Abstract
ZBP-89 regulates Bak to facilitate apoptosis in cancer cells. This study examined if ZBP-89 regulates Bak through an epigenetic mechanism in hepatocellular carcinoma (HCC). We first demonstrated that the expression of Bak was reduced but the levels of DNMT1 and HDAC3 were increased in HCC cancer tissues compared to the corresponding non-cancer tissues. Moreover, there was a negative correlation between Bak expression and DNMT1 levels in HCC. Administration of ZBP-89 downregulated HDAC3 expression and suppressed the activities of HDAC and DNMT, which led to maintenance of histone acetylation status, inhibited the binding of methyl-CpG-binding protein 2 to genomic DNA and demethylated CpG islands in the Bak promoter in HCC cells. Using the xenograft mouse tumor model, we demonstrated that ZBP-89 or inhibitors of either epigenetic enzymes could stimulate Bak expression, induce apoptosis, and arrest tumor growth and that the maximal effort was achieved when ZBP-89 and the enzyme inhibitors was used in combination. Conclusively, ZBP-89 upregulates the expression of Bak by targeting multiple components of the epigenetic pathway in HCC.
Keywords: Hepatocellular Carcinoma, ZBP-89, HDAC, DNMT, Bak
1. Introduction
ZBP-89, a Kruppel-type zinc-finger transcription factor, binds to gene promoter GC-rich sequence to activate or suppress gene transcription which is closely related to cell growth and cell death [1,2]. Previously, we reported that ZBP-89 induced Bak gene expression, a pro-apoptotic protein known to stimulate apoptosis in hepatocellular carcinoma (HCC) cells [3]. In addition to its ability to bind to gene promoters, ZBP-89 also forms protein-protein interactions2. For example, ZBP-89 interacts with histone deacetylases (HDAC) to repress p16 and vimentin gene transcription [2,4], or interacts with p53, GATA-1 and/or FOG-1 to activate gene expression [5,6]. These studies propose a link between ZBP-89 and epigenetic regulation, though the detailed mechanism is not understood. Bak promoter methylation was reported previously [7,8]. Therefore we queried whether the regulation of Bak expression by ZBP-89 is subjected to epigenetic regulation.
HDAC and DNA methyltransferas (DNMT) inhibitors can synergically regulate the same genomic targets [9]. They may synergize when added together, although they activate different gene targets through different mechanisms [6]. An increase in the acetylated histone H3 and histone H4 is closely correlated with gene expression [10]. HDAC3 is regarded as a biomarker of HCC recurrence and therapy target [11,12]. The roles of HDAC and DNMT in the regulation of Bak are known.
CpG dinucleotide islands are usually located in the promoter region of given genes. At these locations, CG dinucleotides that become methylated suppress gene expression. DNA methylation may silence gene expression through either interfering with transcription factor binding or recruiting methylated DNA binding proteins [13,14]. Aberrant genome-wide DNA methylation mediated by DNMT has been thought to be related to tumorigenesis. Increased expression of DNMT in HCC is correlated with poor prognosis and survival [15,16].
Bak expression is suppressed in HCC. We previously reported that ZBP-89 induced Bak gene expression [3]. The aim of this study was to determine whether ZBP-89 regulated Bak gene expression through its ability to modulate HDAC and DNMT
2. Materials and methods
2.1. Chemicals and reagents
Antibodies against ZBP-89, PARP, methyl-CpG-binding protein 2 (MeCP2); protein A/G PLUS-agarose beads, ZBP-89 siRNA, HDAC3 siRNA and DNMT1 siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). HDAC3, HDAC4, DNMT1 and DNMT3a, DNMT3b antibodies were provided by Cayman Chemicals (Ann Arbor, MI). All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
2.2. HCC patient samples, cell lines, siRNA transfection, and adenovirus infection of cells
103 HCC tissue samples and the matched non-cancerous liver tissue samples were obtained from patients with HCC, who underwent surgical resection and the detailed information of these samples was shown in Table S1. The study was approved by the local Clinical Research Ethics Committee. HCC cells, PLC/PRF/5 and HepG2 cells were purchased form ATCC. HepG2 cells are a low tumorigenic cells but they are positive for HBV antigens. PLC/PRF/5 cells positive for HBV show higher tumorigenic ability. MIHA cells are a SV40-immortalized normal liver cell line. 10 µM of each ZBP-89 siRNA, HDAC3 siRNA, or DNMT1 siRNA were transfected into the cells with Fugene reagent (Roche, Indianapolis, IN). The replication-deficient recombinant adenoviral expression vector Ad-ZBP-89 was used according to previous publications [3,5].
2.3. Activities of HDAC and DNMT
The effect of ZBP-89 on HDAC activity in cancer cells was assessed by the Fluor de Lys-Green HDAC Assay Kit (Enzo Life Science, Farmingdale, NY). The experiment was performed according to the manufacturer’s protocol. DNMT was prepared according to a previous publication [17]. The S-adenosyl-L-methionine was used as the methyl group donor. The activity of DNMT was detected by the methyltransferase activity kit (Enzo Life Science, Farmingdale, NY).
2.4. Co-immunoprecipitation (Co-IP) and Western blotting
These experiments were performed according to the previous publications [3,5]. Briefly, cell lysates were incubated overnight with primary antibody after being precleared with protein A/G PLUS-agarose beads. Immunocomplexes were precipitated after incubating with Protein A/G PLUS-agarose beads. Immunocomplexes were released by boiling in 2×SDS sample buffer for Western blot analysis. The positive signals were visualized by ECL reagent kit (GE Healthcare).
2.5. Immunohistochemistry and Immunofluorescence
The immunohistochemical examination was carried out according to our previous publication [3,5]. Briefly, the tissue sections were incubated with a primary antibody followed by a HRP-labeled IgG secondary antibody. The antigen-antibody complex was visualized by the diaminobenzidine method. Phosphate-buffered saline was used as a negative control instead of the primary antibody. For immunofluorescent staining, after the cells were fixed with 4% paraformaldehyde, they were incubated with primary antibody, followed by a FITC- or Rhodamine-labeled secondary antibody. Fluorescent cells were visualized using the Zeiss Axioplan 2 microscope (Zeiss, Germany).
2.6. Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed based on the protocol previously published with some modifications [18]. The following primers were used to detect precipitated sequence:
S1_F1: GGGAGGATGGGAGGTTGGGGG;
S1_R1: AGCCCCTGCCCTCAGGGTTC;
S2_F2:GAGGTGGGGGAGAAGATCAAAGACA,
S2_R2:CGGCACCCGGCTCCAATCAG.
2.7. Sodium bisulfite modification sequencing of Bak promoter
Sodium bisulfite modified sequencing was carried out using the 377DNA sequencer (ABI) and gene promoter primers were designed using MSPPrimer. Totally, 1 µg of genomic DNA was modified and cleared up by EZ DNA modification-Gold kit (Zymo Research, Irvine, CA). The following Bak gene promoter primers were used:
msp_F1: GTTTAGGTTGGAGTGTAGTGGGTTA,
msp_R1: CCAAAATTATACCACTACACTCCTACC;
msp_F2: GTGGTTATAGAGTAATTTTTTTTAGAGGGA,
msp_R2: ACCTACCCAAAAAAACCTTAAACTT;
2.8. Cell proliferation and Apoptosis detection
Cell proliferation was determined by MTT assay as described previously [3,5]. Apoptosis was determined by the Vybrant® Apoptosis Assay Kit (Invitrogen, Carlsbad, CA) and performed according to the introduction of the manufacture [3,5].
2.9. Xenograft animal model
The xenograft tumor model was generated by injecting 2×106 PLC/PRF/5 cells into the left axilla of nude mice and left to grow for 3 weeks. The control group received 0.9% saline by i.p. The Ad-ZBP-89 recombinant adenoviral vector (1×1010 pfu) was administrated by direct injection into tumor tissues. The first injection was done at the beginning of the implantation, and the second at 7 days later. Valproic acid (VPA) and/or zebularine (Zebu) was i.p. injected into mice at 50mg/kg/d and 100 mg/kg/d, respectively, for 7 days [19,20]. At the end of the experiment, the tumor was excised intact and weighted. The expression of Bak in tumor tissues was determined and apoptosis was analyzed by TUNEL [21]. The mouse experiment was approved by the local Animal Research Ethics Committee.
2.10. Statistical analysis
Analyses were performed using SPSS (SPSS Inc, Chicago, IL). Statistical analysis was performed by paired t-test, Mann-Whitney U test or one-way ANOVA. P values of less than 0.05 were considered statistically significant. Results were representative of triplicate experiments and expressed as mean ± SD.
3. Results
3.1. ZBP-89 formed a complex with DNMT1 and HDAC3
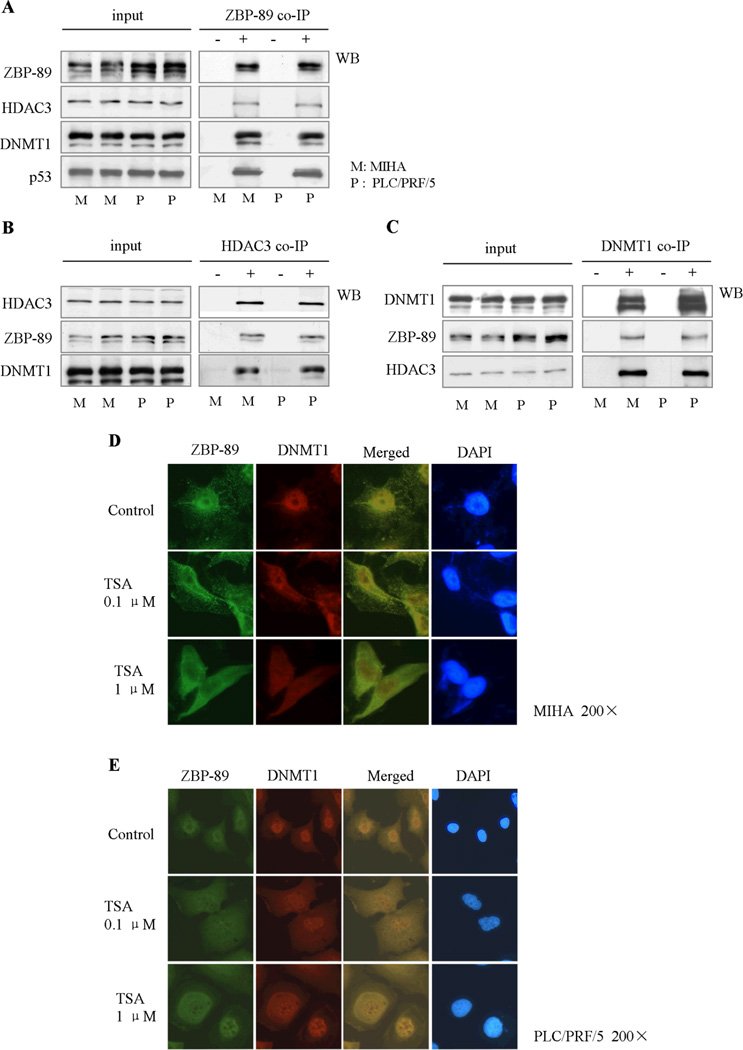
There are a number of epigenetic enzymes which are closely associated with hepato-carcinogenesis, including DNMT1, DNMT3a, DNMT3b, HDAC1, HDAC3 and HDAC4. Therefore, we examined if ZBP-89 could interact with these epigenetic enzymes. Co-IP studies showed that ZBP-89 protein interacted with DNMT1 and HDAC3 but not the rest, ZBP-89 formed a complex with DNMT1 and HDAC3 that could be detected by ZBP-89 antibody as well as DNMT1/HDAC3 antibody (Fig. 1A, B and C). The confocal images showed that ZBP-89 and DNMT1 co-localized primarily in the nucleus (Fig. 1D and E). After treatment with the HDAC inhibitor TSA, ZBP-89 appeared to migrate from the nucleus to the cytoplasm. Some DNMT1 proteins co-migrated with ZBP-89 to the cytoplasm, while some DNMT1 proteins remained in the nucleus.
Fig. 1. ZBP-89 interacts with HDAC3 and DNMT1 to form a complex.
ZBP-89 bound HDAC3 and DNMT1 to form a complex in MIHA and PLC/PRF/5 cells (A). The complex could be precipitated by either HDAC3 antibody or DNMT1 antibody (B, C). Original and merged confocal images showed that the complex formed by ZBP-89 and DNMT1 was primarily located in the nucleus (D, E). However, in the presence of TSA, a HDAC inhibitor, ZBP-89, was mainly found in the cytoplasm, and some DNMT1 was also present in the cytoplasm. “+” represents precipitation with labeled antibodies listed in the left side; “−”represents precipitation with control isotype IgG rather than the main antibodies.
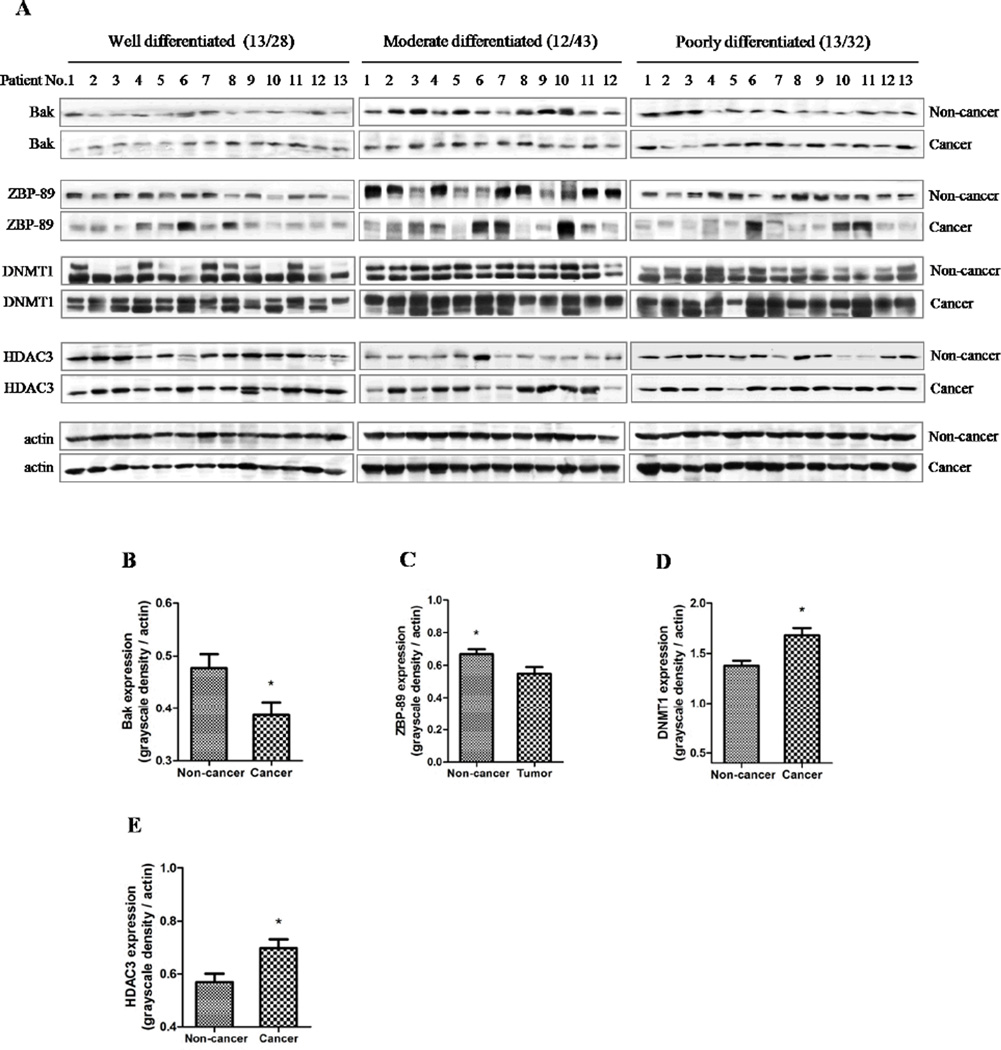
3.2. Expression of Bak, DNMT1 and HDAC3 in HCC tissues
In order to elucidate the relationship between Bak, ZBP-89, and two chromatin-modifying enzymes, Western blot was performed on 103 liver cancer tissues and their non-caner liver tissues (Fig. 2A). We found that the levels of Bak, ZBP-89, DNMT1 and HDAC3 were significantly different between cancer tissues and paired non-cancer tissues (P<0.05, Fig. 2B, C, D and E). The levels of DNMT1 and HDAC3 proteins were much higher in the cancer tissues than the non-cancer tissues, whereas Bak expression was lower in the cancer tissues than in the non-cancer tissues. Furthermore, immunohistochemical staining confirmed that a lower level of ZBP-89, but a higher level of DNMT1 was found in poorly-differentiated HCC (Fig. S1).
Fig. 2. Bak expression is strongly related to ZBP-89, DNMT1 and HDAC3 in HCC.
The expression of Bak, ZBP-89, DNMT1 and HDAC3 in different differentiated HCC samples was examined. The grayscale density of proteins bands was shown in a bar chart, according to different HCC differentiation subtypes. Bak, ZBP-89, DNMT1 and HDAC3 levels were significantly different between cancer tissues and their paired non-cancer tissues (B, C, D and E).
3.3. Inhibition of HDAC and DNMT induced Bak expression and apoptosis
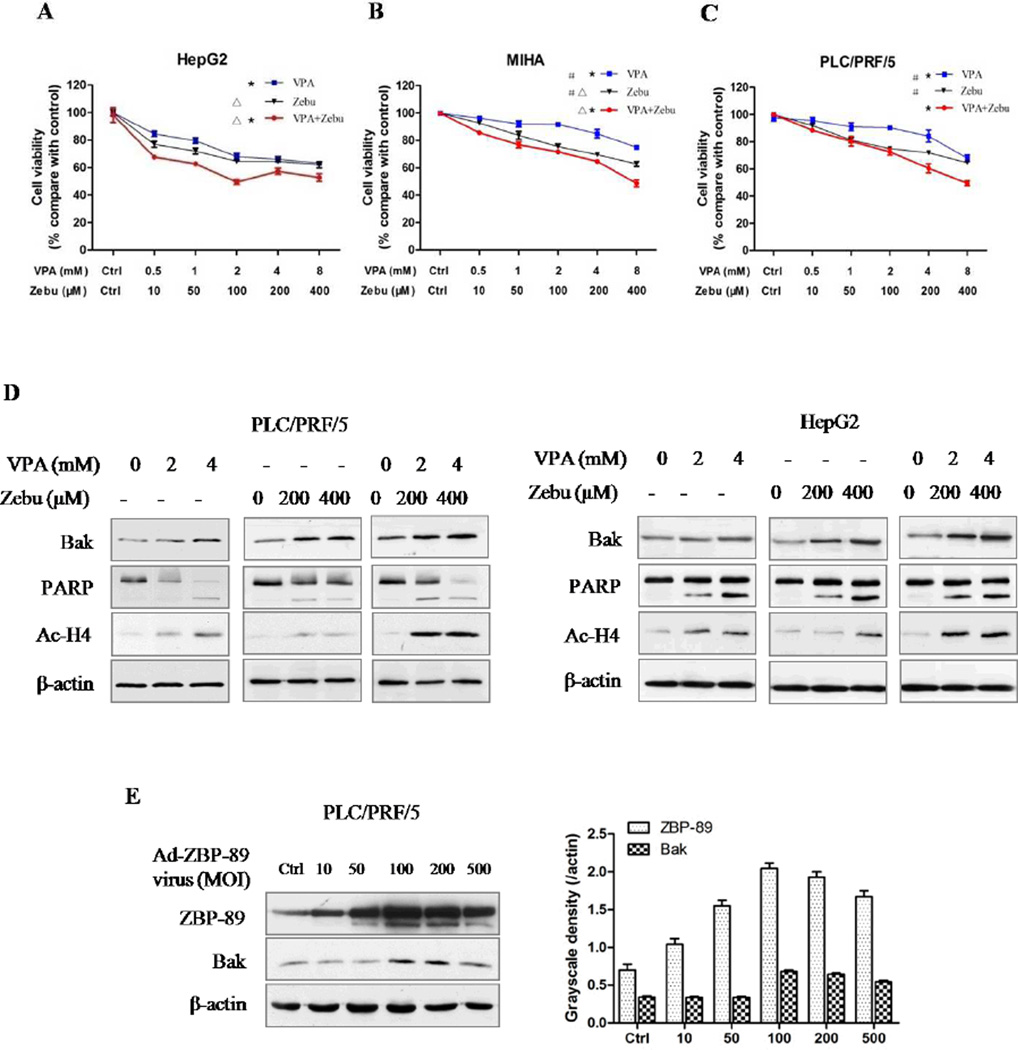
The results showed that VPA and Zebu inhibited the cell proliferation in a time- and dose-dependent manner (Fig. 3A, B and C). Similar results were also obtained when the cells were treated with TSA and 5-aza-dC (data not shown). VPA and Zebu in combination showed the greatest suppression on cell proliferation, compared with either agent alone (P<0.05). Furthermore, both VPA and Zebu induced Bak expression, and activated PARP cleavage-mediated apoptosis in PLC/PRF/5 and HepG2 cells (Fig. 3D, Fig. S2). Both agents also enhanced the acetylation of H4Lys12. These data suggest that Bak expression is regulated by HDAC3 and DNMT1, which is likely related to histone acetylation. In addition, ectopic expression of ZBP-89 clearly induced expression of the pro-apoptotic protein Bak (Fig. 3E).
Fig. 3. HDAC and DNMT inhibitors, and ZBP-89 induce Bak expression.
VPA and Zebu inhibited the proliferation of PLC/PRF/5, HepG2 and MIHA cells. Combination of VPA and Zebu significantly suppressed cells proliferation (A, B and C). Data were shown as Mean±SD. Both VPA acid and Zebu induced Bak expression and cleaved PARP. Treatment of VPA and Zebu increased histone H4 acetylation (D). Ad5-ZBP-89-mediated ZBP-89 overexpression up-regulated Bak expression in a dose-dependent manner after 24 h infection (E). *, Zebu+VPA vs VPA, P<0.05; △, Zebu+VPA vs Zebu, P<0.05; #, Zebu vs VPA, P<0.05, one-way ANOVA analysis.
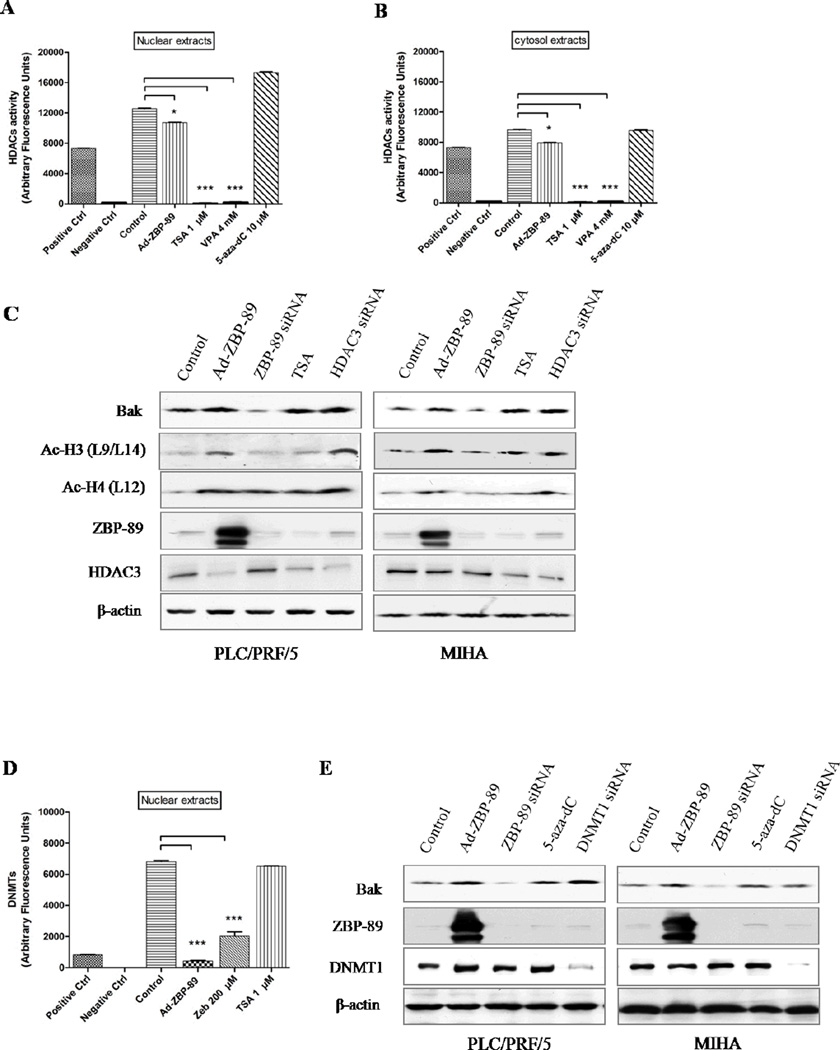
3.4. ZBP-89 inhibited HDAC activity
Both TSA and VPA significantly suppressed the activity of HDAC in both nuclear and cytosol extracts in vitro (P<0.001, Fig. 4A and B). Surprisingly, ZBP-89 overexpression also inhibited HDAC activity (P<0.05). Furthermore, Western blot analysis showed that ZBP-89 could maintain acetylation at histones H3 lysine 9/14 and H4 lysine 12 (Fig. 4C, Lane 2 and 3, Fig. S3).
Fig. 4. ZBP-89 inhibits HDAC and DNMT activities, and increases histone acetylation level.
Histone deacetylases activity analysis showed that adenovirus-mediated ZBP-89 overexpression inhibited HDAC activity in both nuclear and cytosol extracts (A and B). TSA (1 µM) significantly suppressed HDAC activity in vitro. Interestingly. Western blot analysis showed that ZBP-89 increased histone H3 acetylation (Lys9/Lys14) and histone H4 acetylation (Lys12), and decreased HDAC3 protein level (C, Lanes 2 and 3). HDAC3 siRNA upregulated the level of Bak (C, Row 1) and increased histone H3 and H4 acetylation in PLC/PRF/5 and MIHA cells (C, Rows 2 and 3). The overexpression of ZBP-89 significantly inhibited DNMT1 activity in nuclear extracts (D). The activity was also suppressed by DNMT inhibitor Zebu. The downregulation of DNMT1 expression by siRNA increased Bak expression (E Lane 1). ZBP-89 siRNA decreased Bak expression in both cell lines. Data were shown as mean ± SD in triplicate experiments. *P<0.05, ***P<0.001.
3.5. ZBP-89 suppressed DNMT activity and demethylated methyl-CpG islands
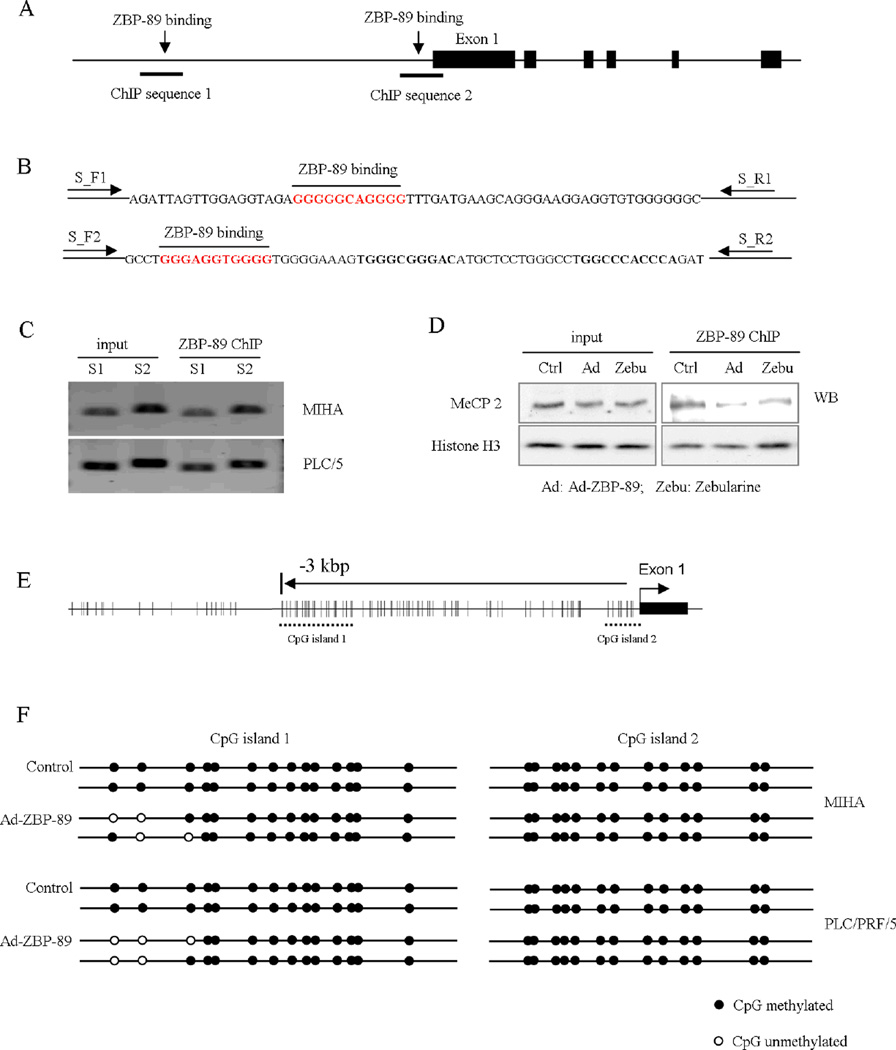
The overexpression of ZBP-89 significantly inhibited DNMT activity in the nuclear extract (P<0.001, Fig. 4D). DNMT1 has been reported to bind DNA at CpG islands [22]. Thus, the finding suggests that ZBP-89 might interfere with the promoter CpG methylation by decreasing DNMT1 activity. Two ZBP-89 DNA binding sites, GGGGGCAGGGG and GGGAGGTGGGG [23], were identified (Fig. 5A and B). Chromatin precipitation method was used to testify the ZBP-89 binding sites. Results showed that 204 bp S1 sequence which is 3 kbp away from exon1, and 228 bp S2 which is near exon1, were precipitated by ZBP-89 antibody and amplified by primers (Fig. 5C). Furthermore, using Co-IP, we measured the precipitated MeCP2, a protein that binds to promoter regions [24,25]. The precipitated products without proteinase K treatment were analyzed by SDS-PAGE and Western blotting, and the result showed that ZBP-89 overexpression inhibited MeCP2 binding to genomic DNA (Fig. 5D). This finding indicated that ZBP-89 might modulate Bak expression by decreasing MeCP2 binding to DNA. Using two computer programs (http://www.mspprimer.org/cgi-mspprimer/design.cgi and http://www.urogene.org/methprimer/index1.html), we found two potential CpG islands in human Bak promoter (Fig. 5E). Bisulfite modification sequencing was performed on these 2 CpG islands and showed that ZBP-89 could demethylate them (Fig. 5F). In the first CpG island, ZBP-89 demethylated methyl-CpGs, but the second CpGs island was not affected.
Fig. 5. ZBP-89 demethylates methyl-CpG dinucleotide.
Schematic diagram of Bak promoter and ZBP-89 binding sites (A). ChIP experiment showed ZBP-89 binding to two regions. One is very close to exon 1 and the other is 3 kbp away form transcription start site (B). 204 bp S1 sequence which is 3 kbp away from exon1, and 228 bp S2 which is near exon1, were precipitated by ZBP-89 antibody and amplified by primers (C). MeCP2 binds to chromatin in a methylation-dependent manner and is associated with a core repressor complex to silence gene transcription. Results showed that adenovirus-mediated ZBP-89 overexpression decreased MeCP2 protein binding to chromatin (D). CpG islands in Bak gene promoter were predicted by online software and 2 potential CpG islands in Bak promoter were found (E). When ZBP-89 was overexpressed, some methylated CpG regions became demethylation (F).
3.6. Downregulation of HDAC3 and DNMT1 enhanced Bak expression
Although the activities of HDAC3 and DNMT1 are affected by ZBP-89, it is still unknown if they directly participate in Bak regulation. We therefore used HDAC3 siRNA and DNMT1 siRNA to knockdown HDAC3 and DNMT1 respectively. It showed that the downregulation of HDAC3 and DNMT1 enhanced Bak expression (Fig 4C and 4E).
3.7. ZBP-89 enhanced Bak expression, induced apoptosis and inhibited tumor growth
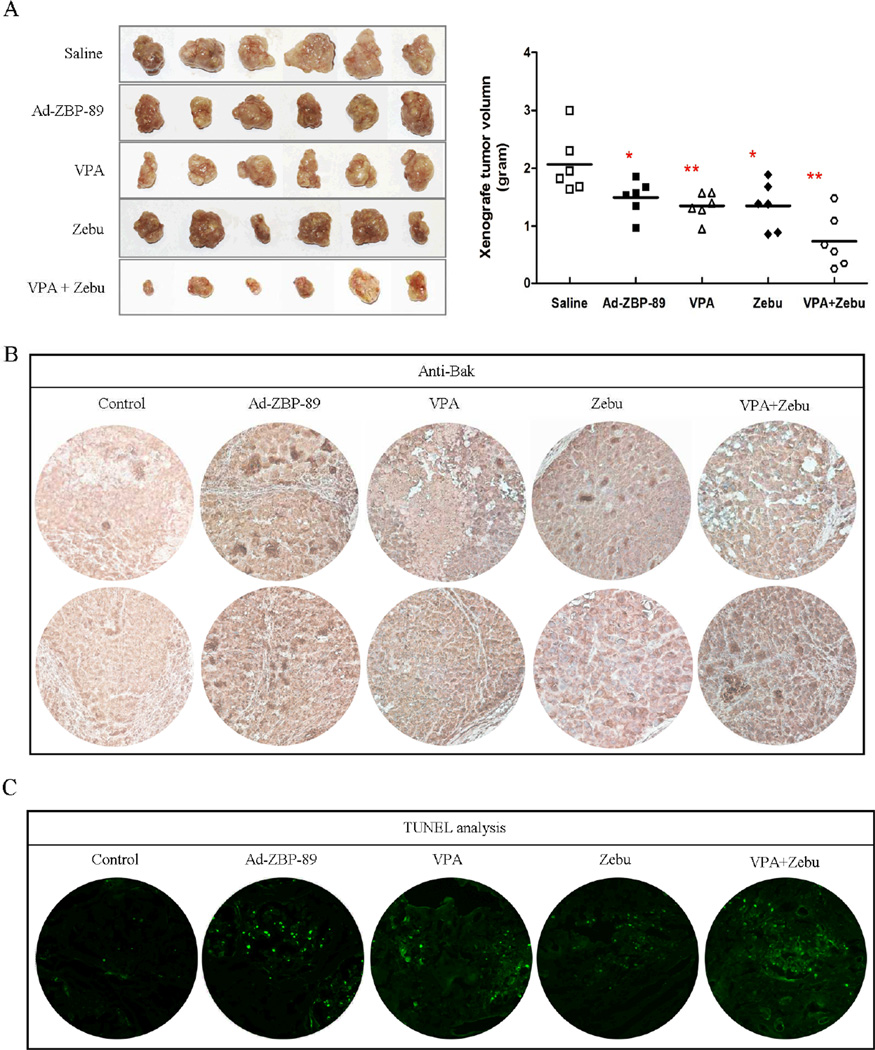
Thirty nude mice were randomly divided into 5 groups, saline, Ad-ZBP-89, VPA, Zebu, and VPA plus Zebu. The significant inhibition of tumor growth was observed in mice treated with Ad-ZBP-89, VPA and Zebu, compared with the saline control (Fig. 6A). The most obvious inhibition was observed in mice treated with VPA and Zebu in combination. The expression of Bak was enhanced by ZBP-89, VPA and Zebu and the TUNEL positive cells were much more in tumor tissues receiving ZBP-89, VPA and Zebu (Fig. 6B and C).
Fig. 6. ZBP-89, VPA and Zebu inhibit xenograft tumor growth.
The tumor-bearing mice were given saline, Ad-ZBP-89, VPA, Zebu and VPA plus Zebu. Two weeks after the treatment, mice were sacrificed by cervical dislocation. Xenograft tumors were resected and weighed to determine the growth of tumor (A). The expression of Bak was examined by immunohistochemical staining (B). The apoptosis was analyzed by TUNEL assay (C). *P<0.05; **P<0.01. All tests were analyzed using Mann-Whitney U test and compared with saline control group.
4. Discussion
The involvement of pro-apoptotic Bak in HCC development, progression and treatment has been documented. The level of Bak is reduced or even non-detectable in HCC cells [26,27]. The reduction of Bak contributes to the development and progression of HCC [28,29]. The significance of Bak in HCC cells is further supported by several experiments in which different agents induce apoptosis in HCC cells by stimulating Bak expression [30–33]. Although the significance of Bak in HCC has been reported, the mechanism responsible for its regulation in HCC is largely unknown. Our previous study has indicated that ZBP-89 may target a certain region of Bak promoter to induce its expression and subsequently apoptosis [3]. In this study we further demonstrated that ZBP-89 stimulated Bak expression through an epigenetic mechanism in HCC.
Sodium butyrate is known to promote ZBP-89-mediated p21waf1 activation in human colorectal cancer cells [34]. ZBP-89 may recruit HDAC1 to the vimentin promoter to repress the expression of vimentin in human cervical cancer HeLa cells [2]. Furthermore, ZBP-89 and HDAC3 may form a complex to help HDAC3 bind to the p16 promoter, resulting in p16 downregulation in human lung cancer cells [4]. To our best knowledge, there is no report on effect of ZBP-89 on the epigenetic regulation of Bak. In the present study, we have shown the involvement of ZBP-89 in the epigenetic regulation of Bak in HCC. First, our Co-IP experiments showed that ZBP-89 protein could form a complex with DNMT1 and HDAC3, and this finding was supported by the confocal examination showing that ZBP-89 and DNMT1 were co-localized primarily in the nucleus. Interestingly, ZBP-89 along with a portion of DNMT1 migrated from the nucleus to the cytoplasm when HCC cells were treated with HDAC inhibitor TSA. Second, ZBP-89 could inhibit the activities of HDAC and DNMT. It was noted that such an inhibitory effect of ZBP-89 on DNMT was even much greater than the classic DNMT inhibitor, Zebu. However, the inhibition of HDAC by ZBP-89 was much weaker than the traditional HDAC inhibitors TSA and VPA. Third, ZBP-89 could maintain histone H3 lysine 9/14 residues and histone H4 lysine 12 residue acetylation status. Fourth, ZBP-89 could significantly inhibit the activity of DNMT that participate in DNA CpG islands de novo methylation. Finally, ZBP-89 could inhibit MeCP2 binding to genomic DNA and partially demethylate Bak promoter CpG islands. These findings evidently indicate that ZBP-89 is able to target multiple points in the epigenetic pathway to upregulate the expression of Bak in HCC.
Our study confirms that the level of Bak is reduced in HCC, especially in the poorly-differentiated HCC. However, a gradual decline in Bak with a corresponding increase in DNMT1 and HDAC3 was not found to be correlated with tumor progression from well/moderate differentiated to poorly differentiated. We do not have a good explanation for this. But it is possible that there are some unknown relevant co-factors in poorly differentiated HCC but not in well/moderately differentiated HCC. The size of the samples in each subgroup may not large enough to render a significant result. Nevertheless, ZBP-89 is able to promote the expression of Bak in HCC cells. Importantly, we have further demonstrated that the expression of Bak is controlled by epigenetic elements in HCC. First, the expression of Bak in liver tissues of HCC is negatively associated with two important epigenetic enzymes, DNMT1 and HDAC3, whose levels are increased in the HCC tumor tissues. Second, the inhibition of either DNMT1 or HDAC3 induces Bak expression and the maximal inhibition is achieved when both enzymes are simultaneously suppressed. These results clearly indicate that both DNMT1 and HDAC3 participate in the expression of Bak in HCC. Third, two CpG islands were located in the promoter of Bak gene as the result of our analysis of human Bak gene promoter. Further analysis showed that the Bak gene is methylated on the dinucleotide CpG islands found. It is well known that CpG island hypermethylation may inhibit transcription by interfering with the recruitment and function of basal transcription factors or transcriptional coactivators [24]. The hypermethylation of CpG islands near the transcriptional regulatory region can promote the recruitment of the methyl-CpG binding domain family proteins including MeCP2 to facilitate a repressive chromatin environment, resulting in silencing genes [24]. MeCP2 can also interact with HDAC-containing complexes to contribute to the histone deacetylation-mediated transcriptional repression [25]. These concepts are consistent with our finding that ZBP-89 inhibits MeCP2 binding to chromatin, and permits demethylation of the CpG islands within the Bak gene, and subsequently induces Bak expression. Though we do not have data to show how ZBP-89 inhibits MeCP2 binding to chromatin, it is possible that ZBP-89 may compete with MeCP2 to bind to genomic DNA, and dissociates MeCP2 from DNA (Fig. S5). Finally, our in vivo experiment demonstrated that the administration of ZBP-89 significantly inhibited the growth of xenograft HCC tumor and such a tumor-inhibitory effect was comparable to that of Zebu, a DNMT inhibitor, and VPA, a HDAC inhibitor. Importantly, along with the reduction of the tumor size, there was an increase in Bak expression and occurrence of apoptosis.
In conclusion, we shows that the expression of Bak is reduced but the levels of DNMT1 and HDAC3 are increased in HCC. Bak expression is negatively associated with levels of these enzymes. ZBP-89 upregulates Bak expression via decreasing DNMT1 and HDAC3, inhibiting MeCP2 binding to genomic DNA and demethylating CpG islands in the Bak promoter. The application of ZBP-89 enhances Bak expression, induces apoptosis and inhibits the growth of HCC tumor in vivo.
Supplementary Material
Acknowledgements
The study was supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region (Project No. CUHK 462009), a CUHK direct grant (Ref No: 2010.1.083), a grant from National Natural Science Foundation of China (No. 81101843) and a Public Health Service grant DK R0155732.
Abbreviations list
- 5-aza-dC
5-aza-2’-deoxycytidine
- ChIP
Chromatin immunoprecipitation
- Co-IP
Co-immunoprecipitation
- DNMT
DNA methyltransferase
- HCC
hepatocellular carcinoma
- HDAC
histone deacetylase
- MeCP2
methyl-CpG-binding protein 2
- MIHA
immortalized non-tumorigenic hepatocyte cell line
- VPA
sodium valproic acid
- Zebu
zebularine
Footnotes
Conflict of Interest
All authors do not have the conflict of interest.
Appendix A. Supplementary data
References
- 1.Salmon M, Zehner ZE. The transcriptional repressor ZBP-89 and the lack of Sp1/Sp3, c-Jun and Stat3 are important for the down-regulation of the vimentin gene during C2C12 myogenesis. Differentiation. 2009;77:492–504. doi: 10.1016/j.diff.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y, Zhang X, Salmon M, Zehner ZE. The zinc finger repressor, ZBP-89, recruits histone deacetylase 1 to repress vimentin gene expression. Genes Cells. 2007;12:905–918. doi: 10.1111/j.1365-2443.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 3.To AK, Chen GG, Chan UP, Ye C, Yun JP, Ho RL, Tessier A, Merchant JL, Lai PB. ZBP-89 enhances Bak expression and causes apoptosis in hepatocellular carcinoma cells. Biochim. Biophys. Acta. 2011;1813:222–230. doi: 10.1016/j.bbamcr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Wang X, Xu L, Pan H, Zhu S, Liang Q, Huang B, Lu J. The transcription factor ZBP-89 suppresses p16 expression through a histone modification mechanism to affect cell senescence. FEBS J. 2009;276:4197–4206. doi: 10.1111/j.1742-4658.2009.07128.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang CZ, Chen GG, Merchant JL, Lai PBS. p53G245D influences effects of HDACi on liver cancer cell growth by modulating the cellular localization of ZBP-89. Cell Cycle. 2012;11:322–334. doi: 10.4161/cc.11.2.18758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 7.Pompeia C, Hodge DR, Plass C, Wu YZ, Marquez VE, Kelley JA, Farrar WL. Microarray analysis of epigenetic silencing of gene expression in the KAS-6/1 multiple myeloma cell line. Cancer Res. 2004;64:3465–3473. doi: 10.1158/0008-5472.CAN-03-3970. [DOI] [PubMed] [Google Scholar]
- 8.Ochs K, Kaina B. Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8 independent. Cancer Res. 2000;60:5815–5824. [PubMed] [Google Scholar]
- 9.Gius D, Cui H, Bradbury CM, Cook J, Smart DK, Zhao S, Young L, Brandenburg SA, Hu Y, Bisht KS, Ho AS, Mattson D, Sun L, Munson PJ, Chuang EY, Mitchell JB, Feinberg AP. Distinct effects on gene expression of chemical and genetic manipulation of the cancer epigenome revealed by a multimodality approach. Cancer Cell. 2004;6:361–371. doi: 10.1016/j.ccr.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Nandakumar V, Vaid M, Katiyar SK. (−)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32:537–544. doi: 10.1093/carcin/bgq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lachenmayer A, Toffanin S, Cabellos L, Alsinet C, Hoshida Y, Villanueva A, Minguez B, Tsai HW, Ward SC, Thung S, Friedman SL, Llovet JM. Combination therapy for hepatocellular carcinoma: Additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J. Hepatol. 2012;56:1343–1350. doi: 10.1016/j.jhep.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu LM, Yang Z, Zhou L, Zhang F, Xie HY, Feng XW, Wu J, Zheng SS. Identification of histone deacetylase 3 as a biomarker for tumor recurrence following liver transplantation in HBV-associated hepatocellular carcinoma. PLoS One. 2010;5:e14460. doi: 10.1371/journal.pone.0014460. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Thain A, Jenkins O, Clarke AR, Gaston K. CpG methylation directly inhibits binding of the human papillomavirus type 16 E2 protein to specific DNA sequences. J. Virol. 1996;70:7233–7235. doi: 10.1128/jvi.70.10.7233-7235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- 15.Oh BK, Kim H, Park HJ, Shim YH, Choi J, Park C, Park YN. DNA methyltransferase expression and DNA methylation in human hepatocellular carcinoma and their clinicopathological correlation. Int J. Mol. Med. 2007;20:65–73. [PubMed] [Google Scholar]
- 16.Saito Y, Kanai Y, Nakagawa T, Sakamoto M, Saito H, Ishii H, Hirohashi S. Increased protein expression of DNA methyltransferase (DNMT) 1 is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomas. Int. J. Cancer. 2003;105:527–532. doi: 10.1002/ijc.11127. [DOI] [PubMed] [Google Scholar]
- 17.Frauer C, Rottach A, Meilinger D, Bultmann S, Fellinger K, Hasenoder S, Wang M, Qin W, Soding J, Spada F, Leonhardt H. Different binding properties and function of CXXC zinc finger domains in Dnmt1 and Tet1. PLoS One. 2011;6:e16627. doi: 10.1371/journal.pone.0016627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee TI, Johnstone SE, Young RA. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protoc. 2006;1:729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ecke I, Petry F, Rosenberger A, Tauber S, Monkemeyer S, Hess I, Dullin C, Kimmina S, Pirngruber J, Johnsen SA, Uhmann A, Nitzki F, Wojnowski L, Schulz-Schaeffer W, Witt O, Hahn H. Antitumor effects of a combined 5-aza-2'deoxycytidine and valproic acid treatment on rhabdomyosarcoma and medulloblastoma in Ptch mutant mice. Cancer Res. 2009;69:887–895. doi: 10.1158/0008-5472.CAN-08-0946. [DOI] [PubMed] [Google Scholar]
- 20.Holleran JL, Parise RA, Joseph E, Eiseman JL, Covey JM, Glaze ER, Lyubimov AV, Chen YF, D'Argenio DZ, Egorin MJ. Plasma pharmacokinetics, oral bioavailability, and interspecies scaling of the DNA methyltransferase inhibitor, zebularine. Clin. Cancer Res. 2005;11:3862–3868. doi: 10.1158/1078-0432.CCR-04-2406. [DOI] [PubMed] [Google Scholar]
- 21.Miao J, Chen GG, Chun SY, Yun JP, Chak EC, Ho RL, Lai PB. Adenovirus-mediated tBid overexpression results in therapeutic effects on p53-resistant hepatocellular carcinoma. Int J. Cancer. 2006;119:1985–1993. doi: 10.1002/ijc.22040. [DOI] [PubMed] [Google Scholar]
- 22.Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW, Baylin SB, Vogelstein B. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 23.Woo AJ, Kim J, Xu J, Huang H, Cantor AB. Role of ZBP-89 in human globin gene regulation and erythroid differentiation. Blood. 2011;118:3684–3693. doi: 10.1182/blood-2011-03-341446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 26.Liu LX, Jiang HC, Liu ZH, Zhu AL, Zhou J, Zhang WH, Wang XQ, Wu M. Gene expression profiles of hepatoma cell line BEL-7402. Hepatogastroenterology. 2003;501:496–1501. [PubMed] [Google Scholar]
- 27.Rousseau B, Menard L, Haurie V, Taras D, Blanc JF, Moreau-Gaudry F, Metzler P, Hugues M, Boyault S, Lemiere S, Canron X, Costet P, Cole M, Balabaud C, Bioulac-Sage P, Zucman-Rossi J, Rosenbaum J. Overexpression and role of the ATPase and putative DNA helicase RuvB-like 2 in human hepatocellular carcinoma. Hepatology. 2007;46:1108–1118. doi: 10.1002/hep.21770. [DOI] [PubMed] [Google Scholar]
- 28.Shigematsu S, Fukuda S, Nakayama H, Inoue H, Hiasa Y, Onji M, Higashiyama S. ZNF689 suppresses apoptosis of hepatocellular carcinoma cells through the down-regulation of Bcl-2 family members. Exp. Cell. Res. 2011;317:1851–1859. doi: 10.1016/j.yexcr.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Lee YH, Judge AD, Seo D, Kitade M, Gomez-Quiroz LE, Ishikawa T, Andersen JB, Kim BK, Marquardt JU, Raggi C, Avital I, Conner EA, MacLachlan I, Factor VM, Thorgeirsson SS. Molecular targeting of CSN5 in human hepatocellular carcinoma: a mechanism of therapeutic response. Oncogene. 2011;30:4175–4184. doi: 10.1038/onc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernando J, Sancho P, Fernandez-Rodriguez CM, Lledo JL, Caja L, Campbell JS, Fausto N, Fabregat I. Sorafenib sensitizes hepatocellular carcinoma cells to physiological apoptotic stimuli. J. Cell. Physiol. 2012;227:1319–1325. doi: 10.1002/jcp.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caja L, Sancho P, Bertran E, Iglesias-Serret D, Gil J, Fabregat I. Overactivation of the MEK/ERK pathway in liver tumor cells confers resistance to TGF-{beta}-induced cell death through impairing up-regulation of the NADPH oxidase NOX4. Cancer Res. 2009;69:7595–7602. doi: 10.1158/0008-5472.CAN-09-1482. [DOI] [PubMed] [Google Scholar]
- 32.Wang QF, Chen JC, Hsieh SJ, Cheng CC, Hsu SL. Regulation of Bcl-2 family molecules and activation of caspase cascade involved in gypenosides-induced apoptosis in human hepatoma cells. Cancer Lett. 2002;183:169–178. doi: 10.1016/s0304-3835(01)00828-x. [DOI] [PubMed] [Google Scholar]
- 33.Hu R, Zhai Q, Liu W, Liu X. An insight into the mechanism of cytotoxicity of ricin to hepatoma cell: roles of Bcl-2 family proteins, caspases, Ca(2+)-dependent proteases and protein kinase C. J. Cell. Biochem. 2001;81:583–593. doi: 10.1002/jcb.1076. [DOI] [PubMed] [Google Scholar]
- 34.Bai L, Merchant JL. ATM phosphorylates ZBP-89 at Ser202 to potentiate p21waf1 induction by butyrate. Biochem. Biophys. Res. Commun. 2007;359:817–821. doi: 10.1016/j.bbrc.2007.05.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.