Abstract
Background
We sought to improve lumbar spine bone mineral density (LS-BMD) in long-term survivors of childhood acute lymphoblastic leukemia (ALL) using calcium and cholecalciferol supplementation.
Procedure
This double-blind, placebo-controlled trial randomized 275 participants (median age, 17 (9 –36.1) years) with age- and gender-specific LS-BMD Z-scores < 0 to receive nutritional counseling with supplementation of 1000 mg/day calcium and 800 IU cholecalciferol or placebo for 2 years. The primary outcome was change in LS-BMD assessed by quantitative computerized tomography (QCT) at 24 months. Linear regression models were employed to identify the baseline risk factors for low LS-BMD and to compare LS-BMD outcomes.
Results
Pre-randomization LS-BMD below the mean was associated with male gender (p=0.0024), white race (p=0.0003), lower body mass index (p<0.0001), and cumulative glucocorticoid doses of ≥5,000 mg (p=0.0012). One hundred eighty-eight (68%) participants completed the study; 77% adhered to the intervention. Mean LS-BMD change did not differ between survivors randomized to supplements (0.33±0.57) or placebo (0.28±0.56). Participants aged 9 – 13 years and those 22–35 years had the greatest mean increases in LS-BMD (0.50±0.66 and 0.37±0.23, respectively). Vitamin D insufficiency [serum 25(OH)D <30 ng/mL] found in 296 (75%), was not associated with LS-BMD outcomes (p=0.78).
Conclusion
Cholecalciferol and calcium supplementation provides no added benefit to nutritional counseling for improving LS-BMD among adolescent and young adult survivors of ALL (93% of whom had LS-BMD Z-scores above the mean at study entry).
Keywords: ALL, vitamin D, controlled trial, childhood cancer survivor, cholecalciferol
INTRODUCTION
In addition to nutritional and behavioral factors that adversely impact bone health at presentation of acute lymphoblastic leukemia (ALL), standard chemotherapy agents, including corticosteroids and methotrexate, have negative effects on bone[1,2]. Osteotoxic effects may be compounded by endocrinopathies resulting from exposure to cranial irradiation and alkylating agents[3]. Failure to attain optimal bone mass during adolescence contributes to bone mineral deficits typically associated with aging, which may precipitate early onset osteoporosis during adulthood[4–6]. Thus, long-term survivors of childhood ALL are at risk for morbidity associated with low bone mineral density (BMD) and may benefit from interventions to optimize bone health as they age.
The adverse consequences of low BMD are well recognized [7]. However, the impact of low BMD in aging survivors of childhood cancer has not been established. The degree to which low BMD is reversible remains unclear, or if its persistence results in premature onset of clinically significant bony morbidity. With this uncertainty, clinicians caring for ALL survivors often provide nutritional supplements in an effort to remediate BMD deficits. However, to date, benefits of such therapeutic interventions have not been validated in large cohorts. Thus, we designed this study to determine if nutritional counseling combined with vitamin D and calcium supplementation would result in greater gains in BMD over 2-years when compared to nutritional counseling combined with placebo among adolescent and young adult survivors of childhood ALL.
METHODS
Eligibility, recruitment, and randomization
The study design and details of recruitment and participant enrollment have been described previously (NCT00186901)[8]. Briefly, following approval from the institutional review board, we contacted survivors of childhood ALL treated between 1984 and 1997 on one of three institutional (Total XI-XIII) protocols[9–14], and who were in remission for at least 5 years. Study participants underwent baseline evaluation of liver, renal and endocrine function and assessment of LS-BMD by quantitative computerized tomography (QCT; S1). All participants received educational material summarizing strategies for maintaining a bone healthy lifestyle such as participating in regular weight-bearing exercise, optimizing dietary intake of calcium and vitamin D, and avoiding smoking and excessive alcohol consumption. Those with LS-BMD age-and sex-specific Z-scores of zero or more completed study participation after the baseline assessment and counseling. Those with LS-BMD Z-scores below zero were eligible for randomization.
Informed consent was obtained from adult patients and parents or guardians of minors; patients over the age of 10 years provided assent. Enrollment was coordinated with patient annual evaluation in the multidisciplinary After Completion of Therapy (ACT) Clinic[15].
The intervention
The intervention was designed to optimize peak bone mass during a known period of enhanced bone mineral accretion. Participants randomized to supplement received a prescription for once daily calcium carbonate 1,000 milligrams (mg) and cholecalciferol 800 International Units (IU), providing twice the Recommended Dietary Allowance (RDA) of cholecalciferol at the time the study was designed. Participants, investigators and study staff were blind to randomization arm; only the pharmacy was aware of group assignment. Participants randomized to placebo received identical tablets composed of inert substances. All randomized participants underwent nutritional counseling to encourage recommended daily intake of calcium and cholecalciferol at baseline and every 6 months for 2 years. Educational and promotional materials from the American Dairy Association, and information about non-dairy foods high in calcium and vitamin D were utilized during counseling; refresher materials were mailed 3, 9, 15 and 21 months after enrollment (S2). Compliance was monitored by pill counting at 6-, 12-, 18-and 24-months and by monitoring urinary calcium every 6 months. Participants received small incentives for meeting calcium and vitamin D intake targets.
Evaluation of bone mineral density
The primary outcome of interest was QCT-determined BMD. Lumbar spine (LS) vertebral trabecular BMD was determined with a Siemens Somatom-Plus spiral CT scanner (Siemens, Iselin, NY) and Mindways QCT calibration phantoms and software (Mindways Software, Inc., Austin, TX), as previously reported[16,17].
We classified diminished LS-BMD as an age- and sex- specific Z-score below zero. Patients were referred for endocrine evaluation if their LS-BMD Z-score was ≤ −2.0. Participants with medically controlled endocrinopathies were allowed to remain on study at the discretion of a pediatric endocrinologist.
Other factors of interest
Patient treatment was obtained from medical records by trained abstractors and included radiation and chemotherapeutic doses, age at leukemia treatment [9–14], gender, race, and age at study enrollment. Threshold values characterizing chemotherapy cumulative doses were: 1) cyclophosphamide < 7,500 mg per meter squared (mg/m2) or ≥ 7,500 mg/m2; 2) methotrexate < 10,000 mg/m2, 10,000–19,999 mg/m2 or ≥ 20,000 mg/m2; and 3) glucocorticoids in prednisone equivalent dose < 5,000 mg/m2 or ≥ 5,000 mg/m2 (assuming 0.75 mg of dexamethasone = 5 mg prednisone). Age at diagnosis was dichotomized at the median as younger than 4.6 and 4.6 years of age or older. Race was classified as white or non-white. Age at study enrollment was classified into 9–13, 14–17, 18–21, and 22–36 years.
Medical history and clinical information were obtained from questionnaire responses, physical examination findings, or laboratory testing (S1). We considered dietary intake, physical activity status, use of hormone replacement therapy, body habitus, bone age[18], pubertal status, and hepatic, renal, thyroid, parathyroid and gonadal function. Usual intake of 110 food items was assessed with the 1998 Block Food Frequency Questionnaire[19,20]; physical activity status was determined by asking about frequency, type and duration of strenuous physical activity[21]. Body mass index (BMI) was used to characterize body habitus. Pubertal status was determined by evaluating Tanner stage (female breast development, testicular volume males,) and was categorized as Stages I–III or IV–V[22]. High pressure liquid chromatography coupled to a mass spectrometer detector (LC/MS) was used to determine serum 25(OH)D. The inter-assay coefficient of variation (CV) is < 5% across measurements.
Statistical methods
Descriptive statistics were calculated for demographic, treatment, and lifestyle factors to describe the baseline population. Demographic and treatment variables were compared between males and females, and between the two study groups with Wilcoxon rank sum tests or Chi-square statistics. Linear regression models were employed to evaluate baseline risk factors for low BMD and to compare LS-BMD change between the supplement and placebo groups at 24 months. Analyses were conducted using SAS 9.2 (Cary, N.C.) and R 2.13.2. Statistical significance was set at p ≤ 0.05.
RESULTS
Characteristics of the study population
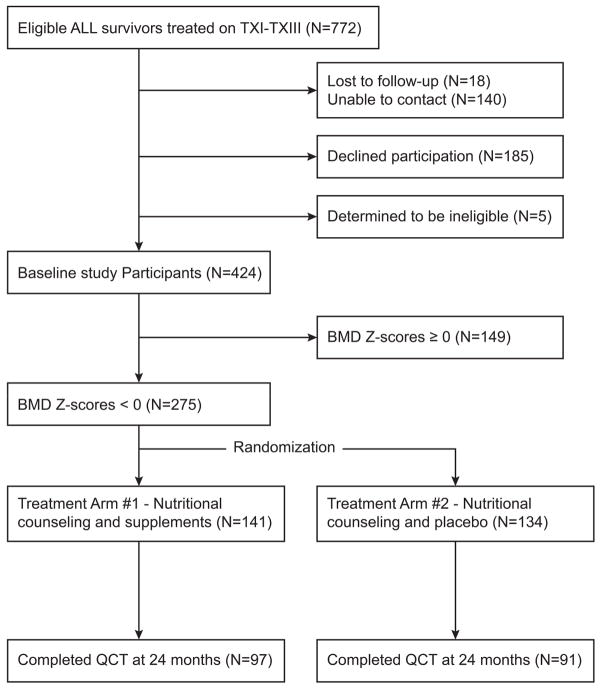
We identified 772 potentially eligible survivors of childhood ALL treated on Total Therapy Protocols XI-XIII (Figure 1).[9–14] Of 614 (79.5%) individuals contacted, 429 (69.8%) agreed to participate. Five were ineligible (one with central nervous system relapse, one with inability to swallow pills, three less than 5 years from start of ALL treatment); the final cohort included 424 participants. Table I shows participant baseline characteristics.
Figure 1.
Consort Diagram
Table I.
Characteristics of the baseline cohort
| Female (N= 206) | Male (N= 218) | Overall (N=424) | ||
|---|---|---|---|---|
| Characteristics | Category | Frequency (%) | ||
| Race | White | 178 (86.4) | 187 (85.8) | 365 (86.1) |
| Non-White | 28 (13.6) | 31 (14.2) | 59 (13.9) | |
| QCT Z-SCORE | Above 0 | 85 (41.3) | 60 (27.5) | 145 (34.2) |
| 0 to -1 | 71 (34.5) | 77 (35.3) | 148 (34.9) | |
| -1 to -2 | 40 (19.4) | 62 (28.4) | 102 (24.1) | |
| Below -2 | 10 (4.9) | 19 (8.7) | 29 (6.8) | |
| Tanner Stage | I | 6 (2.9) | 20 (9.2) | 26 (6.1) |
| II | 11 (5.3) | 15 (6.9) | 26 (6.1) | |
| III | 22 (10.7) | 14 (6.4) | 36 (8.5) | |
| IV | 39 (18.9) | 37 (17.0) | 76 (17.9) | |
| V | 128 (62.1) | 132 (60.6) | 260 (61.3) | |
| Treatment protocol | Total XI | 76 (36.9) | 64 (29.4) | 140 (33.0) |
| Total XII | 39 (18.9) | 45 (20.6) | 84 (19.8) | |
| Total XIIIA | 42 (20.4) | 52 (23.9) | 94 (22.2) | |
| Total XIIIB | 49 (23.8) | 57 (26.1) | 106 (25.0) | |
| Radiation dose | ≥24 Gy | 13 (6.3) | 22 (10.1) | 35 (8.3) |
| 1–23 Gy | 61 (29.6) | 57 (26.1) | 118 (27.8) | |
| None | 132 (64.1) | 139 (63.8) | 271 (63.9) | |
| Cyclophosphamide | <7,500 mg/ m2 | 104 (50.5) | 92 (42.2) | 196 (46.2) |
| ≥7,500 mg/ m2 | 102 (49.5) | 126 (57.8) | 228 (53.8) | |
| Glucocorticoids* | <5,000 mg | 41 (19.9) | 44 (20.2) | 85 (20.0) |
| ≥5,000 mg | 165 (80.1) | 174 (79.8) | 339 (80.0) | |
| Methotrexate | <10,000 mg/ m2 | 79 (38.3) | 71 (32.6) | 150 (35.4) |
| 10,000–19,999 mg/ m2 | 43 (20.9) | 46 (21.1) | 89 (21.0) | |
| 20,000+ mg/m2 | 84 (40.8) | 101 (46.3) | 185 (43.6) | |
| Smoking status | No | 186 (90.3) | 177 (81.2) | 363 (85.6) |
| Yes | 20 (9.7) | 41 (18.8) | 61 (14.4) | |
| Exercise frequency | <1 time/week | 54 (26.2) | 26 (11.9) | 80 (18.9) |
| 1 to 2 times/week | 52 (25.2) | 42 (19.3) | 94 (22.2) | |
| 3 to 4 times/week | 58 (28.2) | 70 (32.1) | 128 (30.2) | |
| 5+ times/ week | 42 (20.4) | 80 (36.7) | 122 (28.8) | |
| Median (range) | ||||
| Age at diagnosis | Years | 4.6 (0.6, 18.7) | 4.6 (0.2, 18.8) | 4.6 (0.2 , 18.8) |
| Age at study entry | Years | 17.0 (9.4, 36.1) | 17.1 (9.0, 35.4) | 17.0 (9.0 , 36.1) |
| Survival time† | Years | 9.0 (4.6, 18.6) | 7.9 (4.7, 19.1) | 8.4 (4.6 , 19.1) |
| Height | cm | 159.4 (127.5, 180.1) | 171.0 (124.6, 192.6) | 162.9 (124.6 , 192.6) |
| Height Z-score | -0.2(−2.8,2.6) | −0.06(−4.5,2.9) | −0.08(−4.5,2.9) | |
| Weight | kg | 60.2 (31.8, 155.3) | 67.8 (28.7, 172.6) | 63.3 (28.7 , 172.6) |
| Weight Z-score | 0.8(−4.23.8) | 0.9(−5.2,3.8) | 0.9(−5.2,3.8) | |
| Body mass index | kg/m2 | 23.9 (15.2, 53.8) | 24.0 (14.2, 54.1) | 24.0 (14.2 , 54.1) |
| BMI Z-score | 0.97 (−2.55,2.61) | 1.03 (−2.17,3.20) | 0.98(−2.55,3.20) | |
| Serum 25(OH)D | ng/mL | 23.0 (5.0,72.0) | 24.0 (5.0,81.0) | 24.0 (5.0,81.0) |
| Dietary calcium | mg | 786.0 (129.4,2276.2) | 828.3 (159.8,2420.0) | 790.2 (129.4 , 2420.0) |
| Dietary Vitamin D | Mg | 123.9(12.1,554.8) | 156.6(16.5,630.5) | 135.2(12.1,630.5) |
| Dietary fiber | gm | 13.3 (3.2,58.1) | 14.9 (3.8,33.3) | 13.5 (3.2,58.1) |
| Dietary magnesium | Mg | 245.3 (60.0,593.3) | 265.7 (93.4,539.4) | 253.4 (60.0,593.3) |
| Moderate/vigorous physical activity | Minutes/week | 68.33 (0,1257.33) | 167.93 (0,768.23) | 109.0(0,1257.33) |
Prednisone equivalent dose.
Survival time = time from completion of ALL therapy to entry into intervention study; cm = centimeters; Gy = Gray; IU = international units; kcal = kilocalories; kg = kilograms;, mg = milligrams; m2= meter squared; QCT = Quantitative Computed Tomography; SD = standard deviation; BMI=Body mass index.
Serum 25(OH)D levels were assayed in 387 of 424 participants (91%); most were insufficient or deficient: 291 (69%) with <30 ng/mL, 114 (27%) with ≤ 20 ng/mL, and 20 (5%) with ≤10 ng/mL. The median age- and gender-specific LS-BMD Z-score was −0.3 (−3.7 to 3.2) for females and −0.6 (−3.9 to 5.1) for males. We found no association with baseline vitamin D and LS-BMD Z-score (p= 0.7) after adjusting for age, gender and race.
At baseline, 279 individuals had LS-BMD Z-scores less than zero and were eligible for randomization; four elected not to participate in the intervention arm. Thus, the final cohort included 275 patients; 141 randomized to receive calcium and vitamin D supplements, and 134 to receive placebo. Table II characterizes the participants by treatment group. There were no differences between the two groups by gender, race, Tanner stage, original treatment protocol, treatment modality or dose, or by smoking or physical activity status. Serum 25(OH)D levels were similar between groups. No participant had compromised hepatic or renal function. Thirty-two (11.6%) of randomized participants had at least one documented endocrinopathy: somatotropin deficiency (8.0%), hypothyroidism (8.6%), and adrenocortitropic hormone deficiency (1.7%) (Table III). The supplement group included 12 (8.1%) and the placebo group 3 (2.2%) patients with history of hypothyroidism (p=0.03). All had been treated previously, or were receiving hormonal therapy. Twenty-nine participants with LS-BMD Z-scores ≤ −2 were referred to endocrinology. None were treated with bisphosphonates, none were excluded from the study for low LS-BMD Z-scores. Median baseline LS-BMD values were 156.3 mg/cm3 (58.5 to 201.3) for the supplement group and 159.6 mg/cm3 (83.0 to 197.2) for the placebo group (P = 0.44). Median baseline LS-BMD Z-scores were –1.0 for both groups.
Table II.
Characteristics of the treatment versus placebo groups at baseline.
| PLACEBO (N= 134) | SUPPLEMENT (N= 141) | |||
|---|---|---|---|---|
| Characteristics | Levels | Frequency (%) | P* | |
| Gender | Female | 56 (41.8) | 63 (44.7) | 0.63 |
| Male | 78 (58.2) | 78 (55.3) | ||
| Race | White | 13 (9.7) | 12 (8.5) | 0.73 |
| Non-White | 121 (90.3) | 129 (91.5) | ||
| QCT Z-Score | 0 to -1 | 71 (25.8) | 76 (27.6) | 0.94 |
| -1 to -2 | 48 (17.5) | 51 (18.6) | ||
| Below -2 | 15 (5.5) | 14 (5.1) | ||
| Tanner Stage | I | 11 (8.2) | 10 (7.1) | 0.8 |
| II | 9 (6.7) | 12 (8.5) | ||
| III | 9 (6.7) | 13 (9.2) | ||
| IV | 22 (16.4) | 27 (19.1) | ||
| V | 83 (61.9) | 79 (56.0) | ||
| Treatment protocol | Total XI | 39 (29.1) | 46 (32.6) | 0.24 |
| Total XII | 19 (14.2) | 19 (13.5) | ||
| Total XIII | 42 (31.3) | 30 (21.3) | ||
| Radiation dose | ≥24 Gray | 11 (8.2) | 13 (9.2) | 0.07 |
| 1 to 23 Gray | 23 (17.2) | 40 (28.4) | ||
| None | 100 (74.6) | 88 (62.4) | ||
| Cyclophosphamide dose | <7,500 mg/m2 | 60 (44.8) | 64 (45.4) | 0.92 |
| ≥7,500 mg/m2 | 74 (55.2) | 77 (54.6) | ||
| Glucocorticoid dose† | <5,000 mg | 20 (14.9) | 19 (13.5) | 0.73 |
| ≥5,000 mg | 114 (85.1) | 122 (86.5) | ||
| Methotrexate | <10,000 mg/m2 | 43 (32.1) | 47 (33.3) | 0.76 |
| 10,000 to 20,000 mg/m2 | 21 (15.7) | 26 (18.4) | ||
| ≥20,000 mg/m2 | 70 (52.2) | 68 (48.2) | ||
| Endocrinopathy | Somatotropin deficiency | 5 (3.7) | 9 (6.1) | 0.36 |
| Hypothyroidism | 3 (2.2) | 12 (8.1) | 0.03 | |
| Adrenal insufficiency | 1 (0.7) | 2 (1.4) | 0.62 | |
| Exercise frequency | <1 time/week | 21 (15.7) | 23 (16.3) | 0.63 |
| 1 to 2 times/week | 29 (21.6) | 29 (20.6) | ||
| 3 to 4 times/week | 37 (27.6) | 48 (34.0) | ||
| 5+ times/week | 47 (35.1) | 41 (29.1) | ||
| Smoking status | No | 111 (82.8) | 124 (87.9) | 0.23 |
| Yes | 23 (17.2) | 17 (12.1) | ||
| Characteristics | Median (range) | P | ||
| Age at diagnosis | Years | 4.6 (1.0, 16.9) | 4.7 (0.7, 17.4) | 0.70 |
| Age at study entry | Years | 17.2 (9.4, 33.5) | 16.6 (9.4, 35.3) | 0.39 |
| Survival time‡ | Years | 7.2 (4.6, 19.1) | 7.1 (5.0, 18.2) | 0.88 |
| Height | cm | 163.2 (127.8, 190.6) | 160.6 (124.6, 191.0) | 0.18 |
| Weight | kg | 59.6 (30.6, 116.6) | 63.0 (29.5, 136.6) | 0.87 |
| Body mass index | kg/m2 | 22.7 (14.2, 39.0) | 23.8 (15.2, 39.7) | 0.13 |
| Serum 25(OH)D | ng/mL | 24.0 (5.0, 72.0) | 24.0 (5.0, 51.0) | 0.83 |
| Dietary calcium | mg | 801.9 (159.8, 2420.0) | 793.4 (129.4, 1997.4) | 0.54 |
| Dietary vitamin D | 123.9 (25.5, 493.0) | 132.9 (12.1, 554.8) | 0.38 | |
| Dietary fiber | gm | 15.1 (3.2, 29.2) | 13.3 (4.4, 34.3) | 0.47 |
| Dietary magnesium | Mg | 262.1 (89.5, 539.4) | 224.2 (66.9, 530.8) | 0.20 |
| LS-BMD (QCT) | mg/cm3 | 159.6 (83.0, 197.2) | 156.3 (58.5, 201.3) | 0.44 |
| LS-BMD (QCT) Z- score | −0.95 (−3.09, −0.05) | −0.97 (−3.94, −0.04) | 0.86 | |
| Moderate/vigorous physical activity | Minutes/week | 139.4 (10, 533.7) | 103.7 (10, 484.0) | 0.47 |
P-values were calculated from the Pearson’s Chi-square test for categorical variables, and from the Wilcoxon rank sum test for continuous variables.
Prednisone equivalent dose;
Survival time = time from completion of ALL therapy to entry into intervention study; cm=centimeters; IU=international units; kcal=kilocalories; kg=kilograms; mg=milligrams; m2=square meters; QCT=Quantitative Computed Tomography; SD=standard deviations; LS=Lumbar Spine; BMD=Bone Mineral Density; MVW=moderate and vigorous activity per week.
Table III.
Risk factor analysis for LS-BMD QCT Z-scores at baseline
| Coefficients | 95% C.I. | P-values | |
|---|---|---|---|
| Intercept | -2.13 | (−2.9,−1.36) | <0.0001 |
| Survival time (years) | 0.01 | (−0.05,0.06) | 0.84 |
| Age at study entry, years | 0.01 | (−0.01,0.04) | 0.37 |
| Gender: female vs. male | 0.38 | (0.15,0.6) | 0.001 |
| Race: non-white vs. white | 0.58 | (0.28,0.89) | 0.0002 |
| Body mass index (kg/m2) | 0.05 | (0.03,0.07) | <0.0001 |
| Tanner stage* | −0.05 | (−0.17,0.07) | 0.43 |
| Smoking (no vs. yes) | 0.23 | (−0.08,0.54) | 0.15 |
| Moderate/vigorous physical activity minutes/week | 0.0007 | (−0.0001,0.0015) | 0.11 |
| Cranial radiation exposure <24 versus >≥24 Gray | 0.3 | (−0.08,0.69) | 0.13 |
| Cyclophosphamide dose (mg/m2): <7.500 vs. ≥7.500 | −0.29 | (−0.55,−0.05) | 0.02 |
| Glucocorticoid dose (mg/m2): <5,000 vs. ≥5,000 | 0.72 | (0.29,1.14) | 0.001 |
| Methotrexate dose (mg/m2): | 0.33 | ||
| <10,000 vs. 10,000–19,999 | −0.08 | (−0.57,0.4) | |
| 20,000+ vs. 10,000–19,999 | −0.29 | (−0.69,0.11) | |
| R square | 0.22 | ||
| Adjusted R square | 0.2 |
Tanner stage was treated as a continuous variable in the linear regression model. Sample size = 422; model DF=13; one participant lacked recorded height or weight at study entry and one participant lacked physical activity data.
Among 275 individuals randomized, 188 (68.4%) remained on study and completed the 24-month evaluation and were included in final analyses. The 87 individuals who did not complete the study included 43 in supplement and 44 in placebo groups. Survivors not completing did not differ from those who completed the study by gender, age at diagnosis, treatment protocol, or baseline LS-BMD Z-score. However, those not completing the study were slightly older (median age 18 years) than those remaining on study (median age 16 years; P < 0.003).
Demographic, treatment and lifestyle factors associated with low BMD prior to randomization
Table III shows associations between demographic, treatment and lifestyle variables, and LS-BMD Z-scores among 424 participants who completed pre-randomization baseline assessments. This model explained 20% of the variance in LS-BMD. Model fit was not improved by adding endocrine status, dietary intake, or measures of vitamin D and calcium. Female gender, non-white race, higher BMI, and lower glucocorticoid doses were associated with higher LS-BMD Z-scores. In the adjusted model, females had BMD Z-scores 0.35 standard deviations (SD) higher than males; non-whites had LS-BMD Z-scores 0.58 SD higher than whites; and a 5 kg/m2 increase in BMI was associated with an increase in LS-BMD Z-score of 0.25 SD. Survivors exposed to cumulative doses of glucocorticoids < 5,000 mg/m2 had, on average, LS-BMD Z-scores0.71 SD higher than those exposed to doses ≥ 5,000 mg/m2.
Nutritional intake of calcium or vitamin D either at baseline or at 2 years did not differ between groups (n = 106; supplement group n = 62 and placebo group n = 44) (S3). Dietary calcium intake increased by a median of 906.5 and 840.3 mg/d and dietary vitamin D intake increased by a median of 147.0 and 185.8 IU/d in placebo and supplement groups, respectively.
Compliance with diet and the dietary intervention
Among those randomized and who completed the study, 213 (77%) took their medication over the entire 2-years. Of these, 25 did not return for their final QCT, but completed all other study documentation. Sixty-two (23%) stopped taking their supplement (n = 22) or placebo (n = 40). Reasons for stopping included renal stones (n = 3), elevated calcium creatinine ratios (n = 3), initiation of open label calcium by a physician (n = 8), and non-compliance (n = 49). Among those who were non-compliant, 46 reported that they did not like the taste. Those who returned for their final evaluation were included in analysis, regardless of compliance.
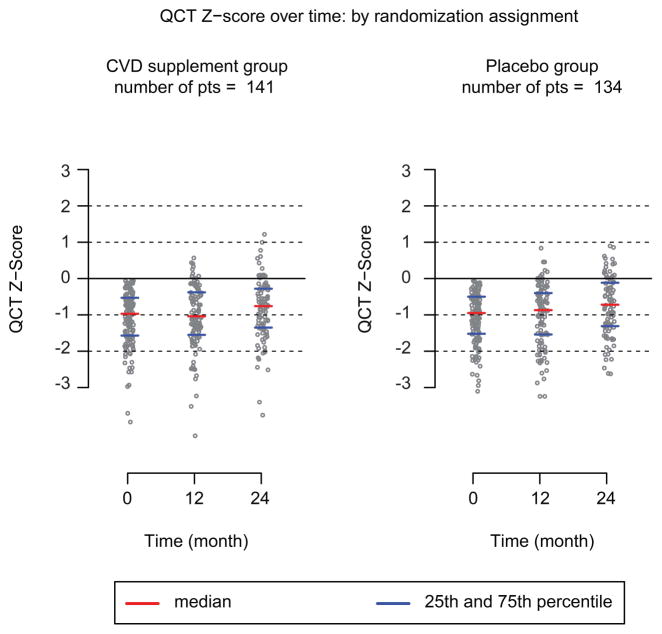
Effects of vitamin D and calcium supplementation on BMD
Table IV and Figure 2 show the impact of 2 years of vitamin D and calcium supplementation on BMD among 188 participants who completed their QCT at 24-months. After adjusting for baseline LS-BMD Z-score, age, gender, race, radiation dose, and chemotherapy, there were no differences in mean LS-BMD values or mean LS-BMD Z-scores between supplement and placebo groups. This lack of association between the intervention and control groups persisted in a model that also adjusted for dietary intake of vitamin D and calcium. Baseline LS-BMD Z-scores and age at start of the study consistently predicted LS-BMD Z-scores at 24 months. A 1 SD higher baseline LS-BMD Z-score was associated with a 0.83 greater LS-BMD Z-score at the 2-year evaluation. Participants 13–17 years and 18–21 years of age at the start of the study had smaller overall changes in LS-BMD Z-score than those aged 22–35 years (−0.2 to −0.4 SDs). Participants aged 9 – 13 years had the greatest increase in LS-BMD Z-score (0.39). Though not reaching statistical significance, among those with baseline Z-scores below -2, individuals who received the supplement appeared to have a bigger increase in BMD (n = 14; median change +13.50 mg/cm3;range 1.1 to 45.9) than those who received the placebo (n = 15, median change +3.05 mg/cm3; range −15.4 to 28.4; P = 0.15). However, among those with a baseline Z-scores between −1 and −2, those who received the supplements had a similar median 24-month change in BMD (n = 51; median change +11.4 mg/cm3; range −16.2 to 86.4) as those who received placebo (n=48; median change +6.8 mg/cm3; range17.1 to 76.2; P = 0.72)). We also performed a subset analysis of only those who completed the study and the conclusion remained the same.
Table IV.
Multivariable Linear Regression for LS-BMD QCT Z-score and LS-BMD QCT Z-score change at 24 months*
| LS-BMD QCT Z-score | LS-BMD QCT Z-score change | |||||
|---|---|---|---|---|---|---|
| Coefficient | 95% C.I. | P-value | Coefficient | 95% C.I. | P-value | |
| Intercept | 0.22 | (−0.43,0.88) | 0.50 | 0.38 | (−0.28,1.04) | 0.26 |
| Bone mineral density LS-BMD QCT Z-Score at baseline† | 0.85 | (0.74,0.96) | <.0001 | |||
| Supplement vs. placebo | 0.008 | (−0.15,0.16) | 0.92 | 0.03 | (−0.13,0.19) | 0.70 |
| Survival time (years) | 0.01 | (−0.03,0.05) | 0.70 | 0.0045 | (−0.04,0.05) | 0.83 |
| Age at study entry (years) | 0.003 | 0.01 | ||||
| 9–12 vs. 22–35 | 0.17 | (−0.17,0.51) | 0.15 | (−0.2,0.49) | ||
| 13–17 vs. 22–35 | −0.26 | (−0.51,0.00) | −0.23 | (−0.49,0.03) | ||
| 18–21 vs. 22–35 | −0.28 | (−0.55,−0.01) | −0.25 | (−0.52,0.03) | ||
| Gender: female vs. male | 0.15 | (−0.01,0.32) | 0.07 | 0.14 | (−0.02,0.31) | 0.09 |
| Race: non-white vs. white | 0.17 | (−0.10,0.44) | 0.20 | 0.15 | (−0.12,0.43) | 0.28 |
| Tanner stage‡ | 0.04 | (−0.05,0.13) | 0.39 | 0.04 | (−0.05,0.13) | 0.36 |
| Moderate/vigorous physical activity minutes/week | −0.0002 | (−0.001,0.0005) | 0.52 | −0.0003 | (−0.0011,0.0004) | 0.42 |
| Cranial radiation exposure <24 vs. ≥24 Gray | −0.08 | (−0.35,0.19) | 0.58 | 0.04 | (−0.24,0.31) | 0.79 |
| Cyclophosphamide dose (mg/m2): <7.500 vs. =7.500 | −0.04 | (−0.22,0.14) | 0.66 | 0.0014 | (−0.18,0.18) | 0.99 |
| Glucocorticoid dose (mg/m2): <5,000 vs. ≥5,000 | −0.24 | (−0.59,0.11) | 0.19 | −0.30 | (−0.66,0.05) | 0.10 |
| Methotrexate dose (mg/m2): | 0.14 | 0.16 | ||||
| <10,000 vs. 10,000–19,999 | −0.37 | (−0.75,0.00) | −0.37 | (−0.75,0) | ||
| 20,000+ vs. 10,000–19,999 | −0.23 | (−0.54,0.08) | −0.20 | (−0.51,0.11) | 0.16 | |
| R square | 0.65 | 0.09 | ||||
| Adjusted R Square§ | 0.62 | 0.16 | ||||
Participants who failed to undergo the 24 month QCT scan were excluded from analysis (87 of 275, excluded). Sample size = 188; model DF=14. Out of the 275 randomized patients, 213 (77%) took their medication over the entire 2-years, but only 188 patients completed the 24 month QCT scan. 135 patients not only were compliant with their meds but also completed the 24 month QCT scan. A subset analysis was performed for these 135 patients and the effect of calcium and vitamin D supplement on 24 months QCT Z-score remained insignificant (p-value=0.80).
We found no interaction effect between baseline LS-BMD (QCT Z-score) and intervention (placebo vs. supplement) and the 24-month QCT Z-score (p= 0.94) in participants as a whole or in those who were both compliant with medications as well as having completed the 24 month QCT (p=0.80).
Tanner stage was treated as a continuous variable.
The adjusted R-Square is an adjustment of the R-squared that penalizes the addition of extraneous predictors to the model. Adjusted R-squared is computed using the formula: , where k is the number of predictors and N is the sample size. Here k is the number of estimated coefficients not counting the intercept. The effects of serum vitamin D on LS-BMD QCT score were not significant (p=0.78). An analysis taking into account baseline values of dietary Vitamin D and Calcium did not change the association between treatment group (calcium and cholecalciferol supplementation versus placebo) and LS-BMD QCT z-score at 24 months (p=0.60). An analysis was performed including a history of hypothyroidism in the model. The effects of hypothyroidism were not significant (p=0.46).
Figure 2.
Quantitative computed tomography (QCT) Z-score at follow-up periods of 12-month and at 24-month are linearly associated with baseline Z-score.
DISCUSSION
This randomized double-blind intervention indicates that nutritional counseling, vitamin D, and calcium supplementation for 2 years offers no benefit above nutritional counseling alone to BMD accretion among survivors of ALL. The most important factors—accounting for about 60% of variability in change in LS-BMD Z-score—were LS-BMD at baseline and age at study enrollment. Our finding of L-S BMD at the time of diagnosis being strongly predictive of LS-BMD over time supports the similar finding by Rayar et al during consolidation therapy[23]. While, on average, participants gained LS-BMD over the study period, they failed to attain normal LS-BMD. Those aged 9–13 years (compared to those 22–35 years) had the greatest LS-BMD improvements. Although few study participants (7%) displayed BMD Z-scores more than 2 SDs below the mean, descriptive data indicate that these survivors might benefit most from a BMD-enhancing intervention.
BMD deficits in survivors of childhood ALL constitute a potentially important health risk throughout adulthood. At 11 years after therapy, nearly 66% of the study cohort had LS-BMD Z-scores below the mean; 31% had LS-BMD deficits more than 1 SD, and 7% had LS-BMD deficits more than 2 SDs below the mean. Factors associated with low LS-BMD Z-scores were male gender, glucocorticoid exposure of at least 5,000 mg/m2, low BMI, and white race.
Achievement of normal BMD is affected by genetic factors[24–28], physical activity[29–33], vitamin D status[34], pubertal status[32], and treatment for ALL[35–38]. The relative influence of these factors on BMD in survivors of childhood ALL has been reported in small cohorts, possibly masking the individual and interactive effects of the unique variables. The data presented here provide insights to guide the development of new interventions directed at optimizing peak bone mass among ALL survivors. Simply providing standard nutritional supplementation appears to be insufficient. Different and more aggressive approaches are necessary and should consider degree of BMD deficit and attained age. Additionally, failure of these survivors to achieve optimal bone mass 5 or more years from their original treatment indicates a critical need to determine if low BMD in this population is associated with fracture risk during aging.
Weight-bearing exercise is a key factor in developing peak BMD, regardless of age or gender.[39] Although physical activity levels varied in our cohort[21], this factor was unassociated with BMD. Recent literature indicates that a jumping exercise of 10 minutes per day, 2–3 times per week, for 7 to 16 months is needed to improve bone health in children[40–44]. Since we did not measure the amount of jumping by our participants as a part of daily physical activity, we could not evaluate this association.
Vitamin D deficiency/insufficiency was prevalent but BMD at enrollment was unrelated to circulating 25(OH)D or calcium status. In vitamin D replete patients, adding supplementation of 800 IU/day cannot be expected to impact bone health. If vitamin D deficiency is severe, this dosing could be inadequate to compensate for such deficits. Unfortunately, there are no universally accepted standards for therapeutic levels of serum 25(OH)D, particularly in children and adolescents.[45,46] The wide range of vitamin D intake necessary to maintain serum 25(OH)D levels in 20 to 40 year olds has been reported as 7.2 to 41.1 μg/day, depending on sun exposure[47]. Emerging investigations should define clinical parameters for children and adolescents [46,48].
The study protocol was designed during 2007, according to the contemporary standard of care for healthy adolescents and young adults, and at a time when the therapeutic threshold of vitamin D status for BMD accretion was under debate[45]. Our prevalence data resemble those for a similar ALL survivor cohort[49], and are comparable to the prevalence of vitamin D insufficiency/deficiency in the general population[46]. In another population-based pediatric study, 15% of participants displayed serum total 25(OH)D levels below 15 ng/mL, and 61% had levels between 15 and 29 ng/mL[50]. Our results are similar to those of Diaz et al.[48], who found no impact on bone mass after 12-months administration of calcitriol in a prospective, randomized study of 24 patients with newly diagnosed ALL. Other than vitamin D deficiency, only 11.6% (32 of 275) of our cohort had endocrinopathies that could adversely impact bone mineralization; all were well controlled with hormonal therapy. We found no associations between any endocrinopathy and BMD in our study.
There are several limitations of this study. First, our results cannot be extrapolated to a population of ALL survivors with a sufficient vitamin D status, or to a population of survivors who were completely compliant with their prescribed intervention. Second, not all of our randomized study participants completed their final QCT evaluation. This could bias our results. Third, we utilized a generic assessment of physical activity that did not specifically evaluate the jumping component of weight-bearing exercise known to facilitate bone mineralization. Future studies should consider incorporation of longitudinal assessment of serum 25(OH)D to monitor compliance with supplementation. Nevertheless, the strengths of this study are the analysis of validated therapeutic exposures for a large clinically well-characterized cohort of pediatric ALL survivors treated at a single institution. Randomization was effective. The double-blind, placebo-controlled, longitudinal design of this trial strengthens the rigorous data analysis. Patient adherence to the intervention among the groups was comparable and reasonable.
In summary, an intervention of nutritional counseling combined with vitamin D and calcium supplements for 2 years did not significantly increase BMD, compared to nutritional counseling with placebo. Whether alternative strategies of individualizing calcium and vitamin D doses, perhaps in combination with physical activity, would have resulted in an increase in BMD in this population is unknown.
Supplementary Material
Acknowledgments
Supported in part by American Lebanese Syrian Associated Charities (S.K., K.N., C-H.P., M.H.), NIH grant R21 HD059292 (K.N., S.K., M.H., R.F.), NIH GM 92666 (MVR, CHP), Gabrielle’s Angel Foundation (K.N., S.K., M.H., R.F.), and Le Bonheur Foundation of Memphis (R.F.). The authors thank Kimberly Johnson for data compilation and management, Dippin’ Dots™ LLC for incentives, Smith-Kline Beecham for calcium and placebo, and especially the hundreds of patients and families who participated. The authors also thank Dr. Shesh Rai for contributions to the study design, Ms. Sandra Gaither and Ms. Kathy Laub for manuscript preparation, CT technologists for processing the QCTs and the hundreds of patients and families who participated in this study.
FUNDING: This work was supported by the National Institutes of Health [P30 CA-21765]; a Center of Excellence grant from the State of Tennessee; the Le Bonheur Foundation (Memphis TN); and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
CONFLICT OF INTEREST
Consultant or Advisory Role (unrelated to this work): Robert Ferry, Ipsen (C) Research Funding (unrelated to this work): Robert Ferry, MacroGenics, Eli Lilly, Bristol-Myers Squibb, Novo Nordisk A/S, Ipsen, Diamyd Therapeutics AB, Pfizer, Tolerx, GlaxoSmithKline, Takeda, Endo Pharmaceuticals
All other authors have no disclosures related to this work.
References
- 1.Fan C, Foster BK, Wallace WH, et al. Pathobiology and prevention of cancer chemotherapy-induced bone growth arrest, bone loss, and osteonecrosis. Current molecular medicine. 2011;11(2):140–151. doi: 10.2174/156652411794859223. [DOI] [PubMed] [Google Scholar]
- 2.Georgiou KR, Scherer MA, Fan CM, et al. Methotrexate chemotherapy reduces osteogenesis but increases adipogenic potential in the bone marrow. Journal of cellular physiology. 2012;227(3):909–918. doi: 10.1002/jcp.22807. [DOI] [PubMed] [Google Scholar]
- 3.Chaiban J, Muwakkit S, Arabi A, et al. Modeling pathways for low bone mass in children with malignancies. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2009;12(4):441–449. doi: 10.1016/j.jocd.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2000;11(12):985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 5.Matkovic V, Jelic T, Wardlaw GM, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model The Journal of clinical investigation. 1994;93(2):799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver H, Jameson KA, Sayer AA, et al. Growth in early life predicts bone strength in late adulthood: the Hertfordshire Cohort Study. Bone. 2007;41(3):400–405. doi: 10.1016/j.bone.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Current rheumatology reports. 2010;12(3):186–191. doi: 10.1007/s11926-010-0097-y. [DOI] [PubMed] [Google Scholar]
- 8.Rai SN, Hudson MM, McCammon E, et al. Implementing an intervention to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia: BONEII, a prospective placebo-controlled double-blind randomized interventional longitudinal study design. Contemporary clinical trials. 2008;29(5):711–719. doi: 10.1016/j.cct.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans WE, Relling MV, Rodman JH, et al. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. The New England journal of medicine. 1998;338(8):499–505. doi: 10.1056/NEJM199802193380803. [DOI] [PubMed] [Google Scholar]
- 10.Kishi S, Griener J, Cheng C, et al. Homocysteine, pharmacogenetics, and neurotoxicity in children with leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(16):3084–3091. doi: 10.1200/JCO.2003.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24(2):371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104(9):2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 13.Rivera GK, Pui CH, Santana VM, et al. Progress in the treatment of adolescents with acute lymphoblastic leukemia. Cancer. 1993;71(10 Suppl):3400–3405. doi: 10.1002/1097-0142(19930515)71:10+<3400::aid-cncr2820711744>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Rivera GK, Raimondi SC, Hancock ML, et al. Improved outcome in childhood acute lymphoblastic leukaemia with reinforced early treatment and rotational combination chemotherapy. Lancet. 1991;337(8733):61–66. doi: 10.1016/0140-6736(91)90733-6. [DOI] [PubMed] [Google Scholar]
- 15.Hudson MM, Hester A, Sweeney T, et al. A model of care for childhood cancer survivors that facilitates research. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2004;21(3):170–174. doi: 10.1177/1043454204264388. [DOI] [PubMed] [Google Scholar]
- 16.Cann CE. Quantitative CT for determination of bone mineral density: a review. Radiology. 1988;166(2):509–522. doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 17.Kaste SC, Tong X, Hendrick JM, et al. QCT versus DXA in 320 survivors of childhood cancer: association of BMD with fracture history. Pediatric blood & cancer. 2006;47(7):936–943. doi: 10.1002/pbc.20854. [DOI] [PubMed] [Google Scholar]
- 18.Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist. Palo Alto, CA: Stanford University Press; 1969. [Google Scholar]
- 19.Block G, Woods M, Potosky A, et al. Validation of a self-administered diet history questionnaire using multiple diet records. Journal of clinical epidemiology. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 20.Boucher B, Cotterchio M, Kreiger N, et al. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public health nutrition. 2006;9(1):84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 21.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Canadian journal of public health Revue canadienne de sante publique. 1986;77(5):359–362. [PubMed] [Google Scholar]
- 22.Tanner JM. The measurement of maturity. Transactions European Orthodontic Society. 1975:45–60. [PubMed] [Google Scholar]
- 23.Rayar MS, Nayiager T, Webber CE, et al. Predictors of bony morbidity in children with acute lymphoblastic leukemia. Pediatric blood & cancer. 2012;59(1):77–82. doi: 10.1002/pbc.24040. [DOI] [PubMed] [Google Scholar]
- 24.Jones TS, Kaste SC, Liu W, et al. CRHR1 polymorphisms predict bone density in survivors of acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(18):3031–3037. doi: 10.1200/JCO.2007.14.6399. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell BD, Yerges-Armstrong LM. The genetics of bone loss: challenges and prospects. The Journal of clinical endocrinology and metabolism. 2011;96(5):1258–1268. doi: 10.1210/jc.2010-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ralston SH, Uitterlinden AG. Genetics of osteoporosis. Endocrine reviews. 2010;31(5):629–662. doi: 10.1210/er.2009-0044. [DOI] [PubMed] [Google Scholar]
- 27.te Winkel ML, van Beek RD, de Muinck Keizer-Schrama SM, et al. Pharmacogenetic risk factors for altered bone mineral density and body composition in pediatric acute lymphoblastic leukemia. Haematologica. 2010;95(5):752–759. doi: 10.3324/haematol.2009.016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng HF, Spector TD, Richards JB. Insights into the genetics of osteoporosis from recent genome-wide association studies. Expert reviews in molecular medicine. 2011;13:e28. doi: 10.1017/S1462399411001980. [DOI] [PubMed] [Google Scholar]
- 29.Callreus M, McGuigan F, Ringsberg K, et al. Self-reported recreational exercise combining regularity and impact is necessary to maximize bone mineral density in young adult women : A population-based study of 1,061 women 25 years of age. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012 doi: 10.1007/s00198-011-1886-5. [DOI] [PubMed] [Google Scholar]
- 30.Jarfelt M, Fors H, Lannering B, et al. Bone mineral density and bone turnover in young adult survivors of childhood acute lymphoblastic leukaemia. European journal of endocrinology / European Federation of Endocrine Societies. 2006;154(2):303–309. doi: 10.1530/eje.1.02092. [DOI] [PubMed] [Google Scholar]
- 31.Marinovic D, Dorgeret S, Lescoeur B, et al. Improvement in bone mineral density and body composition in survivors of childhood acute lymphoblastic leukemia: a 1-year prospective study. Pediatrics. 2005;116(1):e102–108. doi: 10.1542/peds.2004-1838. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Lopez FR, Chedraui P, Cuadros-Lopez JL. Bone mass gain during puberty and adolescence: deconstructing gender characteristics. Current medicinal chemistry. 2010;17(5):453–466. doi: 10.2174/092986710790226138. [DOI] [PubMed] [Google Scholar]
- 33.Vicente-Rodriguez G. How does exercise affect bone development during growth? Sports Med. 2006;36(7):561–569. doi: 10.2165/00007256-200636070-00002. [DOI] [PubMed] [Google Scholar]
- 34.Sonneville KR, Gordon CM, Kocher MS, et al. Vitamin D, Calcium, and Dairy Intakes and Stress Fractures Among Female Adolescents. Archives of pediatrics & adolescent medicine. 2012 doi: 10.1001/archpediatrics.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benmiloud S, Steffens M, Beauloye V, et al. Long-term effects on bone mineral density of different therapeutic schemes for acute lymphoblastic leukemia or non-Hodgkin lymphoma during childhood. Hormone research in paediatrics. 2010;74(4):241–250. doi: 10.1159/000313397. [DOI] [PubMed] [Google Scholar]
- 36.Gilsanz V, Carlson ME, Roe TF, et al. Osteoporosis after cranial irradiation for acute lymphoblastic leukemia. The Journal of pediatrics. 1990;117(2 Pt 1):238–244. doi: 10.1016/s0022-3476(05)80536-0. [DOI] [PubMed] [Google Scholar]
- 37.Hoorweg-Nijman JJ, Kardos G, Roos JC, et al. Bone mineral density and markers of bone turnover in young adult survivors of childhood lymphoblastic leukaemia. Clinical endocrinology. 1999;50(2):237–244. doi: 10.1046/j.1365-2265.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 38.Kaste SC, Jones-Wallace D, Rose SR, et al. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2001;15(5):728–734. doi: 10.1038/sj.leu.2402078. [DOI] [PubMed] [Google Scholar]
- 39.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 40.Gunter K, Baxter-Jones AD, Mirwald RL, et al. Impact exercise increases BMC during growth: an 8-year longitudinal study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23(7):986–993. doi: 10.1359/JBMR.071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macdonald HM, Kontulainen SA, Khan KM, et al. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(3):434–446. doi: 10.1359/jbmr.061205. [DOI] [PubMed] [Google Scholar]
- 42.MacKelvie KJ, Khan KM, Petit MA, et al. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics. 2003;112(6 Pt 1):e447. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 43.Maggio AB, Rizzoli RR, Marchand LM, et al. Physical activity increases bone mineral density in children with type 1 diabetes. Medicine and science in sports and exercise. 2012;44(7):1206–1211. doi: 10.1249/MSS.0b013e3182496a25. [DOI] [PubMed] [Google Scholar]
- 44.Meyer U, Romann M, Zahner L, et al. Effect of a general school-based physical activity intervention on bone mineral content and density: a cluster-randomized controlled trial. Bone. 2011;48(4):792–797. doi: 10.1016/j.bone.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to Endocrine Society vitamin D guideline. The Journal of clinical endocrinology and metabolism. 2012;97(4):1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skversky AL, Kumar J, Abramowitz MK, et al. Association of glucocorticoid use and low 25-hydroxyvitamin D levels: results from the National Health and Nutrition Examination Survey (NHANES): 2001–2006. The Journal of clinical endocrinology and metabolism. 2011;96(12):3838–3845. doi: 10.1210/jc.2011-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cashman KD, Hill TR, Lucey AJ, et al. Estimation of the dietary requirement for vitamin D in healthy adults. The American journal of clinical nutrition. 2008;88(6):1535–1542. doi: 10.3945/ajcn.2008.26594. [DOI] [PubMed] [Google Scholar]
- 48.Diaz PR, Neira LC, Fischer SG, et al. Effect of 1,25(OH)2-vitamin D on bone mass in children with acute lymphoblastic leukemia. Journal of pediatric hematology/oncology. 2008;30(1):15–19. doi: 10.1097/MPH.0b013e318159a522. [DOI] [PubMed] [Google Scholar]
- 49.Simmons JH, Chow EJ, Koehler E, et al. Significant 25-hydroxyvitamin D deficiency in child and adolescent survivors of acute lymphoblastic leukemia: treatment with chemotherapy compared with allogeneic stem cell transplant. Pediatric blood & cancer. 2011;56(7):1114–1119. doi: 10.1002/pbc.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melamed ML, Kumar J. Low levels of 25-hydroxyvitamin D in the pediatric populations: prevalence and clinical outcomes. Pediatric health. 2010;4(1):89–97. doi: 10.2217/phe.09.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.