DOI] [PubMed] [Google Scholar]
- 55.Hager M, Haufe H, Kemmerling R, Hitzl W, Mikuz G, Moser PL, Kolbitsch C. Increased activated Akt expression in renal cell carcinomas and prognosis. J Cell Mol Med. 2009;13:2181–2188. doi: 10.1111/j.1582-4934.2008.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho D, Signoretti S, Regan M, Mier JW, Atkins MB. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res. 2007;13:758–763. doi: 10.1158/1078-0432.CCR-06-1986. [DOI] [PubMed] [Google Scholar]
- 57.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I. et al. Temsirolimus, interferon-alpha or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 58.Zheng B, Jeong JH, Asara JM, Yuan YY, Granter SR, Chin L, Cantley LC. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–247. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodard J, Platanias LC. AMP-activated kinase (AMPK)-generated signals in malignant melanoma cell growth and survival. Biochem Biophys Res Commun. 2010;398(1):135–139. doi: 10.1016/j.bbrc.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grenader T, Goldberg A, Shavit L. Metformin as an addition to conventional chemotherapy in breast cancer. J Clin Oncol. 2009;35:e259. doi: 10.1200/JCO.2009.25.4110. [DOI] [PubMed] [Google Scholar]
- 61.Hou G, Zhang S, Zhang X, Wang P, Hao X, Zhang J. Clinical pathological characteristics and prognostic analysis of 1,013 breast cancer patients with diabetes. Breast Cancer Res Treat. 2013;137(3):807–816. doi: 10.1007/s10549-012-2404-y. [DOI] [PubMed] [Google Scholar]
- 62.Cazzaniga M, Bonanni B, Guerrieri-Gonzaga A, Decensi A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol Biomarkers Prev. 2009;18(3):701–705. doi: 10.1158/1055-9965.EPI-08-0871. [DOI] [PubMed] [Google Scholar]
- 63.Lewis JD, Capra AM, Achacoso NS. Medical therapy for diabetes is associated with increased use of lower endoscopy. Pharmacoepidemiol Drug Saf. 2007;16:1195–1202. doi: 10.1002/pds.1441. [DOI] [PubMed] [Google Scholar]
- 64.Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH, Kim WH. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer. 2012;131(3):752–759. doi: 10.1002/ijc.26421. [DOI] [PubMed] [Google Scholar]
- 65.Lee JH, Jeon SM, Hong SP, Cheon JH, Kim TI, Kim WH. Metformin use is associated with a decreased incidence of colorectal adenomas in diabetic patients with previous colorectal cancer. Dig Liver Dis. 2012;44(12):1042–1047. doi: 10.1016/j.dld.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Spillane S, Bennett K, Sharp L, Barron T. A cohort study of metformin exposure and survival in patients with stage I-III colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1364–1373. doi: 10.1158/1055-9965.EPI-13-0347. [DOI] [PubMed] [Google Scholar]
- 67.Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, Kip KE. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34(10):2323–2328. doi: 10.2337/dc11-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 69.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings RM, Stricker BH. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35:119–124. doi: 10.2337/dc11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monami M, Colombi C, Balzi D, Dicembrini I, Giannini S, Melani C, Vitale V, Romano D, Barchielli A, Marchionni N, Rotella CM, Mannucci E. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. 2011;34(1):129–131. doi: 10.2337/dc10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geraldine N, Marc A, Carla T, Chantal M, Stefaan B, Welcome W, Frank B. Relation between diabetes, metformin treatment and the occurrence of malignancies in a Belgian primary care setting. Diabetes Res Clin Pract. 2012;97:331–336. doi: 10.1016/j.diabres.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DP, Chen CC. Anti-diabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer. 2012;13:143–148. doi: 10.1016/j.cllc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 76.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL, Vauthey JN. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bodmer M, Meier C, Krähenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–1308. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bosco JL, Antonsen S, Sorensen HT, Pedersen L, Lash TL. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev. 2011;20:101–111. doi: 10.1158/1055-9965.EPI-10-0817. [DOI] [PubMed] [Google Scholar]
- 79.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol. 2011;123:200–204. doi: 10.1016/j.ygyno.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 80.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of anti-diabetic agents and the risk of pancreatic cancer: a case control analysis. Am J Gastroenterol. 2012;107:620–626. doi: 10.1038/ajg.2011.483. [DOI] [PubMed] [Google Scholar]
- 81.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hernandez-Diaz S, Adami HO. Diabetes therapy and cancer risk: causal effects and other plausible explanations. Diabetologia. 2010;53:802–808. doi: 10.1007/s00125-010-1675-2. [DOI] [PubMed] [Google Scholar]
- 83.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33(2):322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He XX, Tu SM, Lee MH, Yeung SC. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann Oncol. 2011;22:2640–2645. doi: 10.1093/annonc/mdr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang YX. Do diabetes drugs modify the risk of pancreatic cancer? Gastroenterology. 2009;137:412–415. doi: 10.1053/j.gastro.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 87.Rizos CV, Elisaf MS. Metformin and cancer. Eur J Pharmacol. 2013;705(1-3):96–108. doi: 10.1016/j.ejphar.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 88.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35(12):2665–2673. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J Clin Oncol. 2009;27:3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- 90.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 91.LeRoith D, Baserga R, Helman L, Roberts CT Jr. Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–59. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 92.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 93.Denley A, Carroll JM, Brierley GV, Cosgrove L, Wallace J, Forbes B, Roberts CT Jr. Differential activation of insulin receptor substrates 1 and 2 by insulin-like growth factor-activated insulin receptors. Mol Cell Biol. 2007;27:3569–3577. doi: 10.1128/MCB.01447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 95.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 96.Ulisse S, Baldini E, Sorrenti S, D'Armiento M. The urokinase plasminogen activator system: a target for anticancer therapy. Curr Cancer Drug Targets. 2009;9:32–71. doi: 10.2174/156800909787314002. [DOI] [PubMed] [Google Scholar]
- 97.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhuang Y, Miskimins WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3:18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


