Abstract
Retrotransposons have played a central role in human genome evolution. The accumulation of heritable L1, Alu and SVA retrotransposon insertions continues to generate structural variation within and between populations, and can result in spontaneous genetic disease. Recent works have reported somatic L1 retrotransposition in tumours, which in some cases may contribute to oncogenesis. Intriguingly, L1 mobilization appears to occur almost exclusively in cancers of epithelial cell origin. In this review, we discuss how L1 retrotransposition could potentially trigger neoplastic transformation, based on the established correlation between L1 activity and cellular plasticity, and the proven capacity of L1‐mediated insertional mutagenesis to decisively alter gene expression and functional output.
Keywords: Alu, cancer genomics, EMT, L1, LINE‐1, oncogenesis, plasticity, retrotransposon, SVA, tumorigenesis
Abbreviations
- L1
long interspersed element 1
- ME
mobile genetic element
Introduction
Mobile genetic elements (MEs) are found in nearly all eukaryotic genomes. MEs can be divided into two major classes, transposons and retrotransposons. Transposons use a ‘cut‐and‐paste’ process to relocate in genomic DNA, whereas retrotransposons mobilize through an RNA intermediate in a ‘copy‐and‐paste’ mechanism termed retrotransposition. ME sequences account for at least 45% of human DNA 1, with some estimates ranging as high as 66% 2, mainly due to the activity of the long interspersed element 1 (LINE‐1 or L1) retrotransposon family. L1 is present in all mammals and, in humans, it is the only retrotransposon that remains capable of autonomous mobilization 3. A retrotransposition‐competent L1 is ~ 6 kb in length 5. The core L1 sequence comprises a bicistronic ORF that encodes two proteins, ORF1p and ORF2p, which are essential for L1 mobilization. ORF1p is a 40 kDa protein with nucleic acid binding activity 7 and ORF2p is a 150 kDa protein with demonstrated endonuclease and reverse transcriptase activities 9. The L1 5′‐UTR harbours an internal promoter 11, as well as an antisense promoter of unclear function 12. New L1 insertions are typically flanked by target‐site duplications, a hallmark of the L1 integration process 13.
L1 retrotransposition begins with the transcription of a full‐length mRNA from the L1 internal promoter. This mRNA is transported to the cytoplasm and translated, giving rise to the L1‐encoded proteins. ORF1p and ORF2p bind to their encoding L1 mRNA in a phenomenon termed cis preference, forming the L1 ribonucleoprotein particle 7. The L1 ribonucleoprotein particle gains access to the nucleus by a mechanism that is not completely understood, but can occur independently of nuclear envelope breakdown during cell division 19. Inside the nucleus, L1 integration occurs by a mechanism termed target‐site primed reverse transcription 20. During target‐site primed reverse transcription, ORF2p endonuclease activity produces a single‐stranded nick in genomic DNA, exposing a free 3′ hydroxyl residue that serves as a primer from which the ORF2p reverse transcriptase activity synthesizes a cDNA copy of its associated L1 mRNA 9. Despite the marked cis preference of L1 proteins for their encoding mRNA 16, other cellular RNAs can be mobilized in trans by the L1 enzymatic machinery. These sequences include the non‐autonomous retrotransposons Alu and SVA, as well as protein‐coding mRNAs, the reverse transcription of which gives rise to processed pseudogenes 14. Thus, L1 has played a pivotal role in human genome evolution.
Of ~ 500 000 L1 copies in the human genome, the vast majority have been rendered immobile by 5′ truncation, internal deletions and other mutations 1. As a consequence, only 80–100 retrotransposition‐competent L1s, as well as an estimated 2000–3000 Alu and < 100 SVA copies, are found per individual 25. These elements continue to drive pervasive genetic variation in human populations 28. Spontaneous and inherited occurrences of insertional mutagenesis mediated by L1 have been observed in > 100 diseases, including diabetes, haemophilia and cancer 4. Presumably to limit deleterious mobilization events, eukaryotic cells have developed several defence mechanisms that affect various stages of the retrotransposition process (Fig. 1). Foremost among these is the methylation of retrotransposon promoters to enforce transcriptional repression, as seen in numerous spatiotemporal and environmental contexts in which methylation of the canonical L1 promoter is inversely correlated with its expression 38. Numerous epigenetic modifiers participate in retrotransposon silencing, including the DNA methyltransferase‐like protein Dnmt3L, which is critical for Dnmt3A‐mediated methylation of retroelements in primordial germ cells 41. Suppression of retrotransposition is also reinforced in germ cells by small RNAs, including the Piwi‐interacting RNA silencing pathway 43. Interestingly, abrogated retrotransposon promoter methylation due to methyltransferase and Piwi‐interacting RNA inactivation has been described in association with spermatogenic disorders, illustrating the evolutionary importance of these suppression mechanisms 41. Piwi‐interacting RNAs, along with other small RNAs, including repeat‐associated small interfering RNAs and micro‐RNAs, also act to degrade retrotransposon transcripts via RNA interference 46. Insights gained from human cancer cells 52, as well as other eukaryotes, suggest that RNA interference is a highly conserved defence against retrotransposition, particularly in germ cells 50.
Figure 1.
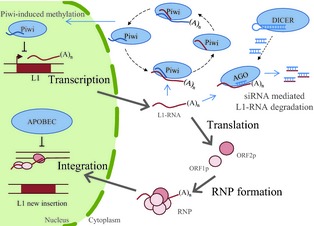
L1 retrotransposition and silencing pathways. L1 mobilization requires the key steps of transcription, mRNA export to the cytoplasm, translation, ribonucleoprotein particle formation, entry into the nucleus and, finally, integration. The Piwi‐induced methylation silencing pathway involves a selective amplification cycle fuelled by Piwi‐mediated cleavage of L1 transcripts. The repeat‐associated small interfering RNA degradation pathway is regulated by the generation of siRNAs from dsRNAs by Dicer and the fragmentation of L1 RNAs by AGO family proteins. L1 integration is also inhibited by several host factors, including members of the APOBEC3 family. Proteins and RNAs implicated in L1‐silencing pathways are represented in blue. L1 RNAs and proteins are represented in pink.
Epigenetic and post‐transcriptional suppression of retrotransposition are complemented by host factors that target L1 target‐site primed reverse transcription intermediates during the generation of new insertions. The exonuclease Trex1, for instance, metabolizes reverse transcribed retrotransposon DNA 56. Numerous studies have reported restriction of L1 mobilization in cultured cells by members of the APOBEC3 (A3) family of cytidine deaminases 57, although deaminase‐dependent and ‐independent modes of action likely play roles in retroelement restriction by different A3 factors. Notably, two studies recently reported a pan‐cancer APOBEC3 mutagenesis signature 64, indicating that APOBEC3 deaminases can target genomic DNA and suggesting a role for APOBEC3‐mediated deamination in the accumulation of mutations during oncogenesis. Thus, paradoxically, APOBEC3 activity might protect cells from potentially oncogenic retrotransposition events, yet exact a mutagenic toll of its own if not tightly regulated. Another factor, the putative RNA helicase MOV10, has been demonstrated to restrict L1 retrotransposition in cultured cells 66. MOV10 associates with the key RNA‐induced silencing complex component AGO2 and the L1 ribonucleoprotein particle, and may thereby degrade, or block the translation of, L1 mRNAs 69. This relationship, along with the evolutionary conservation of RNA interference in germ cells, underlines how host genome suppression of retrotransposons gains efficacy through redundant organization, but also vulnerability due to interdependence. Therefore, the absence or reduction of at least some retrotransposition defence mechanisms post fertilization, such as Piwi‐interacting RNAs 70, may disproportionately accentuate somatic cell retrotransposition, a view supported by fewer occurrences of L1 retrotransposition in gametes compared with soma 71.
It follows that L1‐mediated mutagenesis may contribute meaningfully to cancer, a disease driven by mutations in somatic cells; indeed, the idea that retrotransposition could often be involved in tumorigenesis is not new 74. Numerous cancers exhibit pronounced L1 activation 32. Nonetheless, a range of key issues remain unresolved, including the most obvious question of whether L1 mobilization causes cancer, or vice versa. Here, we summarize some of what is known of L1 activity during oncogenesis and tumour progression, and propose an explanation for why L1 retrotransposition appears to be a common feature of epithelial cancers.
Environmental triggers common to L1 activity and oncogenesis
Cancer encompasses a broad group of more than 200 diseases that involve the uncontrolled growth of cells leading to tumour formation, as well as several other common hallmarks 86. At the molecular level, cancer is a complex disease attributed to the accumulation of multiple risk factors, from genetic predisposition to environmental factors such as diet, lifestyle and exposure to toxic compounds 88. Epidemiological twin studies suggest that environment influences cancer aetiology far more decisively than genetics 89. For instance, the contribution of environmental factors to sporadic cancers ranges from 58 to 82%, versus the highest genetic contribution of 27–42% for colorectal, breast and prostate cancers 89. Interestingly, inherited risk far exceeds the frequency of mutations already reported in cancer genes, suggesting that other contributing mechanisms or types of genetic alteration, such as rare variants and retrotransposition events occurring in noncoding genomic regions, may contribute to cancer development.
Differentiating those mutations that cause oncogenesis (‘drivers’) versus those mutations accumulating in a deregulated genomic environment during the course of oncogenesis (‘passengers’) is a long‐standing challenge in cancer genomics. Furthermore, L1 insertional mutagenesis is only one among a constellation of different types of genetic aberrations that frequently underpin cancer. It is nevertheless intriguing that L1 insertional mutagenesis occurs in tumours 76, cancer cell lines 91 and during development 71. Somatic L1 retrotransposition can occur in both dividing and nondividing cells 19, generating mosaicism and, potentially, tumorigenic mutations. L1 mRNAs are present in differentiated tissues such as brain, kidney, liver and heart 77, and L1 mobilization has, to date, been identified in liver and brain tissue 80. Given the substantial, predominantly deleterious effects of intragenic L1 insertions upon host gene expression 79, L1 insertions may be more likely, on a per mutation basis, to have an impact on tumorigenesis than other genetic aberrations observed in cancer.
Several carcinogenic environmental factors 98 have been demonstrated to increase ME activity in cultured cells 99. For instance, benzopyrenes have been identified as a risk factor for lung, colorectal and breast cancer 100, and have been demonstrated to increase L1 mobilization in HeLa cells 103. Nickel exposure is a risk factor for lung and breast cancer 104, and has likewise been shown to induce L1 mobilization 106. Tumours often show increased levels of free radicals involved in oxidative stress 107; again, oxidative stress has been shown to increase L1 mobilization 83. Oxidative stress and DNA damage occurring as part of senescence can increase chromosomal instability and retrotransposon activity 108, thereby contributing to genomic mosaicism associated with cancer development 109. Hence, if we take as given that retrotransposition is a stochastic process, and that most somatic cells present a basal L1 activity that has evaded silencing 77, it is plausible that environmental factors increase the probability of a somatic L1 insertion affecting an oncogenic locus, thereby triggering neoplastic transformation.
Mapping retrotransposition in cancer genomes
Despite the large effect size of many intragenic L1 insertions, their relative importance to oncogenesis versus the cornucopia of other mutations usually observed in a tumour is unclear. Cancer genome sequencing typically reveals a host of somatic cell mutations, including tens or hundreds of thousands of single nucleotide variants, as well as insertions, deletions, translocations, rearrangements and other more exotic mutations, all found within a given tumour 111. Are a few additional L1 insertions important in this context? One route to address this question is to map the locations of L1 insertions in cancer genomes and ascertain which protein‐coding genes are affected by mutations, following the same logic developed for transposon mutagenesis screens in mouse models of cancer 112. The first success in mapping a bona fide somatic L1 insertion in a tumour occurred > 20 years ago, with the discovery by Miki et al. of an exonic L1, flanked by target‐site duplications and integrated in the APC gene of a colorectal cancer patient 76. Because APC is the pre‐eminent tumour suppressor gene in colorectal cancer caused by familial adenomatous polyposis 113, it is reasonable to conclude that, in this case, a single L1 insertion was sufficient to drive oncogenesis.
Spurred by this paradigm, and aided by the advent of high‐throughput sequencing, several exemplar strategies have been developed in recent years to map endogenous somatic retrotransposition events in tumours (for detailed reviews see 115). The first of these methods, presented by Iskow et al., digested genomic DNA using restriction enzymes that recognize the 3′‐end of L1 and Alu and linked adapters to the resultant fragments to obtain retrotransposon libraries by PCR. Sequencing of these libraries revealed nine de novo L1 insertions in lung tumours 32. More recently, Lee et al. used a computational method to analyse paired‐end whole‐genome sequence data, from tumour and matching blood, searching for paired‐end‐reads mapping to unique genome locations and a distal ME. Using this strategy, they identified 194 de novo ME insertions in colorectal, ovarian and prostate tumours 79. Newly detected somatic insertions were located preferentially in tumour suppressor genes, where integration of an intronic L1 typically inhibited transcription, as expected 79. Similarly, Solyom et al. analysed colorectal tumours and matching tissues by hemi‐nested PCR coupled to sequencing (L1‐seq) 29 and detected 69 de novo L1 insertions, including in the introns of genes previously reported to be involved in cancer 81.
More recently, we identified 12 de novo L1 insertions in a cohort of hepatocellular carcinoma patients using retrotransposon capture sequencing, a hybridization‐based approach to enrich DNA for recent L1, Alu and SVA insertions, followed by deep sequencing 80. Interestingly, one somatic L1 insertion was shown to activate a putative oncogene through ablation of a negative feedback loop 80. This model might explain why expression increases for at least some genes harbouring tumour‐specific L1 insertions 79. Retrotransposon capture sequencing also revealed a validated somatic L1 insertion in nontumour liver, as well as germline L1 and Alu insertions in the tumour suppressor gene MCC that would, by definition, precede tumorigenesis 80.
As these works demonstrate, cancer is arguably the most promising immediate context in which to assess the phenotypic effects of somatic retrotransposition in vivo. In other tissues, such as the brain, the majority of mosaicism due to L1 mobilization is thought to occur late in differentiation 94, meaning that each individual insertion is present in a handful of cells and necessitating single cell or deep targeted sequencing to detect somatic L1 insertions 95. By contrast, some tumour cells containing new L1 insertions are likely to undergo clonal expansion, meaning that the mutations they contain can reach sufficient abundance to be detected even via standard whole‐genome sequencing 79. Extensive cancer gene catalogues 118 somewhat simplify the process of linking mutations to tumorigenesis, and these predictions can be validated in vitro and in vivo using cancer cell lines and animal models, respectively. Nonetheless, tumours do present challenges in retrotransposon mapping. Cellular heterogeneity can obscure subclonal mutations that may have been important in oncogenesis, but not tumour growth. Another issue is that wholesale genetic aberration is commonplace in cancer genomes, leading to difficulties in distinguishing retrotransposition from other structural variation involving retrotransposons, such as rearrangements. As we have observed, this latter problem is surmountable through stringent parameters in calling de novo insertions, and yet can still require extensive validation via PCR and capillary sequencing 80. Despite these challenges, and the numerous unanswered questions remaining in the area, the recent studies discussed above have nevertheless demonstrated that: (a) ME activation can reduce the tumour suppressor capacity of somatic cells, and (b) oncogenesis can be driven by individual ME insertions.
Do cancer stem cells promote L1 mobilization in epithelial tumours?
In‐depth analysis of multiple cancer types has, to date, revealed somatic L1 retrotransposition only in cancers of epithelial origin 32. One explanation for this observation is that epithelial cells are demonstrably more ‘plastic’ than other differentiated potential tumour progenitors. Epithelial cells can be transformed to yield cancer stem cells 119 and can also be reprogrammed into induced pluripotent stem cells via deliberate activation of a mesenchymal to epithelial transition 120. Interestingly, metastasis is more prevalent in epithelial cancers than in other tumour aetiologies and is thought to involve cells that have lost epithelial features and acquired a migratory phenotype 122 via an epithelial to mesenchymal transition, the reverse process of mesenchymal to epithelial transition 123. Thus, to speculate, the basal plasticity of some epithelial cells may endow tumours with greater aggressiveness and evolutionary flexibility (Fig. 2), based on the provision of cellular plasticity by cancer stem cells resident in epithelial tumours 124.
Figure 2.
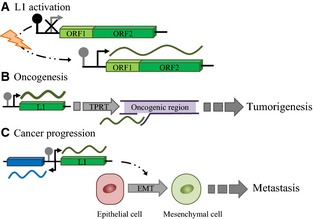
Hypotheses for L1 involvement in tumorigenesis and cancer progression. (A) Environmental factors initially cause cells to change the methylation status of the L1 promoter, activating full‐length L1 transcription. (B) This is followed by de novo L1 retrotransposition into an oncogenic region, resulting in tumorigenesis. (C) Once the tumour is established, the canonical L1 promoter is increasingly hypomethylated, potentially activating the antisense promoter and nearby genes. Moreover, L1 promoter hypomethylation appears to be correlated with an epithelial to mesenchymal transition that could eventually lead to metastasis.
The question of how a cell turns into a cancer stem cell in vivo remains unresolved. Two likely possibilities are that cancer stem cells are naturally reprogrammed from either resident tissue stem cells or from differentiated cells (Fig. 3). In the first case, a stem cell might suffer an oncogenic mutation or ‘lesion’ that yields a tumour cell rather than a normal differentiated cell 125. In the second case, an oncogenic mutation might cause a differentiated cell to reprogramme towards a tumour cell‐like state 126. Given that differentiated epithelial cells are sufficiently plastic to be reprogrammable and that, to date, only epithelial tumours have been demonstrated to accommodate L1 mobilization, it is tempting to conclude that these cancers are primarily caused by reprogrammed differentiated cells, rather than resident tissue stem cell populations. Although, to our knowledge, L1 activity has not been assessed in cancer stem cells, it is established that numerous other stem cell types, from embryonic stem cells to neural progenitor cells, are permissive for L1 mobilization 71. Indeed, directed reprogramming of epithelial cells in vitro to obtain induced pluripotent stem cells activates L1 mobilization 127. Thus, although the relationships between cancer stem cells, epithelial cells and L1 mobilization are somewhat circumstantial, it is reasonable to propose that the plasticity of epithelial tumours explains their specific support of L1 mobilization, increasing the probability that cancer stem cells contain oncogenic L1 driver mutations or are, at the very least, permissive of L1 activity.
Figure 3.
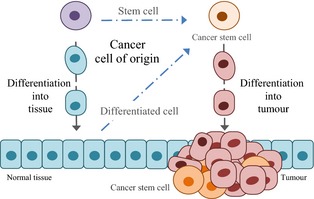
Schematic representation of tumorigenesis in epithelial cancers. A stem cell undergoes differentiation, giving rise to normal tissue. If mutation of an oncogenic region occurs, a normal stem cell can turn into a cancer stem cell. This cancer stem cell can also be generated from a differentiated cell via a mesenchymal to epithelial transition. Once established, cancer stem cells can differentiate into the various cell types that form a tumour.
Conclusions
A clear correlation has been established between L1 mobilization and cancer. However, determining how frequently L1 activity is a cause rather than a consequence of oncogenesis presents a difficult challenge that will require extensive study. L1 mobilization and cancer are both heavily influenced by environment, and it is clear that tumours often contain de novo L1 insertions, some of which map to cancer genes. Yet, most of the pathways leading to L1 activation in cancer remain unknown. For instance, are the key transcription factors known to regulate L1 expression, such as SOX2, RUNX3 and YY1 128 perturbed by other mutations, enabling L1 activation? Or, from another perspective, are retrotransposons simply de‐repressed by abrogation of genome‐wide surveillance mechanisms, such as DNA methylation, in tumour cells? Although critical information is lacking, particularly experimental evidence of L1 activity in cancer progenitor cells, insights gained from pluripotent and other highly plastic cells suggest that retrotransposons opportunistically exploit any weakness or alteration in the cellular systems required for their suppression 94. This, combined with the recognition that epithelial cancers specifically support L1 mobilization, leads to a plausible model in which L1 activation is due to epigenetic or other perturbations of retrotransposon suppression by cancer stem cells.
Even if found to rarely drive oncogenesis, L1 activity may be useful as a diagnostic tool for malignancy and metastasis. Various studies suggest that detectable levels of L1 mRNA and proteins are associated with poor cancer prognosis 84, whereas L1 promoter hypomethylation can indicate problematic genome‐wide epigenetic deregulation 135. From a clinical perspective, it would also be useful to establish whether all tumour cells from a given neoplasm, or just a subset of cells, present high L1 activity, and whether this heterogeneity assists tumour cell evolution in response to chemotherapy or radiotherapy. Finally, it is unknown whether blocking L1 mobilization, for example, using reverse transcriptase inhibitors 19, would in any way affect cancer progression or prognosis. In this regard, and despite perhaps being coincidental, it is interesting that cancer is not thought to occur in the naked mole rat, one of the few mammals that does not maintain active MEs within its genome 139. A key experiment might, therefore, be to inhibit L1 retrotransposition, potentially in an established animal model of cancer, to assess the contribution of L1 to oncogenesis, tumour growth and metastasis. Such in vivo approaches would complement future, larger scale surveys of retrotransposition in human tumours based on high‐throughput sequencing and, potentially, go further in elucidating the origins and importance of L1 activity in cancer.
Acknowledgements
GJF is supported by an Australian NHMRC Career Development Fellowship (GNT1045237), NHMRC Project Grants GNT1042449, GNT1045991 and GNT1052303, and the European Union's Seventh Framework Programme (FP7/2007–2013) under grant agreement No. 259743 underpinning the MODHEP consortium.
References
- 1.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh Wet al (2001) Initial sequencing and analysis of the human genome. Nature 409, 860–921 [DOI] [PubMed] [Google Scholar]
- 2.de Koning APJ, Gu W, Castoe TA, Batzer MA & Pollock DD (2011) Repetitive elements may comprise over two‐thirds of the human genome. PLoS Genet 7, e1002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordaux R & Batzer MA (2009) The impact of retrotransposons on human genome evolution. Nat Rev Genet 10, 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazazian HH Jr, Wong C, Youssoufian H, Scott AF, Phillips DG & Antonarakis SE (1988) Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature 332, 164–166 [DOI] [PubMed] [Google Scholar]
- 5.Dombroski BA, Mathias SL, Nanthakumar E, Scott AF & Kazazian HH Jr (1991) Isolation of an active human transposable element. Science 254, 1805–1808 [DOI] [PubMed] [Google Scholar]
- 6.Scott AF, Schmeckpeper BJ, Abdelrazik M, Comey CT, O'Hara B, Rossiter JP, Cooley T, Heath P, Smith KD & Margolet L (1987) Origin of the human L1 elements: proposed progenitor genes deduced from a consensus DNA sequence. Genomics 1, 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hohjoh H & Singer MF (1996) Cytoplasmic ribonucleoprotein complexes containing human LINE‐1 protein and RNA. EMBO J 15, 630–639 [PMC free article] [PubMed] [Google Scholar]
- 8.Hohjoh H & Singer MF (1997) Sequence‐specific single‐strand RNA binding protein encoded by the human LINE‐1 retrotransposon. EMBO J 16, 6034–6043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Q, Moran JV, Kazazian HH Jr & Boeke JD (1996) Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell 87, 905–916 [DOI] [PubMed] [Google Scholar]
- 10.Mathias SL, Scott AF, Kazazian HH Jr, Boeke JD & Gabriel A (1991) Reverse transcriptase encoded by a human transposable element. Science 254, 1808–1810 [DOI] [PubMed] [Google Scholar]
- 11.Swergold GD (1990) Identification, characterization, and cell specificity of a human LINE‐1 promoter. Mol Cell Biol 10, 6718–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speek M (2001) Antisense promoter of human L1 retrotransposon drives transcription of adjacent cellular genes. Mol Cell Biol 21, 1973–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimaldi G, Skowronski J & Singer MF (1984) Defining the beginning and end of KpnI family segments. EMBO J 3, 1753–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esnault C, Maestre J & Heidmann T (2000) Human LINE retrotransposons generate processed pseudogenes. Nat Genet 24, 363–367 [DOI] [PubMed] [Google Scholar]
- 15.Kulpa DA & Moran JV (2005) Ribonucleoprotein particle formation is necessary but not sufficient for LINE‐1 retrotransposition. Hum Mol Genet 14, 3237–3248 [DOI] [PubMed] [Google Scholar]
- 16.Kulpa DA & Moran JV (2006) Cis‐preferential LINE‐1 reverse transcriptase activity in ribonucleoprotein particles. Nat Struct Mol Biol 13, 655–660 [DOI] [PubMed] [Google Scholar]
- 17.Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, Kazazian HH, Boeke JD & Moran JV (2001) Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol 21, 1429–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin SL (1991) Ribonucleoprotein particles with LINE‐1 RNA in mouse embryonal carcinoma cells. Mol Cell Biol 11, 4804–4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubo S, Seleme MC, Soifer HS, Perez JL, Moran JV, Kazazian HH Jr & Kasahara N (2006) L1 retrotransposition in nondividing and primary human somatic cells. Proc Natl Acad Sci USA 103, 8036–8041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luan DD, Korman MH, Jakubczak JL & Eickbush TH (1993) Reverse transcription of R2Bm RNA is primed by a nick at the chromosomal target site: a mechanism for non‐LTR retrotransposition. Cell 72, 595–605 [DOI] [PubMed] [Google Scholar]
- 21.Cost GJ, Feng Q, Jacquier A & Boeke JD (2002) Human L1 element target‐primed reverse transcription in vitro. EMBO J 21, 5899–5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewannieux M, Esnault C & Heidmann T (2003) LINE‐mediated retrotransposition of marked Alu sequences. Nat Genet 35, 41–48 [DOI] [PubMed] [Google Scholar]
- 23.Garcia‐Perez JL, Doucet AJ, Bucheton A, Moran JV & Gilbert N (2007) Distinct mechanisms for trans‐mediated mobilization of cellular RNAs by the LINE‐1 reverse transcriptase. Genome Res 17, 602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raiz J, Damert A, Chira S, Held U, Klawitter S, Hamdorf M, Lower J, Stratling WH, Lower R & Schumann GG (2012) The non‐autonomous retrotransposon SVA is trans‐mobilized by the human LINE‐1 protein machinery. Nucleic Acids Res 40, 1666–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett EA, Keller H, Mills RE, Schmidt S, Moran JV, Weichenrieder O & Devine SE (2008) Active Alu retrotransposons in the human genome. Genome Res 18, 1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouha B, Schustak J, Badge RM, Lutz‐Prigge S, Farley AH, Moran JV & Kazazian HH Jr (2003) Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci USA 100, 5280–5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills RE, Bennett EA, Iskow RC & Devine SE (2007) Which transposable elements are active in the human genome? Trends Genet 23, 183–191 [DOI] [PubMed] [Google Scholar]
- 28.Beck CR, Collier P, Macfarlane C, Malig M, Kidd JM, Eichler EE, Badge RM & Moran JV (2010) LINE‐1 retrotransposition activity in human genomes. Cell 141, 1159–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ewing AD & Kazazian HH Jr (2010) High‐throughput sequencing reveals extensive variation in human‐specific L1 content in individual human genomes. Genome Res 20, 1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewing AD & Kazazian HH Jr (2011) Whole‐genome resequencing allows detection of many rare LINE‐1 insertion alleles in humans. Genome Res 21, 985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang CR, Schneider AM, Lu Y, Niranjan T, Shen P, Robinson MA, Steranka JP, Valle D, Civin CI, Wang Tet al (2010) Mobile interspersed repeats are major structural variants in the human genome. Cell 141, 1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iskow RC, McCabe MT, Mills RE, Torene S, Pittard WS, Neuwald AF, Van Meir EG, Vertino PM & Devine SE (2010) Natural mutagenesis of human genomes by endogenous retrotransposons. Cell 141, 1253–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witherspoon DJ, Zhang Y, Xing J, Watkins WS, Ha H, Batzer MA & Jorde LB (2013) Mobile element scanning (ME‐Scan) identifies thousands of novel Alu insertions in diverse human populations. Genome Res 23, 1170–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seleme MC, Vetter MR, Cordaux R, Bastone L, Batzer MA & Kazazian HH Jr (2006) Extensive individual variation in L1 retrotransposition capability contributes to human genetic diversity. Proc Natl Acad Sci USA 103, 6611–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck CR, Garcia‐Perez JL, Badge RM & Moran JV (2011) LINE‐1 elements in structural variation and disease. Annu Rev Genomics Hum Genet 12, 187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaer K & Speek M (2013) Retroelements in human disease. Gene 518, 231–241 [DOI] [PubMed] [Google Scholar]
- 37.Hancks DC & Kazazian HH Jr (2012) Active human retrotransposons: variation and disease. Curr Opin Genet Dev 22, 191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baba Y, Huttenhower C, Nosho K, Tanaka N, Shima K, Hazra A, Schernhammer ES, Hunter DJ, Giovannucci EL, Fuchs CSet al (2010) Epigenomic diversity of colorectal cancer indicated by LINE‐1 methylation in a database of 869 tumors. Mol Cancer 9, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muotri AR, Marchetto MC, Coufal NG, Oefner R, Yeo G, Nakashima K & Gage FH (2010) L1 retrotransposition in neurons is modulated by MeCP2. Nature 468, 443–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perng W, Mora‐Plazas M, Marin C, Rozek LS, Baylin A & Villamor E (2013) A prospective study of LINE‐1 DNA methylation and development of adiposity in school‐age children. PLoS ONE 8, e62587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourc'his D & Bestor TH (2004) Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 431, 96–99 [DOI] [PubMed] [Google Scholar]
- 42.Kato Y, Kaneda M, Hata K, Kumaki K, Hisano M, Kohara Y, Okano M, Li E, Nozaki M & Sasaki H (2007) Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet 16, 2272–2280 [DOI] [PubMed] [Google Scholar]
- 43.Aravin A, Gaidatzis D, Pfeffer S, Lagos‐Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi‐Miyagawa S, Nakano Tet al (2006) A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207 [DOI] [PubMed] [Google Scholar]
- 44.Aravin AA, Sachidanandam R, Bourc'his D, Schaefer C, Pezic D, Toth KF, Bestor T & Hannon GJ (2008) A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell 31, 785–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heyn H, Ferreira HJ, Bassas L, Bonache S, Sayols S, Sandoval J, Esteller M & Larriba S (2012) Epigenetic disruption of the PIWI pathway in human spermatogenic disorders. PLoS ONE 7, e47892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heras SR, Macias S, Plass M, Fernandez N, Cano D, Eyras E, Garcia‐Perez JL & Caceres JF (2013) The Microprocessor controls the activity of mammalian retrotransposons. Nat Struct Mol Biol 20, 1173–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macias S, Plass M, Stajuda A, Michlewski G, Eyras E & Caceres JF (2012) DGCR8 HITS‐CLIP reveals novel functions for the Microprocessor. Nat Struct Mol Biol 19, 760–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sijen T & Plasterk RH (2003) Transposon silencing in the Caenorhabditis elegans germ line by natural RNAi. Nature 426, 310–314 [DOI] [PubMed] [Google Scholar]
- 49.Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi‐Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano Tet al (2008) Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 453, 539–543 [DOI] [PubMed] [Google Scholar]
- 50.Chow JC, Ciaudo C, Fazzari MJ, Mise N, Servant N, Glass JL, Attreed M, Avner P, Wutz A, Barillot Eet al (2010) LINE‐1 activity in facultative heterochromatin formation during X chromosome inactivation. Cell 141, 956–969 [DOI] [PubMed] [Google Scholar]
- 51.Levin HL & Moran JV (2011) Dynamic interactions between transposable elements and their hosts. Nat Rev Genet 12, 615–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nuo Y & Haig HK (2006) L1 retrotransposition is suppressed by endogenously encoded small interfering RNAs in human cultured cells. Nat Struct Mol Biol 13, 763–771 [DOI] [PubMed] [Google Scholar]
- 53.Czech B, Malone CD, Zhou R, Stark A, Schlingeheyde C, Dus M, Perrimon N, Kellis M, Wohlschlegel JA, Sachidanandam Ret al (2008) An endogenous small interfering RNA pathway in Drosophila. Nature 453, 798–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malone CD & Hannon GJ (2009) Small RNAs as guardians of the genome. Cell 136, 656–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slotkin RK, Vaughn M, Borges F, Tanurdzic M, Becker JD, Feijo JA & Martienssen RA (2009) Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136, 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stetson DB, Ko JS, Heidmann T & Medzhitov R (2008) Trex1 prevents cell‐intrinsic initiation of autoimmunity. Cell 134, 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bogerd HP, Wiegand HL, Hulme AE, Garcia‐Perez JL, O'Shea KS, Moran JV & Cullen BR (2006) Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc Natl Acad Sci USA 103, 8780–8785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H, Lilley CE, Yu Q, Lee DV, Chou J, Narvaiza I, Landau NR & Weitzman MD (2006) APOBEC3A is a potent inhibitor of adeno‐associated virus and retrotransposons. Curr Biol 16, 480–485 [DOI] [PubMed] [Google Scholar]
- 59.Muckenfuss H, Hamdorf M, Held U, Perkovic M, Lower J, Cichutek K, Flory E, Schumann GG & Munk C (2006) APOBEC3 proteins inhibit human LINE‐1 retrotransposition. J Biol Chem 281, 22161–22172 [DOI] [PubMed] [Google Scholar]
- 60.Kinomoto M, Kanno T, Shimura M, Ishizaka Y, Kojima A, Kurata T, Sata T & Tokunaga K (2007) All APOBEC3 family proteins differentially inhibit LINE‐1 retrotransposition. Nucleic Acids Res 35, 2955–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stenglein MD & Harris RS (2006) APOBEC3B and APOBEC3F inhibit L1 retrotransposition by a DNA deamination‐independent mechanism. J Biol Chem 281, 16837–16841 [DOI] [PubMed] [Google Scholar]
- 62.OhAinle M, Kerns JA, Li MM, Malik HS & Emerman M (2008) Antiretroelement activity of APOBEC3H was lost twice in recent human evolution. Cell Host Microbe 4, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dang Y, Wang X, Esselman WJ & Zheng YH (2006) Identification of APOBEC3DE as another antiretroviral factor from the human APOBEC family. J Virol 80, 10522–10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burns MB, Temiz NA & Harris RS (2013) Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat Genet online in advance of publication. [DOI] [PMC free article] [PubMed]
- 65.Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, Kiezun A, Kryukov GV, Carter SL, Saksena Get al (2013) An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet online in advance of publication. [DOI] [PMC free article] [PubMed]
- 66.Arjan‐Odedra S, Swanson CM, Sherer NM, Wolinsky SM & Malim MH (2012) Endogenous MOV10 inhibits the retrotransposition of endogenous retroelements but not the replication of exogenous retroviruses. Retrovirology 9, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodier JL, Cheung LE & Kazazian HH Jr (2012) MOV10 RNA helicase is a potent inhibitor of retrotransposition in cells. PLoS Genet 8, e1002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodier JL, Cheung LE & Kazazian HH Jr (2013) Mapping the LINE1 ORF1 protein interactome reveals associated inhibitors of human retrotransposition. Nucleic Acids Res 41, 7401–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu C, Zhang X, Huang F, Yang B, Li J, Liu B, Luo H, Zhang P & Zhang H (2012) APOBEC3G inhibits microRNA‐mediated repression of translation by interfering with the interaction between Argonaute‐2 and MOV10. J Biol Chem 287, 29373–29383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuramochi‐Miyagawa S, Kimura T, Yomogida K, Kuroiwa A, Tadokoro Y, Fujita Y, Sato M, Matsuda Y & Nakano T (2001) Two mouse piwi‐related genes: miwi and mili. Mech Dev 108, 121–133 [DOI] [PubMed] [Google Scholar]
- 71.Garcia‐Perez JL, Marchetto MC, Muotri AR, Coufal NG, Gage FH, O'Shea KS & Moran JV (2007) LINE‐1 retrotransposition in human embryonic stem cells. Hum Mol Genet 16, 1569–1577 [DOI] [PubMed] [Google Scholar]
- 72.Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM & Kazazian HH Jr (2009) L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev 23, 1303–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freeman P, Macfarlane C, Collier P, Jeffreys AJ & Badge RM (2011) L1 hybridization enrichment: a method for directly accessing de novo L1 insertions in the human germline. Hum Mutat 32, 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belancio VP, Hedges DJ & Deininger P (2008) Mammalian non‐LTR retrotransposons: for better or worse, in sickness and in health. Genome Res 18, 343–358 [DOI] [PubMed] [Google Scholar]
- 75.Rodic N & Burns KH (2013) Long interspersed element‐1 (LINE‐1): passenger or driver in human neoplasms? PLoS Genet 9, e1003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miki Y, Nishisho I, Horii A, Miyoshi Y, Utsunomiya J, Kinzler KW, Vogelstein B & Nakamura Y (1992) Disruption of the APC gene by a retrotransposal insertion of L1 sequence in a colon cancer. Cancer Res 52, 643–645 [PubMed] [Google Scholar]
- 77.Faulkner GJ, Kimura Y, Daub CO, Wani S, Plessy C, Irvine KM, Schroder K, Cloonan N, Steptoe AL, Lassmann Tet al (2009) The regulated retrotransposon transcriptome of mammalian cells. Nat Genet 41, 563–571 [DOI] [PubMed] [Google Scholar]
- 78.Belancio VP, Roy‐Engel AM, Pochampally RR & Deininger P (2010) Somatic expression of LINE‐1 elements in human tissues. Nucleic Acids Res 38, 3909–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee E, Iskow R, Yang L, Gokcumen O, Haseley P, Luquette LJ 3rd, Lohr JG, Harris CC, Ding L, Wilson RKet al (2012) Landscape of somatic retrotransposition in human cancers. Science 337, 967–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shukla R, Upton KR, Munoz‐Lopez M, Gerhardt DJ, Fisher ME, Nguyen T, Brennan PM, Baillie JK, Collino A, Ghisletti Set al (2013) Endogenous retrotransposition activates oncogenic pathways in hepatocellular carcinoma. Cell 153, 101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solyom S, Ewing AD, Rahrmann EP, Doucet T, Nelson HH, Burns MB, Harris RS, Sigmon DF, Casella A, Erlanger Bet al (2012) Extensive somatic L1 retrotransposition in colorectal tumors. Genome Res 22, 2328–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montoya‐Durango DE & Ramos KS (2010) L1 retrotransposon and retinoblastoma: molecular linkages between epigenetics and cancer. Curr Mol Med 10, 511–521 [DOI] [PubMed] [Google Scholar]
- 83.Giorgi G, Marcantonio P & Del Re B (2011) LINE‐1 retrotransposition in human neuroblastoma cells is affected by oxidative stress. Cell Tissue Res 346, 383–391 [DOI] [PubMed] [Google Scholar]
- 84.Chen L, Dahlstrom JE, Chandra A, Board P & Rangasamy D (2012) Prognostic value of LINE‐1 retrotransposon expression and its subcellular localization in breast cancer. Breast Cancer Res Treat 136, 129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang J, Lunyak VV & Jordan IK (2012) Chromatin signature discovery via histone modification profile alignments. Nucleic Acids Res 40, 10642–10656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanahan D & Weinberg RA (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 87.Hanahan D & Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 88.Tomatis LA, International Agency for Research on Cancer (1990) Cancer: Causes, Occurrence, and Control. International Agency for Research on Cancer, New York [Google Scholar]
- 89.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A & Hemminki K (2000) Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343, 78–85 [DOI] [PubMed] [Google Scholar]
- 90.Sorensen TI, Nielsen GG, Andersen PK & Teasdale TW (1988) Genetic and environmental influences on premature death in adult adoptees. N Engl J Med 318, 727–732 [DOI] [PubMed] [Google Scholar]
- 91.Garcia‐Perez JL, Morell M, Scheys JO, Kulpa DA, Morell S, Carter CC, Hammer GD, Collins KL, O'Shea KS, Menendez Pet al (2010) Epigenetic silencing of engineered L1 retrotransposition events in human embryonic carcinoma cells. Nature 466, 769–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moran JV, Holmes SE, Naas TP, DeBerardinis RJ, Boeke JD & Kazazian HH Jr (1996) High frequency retrotransposition in cultured mammalian cells. Cell 87, 917–927 [DOI] [PubMed] [Google Scholar]
- 93.Ostertag EM, Prak ET, DeBerardinis RJ, Moran JV & Kazazian HH Jr (2000) Determination of L1 retrotransposition kinetics in cultured cells. Nucleic Acids Res 28, 1418–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coufal NG, Garcia‐Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, Morell M, O'Shea KS, Moran JV & Gage FH (2009) L1 retrotransposition in human neural progenitor cells. Nature 460, 1127–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baillie JK, Barnett MW, Upton KR, Gerhardt DJ, Richmond TA, De Sapio F, Brennan PM, Rizzu P, Smith S, Fell Met al (2011) Somatic retrotransposition alters the genetic landscape of the human brain. Nature 479, 534–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Evrony GD, Cai X, Lee E, Hills LB, Elhosary PC, Lehmann HS, Parker JJ, Atabay KD, Gilmore EC, Poduri Aet al (2012) Single‐neuron sequencing analysis of l1 retrotransposition and somatic mutation in the human brain. Cell 151, 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han JS, Szak ST & Boeke JD (2004) Transcriptional disruption by the L1 retrotransposon and implications for mammalian transcriptomes. Nature 429, 268–274 [DOI] [PubMed] [Google Scholar]
- 98.Boffetta P & Nyberg F (2003) Contribution of environmental factors to cancer risk. Br Med Bull 68, 71–94 [DOI] [PubMed] [Google Scholar]
- 99.Fornace AJ Jr & Mitchell JB (1986) Induction of B2 RNA polymerase III transcription by heat shock: enrichment for heat shock induced sequences in rodent cells by hybridization subtraction. Nucleic Acids Res 14, 5793–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Denissenko MF, Pao A, Tang M & Pfeifer GP (1996) Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science 274, 430–432 [DOI] [PubMed] [Google Scholar]
- 101.Tabatabaei SM, Heyworth JS, Knuiman MW & Fritschi L (2010) Dietary benzo[a]pyrene intake from meat and the risk of colorectal cancer. Cancer Epidemiol Biomark Prev 19, 3182–3184 [DOI] [PubMed] [Google Scholar]
- 102.Rathore K & Wang HC (2013) Mesenchymal and stem‐like cell properties targeted in suppression of chronically‐induced breast cell carcinogenesis. Cancer Lett 333, 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stribinskis V & Ramos KS (2006) Activation of human long interspersed nuclear element 1 retrotransposition by benzo(a)pyrene, an ubiquitous environmental carcinogen. Cancer Res 66, 2616–2620 [DOI] [PubMed] [Google Scholar]
- 104.Beveridge R, Pintos J, Parent ME, Asselin J & Siemiatycki J (2010) Lung cancer risk associated with occupational exposure to nickel, chromium VI, and cadmium in two population‐based case‐control studies in Montreal. Am J Ind Med 53, 476–485 [DOI] [PubMed] [Google Scholar]
- 105.Al‐Qubaisi MS, Rasedee A, Flaifel MH, Ahmad SH, Hussein‐Al‐Ali S, Hussein MZ, Eid EE, Zainal Z, Saeed M, Ilowefah Met al (2013) Cytotoxicity of nickel zinc ferrite nanoparticles on cancer cells of epithelial origin. Int J Nanomed 8, 2497–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El‐Sawy M, Kale SP, Dugan C, Nguyen TQ, Belancio V, Bruch H, Roy‐Engel AM & Deininger PL (2005) Nickel stimulates L1 retrotransposition by a post‐transcriptional mechanism. J Mol Biol 354, 246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toyokuni S, Okamoto K, Yodoi J & Hiai H (1995) Persistent oxidative stress in cancer. FEBS Lett 358, 1–3 [DOI] [PubMed] [Google Scholar]
- 108.Maxwell PH, Burhans WC & Curcio MJ (2011) Retrotransposition is associated with genome instability during chronological aging. Proc Natl Acad Sci USA 108, 20376–20381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Laurie CC, Laurie CA, Rice K, Doheny KF, Zelnick LR, McHugh CP, Ling H, Hetrick KN, Pugh EW, Amos Cet al (2012) Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat Genet 44, 642–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jacobs KB, Yeager M, Zhou W, Wacholder S, Wang Z, Rodriguez‐Santiago B, Hutchinson A, Deng X, Liu C, Horner MJet al (2012) Detectable clonal mosaicism and its relationship to aging and cancer. Nat Genet 44, 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, Varela I, Lin ML, Ordonez GR, Bignell GRet al (2010) A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463, 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Starr TK, Allaei R, Silverstein KA, Staggs RA, Sarver AL, Bergemann TL, Gupta M, O'Sullivan MG, Matise I, Dupuy AJet al (2009) A transposon‐based genetic screen in mice identifies genes altered in colorectal cancer. Science 323, 1747–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P,McKechnie Det al (1991) Identification of FAP locus genes from chromosome 5q21. Science 253, 661–665 [DOI] [PubMed] [Google Scholar]
- 114.Kinzler KW, Nilbert MC, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hamilton SR, Hedge P, Markham Aet al (1991) Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science 251, 1366–1370 [DOI] [PubMed] [Google Scholar]
- 115.Faulkner GJ (2011) Retrotransposons: mobile and mutagenic from conception to death. FEBS Lett 585, 1589–1594 [DOI] [PubMed] [Google Scholar]
- 116.Ray DA & Batzer MA (2011) Reading TE leaves: new approaches to the identification of transposable element insertions. Genome Res 21, 813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lim L, Balakrishnan A, Huskey N, Jones KD, Jodari M, Ng R, Song G, Riordan J, Anderton B, Cheung STet al (2013) MiR‐494 within an oncogenic MicroRNA megacluster regulates G1/S transition in liver tumorigenesis through suppression of MCC hepatology, in press. [DOI] [PMC free article] [PubMed]
- 118.Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, Rahman N & Stratton MR (2004) A census of human cancer genes. Nat Rev Cancer 4, 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang ZA, Mitrofanova A, Bergren SK, Abate‐Shen C, Cardiff RD, Califano A & Shen MM (2013) Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell‐of‐origin model for prostate cancer heterogeneity. Nat Cell Biol 15, 274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F & Zhuang Qet al (2010) A mesenchymal‐to‐epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 7, 51–63 [DOI] [PubMed] [Google Scholar]
- 121.Takahashi K & Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- 122.Gotzmann J, Mikula M, Eger A, Schulte‐Hermann R, Foisner R, Beug H & Mikulits W (2004) Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res 566, 9–20 [DOI] [PubMed] [Google Scholar]
- 123.De Craene B & Berx G (2013) Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 13, 97–110 [DOI] [PubMed] [Google Scholar]
- 124.Reya T, Morrison SJ, Clarke MF & Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 [DOI] [PubMed] [Google Scholar]
- 125.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW & Gilbertson RJ (2009) Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457, 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux Get al (2013) Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem‐cell‐like properties. Cell 152, 25–38 [DOI] [PubMed] [Google Scholar]
- 127.Wissing S, Munoz‐Lopez M, Macia A, Yang Z, Montano M, Collins W, Garcia‐Perez JL, Moran JV & Greene WC (2012) Reprogramming somatic cells into iPS cells activates LINE‐1 retroelement mobility. Hum Mol Genet 21, 208–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Athanikar JN, Badge RM & Moran JV (2004) A YY1‐binding site is required for accurate human LINE‐1 transcription initiation. Nucleic Acids Res 32, 3846–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M & Gage FH (2009) Wnt‐mediated activation of NeuroD1 and retro‐elements during adult neurogenesis. Nat Neurosci 12, 1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang N, Zhang L, Zhang Y & Kazazian HH Jr (2003) An important role for RUNX3 in human L1 transcription and retrotransposition. Nucleic Acids Res 31, 4929–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Chan AT, Schernhammer ES, Giovannucci EL & Fuchs CS (2008) A cohort study of tumoral LINE‐1 hypomethylation and prognosis in colon cancer. J Natl Cancer Inst 100, 1734–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pattamadilok J, Huapai N, Rattanatanyong P, Vasurattana A, Triratanachat S, Tresukosol D & Mutirangura A (2008) LINE‐1 hypomethylation level as a potential prognostic factor for epithelial ovarian cancer. Int J Gynecol Cancer 18, 711–717 [DOI] [PubMed] [Google Scholar]
- 133.Shuangshoti S, Hourpai N, Pumsuk U & Mutirangura A (2007) Line‐1 hypomethylation in multistage carcinogenesis of the uterine cervix. Asian Pac J Cancer Prev 8, 307–309 [PubMed] [Google Scholar]
- 134.Yegnasubramanian S, Haffner MC, Zhang Y, Gurel B, Cornish TC, Wu Z, Irizarry RA, Morgan J, Hicks J, DeWeese TLet al (2008) DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res 68, 8954–8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aporntewan C, Phokaew C, Piriyapongsa J, Ngamphiw C, Ittiwut C, Tongsima S & Mutirangura A (2011) Hypomethylation of intragenic LINE‐1 represses transcription in cancer cells through AGO2. PLoS ONE 6, e17934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cruickshanks HA & Tufarelli C (2009) Isolation of cancer‐specific chimeric transcripts induced by hypomethylation of the LINE‐1 antisense promoter. Genomics 94, 397–406 [DOI] [PubMed] [Google Scholar]
- 137.Matlik K, Redik K & Speek M (2006) L1 antisense promoter drives tissue‐specific transcription of human genes. J Biomed Biotechnol 2006, 71753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dai L, Huang Q & Boeke JD (2011) Effect of reverse transcriptase inhibitors on LINE‐1 and Ty1 reverse transcriptase activities and on LINE‐1 retrotransposition. BMC Biochem 12, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buffenstein R (2008) Negligible senescence in the longest living rodent, the naked mole‐rat: insights from a successfully aging species. J Comp Physiol B 178, 439–445 [DOI] [PubMed] [Google Scholar]
- 140.Kim EB, Fang X, Fushan AA, Huang Z, Lobanov AV, Han L, Marino SM, Sun X, Turanov AA, Yang Pet al (2011) Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature 479, 223–227 [DOI] [PMC free article] [PubMed] [Google Scholar]


