Abstract
Nephronophthisis (NPHP) is an autosomal recessive cystic kidney disease, which represents the most frequent genetic cause for end-stage renal disease up to the third decade of life. Nephronophthisis is caused by mutations in eleven different genes called nephrocystins (NPHP1-11, NPHP1L). With an increasing number of identified genes our knowledge of nephronophthisis is changing and improving our understanding of the pathomechanisms in nephronophthisis. Recent publications described ciliary expression of nephrocystins together with other cystoproteins like polycystins 1 and 2, and fibrocystin. These findings have shifted our focus to a pathomechanism involving defects in ciliary function (ciliopathy) and planar cell polarity (PCP). In addition, discoveries of new nephrocystin genes have shown that the disease spectrum of nephronophthisis is much broader than previously anticipated. Different forms of mutations within the same NPHP gene can cause different disease severity. In this review we will highlighten the different hypotheses concerning the pathomechanisms for nephronophthisis and we will underline the clinical variability of nephronophthisis. The clinical spectrum has become even more complex with the possibility of oligogenicity in NPHP.
Keywords: nephronophthisis, cystic kidney disease, ciliopathy, Senior-Loken syndrome, Joubert syndrome, Meckel-Gruber syndrome, molecular genetics
INTRODUCTION
Nephronopthisis (NPHP) was first described in 1945 by Smith and Graham and 6 years later by Fanconi et al . [1,2]. Whereas Smith and Graham called this disease “medullary cystic kidney disease”, Fanconi et al. introduced later the term “familial juvenile nephronophthisis” [1,2]. The term “nephronophthisis” derives from the Greek and means “disintegration of nephrons”, which is one aspect of the histopathology. NPHP is an autosomal recessive tubulointerstitial nephropathy and is one of the most frequent genetic disorders causing end-stage renal disease (ESRD) in children and adolescents [3]. The most frequent form of NPHP, called NPHP type 1, is characterizied by ESRD at a mean age of 13 years [4]. Symptoms are very subtle and may start as early as 6 years of age. They consist of polyuria, polydipsia, secondary enuresis, growth retardation and anemia [5]. In addition, NPHP has a rare infantile form with age of onset of ESRD prior 4 years of age and an adolescent form with a median age of onset of ESRD of 19 years [6]. Renal ultrasound shows initially normal kidney size, increased echogenicity, poor corticomedullary differentiation and corticomedullary cysts (Figure 1) and later smaller, atrophic kidneys with increased echogenicity are found [7]. Imaging at a later stage of disease reveals small, atrophic kidneys and a more prominent cyst development. Histological findings in NPHP are tubular atrophy with thickened or thinned tubular basement membrane, cysts at the corticomedullary border and diffuse interstitial fibrosis (Figure 2) [8,9]. The histological characteristics of the infantile form of NPHP differ from the ones seen in juvenile NPHP. Infanitile NPHP combines features of NPHP (e.g. tubular cell atrophy, tubular cysts and interstitial fibrosis) with features of polycystic kidney disease (e.g. enlarged kidneys, widespread cyst development) [10,11]. Renal biopsy or mutation analysis is required for definitive diagnosis of NPHP. Over 300 cases of NPHP have been published [8]. In 10–15% of NPHP patients extrarenal symptoms are found, which include retinal degeneration (Senior-Loken syndrome), cerebellar vermis aplasia (Joubert syndrome), liver fibrosis, oculomotor apraxia (Cogan syndrome) and cone-shaped epiphysis [12]. A large variety of different syndromes have been published in association with NPHP (Table 1). One of the more prominent syndromes associated with NPHP is the severe perinatal lethal Meckel-Gruber syndrome, which includes occipital encephalocele, polydactyly, microphthalmia, and liver fibrosis among other developmental abnormalities [13]. The incidence of NPHP varies largely from 1:50,000 in Canada to approximately 1 in a million in the United States. In Finland the incidence of NPHP is reported as 1 in 61800 [5,7,14]. Finally, NPHP has also been diagnosed in adults with renal failure occurring later in life [15].
Fig 1. Renal ultrasound in NPHP.
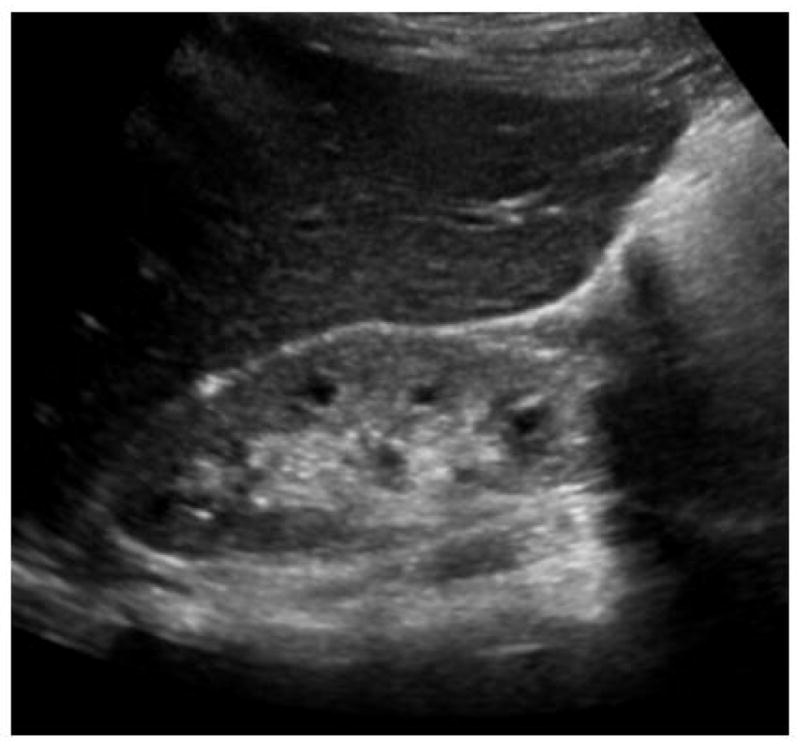
The renal ultrasound shows smaller bilateral kidneys, increased echogenicity (compare to abnormally lower echogenicity of liver), decreased cortico-medullary differentiation, and cortico-medullary cyst formation.
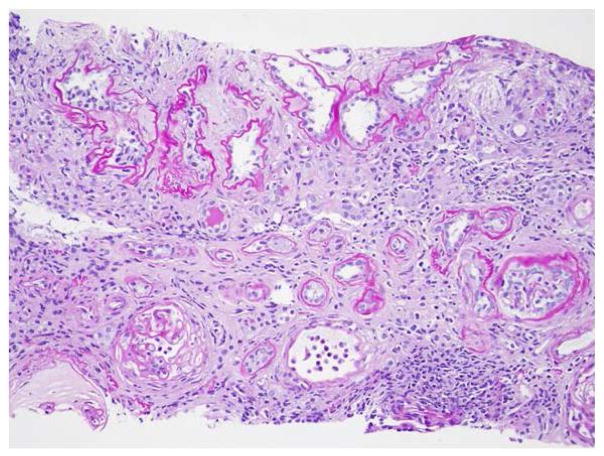
Fig 2. Renal histopathology in NPHP.
Renal histopathology in NPHP is characterized by the triad of tubular cysts, tubular basement membrane disruption, and interstitial fibrosis with interstitial cell infiltration. PAS staining, magnification 20x.
Table 1.
Extrarenal manifestations associated with NPHP and resulting syndromes associated with NPHP mutations.
| Ophthalmologic disorder | Syndrome |
|---|---|
| Retinitis pigmentosa | Senior-Loken syndrome (SLSN) |
| Arima syndrome (cerebro-oculo-hepato-renal syndrome) | |
| Alstrom (RP, obesity, DM type 2, hearing impairment) | |
| RHYNS (RP, hypopituitarism, skeletal dysplasia) | |
| Oculomotor apraxia | Cogan syndrome |
| Nystagmus | Joubert syndrome/Joubert syndrome related disorders |
| Coloboma | Joubert syndrome/Joubert syndrome related disorders |
| Skeletal disorder | |
| Short ribs | Jeune syndrome/asphyxiating thoracic dystrophy |
| Cone-shaped epiphysis | Mainzer-Saldino syndrome |
| Postaxial polydactyly | Joubert syndrome/Joubert syndrome related disorders |
| Bardet-Biedl syndrome(NPH P, RP, obesity, deafness) | |
| Ellis van Creveld | |
| Skeletal dysplasia | Sensenbrenner syndrome / cranioectodermal dysplasia |
| Ellis van Creveld | |
| Neurological disorder | |
| Encephalocele | Meckel-Gruber syndrome (occipital encephalocele, NPHP) |
| Vermis aplasia | Joubert syndrome/Joubert syndrome related disorders |
| Hypopituitarism | RHYNS (RP, hypopituitarism, skeletal dysplasia) |
| Hepatic disorder | |
| Liver fibrosis | Boichis syndrome |
| Meckel-Gruber syndrome (occipital encephaolocele, NPHP) | |
| Arima syndrome (cerebro-oculo-hepato-renal syndrome) | |
| Joubert syndrome/Joubert syndrome related disorders | |
| Others | |
| Situs inversus | |
| Cardiac malformation | |
| Bronchiectasis | |
| Ulcerative colitis | |
RP, retinitis pigmentosa/retinal degeneration; DM, diabetes mellitus; NPHP, nephronophthisis
Initially, patients with NPHP were published under the term “medullary cystic kidney disease” (MCKD). Today MCKD refers to an autosomal dominant cystic kidney disease with hypertension and hyperuricemia, which shares the histology of NPHP [3]. Therefore, NPHP and MCKD have been combined to the “nephronophthisis – MCKD disesase complex” [8]. MCKD type 2 is caused by mutations in the Uromodulin (UMOD) gene [16]. Eleven different genes, that if mutated cause NPHP have been identified by positional cloning (NPHP1-11, NPHP1L) (Tab. 2). The most frequent mutation in NPHP is a homozygous deletion of NPHP1, which causes approximately 20% of cases with the isolated renal form of NPHP, whereas the mutations in the other genes contribute to less than three percent each [12]. In approximately 70% of all individuals with NPHP the causative gene is still unknown [12]. All eleven NPHP genes are sufficient to cause NPHP by two mutations in a single recessive gene. The type of gene mutated as well as the nature of mutations determine the severity of the phenotype regarding age of onset and extent of organ involvement. In addition, modifier effects have been suggested [17].
In this review we want to emphasize the changes in the understanding of the pathophysiology of NPHP. We will also outline the widening spectrum of phenotypes.
Additional phenotypes associated with nephronophthisis
Oculomotor apraxia type Cogan
Oculomotor apraxia (OMA) type Cogan [OMIM %257550] is characterized by an impaired horizontal gaze and nystagmus. As a result the affected individual has to move the head by jerky head movements in order to follow objects. OMA is a rare ocular sign found in NPHP patients with NPHP1 and NPHP4 mutations [12,18]. It is also encountered in Joubert syndrome. Cerebellar vermis aplasia has been published in association with OMA [19].
Nephronophthisis withretinitis pigmen tosis (Senior Loken syndrome)
About 10–15% of patients with NPHP have retinal degeneration, also called retinitis pigmentosa (RP) [20,21]. RP can result in early and severe visual impairment. Early onset of RP resembles Leber’s congenital amaurosis (LCA), whereas late onset is characterized by night blindness and progressive visual loss. RP is diagnosed by fundoscopy and electroretinography. The association of retinitis pigmentosa and NPHP is called Senior-Loken syndrome (SLSN) [OMIM #266900, %606995, #606996, #609254, #610189]. The pathomechanism of the retinopathyis currently unknown but may be related to the function of the connecting cilium and centrosomes of photoreceptors, where nephrocystin proteins are expressed [12,17,22,23]. The frequency of RP with NPHP can range from 6–100%, depending on the NPHP mutation (e.g. 6% for NPHP1, 10% for NPHP2/INV, 100% for NPHP5 and NPHP6) [12]. Retinal symptoms due to NPHP1 deletions usually present with a milder phenotype. SLSN is also found in a few patients with NPHP1 to NPHP4 mutations. Several genes causing NPHP (NPHP1, NPHP8/RPGRIP1L) and NPHP-related phenotypes (AHI1 in Joubert syndrome) are involved in photoreceptor development and act as modifiers of retinal degeneration [24–26].
Cerebellar vermis aplasia with NPHP(Joubert syndrome)
Joubert syndrome (JS) [OMIM %213300] is an autosomal recessive developmental disorder, consisting of cerebellar vermis aplasia (revealed in magnetic resonance brain imaging (MRI) as “molar tooth sign”) (Fig. 3), cerebellar ataxia, hypotonia, oculomotor apraxia, neonatal tachypnea, mental retardation, and retinal degeneration [27]. NPHP is found in 17–27% of JS patients [28]. Additional associated symptoms include liver fibrosis, ocular coloboma, and polydactyly [27]. JS is also called CORS (cerebello-oculo-renal syndrome). JS is caused by mutations in NPHP6/CEP290, which encodes nephrocystin-6 and NPHP8/RPGRIP1L, which encodes nephrocystin-8 [29–31]. Additional mutations in JS were found in other genes including AHI1, MKS3, ARL13B, CC2DA2, INPP5E, and TMEM216 [32–38]. Rare mutations in NPHP1 and NPHP4 were published in JS [39,40]. Cerebral symptoms due to NPHP1 deletions usually present with a milder phenotype.
Fig 3. The “molar tooth sign” is a classical neuroradiological characteristic of JS.
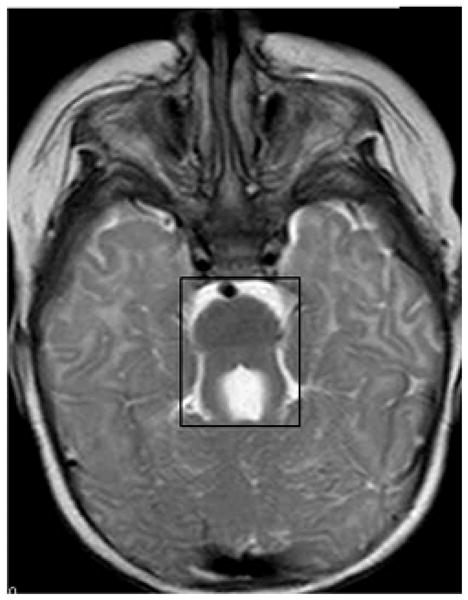
The “molar tooth sign” (shown in box) on a brain magnetic resonance imaging (MRI). Brain MRI axial image at the level of the superior cerebellar peduncles of a JS patient. The “molar tooth sign” is characterized by cerebellar vermis aplasia, thickened and elongated superior cerebellar peduncles, and a deepened interpeduncular fossa.
Meckel-Gruber syndrome
Meckel-Gruber syndrome (MKS) is characterized by renal cystic dysplasia, occipital encephalocele, microphthalmia and other central nervous system malformations, polydactyly, situs inversus, bile duct proliferation and pulmonary hypoplasia. Like all other forms of NPHP, MKS is inherited in an autosomal recessive mode. Newborn with MKS rarely survive longer than two weeks. Recently, a strong allelism has been described in MKS where two truncating mutations (nonsense, frame-shift or splice site mutations) in the genes MKS1, MKS3, NPHP3, NPHP6/CEP290, and NPHP8/RPGRIP1L cause MKS, whereas the presence of at least one missense mutation causes the milder phenotype of JS or SLSN [17,30,41–43]. The defects in MKS represent developmental defects whereas in NPHP and SLSN defects of retina and kidney are degenerative in nature.
Liver fibrosis
NPHP-like ciliopathies have been described together with periportal liver fibrosis in a few cases. Hepatomegaly, portal fibrosis and bile duct proliferation were described in a patient with NPHP3 mutation [44]. Liver fibrosis is also found in Arima syndrome (cerebro-oculo-hepato-renal syndrome) and Meckel syndrome. Very recently, mutations in MKS3/TMEM67 was found to represent a major gene mutated in NPHP-like ciliopathies that exhibit a liver fibrosis phenotype [45]. In this context two truncating mutations caused MKS with biliary duct dysplasia, whereas the presence of at least one missense mutation among the two alleles caused only NPHP-like degenerative liver fibrosis. A similar genotype-phenotype correlation has been described for NPHP6/CEP290and MKS1 [12 ].
Skeletal defects
Skeletal symptoms associated with NPHP are rare. The most frequent skeletal manifestation of NPHP are cone-shaped epiphyses of the phalanges, also called Mainzer-Saldino syndrome [46]. There can also be an association with cerebellar ataxia, retinal degeneration, and polydactyly [47]. Jeune syndrome (asphyxiating thoracic dysplasia) with short limbs and small thorax and Ellis van Creveld syndrome with short stature, short extremities, and polydactyly canalso occur in association with NPHP[ 48,49].
Cardiac defects
Rare cases of cardiac defects (e.g. ventricular septal defect) have been published in association with infantile NPHP and mutations in NPHP2/inversin and NPHP3 [43,50]. Animal models (in zebrafish and mice) for NPHP2/inversin confirmed the association of cystic kidney disease with cardiac septal defects[50 ].
Diagnosing nephronophthisis
Symptoms and signs in NPHP manifest slowly and are subtle. The history may reveal polyuria, polydipsia or secondary enuresis usually starting around 6 years of age. General symptoms of renal failure may be present such as fatigue, pruritus, nausea, vomiting, uremic gastritis, anemia and growth retardation. A family history of consanguity may hint towards an autosomal recessive disease. The physical exam may reveal any of the associated extrarenal phenotypes or may be unremarkable besides pallor and short stature. Urinalysis may reveal a renal concentration defect (<400 mosm/kg in morning urine). Renal function should be evaluated as well as CBC, liver function tests and coagulation tests. Renal ultrasound may show small kidneys with poor corticomedullary differentiation and corticomedullary cysts, and if present, liver fibrosis. Renal biopsy can be done if the kidneys are not too small and atrophic at the timepoint of diagnosis. However, nowadays molecular genetic analysis is the mainstay for making a definitive diagnosis of an NPHP-like ciliopathy. If there is any indication of cerebellar involvement an MRI may be indicated to rule out the molar tooth sign, which indicates Joubert syndrome. If there is concern for NPHP an ophthalmological exam should be performed to rule out retinal degeneration. There are only two ways to obtain a definitive diagnosis of NPHP: renal biopsy or mutation analysis (www.renalgenes.org). Mutation analysis should be initiated in the context of genetic counseling.
Genes mutated inn ephronophthisis
NPHP1 is located at cell contacts and cilary transition zone
Homozygous deletions of NPHP1 on chromosome 2q13 cause NPHP type 1, the most frequent form of NPHP accounting for about 20% of cases [51,52]. The homozygous NPHP1 deletion is also found in patients with additional ocular motor apraxia (OMA) [18], Senior-Loken syndrome [53] and very rarely in Joubert syndrome, which may be due to an epistatic effect by the AHI1 gene [39,54]. In few patients a heterozygous deletion of NPHP1 was associated with a NPHP1point mutation.
NPHP1 encodes nephrocystin-1, which is located at adherens junctions and focal adhesions of renal epithelial cells. In the human kidney nephrocystin-1 is expressed primarily in collecting duct cells [55]. Interaction of nephrocystin-1 was described with p130cas, focal adhesion kinase 2, tensin, filamin A and B [56–58]. Due to the expression pattern and interaction partners nephrocystin-1 was suspected to play a role in cell-cell and cell-matrix signaling. In addition, interaction was later shown with nephrocystin-2/inversin, nephrocystin-3, nephrocystin-4, and Jouberin, indicating that there is a protein complex of nephrocystins [44,50,59,60]. This complex of proteins may function in multiple intracellular compartments including the cilium, cell-cell adherens junctions and focal adhesions [50,56,57]. When ciliary localization of nephrocystin-2/inversin was discovered, nephrocystin-1 was also identified in cilia [50]. The primary ciliary localization was later refined to the transition zone (e.g. at the base of the cilium) in respiratory and renal epithelium and to the connecting cilium of the photoreceptor [61]. PACS-1 and casein kinase 2 phosphorylation are required for targeting of nephrocystin-1 to the transition zone [62].Due to the expression pattern of nephrocystin-1 in the adherens junctions and focal adhesions and the interaction with integral components of these structures (e.g. p130CAS), nephrocystin-1 was initially thought to result in a defective cell-cell and cell-matrix signaling – which resulted in the “adherens junction/focal adhesion hypothesis” [3]. This hypothesis was later linked to the “ciliary hypothesis” by the finding that nephrocystin-4, an interaction partner of nephrocystin-1, co-localizes with β-catenin at cell-cell contact sites, and to primary cilia in polarized renal epithelial cells but is found in centrosomesin dividing cells [63 ].
Mutations in nephrocystin-2 cause infantile NPHP, situs inversus and cardiac defects
Recessive mutations of nephrocystin-2/inversin were identified as the cause for NPHP2 based on a candidate gene approach and positional cloning [10,50]. Characteristics of NPHP2 are: i) age of onset of ESRD prior to 5 years of age, ii) a renal ultrasound finding ofnormal or enlarged kidneys, iii) possible antenatal presentation with oligohydramnios, iv) renal histology showingan overlap of feat ures characteristic of NPHP and ADPKD, and v)possible association with situs inversus and cardiac abnormalities (VSD) [64]. Retinitis pigmentosa is a rare finding in patients with NPHP2/inversin mutations [65]. Even though nephrocystin-2/inversin mutations are rare (1% of all NPHP patients), the identification of nephrocystin-2/inversin mutations as causing NPHP2 resulted in a major breakthrough concerning our understanding of NPHP: Nephrocystin-2/inversin was found to be co-expressed in primary cilia of renal tubular cells with nephrocystin-1 and interacts with nephrocystin-1 and β-tubulin [50]. β-tubulin represents a major protein of the microtubule axoneme of primary cilia. This discovery was one of the first hints towards a unifying theory of renal cystogenesis, which implies that all genes causing cystic kidney disease are expressed in primary cilia, basal bodies or centrosomes [3,66 ]. Recently, nephrocystin-2/inversin was shown to function as an anchor for NPHP3 and NPHP9/Nek8 in cilia [67]. Further studies of nephrocystin-2/inversin showed a cell cycle-dependent expression of nephrocystin-2/inversin in the mitotic spindle in mitosis, the mid-body in cytokinesis and in cilia, the basal body and centrosomes in the interphase [68]. Cell-cyle-specific expression of nephrocystin-2/inversin in these organelles supported the development of the “planar cell polarity” (PCP) hypothesis of the pathogenesis of NPHP [see below “Planar cell polarity”]. This hypothesis was supported by Simons et al., who demonstrated a role for nephrocystin-2/inversin in the Wnt signaling pathway, which is involved in planar cell polarity [69]. If nephrocystin-2/inversin is defective, the canonical pathway of the Wnt signaling will dominate over the non-canonical form and will disrupt apical-basolateral polarity of the renal epithelial cells. Besides mutations in NPHP2/inversin mutations in NPHP3 and NPHP9/NEK8 were also identified in patients with infantile NPH [70–72].
NPHP3mutations are a rare cause of NPH Pbut maycause a wide sp ectrum of disease
NPHP3 was mapped and identified in one large Venezuelan kindred with NPHP [44]. It encodes nephrocystin-3 which interacts with nephrocystin-1 and inversin [43,44]. Nephrocystin-3, like inversin, may inhibit the canonical Wnt signaling pathway [43]. Moreover, mutations in the murine ortholog Nphp3 cause the renal cystic mouse mutant pcy, which generates a hypomorphic Nphp3 allele [44]. Interestingly, the pcy mouse model responds very well to treatment with a vasopressin-2 receptor antagonist [73]. The Nphp3 knockout mouse model shows situs inversus, congenital heart defects and embryonic lethality, a phenotype very similar to Meckel-Gruber syndrome, thus confirming that complete loss of function mutations cause the developmental phenotype of MKS, whereas missense mutations cause primarily degenerative phenotypes [43]. In humans, mutations in NPHP3 result in a variety of phenotypes ranging from adolescent NPHP, NPHP with liver fibrosis, NPHP with RP, infantile NPHP to Meckel-Gruber syndrome (MKS), dependent on the nature of the mutated alleles [43,44,70,72]. Truncating mutations result in developmental, early-onset phenotypes resembling MKS, whereas non-truncating mutations result in milder degenerative phenotypes with later age of onset.
Nephrocystin-4: Combining the cilia and the cell junction hypothesis
NPHP4 mutations were identified by positional cloning on chromosome 1p36 [40,74]. NPHP4 encodes nephrocystin-4, which also localizes to primary cilia, basal bodies and centrosomes [63]. Nephrocystin-4 interacts with nephrocystin-1 and nephrocystin-8/RPGRIP1L and forms complexes with α-tubulin [30,40]. Recently, nephrocystin-4 and nephrocystin-1 have been shown to associate with PALS1/PATJ and Par6, which is required for epithelial morphogenesis [75]. Mutations in NPHP4 account for about 2% of NPHP and can result in isolated NPHP, NPHP with OMA and SLSN.
NPHP5 mutations cause a retinal-renal phenotype
Homozygous truncating mutations of NPHP5/IQCB1 cause SLSN with early-onset RP in association with NPHP [76]. NPHP5/IQCB1 encodes nephrocystin-5, which contains two IQ calmodulin binding sites and a coiled-coil domain. Nephrocystin-5 interacts directly with calmodulin via the IQ domains and forms a complex with retinitis pigmentosa GTPase regulator (RPGR) [76]. Mutations in RPGR result in X-linked retinitis pigmentosa. Prior to the “ciliary hypothesis” the pathologic basis for retinal involvement in SLSN was not well understood. The strong association of NPHP5/IQCB1 mutations prompted further expression studies and nephrocystin-5 was found to be expressed in the connecting cilia of photoreceptors [76]. This finding supported the ciliary hypothesis and provided a potential pathologic basis for the retinal-renal phenotype of SLSN. The primary cilium of renal epithelial cells corresponds to the connecting cilia of the photoreceptors of the retina [77]. In addition to nephrocystin-5, expression of nephrocystin-6 was also shown in the connecting cilium of the photoreceptors and nephrocystin-5 and 6 were shown to interact with each other [29,78].
NPHP6 mutations cause Joubert syndrome
NPHP6/CEP290 were found to cause JS [29,79]. The gene product, nephrocystin-6, activates and interacts with ATF4 (activating transcription factor 4), a transcription factor which may be involved in cAMP dependent renal cyst formation [73]. Nephrocystin-6 constitutes a part of the centrosomal proteome [29,80]. Similar to the NPHP2/INV and NPHP4 gene products nephrocystin-6 is localized at centrosomes and at the mitotic spindle [29]. Knockdown of the nphp6 ortholog in zebrafish resulted in renal cysts, retinal degeneration and cerebellar malformation and a defect of planar cell polarity, thereby recapitulating the human JS phenotype [29]. NPHP6/CEP290 mutations can also result in JS without renal involvement and in a broader variety of phenotypes ranging from isolated NPHP, SLSN, JS to MKS and BBS [29,81–84]. Interestingly, mutations of NPHP6/CEP290 also cause isolated Leber congenital amaurosis (LCA) and amounts for 21% this disease [85]. The mouse model rd16 has an inframe deletion of 300 amino acids in Nphp6/Cep290, which mimics the RP phenotype without showing brain or kidney abnormalities, resulting in a hypomorphic allele [86].
Increased apoptosis and fibrosis resultsin NPH P7
NPHP7/GLIS2 mutations were identified as causing isolated NPHP in a large Cree Indian kindred. Affected individuals developed renal failure prior to 8 years of age [87]. NPHP7/GLIS2 encodes the Kruppel-like zinc-finger transcription factor “Gli-similar protein 2”. NPHP7/GLIS2 localizes to the primary cilia and the nucleus. A mouse knockout model of Glis2 revealed severe renal atrophy and fibrosis [87]. The kidneys of the Glis2 mutant mice showed upregulation of genes that promote epithelial-to-mesenchymal transition and fibrosis [87]. NPHP7/GLIS2 is related to GLI transcription factors and thereby links the pathogenesis of NPHP to the sonic hedgehog pathway, which is involved in cell fate determination, tissue patterning and maintenance of stem cell pools in postembryonic tissues.
NPHP8/RPGRIP1L mutations cause Joubert syndromean d Meckel-Gruber syndrome
NPHP8/RPGRIP1L mutations were identified by positional cloning as causing Joubert syndrome-like phenotype (cerebro-oculo-renal syndrome [CORS]) [23]. NPHP8/RPGRIP1L encodes the protein RPGRIPL1 (retinitis pigmentosa GTPase regulator interacting protein 1-like) which co-localizes with NPHP4 and NPHP6 at centrosomes and basal bodies [30]. Two missense mutations result in the CORS phenotype, whereas one or more truncating mutations cause the more severe phenotype of Meckel-Gruber syndrome [30,31]. RPGRIP1L was shown to interact with nephrocystin-4 and missense mutations in NPHP8/RPGRIP1L of affected patients reduced the RPGRIP1L interaction with nephrocystin-4 [30,88]. Additional characteristics of affected patients included polydactyly, scoliosis, pituitary agenesis and partial growth deficiency. The corresponding Rpgrip1l (Ftm for fused-toes mouse) knockout mouse exhibits cerebral, renal and hepatic defects similar to CORS and Meckel-Gruber syndrome. Recently, a genotype-phenotype correlation became evident for NPHP3, NPHP6 and NPHP8, in which the presence of two truncating mutations causes the severe, early-onset developmental dysplastic phenotype of MKS with broad organ involvement, whereas at least one missense mutation (of the two recessive mutations) causes a milder, late-onset, degenerative phenotype with more restricted organ involvement.
NPHP8/RPGRIP1L mutations were recently shown to cause retinal degeneration [89]. Missense mutations and the sequence variant (A229T) were found in patients with LCA and retinal degeneration combined with other ciliopathies as BBS, SLSN, JS and MKS.
Linking cilia and cell-cycle defects in NPHP
NPHP9/NEK8 encodes the NEK8 protein (never in mitosis A-related kinase 8), which if mutated causes NPHP type 9. Three highly conserved missense mutationswere found in three different individuals [71]. One patient with a homozygous NPHP9/NEK8 mutation developed infantile NPHP at age of 3 years [71]. In two other patients the second recessive mutation was not identified. One of these two patients had an additional homozygous NPHP5/IQCB1 mutation and RP in addition to NPHP [71]. One of the mutations was found in the RCC1 domain of NEK8. The corresponding jck mouse model, which is characterized by cystic renal disease is caused by a missense mutation (G448V) in the RCC1 domain [90]. Expression studies of all three mutated proteins in medullary collecting duct cells showed defects of centrosomal and ciliary localization of NEK8 [71]. NPHP9/NEK8 is important in the regulation of the cell-cycle, offering a link between nephrocystins and the role of centrosomes for cell-cycle regulation. Interestingly, polycystin-1 and polycystin-2 (the two genes mutated in ADPKD, which are also expressed in primary renal cilia) signaling has also been linked to cell growth regulation involving the JAK-STAT pathway [91–93]. The jck and cpk mice, which represent models for PKD were successfully treated by the cyclin-dependent kinase inhibitor roscovitine, which underlines the involvement of cell-cycle regulation in renal cystic disease [94].
NPHP11/MKS3may cause Joubert syndrome or Meckel-Gruber syndrome
Mutations in NPHP11/MKS3/TMEM67 result in a wide spectrum of NPHP-like ciliopathies ranging from NPHP with liver disease, to JS and Meckel syndrome. NPHP11/MKS3/TMEM67 encodes the protein meckelin, which was found to be expressed in the primary cilia and the plasma membrane [95] Missense mutations in NPHP11/MKS3/TMEM67 were discovered in a population characterized by NPHP and liver fibrosis [45,96]. Four new missense mutations were found in 5 kindreds, resulting in a hypomorphic allele and leading to a milder phenotype than the truncating mutations [45]. Doherty et al. also identified some patients with COACH syndrome (cerebellar vermis hypoplasia, oligophrenia (developmental delay/mental retardation), ataxia, coloboma, and hepatic fibrosis) – a JS related disorder – and found MKS3/TMEM67 mutations in 19/23 families (83% of the cohort) [96]. Because of the strong association of MKS3/TMEM67 mutations and the NPHP plus liver fibrosis phenotype, MKS3/TMEM67 is now also called NPHP11 [45].
NPHP1L – a nephronophthisis like phenotype
NPHP1L/XPNPEP3 was identified by homozygosity mapping in two consanguineous kindreds on chromosome 22. Renal histopathology was consistent with NPHP and a splice site mutation and a 4 bp deletion, causing two loss of function mutations, were discovered [97]. The phenotype included hypertension, cardiomyopathy, renal failure and seizures [97]. A complex-I-defect mitochondropathy with decreased NADH-CoQ-Oxireductase acitivity was discovered. NPHP1L/XPNPEP3 isoform 1 has a N-terminal 79 amnio acid sequence which is responsible for mitochondrial localization and suggests a mitochondrial function of this protein [97]. Because this is the first gene not being consistent with the cilia hypothesis it may only cause a phenocopy of NPHP but may not belong to the family of ciliopathies [ 97].
The “ciliary hypothesis” of NPHP
Ciliary expression of nephrocystins may explain organ involvement in NPHP
So far all proteins of genes, which cause cystic kidney disease are expressed in the primary renal cilium, basal bodies, centrosomes or the mitotic spindle in a cell-cycle dependent fashion [3,66 ]. Even Uromodulin, the gene altered in autosomal dominant medullary cystic kidney disease type 2 (MCKD2), which shares histopathology with autosomal recessive nephronophthisis, was found to be expressed in cilia [98]. The primary cilium is an organelle of almost every cell that projects like an antenna from the cell surface. The primary cilium contains an axoneme, which consists of 9+0 microtubular doublets (in contrast to motile cilia which contain 9+2 microtubular doublets) [3]. The axoneme is assembled by “intraflagellar transport” (IFT) because no protein biosynthesis occurs within the cilium [3]. Cilia are involved in photosensation, mechanosensation, osmotic, olfactory and temperature sensation [3]. The basal body from which the cilium is assembled is located at the root of the cilium and derives from the mother centriole [3].
Nephrocystin-1 and nephrocystin-4 are evolutionary conserved in the nematode C. elegans. Expression of the nephrocystin-1 and nephrocystin-4 orthologs was found in ciliated neurons of the head (amphids) and tail (phasmids) [99]. The expression pattern showed significant overlap with the localization of other cystoprotein orthologs in C. elegans like polycystin-1 (lov-1), polycystin-2 (pkd-2) or multiple orthologs of the BBS proteins [99,100]. Knockdown of the nephrocystin-1 and nephrocystin-4 orthologs resulted in a very similar phenotype compared to the knockout nematodes of the polycystin-1 and polycystin-2 orthologs (lov-1 and pkd-2, respectively) [99]. Nephrocystin-1 and nephrocystin-4 orthologs were found to be required for morphologic integrity, and nephrocystin-4 contributes to the regulation of life span in the nematode [101,102]. For some nephrocystins (nephrocystin-2, -4 and -6) evolutionary conservation reaches back more than 1.5 billion years to a unicellular organism called Chlamydomonas reinhardtii. Nephrocystin-4 and a minimum of six other proteins of the BBS complex are part of the basal body proteome in Ch. reinhardtii, which if mutated causes impaired IFT and defective flagellar propulsion [93,100] .
The function of cilia in NPHP is still not completely resolved. Renal cilia may sense tubular flow of urine [103]. For polycystin-1 and polycystin-2 it was shown that both are able to sense flow resulting in intracellular calcium signaling [103]. Other phenotypes associated with NPHP can also be explained by the ciliary hypothesis. Nephrocystin-5 and nephrocystin-6 were found to be expressed in the connecting cilium of the photoreceptor [29,76]. The connecting cilium is responsible for the daily transport of rhodopsin [3]. Impaired rhodopsin transport results in retinitis pigmentosa. Ciliary expression of nephrocystins has also been published in the central nervous system and the cholangiocytes of the liver, which could explain the association with Joubert syndrome and liver fibrosis, respectively [44,45]. Ciliary involvement was also shown for Jeune syndrome by identification of mutations in the component of intraflagellar transportIFT80 [ 104].
Planar cell polarity(PCP)
The term planar cell polarity (PCP) refers to orientation of cells in a plane perpendicular to apico-basal polarity. In epithelial cells this would be the plane parallel to the basement membrane. PCP is achieved by correct orientation of the mitotic spindle and centrosomes[12, 105]. Maintenance of normal tubular development and morphology is dependent on proper planar cell polarity (PCP) [105]. The PCP hypothesis of renal cystic ciliopathies is based on the finding that the mitotic angle in cells with mutated cystoproteins is altered, which results in abnormal cell divison [105] (Fig. 4). The result of abnormal PCP is that the tubulus are not extending longitudinally but at a certain angle to the longitudinal axis resulting in a dilatation of the tubule and thereby in a cystic structure [105]. Involvement of the non-canonical Wnt pathway is important for maintenance of PCP [69]. If the elongation of tubulues is disrupted postnatally by PCP defects, aberrant morphogenesis leads to tubule cyst formation (Fig.4 ). Planar cell polaritydefects due to malorientation of the mitotic spindle were shown in the pck rat model of human ARPKD, the Hnf1β knockout mouse, and the Kif3a knockout mouse – three rodent models for cystic kidney disease [105,106].
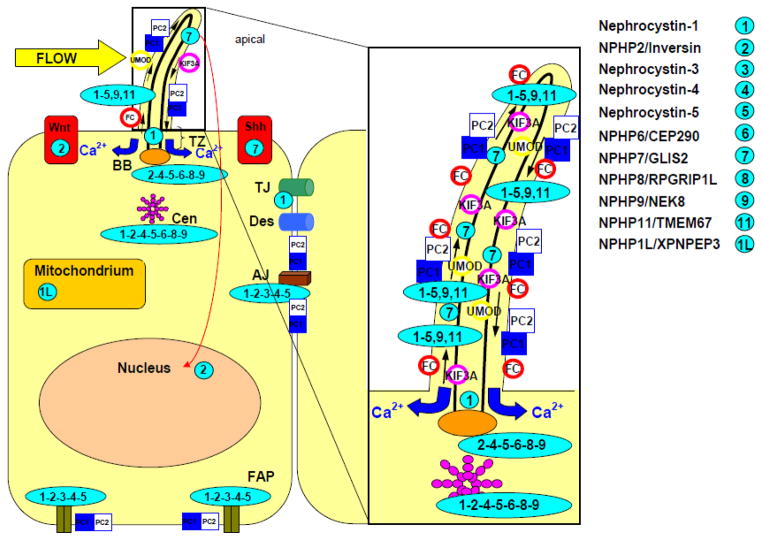
Fig 4. Subcellular localization of the nephrocystins.
Nephrocystins are detected in the primary cilia, basal bodies, the mitotic spindle, focal adhesions and adherens junctions. Most nephrocystins are expressed in the primary cilium (see enlarged box), the basal body (BB) and centrosomes (Cen) in a cell cycle-dependent manner. NPHP1 is expressed in the transition zone (TZ), focal adhesion plaques (FAP), adherens junctions (AJ), and tight junctions (TJ). Arrows in the cilium show the directions of the anterograde and retrograde transport along the microtubule transport. The intraflagellar transport is mediated by kinesin 2, a heterotrimeric protein that is composed of two motor units (Kif3a and Kif3b) and one nonmotor unit (KAP3). Sensory cilia transfer external stimuli. Wnt and hedgehog (Shh) signaling interfere with planar cell polarity by orientation of centrosomes and mitotic spindles. Adapted from Watnick and Germino.66
Modifier genes in NPHP
There is evidence for modifier genes of NPHP [42,55,63,89]. Individuals with a homozygous NPHP1 deletion and an additional heterozygous NPHP6 mutation were identified [54,107]. Modifier genes have also been reported for Joubert syndrome and Meckel-Gruber syndrome. Tory et al . published a combination of mutations in either NPHP1 and AHI1, NPHP6 and AHI1 or NPHP1 and NPHP6 in 28 kindreds with Joubert syndrome [54]. Both publications point out that the additional heterozygous mutation in a second gene may modulate the phenotype of the two recessive mutations in a primary gene in an epistatic way.
Possible approaches totreatment in NPH P
Currently, treatment of NPHP has to focus on the conservative approach of treating end-stage renal disease, providing dialysis and renal transplantation. Even though there is no approved specific treatment available for NPHP at this point there are some promising developments. Possible future treatment might include a vasopressin V2 receptor antagonist, because in the pcy mouse, a model of NPHP type 3, cystogenesis and progression of disease were altered profoundly by treatment with OPC31260 via reduction of cAMP [73]. In addition, there is growing evidence for rapamycin (an mTOR inhibitor) to alleviate cystogenesis [108,109]. Moreover, Roscovitine has shown improvement of cyst growth in jck (the mouse model of NPHP type 9) and cpk mice, which are models for human cystic kidney disease [94].
Outlook
The understanding of NPHP has improved significantly from a solely histopathological entity to the discovery of the NPHP causing genes and molecular mechanisms. Only about 30% of patients with NPH have an identifiable mutation. This means many more NPHP genes are expected to be found. New genes will gives us additional insight about the pathomechanism and how cilia are linked to cyst development. New therapeutic approaches are promising and will hopefully succeed in starting alternative treatment options besides conservative treatment and renal replacement therapy.
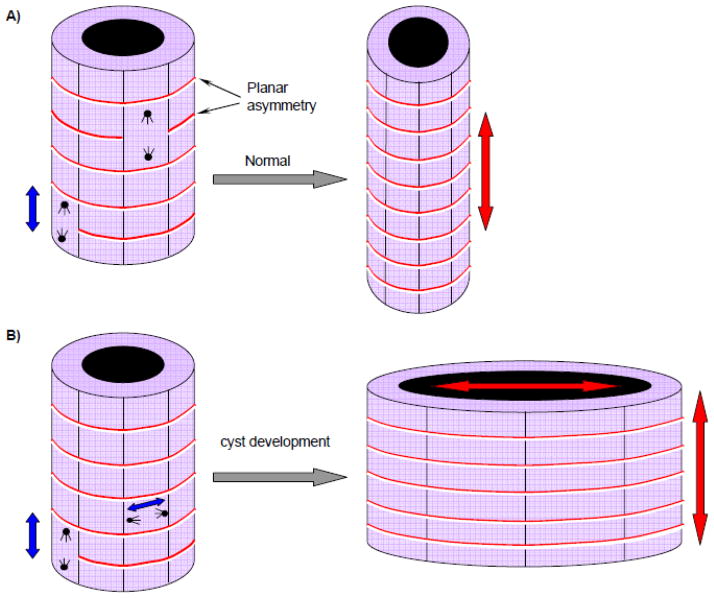
Fig 5. Altered planar cell polarity causes cyst formation.
Correct orientation of the mitotic spindle and centrosomes of renal tubular epithelial cells are especially during development required for proper growth of the longitudinal axis of the tubule (A). If the apical-basolateral polarity is disrupted a dilated tubule or cyst would develop (B). Non-canonical Wnt signaling is involved in proper cell orientation. Urinary flow in the renal tubules could provide signaling via cilia about cellular orientation. Adapted from Germino 2005. 110
Table 2.
Summary of NPHP1-NPHP11 genes, gene products, chromosomal localization, phenotypes, extrarenal symtoms, and mutation frequency of nephrocystins [12,53].
| Gene (protein) | Chromosome | Phenotype (median age at ESRF) | Extrarenal symptoms | Mutation frequency | Interaction partners |
|---|---|---|---|---|---|
| NPHP1 (nephrocystin-1) | 2q13 | NPHP (13yrs) | RP (10%),OMA (2%), JS (rarely) | 23.4% homozygous deletion 2.1% point mutation |
Inversin, nephrocystin- 3, nephrocystin-4, filamin A and B, tensin, β-tubulin, PTK2B |
| NPHP2/INVS (inversin) | 9q31 | Infantile NPHP(<5yrs) | RP (10%), LF, situs inversus, VSD | 1.4% | Nephrocystin-1, calmodulin, catenins, β-tubulin, APC2 |
| NPHP3 (nephrocystin-3) | 3q22 | Infantile and adolescent NPHP | LF, RP (10%), situs inversus, MKS | 0.7% If truncating mutation infantile form |
Nephrocystin-1 |
| NPHP4 (nephrocystin-4) | 1p36 | NPHP (21 yrs) | RP (10%), OMA, LF | 2.6% | Nephrocystin-1, BCAR1, PTK2B |
| NPHP5/IQCB1 (nephrocystin-5) | 3q21 | NPHP (13 years) | Early-onset RP | 3.6% | Calmodulin, RPGR, nephrocystin-6 |
| NPHP6/CEP290 (nephrocystin-6/CEP290) | 12q21 | NPHP | JS, MKS | 1% | ATF4, nephrocystin-5, CC2D2A |
| NPHP7/GLIS2(nephrocystin -7/GLIS2) | 16p | NPHP | _ | 0.1% | _ |
| NPHP8/RPGRIP1L (nephrocystin-8/RPGRIP1L) | 16q | NPHP | JS, MKS | 0.5% | Nephrocystin-1 |
| NPHP9/NEK8 (nephrocystin-9/NEK8) | 17q11 | Infantile NPHP | _ | 0.1% | _ |
| TMEM67/MKS3/NPHP11 (Meckelin/nephrocystin-11) | 8q22.1 | MKS, JS, NPHP+LF | JS, MKS | ||
| NPHP1L/XPNPEP3 (nephrocystin-1L/XPNPEP3) | 22q13 | NPHP | Cardiomyopathy, seizures | 0.1% |
ATF4, activating transcription factor 4;APC2, anap hase-promoting complex 2; BCAR1, breast cancer anti-estrogen resistance 1;CC2D2A, coiled-coil and C2 domain containing 2A;JS, Joubert syndrome; LF, liver fibrosis; MKS, Meckel-Gruber syndrome; OMA,oculomotor apraxia; PTK2B, protein tyrosine kinase 2B; RP, retinitis pigmentosa; RPGR, retinitis pigmentosa GTPase regulator; VSD, ventricular septal defect
Acknowledgments
We thank Dr. Sandy Cope-Yokoyama (Department of Pathology, Children's Medical Center Dallas and UT Southwestern Medical Center, Dallas) for her contribution in regard of the images of renal pathology in nephronophthisis and Dr. Michael Craig Morris (Department of Radiology, Children's Medical Center Dallas and UT Southwestern Medical Center, Dallas) for his contribution of the Joubert syndrome image. F. H. is an investigator of the Howard Hughes Medical Institute, the Frederick G. L. Huetwell professor, and a Doris Duke Distinguished Clinical Scientist. He is supported by grants from the NIH (DK068306, DK064614 and DK069274). M.T. W. is a fellow of the Pediatric Scientist Development Program (PSDP) and was supported by grants from the Koeln Fortune Program Faculty of Medicine, University of Cologne (184/2004), the German Kidney Fund (Deutsche Nierenstiftung), the German Research Foundation (DFG WO 1229/2-1)and a T32 training grant .
References
- 1.Smith C, Grham J. Congenital medullary cysts of the kidneys with severe refractory anemia. Am J Dis Child. 1945;69:369–377. [Google Scholar]
- 2.Fanconi G, Hanhart E, von Albertini A, Uhlinger E, Dolivo G, Prader A. Familial, juvenile nephronophthisis (idiopathic parenchymal contracted kidney) Helv Paediatr Acta. 1951;6:1–49. [PubMed] [Google Scholar]
- 3.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005;6:928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrandt F, Strahm B, Nothwang H-G, Gretz N, Schnieders B, Singh-Sawhney I, Kutt R, Vollmer M Brandis Mmembers of the APN study group . Molecular genetic identification of families with juvenile nephronophthisis type 1: rate of progression to renal failure. Kindey Int. 1997;51:261–269. doi: 10.1038/ki.1997.31. [DOI] [PubMed] [Google Scholar]
- 5.Ala-Mello S, Sankila EM, Koskimies O, de la Chapelle A, Kääriäinen H. Molecular studies in Finnish patients with familial juvenile nephronophthisis exclude a founder effect and support a common mutation causing mechanism. J Med Genet. 1998;35:279–283. doi: 10.1136/jmg.35.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omran H, Fernandez C, Jung M, Häffner K, Fargier B, Villaquiran A, Waldherr R, Gretz N, Brandis M, Rüschendorf F, Reis A, Hildebrandt F. Identification of a new gene locus for adolescent nephronophthisis, on chromosome 3q22 in a large Venezuelan pedigree. Am J Hum Genet. 2000;66:118–127. doi: 10.1086/302705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blowey DL, Querfeld U, Geary D, Warady BA, Alon U. Ultrasound findings in juvenile nephronophthisis. Pediatr Nephrol. 1996;10:22–24. doi: 10.1007/BF00863431. [DOI] [PubMed] [Google Scholar]
- 8.Waldherr R, Lennert T, Weber HP, Födisch HJ, Schärer K. The nephronophthisis complex. A clinicopathologic study in children. Virchows Arch A Pathol Anat Histol. 1982;394:235–254. doi: 10.1007/BF00430668. [DOI] [PubMed] [Google Scholar]
- 9.Zollinger HU, Mihatsch MJ, Edefonti A, Gaboardi F, Imbasciati E, Lennert T. Nephronophthisis (medullary cystic disease of the kidney). A study using electron microscopy, immunofluorescence, and a review of the morphological findings. Helv Paediatr Acta. 1980;35:509–530. [PubMed] [Google Scholar]
- 10.Haider NB, Carmi R, Shalev H, Sheffield VC, Landau D. A Bedouin kindred with infantile nephronophthisis demonstrates linkage to chromosome 9 by homozygosity mapping. Am J Hum Genet. 1998;63:1404–1410. doi: 10.1086/302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnadoux MF, Bacri JL, Broyer M, Habib R. Infantile chronic tubulo-interstitial nephritis with cortical microcysts: variant of nephronophthisis or new disease entity? Pediatr Nephrol. 1989;3:50–55. doi: 10.1007/BF00859626. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, Aligianis IA, Ward CJ, Pasha S, Punyashthiti R, Malik Sharif S, Batman PA, Bennett CP, Woods CG, McKeown C, Bucourt M, Miller CA, Cox P, Algazali L, Trembath RC, Torres VE, Attie-Bitach T, Kelly DA, Maher ER, Gattone VH, 2nd, Harris PC, Johnson CA. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- 14.Ala-Mello S, Koskimies O, Rapola J, Kääriäinen H. Nephronophthisis in Finland: epidemiology and comparison of genetically classified subgroups. Eur J Hum Genet. 1999;7:205–211. doi: 10.1038/sj.ejhg.5200268. [DOI] [PubMed] [Google Scholar]
- 15.Bollée G, Fakhouri F, Karras A, Noël LH, Salomon R, Servais A, Lesavre P, Morinière V, Antignac C, Hummel A. Nephronophthisis related to homozygous NPHP1 gene deletion as a cause of chronic renal failure in adults. Nephrol Dial Transplant. 2006;21:2660–2663. doi: 10.1093/ndt/gfl348. [DOI] [PubMed] [Google Scholar]
- 16.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, Saunier S, Salomon R, Gonzales M, Rattenberry E, Esculpavit C, Toutain A, Moraine C, Parent P, Marcorelles P, Dauge MC, Roume J, Le Merrer M, Meiner V, Meir K, Menez F, Beaufrère AM, Francannet C, Tantau J, Sinico M, Dumez Y, MacDonald F, Munnich A, Lyonnet S, Gubler MC, Génin E, Johnson CA, Vekemans M, Encha-Razavi F, Attié-Bitach T. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–179. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betz R, Rensing C, Otto E, Mincheva A, Zehnder D, Lichter P, Hildebrandt F. Children with ocular motor apraxia type Cogan carry deletions in the gene (NPHP1) for juvenile nephronophthisis. J Pediatr. 2000;136:828–831. [PubMed] [Google Scholar]
- 19.Harris CM, Hodgkins PR, Kriss A, Chong WK, Thompson DA, Mezey LE, Shawkat FS, Taylor DS, Wilson J. Familial congenital saccade initiation failure and isolated cerebellar vermis hypoplasia. Dev Med Child Neurol. 1998;40:775–779. doi: 10.1111/j.1469-8749.1998.tb12347.x. [DOI] [PubMed] [Google Scholar]
- 20.Senior B, Friedmann AI, Braudo JL. Juvenile familial nephropathy with tapetoretinal degeneration: a new oculorenal dystrophy. Am J Ophthalmol. 1961;52:625–633. doi: 10.1016/0002-9394(61)90147-7. [DOI] [PubMed] [Google Scholar]
- 21.Loken AC, Hanssen O, Halvorsen S, Jolster NJ. Hereditary renal dysplasia and blindness. Acta Paediatr. 1961;50:177–184. doi: 10.1111/j.1651-2227.1961.tb08037.x. [DOI] [PubMed] [Google Scholar]
- 22.Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. doi: 10.1080/13816810701537424. [DOI] [PubMed] [Google Scholar]
- 23.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, Sayer JA, Otto EA, Attanasio M, O'Toole JF, Jin G, Shou C, Hildebrandt F, Williams DS, Heckenlively JR, Swaroop A. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang ST, Chiou YY, Wang E, Chien YL, Ho HH, Tsai FJ, Lin CY, Tsai SP, Li H. Essential role of nephrocystin in photoreceptor intraflagellar transport in mouse. Hum Mol Genet. 2009;18:1566–1577. doi: 10.1093/hmg/ddp068. [DOI] [PubMed] [Google Scholar]
- 25.Louie CM, Caridi G, Lopes VS, Brancati F, Kispert A, Lancaster MA, Schlossman AM, Otto EA, Leitges M, Gröne HJ, Lopez I, Gudiseva HV, O'Toole JF, Vallespin E, Ayyagari R, Ayuso C, Cremers FP, den Hollander AI, Koenekoop RK, Dallapiccola B, Ghiggeri GM, Hildebrandt F, Valente EM, Williams DS, Gleeson JG. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet. 2010;42:175–180. doi: 10.1038/ng.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, Maubaret C, Diaz-Font A, Macdonald I, Muzny DM, Wheeler DA, Morgan M, Lewis LR, Logan CV, Tan PL, Beer MA, Inglehearn CF, Lewis RA, Jacobson SG, Bergmann C, Beales PL, Attié-Bitach T, Johnson CA, Otto EA, Bhattacharya SS, Hildebrandt F, Gibbs RA, Koenekoop RK, Swaroop A, Katsanis N. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–745. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parisi MA, Doherty D, Chance PF, Glass IA. Joubert syndrome (and related disorders) (OMIM 213300) Eur J Hum Genet. 2007;15:511–521. doi: 10.1038/sj.ejhg.5201648. [DOI] [PubMed] [Google Scholar]
- 28.Valente EM, Brancati F, Silhavy JL, Castori M, Marsh SE, Barrano G, Bertini E, Boltshauser E, Zaki MS, Abdel-Aleem A, Abdel-Salam GM, Bellacchio E, Battini R, Cruse RP, Dobyns WB, Krishnamoorthy KS, Lagier-Tourenne C, Magee A, Pascual-Castroviejo I, Salpietro CD, Sarco D, Dallapiccola B, Gleeson JG International JSRD Study Group . AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders. Ann Neurol. 2006;59:527–534. doi: 10.1002/ana.20749. [DOI] [PubMed] [Google Scholar]
- 29.Sayer JA, Otto EA, O'Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38(6):674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 30.Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, Gerhardt C, Rattenberry E, Wolf MT, Gubler MC, Martinovic J, Encha-Razavi F, Boddaert N, Gonzales M, Macher MA, Nivet H, Champion G, Berthélémé JP, Niaudet P, McDonald F, Hildebrandt F, Johnson CA, Vekemans M, Antignac C, Rüther U, Schneider-Maunoury S, Attié-Bitach T, Saunier S. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39(7):875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 31.Wolf MT, Saunier S, O'Toole JF, Wanner N, Groshong T, Attanasio M, Salomon R, Stallmach T, Sayer JA, Waldherr R, Griebel M, Oh J, Neuhaus TJ, Josefiak U, Antignac C, Otto EA, Hildebrandt F. Mutational analysis of the RPGRIP1L gene in patients with Joubert syndrome and nephronophthisis. Kidney Int. 2007;72:1520–1526. doi: 10.1038/sj.ki.5002630. [DOI] [PubMed] [Google Scholar]
- 32.Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M, Gascon G, Bodell A, Shugart YY, Ruvolo M, Walsh CA. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–1013. doi: 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- 33.Parisi MA, Doherty D, Eckert ML, Shaw DW, Ozyurek H, Aysun S, Giray O, Al Swaid A, Al Shahwan S, Dohayan N, Bakhsh E, Indridason OS, Dobyns WB, Bennett CL, Chance PF, Glass IA. AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome. J Med Genet. 2006;43:334–339. doi: 10.1136/jmg.2005.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brancati F, Iannicelli M, Travaglini L, Mazzotta A, Bertini E, Boltshauser E, D'Arrigo S, Emma F, Fazzi E, Gallizzi R, Gentile M, Loncarevic D, Mejaski-Bosnjak V, Pantaleoni C, Rigoli L, Salpietro CD, Signorini S, Stringini GR, Verloes A, Zabloka D, Dallapiccola B, Gleeson JG, Valente EM International JSRD Study Group . MKS3/TMEM67 mutations are a major cause of COACH Syndrome, a Joubert Syndrome related disorder with liver involvement. Hum Mutat. 2009;30:E432–442. doi: 10.1002/humu.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorden NT, Arts HH, Parisi MA, Coene KL, Letteboer SJ, van Beersum SE, Mans DA, Hikida A, Eckert M, Knutzen D, Alswaid AF, Ozyurek H, Dibooglu S, Otto EA, Liu Y, Davis EE, Hutter CM, Bammler TK, Farin FM, Dorschner M, Topçu M, Zackai EH, Rosenthal P, Owens KN, Katsanis N, Vincent JB, Hildebrandt F, Rubel EW, Raible DW, Knoers NV, Chance PF, Roepman R, Moens CB, Glass IA, Doherty D. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet. 2008;83:559–571. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attié-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, Otto EA, Hildebrandt F, Glass IA, Logan CV, Johnson CA, Bennett C, Brancati F, Valente EM, Woods CG, Gleeson JG. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, Fazzi E, Travaglini L, Field SJ, Gayral S, Jacoby M, Schurmans S, Dallapiccola B, Majerus PW, Valente EM, Gleeson JG International Joubert Syndrome Related Disorders Study Group. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edvardson S, Shaag A, Zenvirt S, Erlich Y, Hannon GJ, Shanske AL, Gomori JM, Ekstein J, Elpeleg O. Joubert syndrome 2 (JBTS2) in Ashkenazi Jews is associated with a TMEM216 mutation. Am J Hum Genet. 2010;86:93–97. doi: 10.1016/j.ajhg.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, Shaw DW, McDonald R, Eddy A, Chance PF, Glass IA. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet. 2004;75:82–91. doi: 10.1086/421846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mollet G, Salomon R, Gribouval O, Silbermann F, Bacq D, Landthaler G, Milford D, Nayir A, Rizzoni G, Antignac C, Saunier S. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet. 2002;32:300–305. doi: 10.1038/ng996. [DOI] [PubMed] [Google Scholar]
- 41.Kyttälä M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, Paavola-Sakki P, Peltonen L, Kestilä M. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38:155–157. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- 42.Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, Aligianis IA, Ward CJ, Pasha S, Punyashthiti R, Malik Sharif S, Batman PA, Bennett CP, Woods CG, McKeown C, Bucourt M, Miller CA, Cox P, Algazali L, Trembath RC, Torres VE, Attie-Bitach T, Kelly DA, Maher ER, Gattone VH, 2nd, Harris PC, Johnson CA. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- 43.Bergmann C, Fliegauf M, Brüchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kränzlin B, Nürnberg G, Becker C, Grimm T, Girschick G, Lynch SA, Kelehan P, Senderek J, Neuhaus TJ, Stallmach T, Zentgraf H, Nürnberg P, Gretz N, Lo C, Lienkamp S, Schäfer T, Walz G, Benzing T, Zerres K, Omran H. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, Sudbrak R, Antignac C, Gretz N, Walz G, Schermer B, Benzing T, Hildebrandt F, Omran H. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet. 2003;34:455–459. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- 45.Otto EA, Tory K, Attanasio M, Zhou W, Chaki M, Paruchuri Y, Wise EL, Utsch B, Wolf MT, Becker C, Nürnberg G, Nürnberg P, Nayir A, Saunier S, Antignac C, Hildebrandt F. Hypomorphic Mutations in Meckelin (MKS3/TMEM67) cause Nephronophthisis with Liver Fibrosis (NPHP11) J Med Genet online. 2009 doi: 10.1136/jmg.2009.066613. [DOI] [PubMed] [Google Scholar]
- 46.Ellis DS, Heckenlively JR, Martin CL, Lachman RS, Sakati NA, Rimoin DL. Leber's congenital amaurosis associated with familial juvenile nephronophthisis and cone-shaped epiphyses of the hands (the Saldino-Mainzer syndrome) Am J Ophthalmol. 1984;97:233–239. doi: 10.1016/s0002-9394(14)76095-7. [DOI] [PubMed] [Google Scholar]
- 47.Mainzer F, Saldino RM, Ozonoff MB, Minagi H. Familial nephropathy associated with retinitis pigmentosa, cerebellar ataxia and skeletal abnormalities. Am J Med. 1970;49:556–562. doi: 10.1016/s0002-9343(70)80051-1. [DOI] [PubMed] [Google Scholar]
- 48.Donaldson MD, Warner AA, Trompeter RS, Haycock GB, Chantler C. Familial juvenile nephronophthisis, Jeune's syndrome, and associated disorders. Arch Dis Child. 1985;60:426–434. doi: 10.1136/adc.60.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moudgil A, Bagga A, Kamil ES, Rimoin DL, Lachman RS, Cohen AH, Jordan SC. Nephronophthisis associated with Ellis-van Creveld syndrome. Pediatr Nephrol. 1998;12:20–22. doi: 10.1007/s004670050395. [DOI] [PubMed] [Google Scholar]
- 50.Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J, Hanusch H, Brandis M. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet. 1997;17:149–153. doi: 10.1038/ng1097-149. [DOI] [PubMed] [Google Scholar]
- 52.Saunier S, Calado J, Heilig R, Silbermann F, Benessy F, Morin G, Konrad M, Broyer M, Gubler MC, Weissenbach J, Antignac C. A novel gene that encodes a protein with a putative src homology 3 domain is a candidate gene for familial juvenile nephronophthisis. Hum Mol Genet. 1997;6:2317–2323. doi: 10.1093/hmg/6.13.2317. [DOI] [PubMed] [Google Scholar]
- 53.Otto EA, Helou J, Allen SJ, O'Toole JF, Wise EL, Ashraf S, Attanasio M, Zhou W, Wolf MT, Hildebrandt F. Mutation analysis in nephronophthisis using a combined approach of homozygosity mapping, CEL I endonuclease cleavage, and direct sequencing. Hum Mutat. 2008;29:418–426. doi: 10.1002/humu.20669. [DOI] [PubMed] [Google Scholar]
- 54.Tory K, Lacoste T, Burglen L, Morinière V, Boddaert N, Macher MA, Llanas B, Nivet H, Bensman A, Niaudet P, Antignac C, Salomon R, Saunier S. High NPHP1 and NPHP6 mutation rate in patients with Joubert syndrome and nephronophthisis: potential epistatic effect of NPHP6 and AHI1 mutations in patients with NPHP1 mutations. J Am Soc Nephrol. 2007;18:1566–1575. doi: 10.1681/ASN.2006101164. [DOI] [PubMed] [Google Scholar]
- 55.Eley L, Moochhala SH, Simms R, Hildebrandt F, Sayer JA. Nephrocystin-1 interacts directly with Ack1 and is expressed in human collecting duct. Biochem Biophys Res Commun. 2008;371:877–882. doi: 10.1016/j.bbrc.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Benzing T, Gerke P, Höpker K, Hildebrandt F, Kim E, Walz G. Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2. Proc Natl Acad Sci U S A. 2001;98:9784–9789. doi: 10.1073/pnas.171269898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donaldson JC, Dempsey PJ, Reddy S, Bouton AH, Coffey RJ, Hanks SK. Crk-associated substrate p130(Cas) interacts with nephrocystin and both proteins localize to cell-cell contacts of polarized epithelial cells. Exp Cell Res. 2000;256:168–178. doi: 10.1006/excr.2000.4822. [DOI] [PubMed] [Google Scholar]
- 58.Donaldson JC, Dise RS, Ritchie MD, Hanks SK. Nephrocystin-conserved domains involved in targeting to epithelial cell-cell junctions, interaction with filamins, and establishing cell polarity. J Biol Chem. 2002;277:29028–29035. doi: 10.1074/jbc.M111697200. [DOI] [PubMed] [Google Scholar]
- 59.Mollet G, Salomon R, Gribouval O, Silbermann F, Bacq D, Landthaler G, Milford D, Nayir A, Rizzoni G, Antignac C, Saunier S. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet. 2002;32:300–305. doi: 10.1038/ng996. [DOI] [PubMed] [Google Scholar]
- 60.Eley L, Gabrielides C, Adams M, Johnson CA, Hildebrandt F, Sayer JA. Jouberin localizes to collecting ducts and interacts with nephrocystin-1. Kidney Int. 2008;74:1139–1149. doi: 10.1038/ki.2008.377. [DOI] [PubMed] [Google Scholar]
- 61.Fliegauf M, Horvath J, von Schnakenburg C, Olbrich H, Müller D, Thumfart J, Schermer B, Pazour GJ, Neumann HP, Zentgraf H, Benzing T, Omran H. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J Am Soc Nephrol. 2006;17:2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- 62.Schermer B, Höpker K, Omran H, Ghenoiu C, Fliegauf M, Fekete A, Horvath J, Köttgen M, Hackl M, Zschiedrich S, Huber TB, Kramer-Zucker A, Zentgraf H, Blaukat A, Walz G, Benzing T. Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia. EMBO J. 2005;24:4415–4424. doi: 10.1038/sj.emboj.7600885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mollet G, Silbermann F, Delous M, Salomon R, Antignac C, Saunier S. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet. 2005;14:645–656. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- 64.Gagnadoux MF, Bacri JL, Broyer M, Habib R. Infantile chronic tubulo-interstitial nephritis with cortical microcysts: variant of nephronophthisis or new disease entity? Pediatr Nephrol. 1989;3:50–55. doi: 10.1007/BF00859626. [DOI] [PubMed] [Google Scholar]
- 65.O'Toole JF, Otto EA, Frishberg Y, Hildebrandt F. Retinitis pigmentosa and renal failure in a patient with mutations in INVS. Nephrol Dial Transplant. 2006;21:1989–1991. doi: 10.1093/ndt/gfl088. [DOI] [PubMed] [Google Scholar]
- 66.Watnick T, Germino G. From cilia to cyst. Nat Genet. 2003;34:355–356. doi: 10.1038/ng0803-355. [DOI] [PubMed] [Google Scholar]
- 67.Shiba D, Manning DK, Koga H, Beier DR, Yokoyama T. Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton. 2010;67:112–119. doi: 10.1002/cm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgan D, Eley L, Sayer J, Strachan T, Yates LM, Craighead AS, Goodship JA. Expression analyses and interaction with the anaphase promoting complex protein Apc2 suggest a role for inversin in primary cilia and involvement in the cell cycle. Hum Mol Genet. 2002;11:3345–3350. doi: 10.1093/hmg/11.26.3345. [DOI] [PubMed] [Google Scholar]
- 69.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Krönig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tory K, Rousset-Rouvière C, Gubler MC, Morinière V, Pawtowski A, Becker C, Guyot C, Gié S, Frishberg Y, Nivet H, Deschênes G, Cochat P, Gagnadoux MF, Saunier S, Antignac C, Salomon R. Mutations of NPHP2 and NPHP3 in infantile nephronophthisis. Kidney Int. 2009;75:839–847. doi: 10.1038/ki.2008.662. [DOI] [PubMed] [Google Scholar]
- 71.Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol. 2008;19:587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tory K, Rousset-Rouvière C, Gubler MC, Morinière V, Pawtowski A, Becker C, Guyot C, Gié S, Frishberg Y, Nivet H, Deschênes G, Cochat P, Gagnadoux MF, Saunier S, Antignac C, Salomon R. Mutations of NPHP2 and NPHP3 in infantile nephronophthisis. Kidney Int. 2009;75:839–847. doi: 10.1038/ki.2008.662. [DOI] [PubMed] [Google Scholar]
- 73.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 74.Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MT, Schuermann MJ, Becker A, Birkenhäger R, Sudbrak R, Hennies HC, Nürnberg P, Hildebrandt F. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet. 2002;71:1161–1167. doi: 10.1086/344395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delous M, Hellman NE, Gaudé HM, Silbermann F, Le Bivic A, Salomon R, Antignac C, Saunier S. Nephrocystin-1 and nephrocystin-4 are required for epithelial morphogenesis and associate with PALS1/PATJ and Par6. Hum Mol Genet. 2009;18:4711–4723. doi: 10.1093/hmg/ddp434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O'Toole JF, Helou J, Attanasio M, Utsch B, Sayer JA, Lillo C, Jimeno D, Coucke P, De Paepe A, Reinhardt R, Klages S, Tsuda M, Kawakami I, Kusakabe T, Omran H, Imm A, Tippens M, Raymond PA, Hill J, Beales P, He S, Kispert A, Margolis B, Williams DS, Swaroop A, Hildebrandt F. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 77.Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schäfer T, Pütz M, Lienkamp S, Ganner A, Bergbreiter A, Ramachandran H, Gieloff V, Gerner M, Mattonet C, Czarnecki PG, Sayer JA, Otto EA, Hildebrandt F, Kramer-Zucker A, Walz G. Genetic and physical interaction between the NPHP5 and NPHP6 gene products. Hum MolGenet. 2008;17:3655–3662. doi: 10.1093/hmg/ddn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, Fazzi E, Signorini S, Louie CM, Bellacchio E, Bertini E, Dallapiccola B, Gleeson JG. Mutations in CEP290, which encodesa centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38:623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- 80.Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization ofthe human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 81.Frank V, den Hollander AI, Brüchle NO, Zonneveld MN, Nürnberg G, Becker C, Du Bois G, Kendziorra H, Roosing S, Senderek J, Nürnberg P, Cremers FP, Zerres K, Bergmann C. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel-Gruber syndrome. Hum Mutat. 2008;29:45–52. doi: 10.1002/humu.20614. [DOI] [PubMed] [Google Scholar]
- 82.Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, Saunier S, Salomon R, Gonzales M, Rattenberry E, Esculpavit C, Toutain A, Moraine C, Parent P, Marcorelles P, Dauge MC, Roume J, Le Merrer M, Meiner V, Meir K, Menez F, Beaufrère AM, Francannet C, Tantau J, Sinico M, Dumez Y, MacDonald F, Munnich A, Lyonnet S, Gubler MC, Génin E, Johnson CA, Vekemans M, Encha-Razavi F, Attié-Bitach T. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–179. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Helou J, Otto EA, Attanasio M, Allen SJ, Parisi MA, Glass I, Utsch B, Hashmi S, Fazzi E, Omran H, O'Toole JF, Sayer JA, Hildebrandt F. Mutation analysis of NPHP6/CEP290 in patients with Joubert syndrome and Senior-Løken syndrome. J Med Genet. 2007;44:657–663. doi: 10.1136/jmg.2007.052027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Lewis RA, Eyaid W, Banin E, Dollfus H, Beales PL, Badano JL, Katsanis N International Joubert Syndrome Related Disorders Study Group. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40:443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 85.den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KE, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, Hoyng CB, van den Born LI, Rohrschneider K, Cremers FP. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, Sayer JA, Otto EA, Attanasio M, O'Toole JF, Jin G, Shou C, Hildebrandt F, Williams DS, Heckenlively JR, Swaroop A. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Attanasio M, Uhlenhaut NH, Sousa VH, O'Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA, Seelow D, Nürnberg G, Becker C, Chudley AE, Nürnberg P, Hildebrandt F, Treier M. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet. 2007;39:1018–1024. doi: 10.1038/ng2072. [DOI] [PubMed] [Google Scholar]
- 88.Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Märker T, Voesenek K, Kartono A, Ozyurek H, Farin FM, Kroes HY, Wolfrum U, Brunner HG, Cremers FP, Glass IA, Knoers NV, Roepman R. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- 89.Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, Maubaret C, Diaz-Font A, Macdonald I, Muzny DM, Wheeler DA, Morgan M, Lewis LR, Logan CV, Tan PL, Beer MA, Inglehearn CF, Lewis RA, Jacobson SG, Bergmann C, Beales PL, Attié-Bitach T, Johnson CA, Otto EA, Bhattacharya SS, Hildebrandt F, Gibbs RA, Koenekoop RK, Swaroop A, Katsanis N. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–745. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- 91.Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol. 2008;19:469–476. doi: 10.1681/ASN.2006090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Natoli TA, Gareski TC, Dackowski WR, Smith L, Bukanov NO, Russo RJ, Husson H, Matthews D, Piepenhagen P, Ibraghimov-Beskrovnaya O. Pkd1 and Nek8 mutations affect cell-cell adhesion and cilia in cysts formed in kidney organ cultures. Am J Physiol Renal Physiol. 2008;294:F73–83. doi: 10.1152/ajprenal.00362.2007. [DOI] [PubMed] [Google Scholar]
- 93.Mykytyn K, Sheffield VC. Establishing a connection between cilia and Bardet-Biedl Syndrome. Trends Mol Med. 2004;10:106–109. doi: 10.1016/j.molmed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 95.Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, Badano JL, Blair-Reid S, Sriram N, Katsanis N, Attie-Bitach T, Afford SC, Copp AJ, Kelly DA, Gull K, Johnson CA. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet. 2007;16:173–186. doi: 10.1093/hmg/ddl459. [DOI] [PubMed] [Google Scholar]
- 96.Doherty D, Parisi MA, Finn LS, Gunay-Aygun M, Al-Mateen M, Bates D, Clericuzio C, Demir H, Dorschner M, van Essen AJ, Gahl WA, Gentile M, Gorden NT, Hikida A, Knutzen D, Ozyurek H, Phelps I, Rosenthal P, Verloes A, Weigand H, Chance PF, Dobyns WB, Glass IA. Mutations in 3 genes (MKS3, CC2D2A and RPGRIP1L) cause COACH syndrome (Joubert syndrome with congenital hepatic fibrosis) J Med Genet. 2009;47:8–21. doi: 10.1136/jmg.2009.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Toole JF, Liu Y, Davis EE, Westlake CJ, Attanasio M, Otto EA, Seelow D, Nurnberg G, Becker C, Nuutinen M, Kärppä M, Ignatius J, Uusimaa J, Pakanen S, Jaakkola E, van den Heuvel LP, Fehrenbach H, Wiggins R, Goyal M, Zhou W, Wolf MT, Wise E, Helou J, Allen SJ, Murga-Zamalloa CA, Ashraf S, Chaki M, Heeringa S, Chernin G, Hoskins BE, Chaib H, Gleeson J, Kusakabe T, Suzuki T, Isaac RE, Quarmby LM, Tennant B, Fujioka H, Tuominen H, Hassinen I, Lohi H, van Houten JL, Rotig A, Sayer JA, Rolinski B, Freisinger P, Madhavan SM, Herzer M, Madignier F, Prokisch H, Nurnberg P, Jackson P, Khanna H, Katsanis N, Hildebrandt F. Individuals with mutations in XPNPEP3, which encodes a mitochondrial protein, develop a nephronophthisis-like nephropathy. J Clin Invest. 2010;120:791–802. doi: 10.1172/JCI40076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zaucke F, Boehnlein JM, Steffens S, Polishchuk RS, Rampoldi L, Fischer A, Pasch A, Boehm CW, Baasner A, Attanasio M, Hoppe B, Hopfer H, Beck BB, Sayer JA, Hildebrandt F, Wolf MT. Uromodulin is expressed in renal primary cilia and UMOD mutations result in decreased ciliary uromodulin expression. Hum Mol Genet. 2010;19:1985–1997. doi: 10.1093/hmg/ddq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolf MT, Lee J, Panther F, Otto EA, Guan KL, Hildebrandt F. Expression and phenotype analysis of the nephrocystin-1 and nephrocystin-4 homologs in Caenorhabditis elegans. J Am Soc Nephrol. 2005;16:676–687. doi: 10.1681/ASN.2003121025. [DOI] [PubMed] [Google Scholar]
- 100.Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6:194–205. doi: 10.1038/nrg1557. [DOI] [PubMed] [Google Scholar]
- 101.Jauregui AR, Nguyen KC, Hall DH, Barr MM. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J Cell Biol. 2008;180:973–988. doi: 10.1083/jcb.200707090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winkelbauer ME, Schafer JC, Haycraft CJ, Swoboda P, Yoder BK. The C. elegans homologs of nephrocystin-1 and nephrocystin-4 are cilia transition zone proteins involved in chemosensory perception. J Cell Sci. 2005;118:5575–5587. doi: 10.1242/jcs.02665. [DOI] [PubMed] [Google Scholar]
- 103.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 104.Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, Johnson C, Irving M, Elcioglu N, Winey M, Tada M, Scambler PJ. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 105.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38 :21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 106.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoefele J, Wolf MT, O'Toole JF, Otto EA, Schultheiss U, Dêschenes G, Attanasio M, Utsch B, Antignac C, Hildebrandt F. Evidence of oligogenic inheritance in nephronophthisis. J Am Soc Nephrol. 2007;18:2789–2795. doi: 10.1681/ASN.2007020243. [DOI] [PubMed] [Google Scholar]
- 108.Tobin JL, Beales PL. Restoration of renal function in zebrafish models of ciliopathies. Pediatr Nephrol. 2008;23:2095–2099. doi: 10.1007/s00467-008-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Germino GG. Linking cilia to Wnts. Nat Genet. 2005;37:455–457. doi: 10.1038/ng0505-455. [DOI] [PubMed] [Google Scholar]