Abstract
Purpose of review
Lung transplantation for infants and children is an accepted but rarely exercised option for treatment of end-stage lung disease with outcomes equivalent to those for adults. However, widespread misconceptions regarding pediatric outcomes often confound timely and appropriate referral to specialty centers. We present updated information for primary pediatricians to utilize when counseling families with children confronted by progressive end-stage pulmonary or cardiovascular disease.
Recent findings
We provide general guidelines to consider for referral, discuss allocation of organs in children, information regarding standard treatment protocols, and survival outcomes.
Summary
Lung transplantation is a worthwhile treatment option to consider in children with end-stage lung disease. The treatment is complex, but lung transplant provides substantial survival benefit and markedly improved quality of life for children and their families. This timely review provides comprehensive information for pediatricians who are considering options for treatment of children with end stage lung disease.
Keywords: Pediatric lung transplantation, candidate selection, immune suppression, survival, bronchiolitis obliterans
Abbreviations: ABPA, ALI, AMR, AR, ATG, BOS, CD4, CD8, CD20, CD52, CF, CLAD, CNI, EBV, FEV1, HLA, IL2RA, ISHLT, PRA, PTLD, UNOS, NK
Introduction
Since the initial report of lung transplantation in a human being in 1963 (1), incremental advances in surgical technique, immunology, organ procurement, and preservation have enabled lung transplantation to be employed as a viable therapeutic option to address end-stage lung disease in infants and children (2, 3). Globally more than 45,000 lung and 4,400 heart-lung transplants have been performed. Of these, 1875 lung and 667 heart-lung procedures were performed in children (Figure 1). This disparity derives from the smaller number of children relative to adults in the general population as well as the natural history of lung disease in children to improve with growth. Increasing success in the area of pediatric lung transplantation has prompted the establishment of pediatric programs in lung transplantation throughout the world. In 2011, there were 43 medical centers active in pediatric lung transplantation worldwide, with 18 medical centers established in the United States (4).
Figure 1.
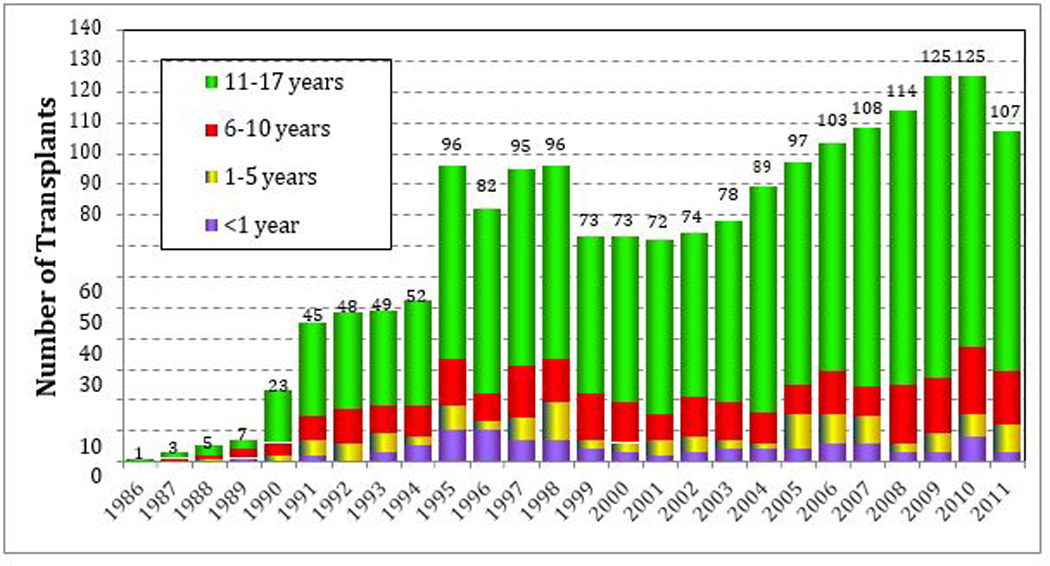
Age distribution of pediatric lung recipients by year of transplant. Age distribution for pediatric lung pediatric lung recipients (transplants from January 1986 to June 2011).(4)
History
The first lung transplantation, reported in 1963, was technically successful for a 58-year old convicted felon who had metastatic bronchial carcinoma. While the transplant was successful, the recipient died of renal failure 18 days later (1). In 1966, the first lobar lung transplantation was performed by Shinoi and colleagues at the Tokyo Medical College. The transplanted lobe remained in place for 18 days, providing essential pulmonary support during a period of time when the patient may have otherwise died due to hypoxemia (5).
Throughout the 1970s, the promise of lung transplantation went unrealized, largely as a result of dehiscense at the bronchial anastomoses. But the advent of a steroid sparing approach by adding cyclosporine to the perioperative immune suppression in lung transplantation improved the integrity of the bronchial anastomoses and post-operative outcomes (6, 7).
The number of children undergoing lung transplantation has increased over the past 15 years. Between 1998 and 2003, approximately 59 children underwent lung transplantation annually. By comparison, in 2011, a total of 107 pediatric procedures were reported by 43 medical centers. The majority of pediatric centers reported performing between 1 and 4 procedures and 5 centers reported doing more than 5 transplant procedures. The majority of pediatric lung recipients are between 11 and 17 years of age, 20% are between 6 and 10 years of age, and 10% of the recipients are between 1 and 5 years old. Between 2007 and 2011, an average of 5 children younger than 1 year of age underwent lung transplantation (4).
In general, the most frequent indication for lung transplantation is cystic fibrosis (CF). While half of all children undergoing lung transplantation at less than age 10 do so as a result of CF, the number increases to almost 70% in older children. In children less than a year of age, congenital heart disease and pulmonary vascular disease remain the leading indication for pediatric lung transplantation (Table 1) (4).
Table 1.
Indications for Transplant for Pediatric Lung Transplants (Transplants From January 1990 to June 2012).*(4)
| Diagnosis | < 1 Year | 1–5 Years | 6–10 Years | 11–17 Years | ||||
|---|---|---|---|---|---|---|---|---|
| Cystic Fibrosis | 1 | 1.0% | 6 | 4.8% | 140 | 53.0% | 916 | 70.6% |
| Idiopathic Pulmonary Arterial Hypertension | 12 | 12.5% | 28 | 22.4% | 23 | 8.7% | 101 | 7.8% |
| Congenital Heart Disease | 16 | 16.7% | 10 | 8.0% | 4 | 1.5% | 11 | 0.8% |
| Pulmonary Vascular Disease | 8 | 8.3% | 7 | 5.6% | 4 | 1.5% | 1 | 0.1% |
| ChILD | 23 | 23.9% | 14 | 12.2% | 14 | 5.3% | 29 | 2.2% |
| Bronchopulmonary Dysplasia | 3 | 3.1% | 3 | 2.4% | 6 | 2.3% | 3 | 0.2% |
| Other | 13 | 13.5% | 6 | 4.8% | 13 | 4.9% | 32 | 2.5% |
Used by permission and adapted from the 16th Official Pediatric Lung Transplant registry, ISHLT
ChILD; diffuse interstitial lung diseases of childhood
Candidates
Lung transplantation is considered in selected children with end stage or progressive lung disease or life-threatening pulmonary vascular disease for which there is no other medical therapy. Regardless of underlying diagnosis, all candidates require: (1) A clear diagnosis and well delineated trajectory of illness such that the child is at high risk of death, despite optimal medical therapy; (2) Reliable access to transplant services and medications after transplantation; and (3) Assurance that patient and support system can and will adhere to the rigorous therapeutic plan before and after the transplant.
Contraindications
Comorbid disorders can complicate the procedure and compromise the outcome. Table 2 includes several relative contraindications, though there is significant variability between centers (8). The experience for patients undergoing lung transplantation directly from ECMO (extra-corporeal membrane oxygenation) is both limited and poor, with an overall survival rate of only 40% (9). Given these dismal outcomes, transplantation from ECMO is an absolute contraindication for lung transplantation in most centers.
Table 2.
Contraindications to Lung Transplantation (8)
| ABSOLUTE | RELATIVE |
|---|---|
| Active malignancy | Pleurodesis |
| Sepsis | Renal insufficiency |
| Active tuberculosis | Markedly abnomal body mass index |
| Severe neuromuscular disease | Mechanical ventilation/tracheostomy |
| Documented, refractory non-adherence | Scoliosis |
| Multiple organ dysfunction | Poorly controlled diabetes mellitus |
| Acquired Immunodeficiency Syndrome | Osteoporosis |
| Hepatitis C with histologic liver disease | Chronic airway infection with multiply resistant organisms |
| ECMO | Fungal infection/colonization |
| Hepatitis B surface antigen positive |
ECMO; extracorporeal membrane oxygenation
Paracorporeal membrane oxygenation has been utilized in a subset of infants and children. The method entails use of a membrane oxygenator interposed between the pulmonary artery (PA) to left atrium (LA) that serves as a pumpless oxygenator to allow for extubation while awaiting lung transplantation. However, there is minimal experience with this technique in pediatrics and thromboembolic complications and cerebral vascular accident have been reported (10, 11).
Survival and outcomes
Despite improvements in clinical outcome, morbidity and mortality associated with lung transplantation remains high. Mortality is greatest in the first year with approximately 15% of all recipients dying due to infection and graft failure (4). Nonetheless, the overall survival rate has improved over the past 30 years. Survival is similar between CF patients and children with other diagnoses, and survival is similar amongst all age pediatric recipients, including infants, when conditional survival to one year is considered (Figure 2). Before 2000, median survival was 3.3 years among all children, but median survival improved substantially to 5.8 years after 2000, and upon conditional analysis limited to survival to one year, pediatric median survival increased to 8.7 years compared to 9.6 years in adults (4, 12). Single lung transplantation is infrequently applied to the pediatric population, as mortality is 40% in the first year and data indicate that the survival of these patients is significantly decreased compared to patients that undergo bilateral sequential lung transplantation (4).
Figure 2.
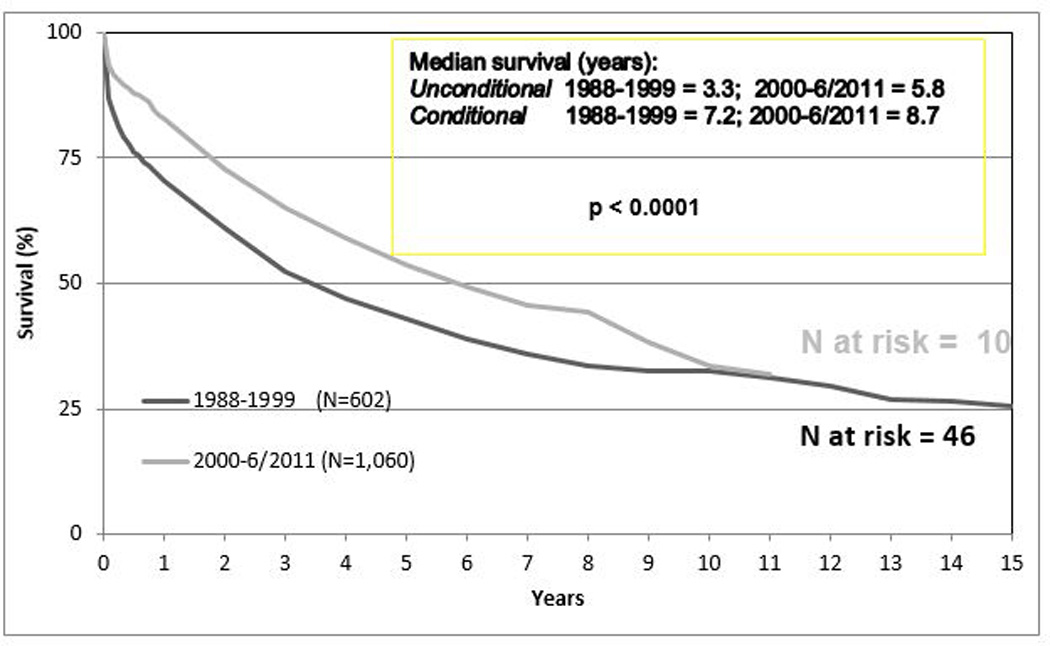
Kaplan-Meir survival curve of lung transplant recipients by time of transplant. (Transplantation between January 1988 and June 2011). There is no difference in length of survival between eras (4).
Extended survival is compromised by chronic lung allograft dysfunction (CLAD), previously termed bronchiolitis obliterans syndrome (BOS). CLAD is associated with both acute and chronic rejection (12, 13) and is characterized by progressive airflow obstruction due to fibrotic obliteration of the small airways, restrictive physiology, or both. Within 5 years, less than 50% of transplant recipients are free from BOS (4) (Figure 3). Significant study has been given to identify clinical tools to minimize both acute and chronic graft dysfunction, yet the etiologies are elusive.
Figure 3.
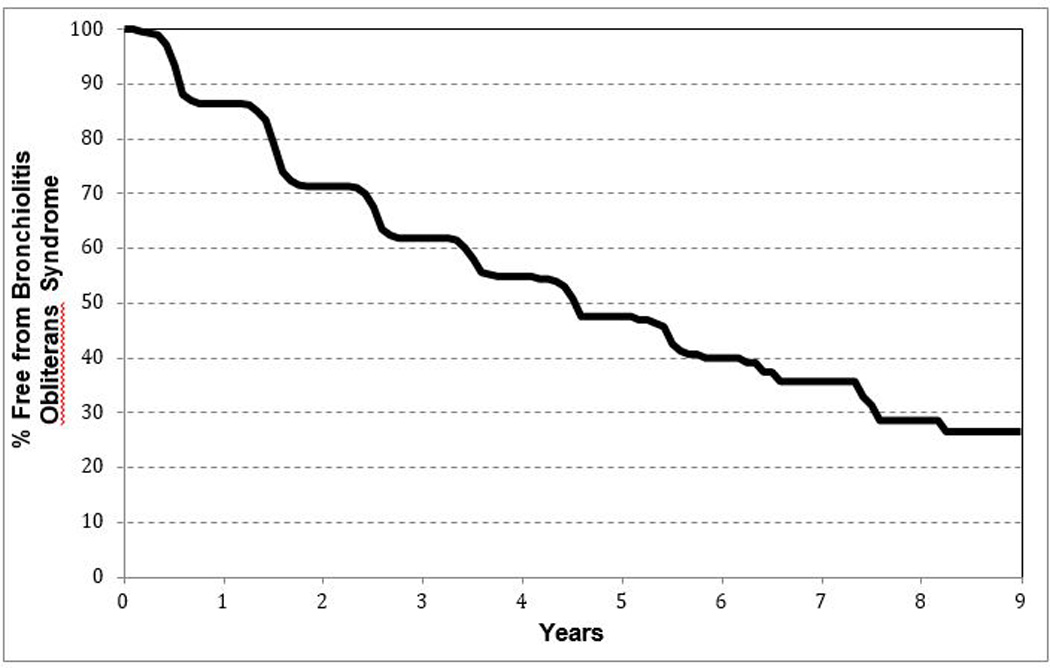
Freedom from bronchiolitis|obliterans for pediatric lung recipients (follow-up: April 1994 to June 2012) (4).
Surgical Approach
Currently, bilateral sequential lung transplant is the most frequently performed lung transplant procedure in children and is performed most frequently via median sternotomy. The mainstem bronchi and left and right pulmonary arteries are connected via end-to-end anastomoses. Two pulmonary veins with intact atrial connections are harvested from each donor lung. Each left atrial patch is sewn onto the recipient heart. This surgical approach minimizes cardiopulmonary bypass time, which reduces the related complications (2, 3, 13, 14).
Though combined heart-lung transplantation had been a favored surgical approach, improved surgical techniques as well as the profound scarcity of donor organs have led to a dramatic decrease in the frequency of heart-lung transplantation. Moreover, right-sided heart failure associated with pulmonary hypertension resolves following lung transplantation, and this has obviated the need for heart and lung transplantation for primary pulmonary hypertension (15, 16). Additionally, there is no difference in survival between patients that undergo bilateral sequential lung transplantation compared to those that undergo heart-lung transplantation. Lung transplantation alone maximizes the benefit from a single donor, thereby benefiting more children (14).
In the 1990’s, living donor lobar lung transplantation was developed as a strategy for transplantation in order to decrease waiting time of severely ill children awaiting lung transplantation, but with the adoption of new lung allocation score in 2005 and improved peri-transplant strategies, waitlist deaths have decreased (17–19). The relative efficacy of the new lung allocation scoring system combined with the technical and ethical challenges associated with living lobar transplantation, have prevented wider adoption of the procedure in the United States (20) (21).
Post-operative Management
Immediate postoperative care is focused on respiratory and hemodynamic management. In the perioperative period, pulmonary care emphasizes reestablishment of functional residual capacity. Aggressive tracheobronchial toilet, chest physiotherapy, and bronchoalveolar lavage will mobilize secretions to ensure patency of the airways. Mechanical ventilation is generally necessary for less than 48 hours, but is prolonged in the event of graft dysfunction. To minimize radical related injury to the lungs, the fraction of inspired oxygen is maintained at less than 60% while maintaining systemic arterial saturation 94% or greater. Optimal ventilator strategy utilizes 5–7 cc/kg tidal volumes and a plateau inspiratory pressure of less than 30 cm of water (22, 23). Sufficient positive end expiratory pressure is used to fully recruit and maintain the functional residual capacity of the newly transplanted lungs. Inhalational nitric oxide has been shown to improve oxygenation in the presence of acute graft dysfunction, likely as a result of enhanced ventilation and perfusion matching.
Hemodynamic status must be closely monitored. Vascular permeability and myocardial function may be adversely affected by cardiopulmonary bypass, necessitating inotropic support in the perioperative period. Hemodynamic instability may be exacerbated by diminished intravascular volume. Central venous pressure monitoring is beneficial in order to optimize cardiac output. Early recognition of compromised renal function is essential as the prescription of all medications excreted and metabolized by the kidneys will need to be promptly altered.
Recipients may experience early severe graft dysfunction as a result of lung injury incurred during or prior to organ harvest. The incidence of early graft dysfunction (PGD) is between 10 and 35%. The clinical presentation of PGD is entirely consistent with acute respiratory distress syndrome as manifested by elevated alveolar-arteriolar gradient, compromised pulmonary compliance, poor ventilation and perfusion matching, and impaired diffusion (24). Most grafts will recover with judicious ventilator management as well as diuretic and pressor support. Extracorporeal membrane oxygenation has been successfully employed as a therapeutic modality (25). Improved surgical techniques and organ perfusate has diminished the severity of early graft dysfunction over the last decade (26, 27).
The post-operative course can be complicated by technical problems associated with the surgery. At many centers the patency of the airway anastomoses is routinely assessed within 24 hours by direct visualization with flexible bronchoscopy. While the vascular anastomoses are more difficult to assess, arterial anastomoses are generally amenable to inspection with nuclear medicine studies. In order to assess the venous anastomoses, transesophageal echocardiography may be necessary. Overall, post-operative bleeding is the most frequent cause for reoperation (15).
Vocal cord paresis or diaphragmatic paresis can complicate virtually any major thoracic surgery, and both derive from injuries to the nerve at the time of surgery. However, the clinical symptoms entailed by these issues are generally not apparent until after extubation. Vocal cord paralysis or paresis results from injury to recurrent laryngeal nerve and phrenic nerve injury leads to diaphragmatic paralysis or paresis. The likelihood of phrenic nerve injury is increased in patients that have had prior thoracic surgery (28, 29). Most of these injuries resolve within several weeks of surgery, but serious consideration should be given to early diaphragmatic plication as the risk of infection in the lung affected by the paretic hemidiaphragm is quite high (30). Vocal cord function may be temporarily compromised following removal of an endotracheal tube even in the absence of true injury, thus, definitive evaluation for it should be deferred at least 72 hours after extubation (28).
Immunobiology
The long-term success of lung transplantation is achieved with the use of immunosuppressive drugs that inhibit rejection of the lung allograft. Immune suppression strategies in lung transplantation generally consist of a triple- drug maintenance regimen comprised of a calcineurin inhibitor (CNI), a T-cell antiproliferative, and corticosteroids (generally, tacrolimus, mycophenolate mofetil/mycophenolic acid, and prednisone). Approximately 60% of pediatric lung transplant centers utilize an induction regimen in the perioperative time period, though data do not support the notion that induction confers either a survival benefit or reduction in the incidence of chronic lung allograft disease (CLAD) (4, 31, 32).
Acute cellular rejection (ACR) of the transplanted lungs occurs in almost half of children that undergo lung transplantation. Episodes of ACR entail activation of the innate and adaptive immune responses and result in recruitment of alloreactive CD4+ and CD8+ T lymphocytes to the lung allograft which magnify the recruitment of neutrophils, eosinophils, B-lymphocytes, macrophages and NK cells, causing lung injury (33).
The clinical manifestations of ACR include fever, dyspnea, and hypoxia. Chest radiograph findings are relatively non-specific but often include perihilar infiltrates and effusions. Airflow obstruction may be detected with spirometry. The patient must be evaluated for both infection and rejection when these signs are detected. This requires bronchoscopy to obtain bronchoalveolar lavage samples and transbronchial biopsies.
Histologic evaluation of the specimens leads to the assignment of a grade: A0 indicates the absence of rejection, and grade A4 indicates severe rejection (34, 35). There is a clear relationship between AR and eventual development of CLAD. Treatment for ACR is initiated with high dose intravenous methylprednisolone and bronchoscopy is later repeated to assess for resolution of abnormal histopathology. For refractory ACR, more aggressive treatment with alemtuzumab (CD52 receptor antagonist), photopheresis, or total lymphoid irradiation may be pursued.
Long Term Surveillance
Monitoring for lung transplant complications requires vigilance. Most centers obtain routine laboratory tests, spirometry and other pulmonary function tests, chest radiographs, and regular bronchoscopic screening. In general, the levels for immunosuppression medications are higher than those used in other solid organ transplants, particularly during the first several months after transplantation, as the incidence of ACR is greatest in the first six months following transplantation. Serum levels of CNIs are monitored frequently to assure appropriate drug levels. Lung function is assessed with spirometry. CLAD is detected with spirometry with a decrease in small airway flows or restrictive indices, and is staged to guide therapy (Table 3) (36). Transbronchial biopsy is most sensitive for the diagnosis of global graft pathology, such as ACR, but for processes such as BO, which tends to manifest with an inhomogenous distribution, open lung biopsy may be indicated to define underlying processes of graft dysfunction. Other diagnostic studies include ventilation/perfusion scans, computed tomography, and comprehensive plethysmography studies.
Table 3.
Pulmonary Function Criteria for BOS staging. (36)
| BOS Stage | FEV1 (% pred) |
|---|---|
| 0p | FEV1 81–90% of best and/or FEF 25–75% is < 75% of best |
| BOS1 | FEV1 66–80% of best |
| BOS2 | FEV1 51–65% of best |
| BOS3 | FEV1 < 50% of best |
BOS – bronchiolitis obliterans syndrome
FEV1 – forced expiratory volume in 1 second, as a percent of the predicted value for age
FEF 25–75% - mid-expiratory flow rate
Infectious Diseases
The compromised immune function superimposed on the difficulties in mobilizing secretions in the perioperative lung transplant recipient predisposes to infection, and any infection of the graft may become life threatening. Thus, the index of suspicion for infection must be high and the threshold for initiating broad-spectrum antibiotic therapy low. Early evaluation with bronchoscopy is essential (37, 38).
As opportunistic infections in the lung transplant population are particularly problematic, prophylactic treatment with antiviral and anti-pneumocystis jirovecii therapeutic agents are standard components of treatment. CMV infection is associated with an increased incidence of both acute and chronic rejection. Prophylactic treatment with gancyclovir has been adopted by most transplant centers (39). With the advent of gene-based diagnostic modalities, and the corresponding enhancement in diagnostic sensitivity, treatment has become more focused. Whether such strategies diminish the incidence of bronchioloitis obliterans is not known (40). Despite effective antiviral therapy, CMV infection remains a source of both morbidity and mortality.
Aspergillosis is a particularly problematic infectious pathogen in the pediatric lung transplant population (41). A significant number of children with cystic fibrosis are colonized with aspergillus, and many have allergic bronchopulmonary aspergillosis (ABPA). Thus, in the post-operative period, CF patients are routinely treated with 1 – 3 months with voriconazole, because aspergillus can become invasive in immune compromised patients, resulting in disseminated infection (42, 43). Thus, children colonized with Aspergillus are treated with anti-fungal therapy prior to transplantation (44, 45) in order to diminish the infectious burden and thereby mitigate the likelihood of disseminated infection in the perioperative period (46).
Post-transplant lymphoproliferative disease (PTLD)
Post-transplant lymphoproliferative disease (PTLD) is a serious, sometimes fatal complication that occurs following lung transplantation. Primary Epstein-Barr virus (EBV) infection, usually acquired from the donor, represents a major risk factor for the development of PTLD following lung transplantation (47). PTLD after lung transplantation (up to 10%) appears to be related to EBV-infected donor lymphocytes that reside in the form of bronchus-associated lymphoid tissue that may contribute to activation in the setting of immune suppression used for lung graft recipients. Data support the use of prophylactic anti-viral therapy in sero-negative patients of positive donors (48). Clinically, PTLD may present with lymphadenopathy, fever, malaise, and often with mass lesions noted on radiologic examination. Diagnosis is directed with quantitative polymerase chain reaction to detect EBV viremia and biopsy of lymph nodes or affected tissues. Treatment includes a reduction in immune suppression and the initiation of biologic agents designed to specifically target CD20 surface markers on B-cells to activate complement-dependent B-cell cytotoxicity (49). Patients with single organ involvement achieve remission in a relatively short time frame.
Bronchiolitis Obliterans Syndrome (BOS)
Beyond the first year, chronic lung allograft dysfunction (CLAD) is the leading cause of death in lung and heart-lung transplant recipients. Until recently, the physiological hallmark of chronic rejection was characterized by an obstructive ventilatory defect marked clinically by a persistent fall in the forced expiratory volume in 1 second (FEV1). This syndrome, BOS, was defined to allow a uniformity of description and grading of severity between transplant centers (Table 3) (36). However, factors other than the fibrotic obliteration, such as bronchiolitis obliterans (BO) of the airway lumen, account for graft loss. Thus, a broader term, CLAD has been adopted to include multiple manifestations of graft dysfunction due to either immunological or non-immunological allograft insults. Injury events associated with BOS include ischemia-reperfusion injury, primary graft dysfunction, diffuse alveolar damage, cellular and antibody-mediated rejection, infection, and/or aspiration of gastric contents (50).
Until recently, CLAD was generally thought to be irreversible. A subgroup of patients with BOS may respond to azithromycin with an improvement of their FEV1 > 10% and stabilization. But a restrictive form of chronic rejection has recently been described, which does not fit the strict definition of BOS as non- obstructive dysfunction is measured. Numerous studies have illustrated the highly variable clinical course in CLAD, ranging from gradual decline over years to sudden-onset, rapidly deteriorating graft function within weeks (51–53). Given this heterogeneity, CLAD phenotypes are distinguished based on changes in total lung capacity, bronchoalveolar lavage cellularity and average rate of decline in lung function leading to the distinction of the restrictive allograft syndrome (RAS) and neutrophilic CLAD subtypes by their potential for response to treatment with azithromycin and extracorporeal photopheresis (ECP) (54). Responders to ECP gain a significant survival benefit, as 2-year mortality in this group is under 3%. Conversely, rapidly progressive CLAD is difficult to treat if surveillance studies do not uncover a treatable cause, and median survival is 2.5 years in this subset. The immunological basis of action in ECP remains incompletely understood, but may be related to upregulation of CD4+CD25+ T-regulatory cells (55–59). More study is necessary to determine the origins of CLAD and in determining which subgroups will best respond to treatment.
Drug interactions
In the perioperative period, most pediatric lung transplant recipients are treated with multiple medications. In initiating or discontinuing therapy careful consideration must be undertaken to consider drug interactions. This is especially true in the context of medications that are metabolized via the cytochrome P450 pathway in the liver. Macrolide antibiotics, antifungal agents, and antiepileptic agents will have significant effects on the metabolism of both tacrolimus and cyclosporine (60). Moreover, standard measures of renal function may provide unjustified reassurance as glomerular filtration rate may be significantly diminished despite relatively normal serum levels of blood urea nitrogen and creatinine (61). Given that children undergoing lung transplantation have been chronically ill for many years prior to the transplantation, the presence of compromised renal function is not surprising. Accordingly, treatment with nephrotoxic agents must be undertaken with careful consideration.
Medical Management
At least half of pediatric lung transplant patients are treated for cystic fibrosis, which confers multi-organ disease. Increased morbidity occurs as a consequence of the anti-rejection medications in CF and in the non-CF recipients as well. Tacrolimus causes nephrotoxicity and is associated with glucose intolerance or the onset of insulin- dependent diabetes mellitus, osteoporosis, and systemic hypertension. Attention must be given to electrolyte balance, hypomagnesemia, and hydration. The nutritional status of most pre- and post-transplant recipients is often compromised. While this tends to improve post-operatively, attention to calorie and protein repletion remains an important determinant of ourcome in children.
Summary
Lung transplantation is an increasingly well-accepted treatment for children with end-stage lung disease resulting from many causes. Improved surgical technique has brought transplantation to a broader set of patients. Nonetheless, long-term survival rates remain significantly lower than for transplantation of other solid organs. In order for lung transplantation to realize its full promise, better understanding of and treatment for acute and chronic rejection and CLAD must occur. Notwithstanding such limitations, outcome of children undergoing lung transplantation has progressively improved. With ongoing and incremental improvements in care, lung transplantation has become more common. Thus, it is essential that providers of pediatric critical care and pulmonary medicine become more familiar with the nuances of care for children undergoing lung transplantation.
Key Points.
Lung transplant outcomes are relatively similar between children and adults.
Lung transplantation is a viable option for children with severe, end-stage lung disease that is likely to be fatal within a relatively short time frame.
For some neonatal lung diseases, lung transplantation is the only treatment option.
Medical management of children that have undergone lung transplantation requires a multi-disciplinary approach and the central involvement of a pediatric pulmonologist with specific expertise and training in lung transplant medicine.
Footnotes
Financial disclosure- The authors of this manuscript have not received donations or any other financial support from any organization that may reflect a conflict of interest relative to the information contained in the present publication.
References
- 1. Hardy J, Webb W, Dalton M, Walker G. Lung homotransplantations in man. JAMA. 1963;186:99–108. doi: 10.1001/jama.1963.63710120001010. The reference represents the first report of lung transplantation in humans. The manuscript is of significant historical interest.
- 2. Starnes V, Marshall S, Lewiston N, Theodore J, Stinson E, Shumway N. Heart-lung transplantation in infants, children, and adolescents. J Ped Surgery. 1991;26:434–438. doi: 10.1016/0022-3468(91)90991-2. The reference is among the first reports of lung transplantation in children, written by pioneers in the field.
- 3.Spray T, Mallory G, Canter C, Huddleston C. Pediatric lung transplantation. J Thoracic Cardiovascular Surgery. 1994;107:990–1000. [PubMed] [Google Scholar]
- 4. Benden C, Edwards LB, Kucheryavaya AY, Christie JD, Dipchand AI, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official pediatric lung and heart-lung transplantation report--2013; focus theme: age. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013 Oct;32(10):989–997. doi: 10.1016/j.healun.2013.08.008. **The work provides a real time update on the state of pediatric lung transplantation.
- 5.Mendeloff EN. The history of pediatric heart and lung transplantation. Pediatric transplantation. 2002;4(6):270–279. doi: 10.1034/j.1399-3046.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M, Lima O, Morgan E HAA, Luk S, Ferdman A, et al. A comparison between cyclosporine A and methylprednisolone plus azathioprine on bronchial healing following canine lung transplantation. J Thoracic Cardiovasc Surgery. 1983;85:821–826. [PubMed] [Google Scholar]
- 7. Cooper J, Pearson F, Patterson G, Todd T, Ginsberg R, Goldberg M. Technique of successful lung transplantation in humans. J Thoracic Cardiovascular Surgery. 1987;93(2):173–181. The paper outlines a substantial surgical advance that overcame a huge technical challenge.
- 8.Faro A, Mallory GB, Visner GA, Elidemir O, Mogayzel PJ, Jr, Danziger-Isakov L, et al. American Society of Transplantation executive summary on pediatric lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007 Feb;7(2):285–292. doi: 10.1111/j.1600-6143.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- 9.Puri V, Epstein D, Raithel SC, Gandhi SK, Sweet SC, Faro A, et al. Extracorporeal membrane oxygenation in pediatric lung transplantation. The Journal of thoracic and cardiovascular surgery. 2010 Aug;140(2):427–432. doi: 10.1016/j.jtcvs.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Hoganson DM, Gazit AZ, Sweet SC, Grady RM, Huddleston CB, Eghtesady P. Neonatal paracorporeal lung assist device for respiratory failure. Ann Thorac Surg. 2013 Feb;95(2):692–694. doi: 10.1016/j.athoracsur.2012.05.128. [DOI] [PubMed] [Google Scholar]
- 11. Hoganson DM, Gazit AZ, Boston US, Sweet SC, Grady RM, Huddleston CB, et al. Paracorporeal lung assist devices as a bridge to recovery or lung transplantation in neonates and young children. The Journal of thoracic and cardiovascular surgery. 2014 Jan;147(1):420–427. doi: 10.1016/j.jtcvs.2013.08.078. * This work demonstrates the potential of using devices to enahnce oxygenation in children with compromised lung function. Improvements in technology may enable broader use of the strategy.
- 12.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report--2013; focus theme: age. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013 Oct;32(10):965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Kurland G, Michelson P. Bronchiolitis obliterans in children. Pediatr Pulmonol. 2005;39(3):193–208. doi: 10.1002/ppul.20145. [DOI] [PubMed] [Google Scholar]
- 14.Bolman RMr, Shumway SJ, Estrin JA, Hertz MI. Lung and heart-lung transplantation. Evolution and new applications. Ann Surg. 1991;214(4):456–468. doi: 10.1097/00000658-199110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcasoy SM, Kotloff RM. Lung transplantation. New England Journal of Medicine. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [Review]. [DOI] [PubMed] [Google Scholar]
- 16.Pasque M, Trulock E, Cooper J, Triantifillou A, Huddleston C, Rosenbloom M, et al. Single lung transplantation for pulmonary hypertension. Single institution experience in 54 patients. Circulation. 1995;92(8):2252–2258. doi: 10.1161/01.cir.92.8.2252. [DOI] [PubMed] [Google Scholar]
- 17.Ivy D. Diagnosis and treatment of severe pediatric pulmonary hypertension. Cardiol Rev. 2001;9(4):227–237. doi: 10.1097/00045415-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Starnes VA, Barr ML, Cohen RG. Lobar transplantation: indications, technique, and outcome. J Thoracic Cardiovascular Surgery. 1994;108(3):403–410. [PubMed] [Google Scholar]
- 19.Starnes VA, Woo MS, MacLaughlin EF, Horn MV, Wong PC, Rowland JM, et al. Comparison of outcomes between living donor and cadaveric lung transplantation in children. Annals of Thoracic Surgery. 1999;68(6):2279–2283. doi: 10.1016/s0003-4975(99)01155-8. [DOI] [PubMed] [Google Scholar]
- 20.Woo MS, MacLaughlin EF, Horn MV, Wong PC, Rowland JM, Barr ML, et al. Living donor lobar lung transplantation: the pediatric experience. Pediatric transplantation. 1998;2(3):185–190. [PubMed] [Google Scholar]
- 21.Thabut G, Christie JD, Mal H, Fournier M, Brugiere O, Leseche GCY, et al. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. American journal of respiratory and critical care medicine. 2013;187(12):1335–1340. doi: 10.1164/rccm.201303-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England journal of medicine. 2000 May 4;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 23.Frank J, Mathay M. Science review: Mechanisms of ventilator induced injury. Critical Care. 2003;7(3):233–241. doi: 10.1186/cc1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JC, Christie JD. Primary graft dysfunction. Proc Am Thorac Soc. 2009 Jan 15;6(1):39–46. doi: 10.1513/pats.200808-082GO. Epub 2009/01/10.eng. [DOI] [PubMed] [Google Scholar]
- 25.Meyers BF, Sundt TM, III, Henry S, Trulock EP, Guthrie T, Cooper JD, et al. Selective use of extracorporeal membrane oxygenation is warranted after lung transplantation. J Cardiovascular Surgery. 2000;120(1):20–26. doi: 10.1067/mtc.2000.105639. [DOI] [PubMed] [Google Scholar]
- 26.Fujino S, Nagahiro AN, Triantafillou AN, Boasquevisque CH, Yano M, D CJ, et al. Inhaled nitric oxide at the time of harvest improves early graft function. Annals of Thoracic Surgery. 1997;63:1383–1389. doi: 10.1016/s0003-4975(97)00236-1. [DOI] [PubMed] [Google Scholar]
- 27.Fujino S, Nagahiro I, Yamashita M, Yano M, Schmid R, J C, et al. Preharvest nitroprusside flush improves posttransplantation lung function. Journal of Heart & Lung Transplantation. 1997;16:1073–1080. [PubMed] [Google Scholar]
- 28.Fan LL, Campbell DN, Clarke DR, Washington RL, Fix E, J, White CW. Paralyzed left vocal cord associated with ligation of patent ductus arteriosus. J Thorac Cardiovasc Surg. 1989;98(4):611–613. [PubMed] [Google Scholar]
- 29.Grocott HP, Clark JA, Homi HM, Sharma A. "Other" neurologic complications after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8(3):213–226. doi: 10.1177/108925320400800304. [DOI] [PubMed] [Google Scholar]
- 30.de Leeuw M, Williams JM, Freedom RM, Williams WG, Shemie SD, McCrindle BW. Impact of diaphragmatic paralysis after cardiothoracic surgery in children. J Thorac Cardiovasc Surg. 1999;118(3):510–517. doi: 10.1016/S0022-5223(99)70190-X. [DOI] [PubMed] [Google Scholar]
- 31.Brock MV, Borja MC, Ferber L, Orens JB, Anzcek RA, Krishnan J, et al. Induction therapy in lung transplantation: a prospective, controlled clinical trial comparing OKT3, anti-thymocyte globulin, and daclizumab. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2001;20(12):1282–1290. doi: 10.1016/s1053-2498(01)00356-4. [DOI] [PubMed] [Google Scholar]
- 32. Sweet SC. Induction therapy in lung transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2013 Jul;26(7):696–703. doi: 10.1111/tri.12115. *The manuscript provides important information surrounding the propriety of induction therapy and also demonstrates the potential of using a collective approach for clinical research in lung transplantation.
- 33.Snyder LD, Palmer SM. Immune mechanisms of lung allograft rejection. Seminars in respiratory and critical care medicine. 2006 Oct;27(5):534–543. doi: 10.1055/s-2006-954610. [DOI] [PubMed] [Google Scholar]
- 34.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2007 Dec;26(12):1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Yousem S, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. A revision of the 1990 Working Formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group (LRSG) J Heart Lung Transplantation. 1996;15:1–15. [PubMed] [Google Scholar]
- 36.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2002 Mar;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 37.Higenbottam T, Stewart S, Penketh A, Wallwork J. The diagnosis of lung rejection and opportunistic infection by transbronchial lung biopsy. Transplant Proc. 1987;19(5):3777–3778. [PubMed] [Google Scholar]
- 38.Guilinger RA, Paradis IL, Dauber JH, Yousem SA, Williams PA, Keenan RJ, et al. The importance of bronchoscopy with transbronchial biopsy and bronchoalveolar lavage in the management of lung transplant recipients. Am J Respir Crit Care Med. 1995;152(6 Pt 1):2037–2043. doi: 10.1164/ajrccm.152.6.8520773. [DOI] [PubMed] [Google Scholar]
- 39.Perreas KG, McNeil K, Charman S, Sharples LD, Wreghitt T, Wallwork J. Extended ganciclovir prophylaxis in lung transplantation. J Heart Lung Transplant. 2005;24(5):583–587. doi: 10.1016/j.healun.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Johansson I, Martensson G, Nystrom U, Nasic S, Andersson R. Lower incidence of CMV infection and acute rejections with valganciclovir prophylaxis in lung transplant recipients. BMC infectious diseases. 2013;13:582. doi: 10.1186/1471-2334-13-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dummer JS, Lazariashvilli N, Barnes J, Ninan M, Milstone AP. A survey of anti-fungal management in lung transplantation. J Heart Lung Transplant. 2004;23(12):1376–1381. doi: 10.1016/j.healun.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 42.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. The Journal of infection. 2012 Nov;65(5):453–464. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Nunley DR, Ohori P, Grgurich WF, Iacono AT, Williams PA, Keenan RJ, et al. Pulmonary aspergillosis in cystic fibrosis lung transplant recipients. Chest. 1998;114(5):1321–1329. doi: 10.1378/chest.114.5.1321. [DOI] [PubMed] [Google Scholar]
- 44.Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance(R) registry and invasive fungal infections: update 2012. Diagnostic microbiology and infectious disease. 2012 Aug;73(4):293–300. doi: 10.1016/j.diagmicrobio.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Avery RK. Prophylactic strategies before solid-organ transplantation. Curr Opin Infect Dis. 2004;17(4):353–356. doi: 10.1097/01.qco.0000136936.13662.74. [DOI] [PubMed] [Google Scholar]
- 46.Minari A, Husni R, Avery RK, Longworth DL, DeCamp M, Bertin M, et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl Infect Dis. 2002;4(4):195–200. doi: 10.1034/j.1399-3062.2002.t01-2-02002.x. [DOI] [PubMed] [Google Scholar]
- 47.Davis JE, Sherritt MA, Bharadwaj M, Morrison LE, Elliott SL, Kear LM, et al. Determining virological, serological and immunological parameters of EBV infection in the development of PTLD. Int Immunol. 2004;16(7):983–989. doi: 10.1093/intimm/dxh099. [DOI] [PubMed] [Google Scholar]
- 48.Malouf MA, Chhajed PN, Hopkins P, Plit M, Turner J, Glanville AR. Anti-viral prophylaxis reduces the incidence of lymphoproliferative disease in lung transplant recipients. J Heart Lung Transplant. 2002;21(5):547–554. doi: 10.1016/s1053-2498(01)00407-7. [DOI] [PubMed] [Google Scholar]
- 49.Pescovitz MD. The use of rituximab, anti-CD20 monoclonal antibody, in pediatric transplantation. Pediatr Transplant. 2004;8(1):9–21. doi: 10.1046/j.1397-3142.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 50.Verleden GM, Vos R, Verleden SE, De Wever W, De Vleeschauwer SI, Willems-Widyastuti A, et al. Survival determinants in lung transplant patients with chronic allograft dysfunction. Transplantation. 2011 Sep 27;92(6):703–708. doi: 10.1097/TP.0b013e31822bf790. [DOI] [PubMed] [Google Scholar]
- 51.Glanville AR. Bronchoscopic monitoring after lung transplantation. Seminars in respiratory and critical care medicine. 2010 Apr;31(2):208–221. doi: 10.1055/s-0030-1249117. [DOI] [PubMed] [Google Scholar]
- 52.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. American journal of respiratory and critical care medicine. 2010 Sep 15;182(6):784–789. doi: 10.1164/rccm.201002-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lama VN, Murray S, Lonigro RJ, Toews GB, Chang A, Lau C, et al. Course of FEV(1) after onset of bronchiolitis obliterans syndrome in lung transplant recipients. American journal of respiratory and critical care medicine. 2007 Jun 1;175(11):1192–1198. doi: 10.1164/rccm.200609-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greer M, Dierich M, De Wall C, Suhling H, Rademacher J, Welte T, et al. Phenotyping established chronic lung allograft dysfunction predicts extracorporeal photopheresis response in lung transplant patients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013 Apr;13(4):911–918. doi: 10.1111/ajt.12155. [DOI] [PubMed] [Google Scholar]
- 55.Morrell MR, Despotis GJ, Lublin DM, Patterson GA, Trulock EP, Hachem RR. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010 Apr;29(4):424–431. doi: 10.1016/j.healun.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 56.Rook AH, Cohen JH, Lessin SR, Vowels BR. Therapeutic applications of photopheresis. Dermatologic clinics. 1993 Apr;11(2):339–347. [PubMed] [Google Scholar]
- 57.Andreu G, Achkar A, Couetil JP, Guillemain R, Heshmati F, Amrein C, et al. Extracorporeal photochemotherapy treatment for acute lung rejection episode. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1995 Jul-Aug;14(4):793–796. [PubMed] [Google Scholar]
- 58.Meloni F, Cascina A, Miserere S, Perotti C, Vitulo P, Fietta AM. Peripheral CD4(+)CD25(+) TREG cell counts and the response to extracorporeal photopheresis in lung transplant recipients. Transplantation proceedings. 2007 Jan-Feb;39(1):213–217. doi: 10.1016/j.transproceed.2006.10.227. [DOI] [PubMed] [Google Scholar]
- 59.Benden C, Speich R, Hofbauer GF, Irani S, Eich-Wanger C, Russi EW, et al. Extracorporeal photopheresis after lung transplantation: a 10-year single-center experience. Transplantation. 2008 Dec 15;86(11):1625–1627. doi: 10.1097/TP.0b013e31818bc024. [DOI] [PubMed] [Google Scholar]
- 60.Buckhart G, Canafax D, Yee G. Cyclosporine monitoring. Drug Intelligence Clinical Pharmacology. 1986;20(9):649–652. doi: 10.1177/106002808602000901. [DOI] [PubMed] [Google Scholar]
- 61.Hellerstein S, Alon U, Warady BA. Creatinine for estimation of glomerular filtration rate. Pediatr Nephrol. 1992;6(6):507–511. doi: 10.1007/BF00866485. [DOI] [PubMed] [Google Scholar]


