Abstract
Ocular swabs collected in Tanzania were evaluated by Amplicor CT and Aptima Combo2 assays for the detection of Chlamydia trachomatis (CT) to determine if pooling could be used to reduce the cost of detection. Pooling would be an accurate method and so far resulted in a cost-savings of 62.2%.
Trachoma, caused by ocular infections of Chlamydia trachomatis (CT), is endemic in 55 countries resulting in approximately 3.8 million cases of blindness and 5.3 million cases of impaired vision throughout Africa and Southeast Asia (WHO, 2007; Goodhew et al., 2012). No defined gold standard test for detecting ocular CT exists however, PCR, considered to be sensitive and specific can be costly at $10 to $15/test (Chidambaram et al., 2006; See et al., 2011; Goodhew et al., 2012). Pooling specimens, when testing for genital CT infections is beneficial in reducing costs and increasing efficiency of testing in low-prevalence populations (Lewis et al., 2012). Previously is has been shown that pooling cervical specimens into pools of 5 or 10 there was 100% sensitivity when compared to individual testing and that cost savings decreased as the specimens per pool increased (Shipitsyna et al., 2007). Ocular specimens collected throughout villages in Tanzania were sent to the Johns Hopkins University (JHU) Research Laboratory (Baltimore, MD) for the detection of CT by the Amplicor CT PCR assay (Amplicor) (Roche Diagnostics; Indianapolis, IN). With approximately 15,000 specimens shipped annually from low prevalence populations, pooling could be an ideal method to decrease cost and time; specifically ocular swab specimens received from villages with an expected prevalence of infection of <10%. Expected prevalence was determined based on trachoma prevalence during the visit of sample collection and/or infection prevalence during prior visits. Pooling on Amplicor was initially analyzed; however, due to the future unavailability of this assay, pooling was also analyzed on the Hologic-GenProbe PANTHER system using the Aptima Combo 2 (AC2) (Hologic Gen-Probe Inc; San Diego, CA) assay to determine if it would be a suitable alternative when testing ocular swabs from Tanzania for CT. An in-house verification of ocular swab specimens was performed to determine if the AC2 assay performed as well as or better than Amplicor in order to establish AC2 as a suitable method for detecting CT in ocular swabs.
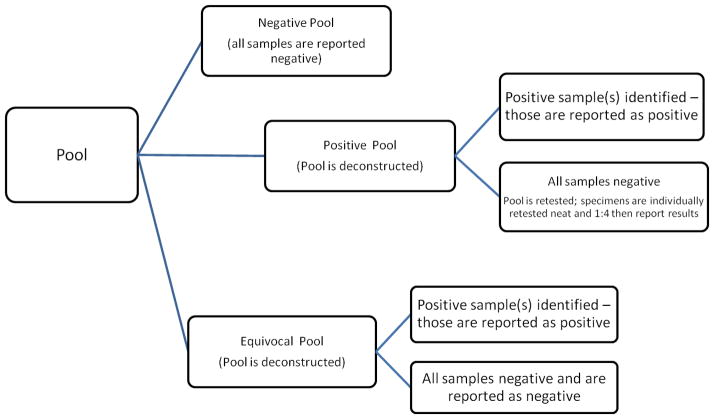
Specimen collection was performed as previously described (Stare et al., 2011). Swabs were shipped frozen in a dry state to JHU. Upon arrival specimens were stored at −80°C until testing. Swabs were rehydrated with 1mL of sterile molecular grade diethylpyrocarbonate (DEPC) water (Quality Biological, Inc. Gaithersburg, MD). Pooling analysis for Amplicor was previously completed on 116 ocular swab specimens that were also tested by Amplicor individually. 29 pools of 4 specimens each were constructed, chronologically from the shipping manifest, using 50uL of each sample for a total pool volume of 200uL. DNA extraction and detection of CT was performed using the Roche MagNA Pure LC extraction robot and the Amplicor PCR assay as previously described (Dalesio et al., 2004; Dize et al., 2013). Positive pools/specimens were defined as those having an optical density (OD), read at A450 nm, of ≥ 0.8, while negatives had an OD of < 0.2. An OD of ≥ 0.2 and < 0.8 was considered equivocal and retested in duplicate. If neither duplicate was positive the specimen was considered negative by Amplicor. Fifty pool sets previously tested using Amplicor were reconstructed and tested by AC2. For reconstruction, 50uL of four samples each, for a total volume of 200uL, was added into a GenProbe UniSex collection tube. Testing and result determination were performed according to manufactures instructions. All samples within a negative pool were considered negative, specimens within positive or equivocal pools were tested individually by the same test used for the pool and results were reported based upon the individual result. (Figure 1)
Figure 1.
Pooling Algorithm for Reporting Results for Roche Amplicor CT PCR Amplicor and GenProbe Aptima Combo 2 (AC2) assays.
Of 29 pools initially evaluated using Amplicor, 3 pools were positive for CT; 2 pools contained one positive specimen each while one pool contained 2 positive specimens. Results were consistent when the samples were previously tested individually. Of the 50 pools analyzed by Amplicor and AC2, Amplicor detected 24 positive, 25 negative and 1 equivocal pools. AC2 found 24 positive and 26 negative pools. The discrepant pool (Amplicor equivocal/AC2 negative) was deconstructed; one sample tested positive (OD= 1.551) on Amplicor; however, all tested negative by AC2. When compared to Amplicor (our previous reference standard), AC2 had a sensitivity of 96%, a specificity of 100%, a NPV of 96.2% and a PPV of 100%. (Table 1)
Table 1.
Pooling Results on the GenProbe Aptima Combo2 (AC2) Assay as Compared to the Roche Amplicor CT PCR (Amplicor) Assay for the detection of Chlamydia trachomatis in ocular swabs.
| No. of specimens with indicated result | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test | No. Of Specimens Tested | Ra+/Cb+ | R−/C− | R+/C− | R−/C+ | % Sensitivity (95% CIc) | % Specificity (95% CI) | NPV (%) | PPV (%) |
| AC2 | 50 | 24 | 25 | 1* | 0 | 96 (77.6 – 99.7) | 100 (83.4 – 100) | 92.6 | 100 |
1 pool was equivocal on Amplicor, after pool deconstruction and individual specimen testing there was one positive specimen in this pool.
R, reference method result (Amplicor)
C, comparative method result
95% Confidence Intervals
Trachoma is the leading cause of preventable disease and the third most common cause of blindness; productivity loss due to visual impairment and trichiasis was found to be 8 billion US$ in the year 2003 (Baltussen et al., 2005; Burton and Mabey, 2009). It is currently unknown how much is spent on molecular testing annually for analyzing ocular swabs for CT; however Shipitsyna et al. (2007) reported that pooling female cervical samples for the detection of CT into pools of five, assay cost per specimen was reduced by 53.3%. It has previously been shown that pooling ocular specimens using DNA amplification tests for the detection of CT, even at a prevalence of 50% pooling two samples can result in cost effectiveness (Diamant et al., 2001). Since March 2012, when pooling was implemented in our laboratory for the detection of CT in ocular swabs by Amplicor, 2814 pools have been tested which translated to 11,583 individual specimens. The cost to perform Amplicor per sample is 11.11 US$. The total cost to run 2814 pools with 1435 retests, due to positive pools, was 47,199.39 US$; however if all specimens were run individually having a 1% retest rate for equivocal tests the total cost would have amounted to 129,974.00 US$. Pooling ocular specimens on Amplicor has resulted in a cost savings of 62.2%. Due to Amplicor no longer being manufactured, the AC2 assay will be used in the future when testing ocular swab specimens from Tanzania for CT. Extensive cost analyses have not yet been performed for AC2, however based on the large cost savings seen with Amplicor we estimate a large cost savings using AC2 as well.
Pooling ocular specimens has been performed previously using Amplicor however; positive pools were not deconstructed to determine individual positives (House et al., 2009). The importance of deconstructing positive pools is underscored by our finding. In a very low prevalence set of 29 pools, three positive pools, one containing two positives; had the pools not been deconstructed, estimated infection prevalence would have been 10% versus the actual prevalence of 14%. Unpooling is a small component of overall testing, and provides improved precision, especially for monitoring return of infection.
Acknowledgments
Funding: This work was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), and the Bill and Melinda Gates Foundation.
The study protocol was approved by the IRB (NA_00018439).
C.A.G. has received honoraria or research support from the following sponsors: Gen-Probe Hologic and Roche Molecular Diagnostics.
References
- Baltussen RM, Sylla M, Frick KD, Mariotti S. Cost-effectiveness of trachoma control in seven world regions. Ophthalmic Epidemiology. 2005;12:91–101. doi: 10.1080/09286580590932761. [DOI] [PubMed] [Google Scholar]
- Burton MJ, Mabey DCW. The global burden of trachoma: A review. PLoS Neglected Tropical Diseases. 2009;3(10):e460. doi: 10.1371/journal.pntd.0000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambaram JD, Alemayehu W, Melese M, Lakew T, Yi E, House J, et al. Effect of a single mass antibiotic distribution on the prevalence of infectious trachoma. JAMA: the Journal of the American Medical Association. 2006;295(10):1142–6. doi: 10.1001/jama.295.10.1142. [DOI] [PubMed] [Google Scholar]
- Dalesio N, Marsiglia V, Quinn A, Quinn TC, Gaydos CA. Performance of the MagNA Pure LC Robot for extraction of Chlamydia trachomatis and Neisseria gonorrhoeae DNA from urine and swab specimens. Journal of Clinical Microbiology. 2004;42(7):3300–2. doi: 10.1128/JCM.42.7.3300-3302.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant J, Benis R, Schachter J, Moncada J, Pang F, Jha HC, et al. Pooling of Chlamydia laboratory tests to determine the prevalence of ocular Chlamydia trachomatis infection. Ophthalmic Epidemiology. 2001;8(2–3):109–117. doi: 10.1076/opep.8.2.109.4156. [DOI] [PubMed] [Google Scholar]
- Dize L, West S, Williams JA, Van Der Pol B, Quinn TC, Gaydos CA. Comparison of the Abbott m2000 Real Time CT Assay and the Cepheid GeneXpert CT/NG Assay to the Roche Amplicor CT Assay for detection of Chlamydia trachomatis in ocular samples from Tanzania. Journal of Clinical Microbiology. 2013;51(5):1611–3. doi: 10.1128/JCM.00519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhew EB, Priest JW, Moss DM, Ahong G, Munoz B, Mkocha H, et al. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Neglected Tropical Diseases. 2012;6(11):e1873. doi: 10.1371/journal.pntd.0001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JL, Ayele B, Porco TC, Shou Z, Hong KC, Gebre T, et al. Assessment of herd protection against trachoma due to repeated mass antibiotic distribution: a cluster-randomised trial. Lancet. 2009;373(9669):1111–8. doi: 10.1016/S0140-6736(09)60323-8. [DOI] [PubMed] [Google Scholar]
- Lewis JL, Lockary VM, Kobic S. Cost savings and increased efficiency using a stratified specimen pooling strategy for Chlamydia trachomatis and Neisseria gonorrhoeae. Sexually Transmitted Diseases. 2012;39(1):46–8. doi: 10.1097/OLQ.0b013e318231cd4a. [DOI] [PubMed] [Google Scholar]
- See CW, Alemayehu W, Melese M, Zhou Z, Porco TC, Shiboski S, et al. How reliable are tests for trachoma?-A latent class approach. Investigative Ophthalmology and Visual Science. 2011;52(9):6133–7. doi: 10.1167/iovs.11-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipitsyna E, Shalepo K, Savicheva A, Unemo M, Domeika M. Pooling samples: the key to sensitive, specific and cost-effective genetic diagnosis of Chlamydia trachomatis in low-resource countries. Acta Dermato-Venereologica. 2007;87:140–3. doi: 10.2340/00015555-0196. [DOI] [PubMed] [Google Scholar]
- Stare D, Harding-Esch E, Munoz B, Bailey R, Mabey D, Holland M, et al. Design and baseline data of a randomized trial to evaluate coverage and frequency of mass treatment with Azithromycin: The partnership for rapid elimination of trachoma (PRET) in Tanzania and The Gambia. Ophthalmic Epidemiology. 2011;18(1):20–9. doi: 10.3109/09286586.2010.545500. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Global initiative for the elimination of avoidable blindness: action plan 2006–2011. Geneva: WHO; 2007. WHO document. [Google Scholar]