Abstract
Objectives
The introduction of highly active antiretroviral therapy (HAART) leads to control of HIV replication to <50 copies/ml, similar to levels in “elite controllers”. Low-level viral replication may be one of the contributing factors to persistent immune activation/inflammation in HAART treated individuals. There are still gaps in our knowledge whether low level replication persists in systemic versus mucosal sites.
Design and Methods
Subjects for this study were recruited from the Women’s Interagency HIV Study. We evaluated 33 “elite controllers” who naturally controlled HIV replication and 33 matched HAART-suppressed recipients. This study employed a sensitive target-capture transcription-mediated-amplification assay to compare low-level virus concentrations in plasma and cervical-vaginal lavage (CVL) samples from HIV+ HAART recipients and “elite controllers”.
Results
The median (IQR) plasma viral load S/Co for “elite controllers” was 10.5 (3.9, 21.1) which was significantly (p<0.001) higher than the S/Co for HAART recipients (2.0 (1, 4.9])). The majority of CVL samples from both groups had undetectable HIV RNA and the proportion of CVL samples with a cutoff >1.0 was not different between “elite controllers” and HAART-suppressed recipients.
Conclusions
This study demonstrated persistent low-level HIV replication in “elite controllers”, suggesting potential value of HAART treatment for these individuals. Absent or very low levels of HIV RNA in CVL indicate very low risk of secondary sexual transmission for both groups.
Introduction
Currently available highly active antiretroviral therapy (HAART) regimens can control HIV replication to <50 copies/mL and improve clinical outcomes (1). The development of more sensitive HIV RNA assays has enabled detection of persistent viral replication in HAART-recipients, but the clinical importance of low-level viremia is not currently understood (2). Persistent low-level virus replication may contribute to ongoing immune activation/inflammation, and to non-HIV co-morbidities including cardiovascular disease, bone disease, and neurocognitive decline (3–4).
Some HIV-infected individuals control HIV replication to <50 copies/mL without HAART (5). These individuals, termed “elite controllers”, represent less than 1% of participants in most HIV cohorts. “Elite controllers” often have innate (e.g., NK cell) and adaptive (e.g., HIV-specific CD8+ T cell) immune responses that contribute to the control of viral replication (6). In addition, various host genetic factors are associated with viral control in “elite controllers”, including polymorphisms of CCR5, number of copies of the CCL3L1 gene and specific HLA haplotypes (7). The level of activation of HIV-specific CD4+ and CD8+ T-cells has been shown to be elevated in “elite controllers” relative to HAART recipients and HIV-uninfected controls, which may reflect ongoing T cell responses stimulated by low level viral replication (8).
Few studies have compared the level of viremia in “elite controllers” to that found among patients on suppressive HAART regimens (9–10). We addressed this question in the Women’s Interagency HIV Study (WIHS), the largest prospective cohort study of HIV-infected and uninfected women in the US. In addition to evaluating low level plasma viremia among “elite controllers” and a matched group of HAART-suppressed women, WIHS provided the unique opportunity to evaluate viral load in cervical-vaginal lavage (CVL) fluids of these subjects. To our knowledge there have been no studies to date that have determined and compared CVL viral load, using highly sensitive methods, among “elite controllers” and HAART-suppressed participants.
Methods
WIHS enrolled 2054 HIV-infected and 569 uninfected women during 1994–95. Another 737 HIV-infected and 406 uninfected women enrolled during 2001–02 (11,12). For this analysis the study population comprised 33 “elite controllers” and 33 matched HAART recipients. “Elite controllers” were HIV-infected, ARV-naïve individuals with plasma viral loads <80 copies/mL (assay available at time of analysis) for ≥2 years. One viral blip (80–1000 copies/mL) was allowed during this 2-year interval. HAART recipients were those with viral loads ≤80 copies/mL for ≥2 years. We used propensity-score methods to match HAART recipients to “elite controllers” on age, race, body mass index, HCV antibody status, CD4 T-cell count, and study visit. We selected for testing ≥3 visits per person during the phenotypic period: the start, end, and alternating visits for each “elite controller”, and the start, end, and median visit for each HAART recipient. We selected CVL samples from 20 HIV+ viremic subjects who did not report using HAART to evaluate the performance of the viral load assay on CVL samples.
Longitudinal plasma and CVL samples were tested for HIV RNA levels using the FDA-approved isothermal TC-TMA assay (Aptima®, Gen-Probe, San Diego, CA). This assay is used to detect very early HIV infection in both blood donor screening and clinical diagnostic settings (13). The assay utilizes 0.5 mL of plasma, and in this study was performed in quadruplicate, testing a total of 2.0 mL of plasma or CVL samples. Replicate testing allows for an improved lower detection limit of ~1 copy/mL, compared to a 50% detection limit of 3.6–14 copies when the assay is performed in singlet. The assay results are reported as a signal/cutoff (S/Co) ratio. The range of the ratio is 0–30, with an S/Co >1.0 classified as HIV RNA “positive”. Panels comprised of HIV-1 clade B virions spiked into plasma and CVL samples were prepared by the NIAID AIDS Clinical Trials Group Viral Quality Assurance (ACTG VQA) laboratory to establish the limits of detection and dynamic range for quantitation of HIV by the replicate TC-TMA assay as well as for quality control during the course of clinical sample testing.
Comparisons of baseline characteristics between phenotypic groups were conducted using chi-square analysis or Fisher’s exact tests for categorical variables and Wilcoxon rank tests for continuous measures. Longitudinal data were analyzed using linear mixed models incorporating left-censoring (14) for the continuous outcomes and GEE models for the categorical outcomes. To normalize distributions, plasma and CVL S/Co data were transformed by base 10 logarithms. Non-parametric trend analyses over time utilized Mann-Kendall tests. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC) and graphics were generated using R package version 2.15.2.
Results
Participant Characteristics at Baseline
Complete data on all study variables were available for 32 “elite controllers” and 31 matched HAART recipients. The groups were well-matched with respect to age, race/ethnicity, HCV status and body mass index at baseline. The median evaluation period was 4 years for “elite controllers” and 4.5 years for HAART recipients (p>0.05 for all matching factors).
TMA Assay Performance
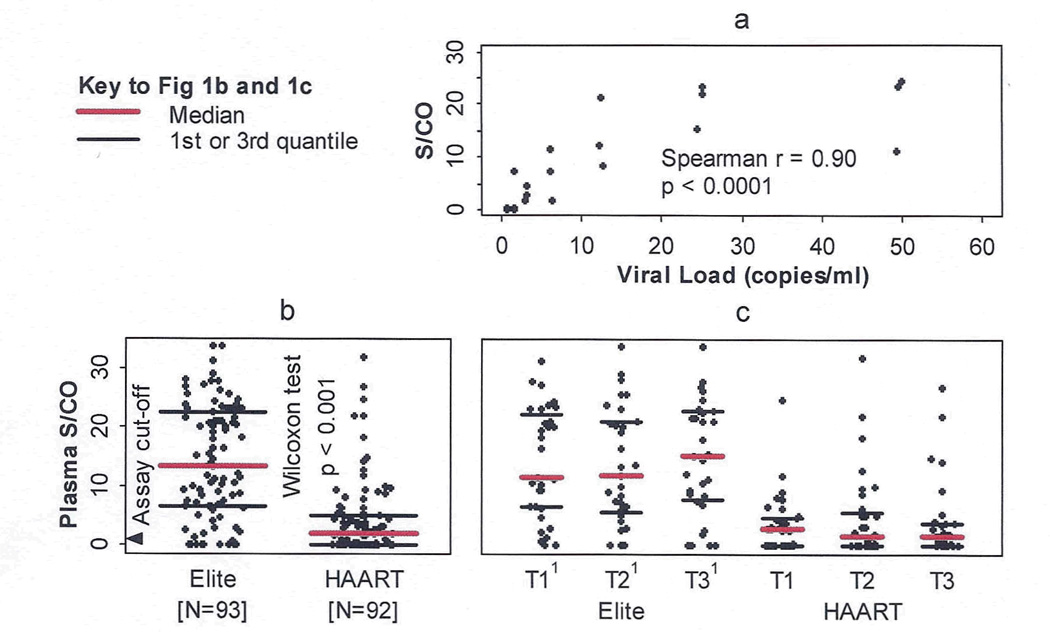
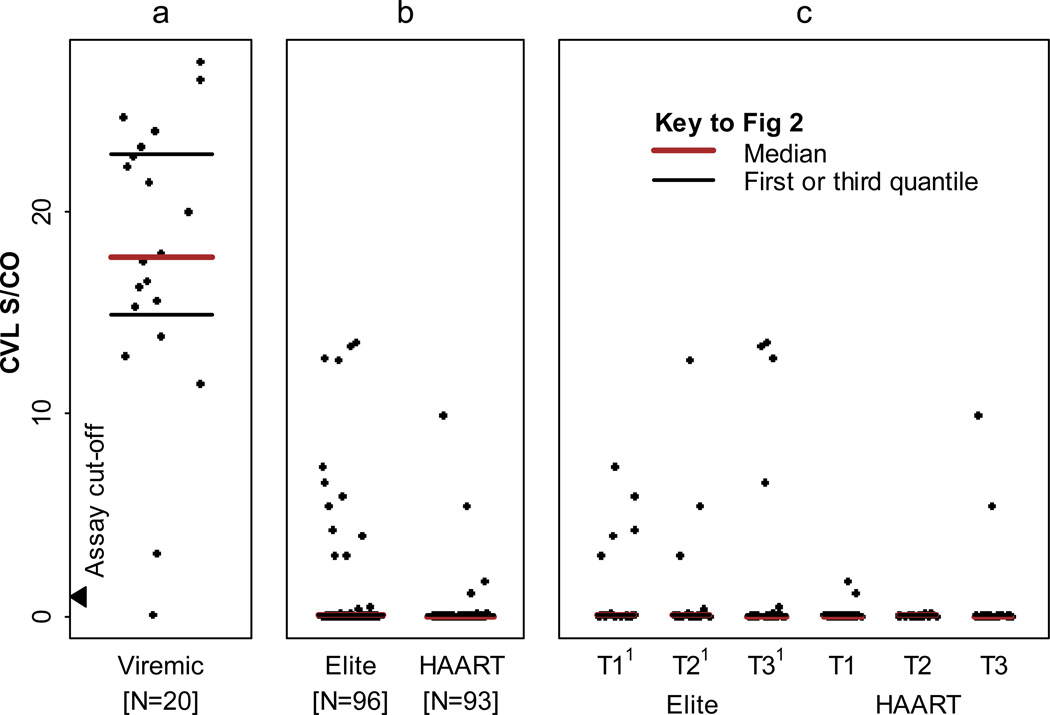
Figure 1a presents the correlation of average S/Co values of 4 replicate TC-TMA assays with estimated viral loads in serially diluted standards prepared by the ACTG VQA laboratory and tested under code. The results demonstrated a lower limit of sensitivity of 1–3 copies/mL and a dynamic range up to 30–50 copies/mL with a highly significant (r=0.90 p=<0.0001) correlation between input viral levels and average S/Co of the replicate TC-TMA assay (Figure 1a). The low level viral load quality control panel demonstrated excellent assay specificity (no signal in replicate TC-TMA testing of non-spiked QC samples), consistent high S/Co values in the 50 copy/mL QC samples, and intermediate reactivity in the 5 copy/mL QC samples. Spiking of non-diluted CVL samples from HIV-uninfected control women with 50 and 500 HIV copies/ml yielded S/Co values ranging from 6.9–8.2 and 21.9–23.0, respectively. CVL samples spiked with 5 HIV copies/mL and non-spiked CVL samples yielded consistently negative results (S/Co values <0.24). We also tested CVL samples from 20 HIV viremic WIHS women who were not on HAART at the time of sampling and had plasma viral loads selected to be representative of the distribution of plasma viremia in untreated women. All except one of these samples tested positive for HIV RNA by TC-TMA, with S/Co values ranging from 0.15 to 27 (Figure 2a).
Fig 1.
(a) Correlation of HIV RNA with average S/CO values of replicate TC-TMA assays. Plasma from an HIV- donor was spiked with serially diluted HIV RNA (copies/ml 0.78 to 50) and tested by TC-TMA in quadruplicate on 3 separated days. (b) Low level plasma HIV RNA levels are increased in elite controllers, compared to HAART suppressors. (c) Longitudinal evaluation of low level plasma HIV RNA. Mann-Kendall test shows no trend exists over time in each of the two groups (p = 0.72 and 0.85 respectively).
1T1, T2, T3: the start, middle, and end visit of phenotypic period respectively
Fig 2.
Low level CVL HIV RNA in three different phenotypic groups. (a) CVL HIV RNA in representative viremic subjects off HAART. (b) Elite controllers compared to HAART suppressors. (c) Logitudinal evaluation of CVL HIV RNA in elite controllers and HAART suppressors.
1T1, T2, T3: the start, middle, and end visit of phenotypic period respectively
HIV RNA Levels in Plasma and CVL
We evaluated 93 plasma samples from 32 “elite controllers” and 92 plasma samples from 31 HAART recipients who had valid plasma TC-TMA results. The median (IQR) plasma viral load S/Co for “elite controllers” was 10.5 (3.9, 21.1) which was significantly (p<0.001) higher than the S/Co for HAART recipients (2.0 (1, 4.9]) (Fig 1b). The proportion of plasma samples that were undetectable (at the assay cutoff of 1.0 copies) was significantly lower among “elite controllers” than among HAART recipients (12% vs 45%, p<0.001). Plasma S/Co levels were stable during the entire period of longitudinal analysis among elite controllers (slope = 0.00, p=0.93), while levels decreased slightly but not significantly in HAART recipients (slope = −0.01, p=0.30) (Figure 1c).
Ninety-six CVL samples from 32 “elite controllers” and 93 CVL samples from 31 HAART recipients had valid CVL TC-TMA results (based on reactive internal control results indicating no inhibition). The majority of CVL samples had undetectable HIV RNA based on the average S/Co of replicates below the assay cutoff of 1.0 (Figure 2b); however S/Co values of individual TC-TMA determinations were above the assay cutoff of 1.0 for 12 (13%) elite CVL samples and 4 (4%) HAART CVL samples. The proportion of CVL replicate samples with S/Co>1.0 from “elite controllers” was not significantly different from HAART recipients (p=0.12).
Discussion
Utilizing the replicate TC-TMA assay approach we found that “elite controllers” demonstrated higher frequency of low-level plasma viral loads compared to HAART recipients with undetectable viral loads in standard assays. Among “elite controllers” the low levels of plasma virus were stable whereas there was a non-significant decline in HAART recipients over the 4-year study period. In contrast we did not find significantly elevated S/Co ratios in CVL specimens from “elite controllers” compared to HAART recipients. Of note detectable levels of HIV RNA were documented in CVL samples from viremic, untreated subjects, validating the sensitivity of the TC TMA assay for CVL samples.
These findings are consistent with previously published studies from two well-defined “elite controller“ cohorts. A study by Hatano and colleagues (10) utilized the same TC-TMA assay as our study and found a mean HIV RNA S/Co of 9.3 among elite- versus 6.3 among HAART recipients. Although their finding was not statistically significant among the “elite controllers” the investigators found that 98% of the samples had detectable RNA, a higher proportion than among HAART recipients. In another study of “elite controllers” from Pereyra and colleagues (6), persistent low-level HIV correlated with levels of HIV-specific antibody. A study by Dinoso et al. (9) reported that six “elite controllers” with detectable viral load demonstrated a higher median viral load copy number (25 copies/mL) compared to nine HAART recipients with detectable viral loads (2 copies/ml).
This low level of HIV detected in “elite controllers” may contribute to immune activation and progressive T-cell loss. Other immune factors may also drive persistent immune activation/inflammation, including co-infecting pathogens (e.g. cytomegalovirus) and products of microbial translocation from the gastrointestinal tract. We have previously demonstrated that “elite controllers” show higher levels of CD8+ T-cell activation compared to HAART recipients (15). Persistent immune activation may contribute to non-HIV co-morbidities including cardiovascular disease. The implication for this finding is the potential benefit of antiretroviral treatment for “elite controllers” (16). Indeed the ACTG has initiated a treatment trial for “elite controllers” with the primary outcome being the impact of HAART on immune activation.
In contrast to the CVL samples of the HIV+ viremic women off HAART, we observed the virtual absence of viral RNA in serial CVL specimens from both “elite controllers” and HAART recipients. These results suggest there is probably a very low risk of secondary sexual transmission of HIV from “elite controllers” and HAART recipient women who are aviremic, which is consistent with studies documenting absence of HIV in semen from HAART recipients with controlled viremia and with clinical studies documenting efficacy of HAART in reducing heterosexual transmission of HIV (17).
This study demonstrates that one can gain insights into the pathogenic significance of persistent HIV replication by comparing “elite controllers” to HAART recipients. These individuals provide a unique opportunity to evaluate how natural control of HIV (i.e., in the absence of antiretroviral therapy) impacts the host immune system and subsequent disease outcomes relative to treatment that is observed in HAART suppressed subjects. This study provides potential new insights into the design of trials to define the role of low-level HIV replication contributing to non-HIV co- morbidities and the potential value of treating “elite controllers” to further suppress viral replication to, or potentially even below, levels seen in HAART suppressed patients.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
U01 AA 020800-NIH/NIAAA(SD) R03 A1076011 and the Zumberge Foundation of the University of Southern California (MV) and Reagents for TC-TMA testing were provided by Novartis/Hologic:Gen-Probe at no cost for this study.
Footnotes
The authors do not have a commercial or other interests that might present a conflict of interest.
Portions of this work were previously presented as an abstract:
Landay A, Golub E, Desai S, Anastos K, Durkin H, Young M, Villacres M, Greenblatt R, Norris P, Busch M, Women’s Interagency HIV Study Group. THPE039 – Low level viral load detection in blood and mucosal sites in “elite controllers” and HAART suppressed women enrolled in the Women’s Interagency HIV Study (WIHS). AIDS 2012. XIX International AIDS Conference July 22–27. Washington, DC.
- Alan Landay, Elizabeth Golub and Michael Busch designed the study and wrote the manuscript.
- JinBing Zhang performed the statistical analysis.
- Val Winkelman performed the TMA assay.
- Seema Desai, Kathryn Anastos, Helen Durkin, Mary Young, Maria Villacres, Ruth Greenblatt and Philip Norris participated in the manuscript writing and review.
References
- 1.Pallela FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuhaus J, Angus B, Kowalska JD, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24:697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Pereyra F, Palmer S, Miura T, et al. Persistent low-level viremia in HIV-1 “elite controllers” and relationship to immunologic parameters. J infect Dis. 2009;200:984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fellay J, Shianna KV, Ge D, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(10):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinoso JB, Kim SY, Siliciano RF, et al. A comparison of viral loads between HIV-1-infected elite suppressors and individuals who receive suppressive highly active antiretroviral therapy. Clin Infect Dis. 2008;47:102–104. doi: 10.1086/588791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83(1):329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clinical and Diagnostic Laboratory Immunology. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkan SE, Melnick SL, Preston-Martin S, et al. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. Epidemiology. 1998;9(2):117–125. [Google Scholar]
- 13.Busch MP, Hecht FM. Nucleic acid amplification testing for diagnosis of acute HIV infection: has the time come? AIDS. 2005;19:1317–1319. doi: 10.1097/01.aids.0000180103.65640.d8. [DOI] [PubMed] [Google Scholar]
- 14.Hughes MD. Analysis and design issues for studies using censored biomarker measurements with an example of viral load measurements in HIV clinical trials. Stat. Med. 2000 Dec 15;19(23):3171–3191. doi: 10.1002/1097-0258(20001215)19:23<3171::aid-sim619>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Hunt PW, Landay AL, Sinclair E, et al. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS one. 2011;6(1):e15924. doi: 10.1371/journal.pone.0015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatano H, Yukl SA, Ferre AL, Graf EH, Somsouk M, Sinclair E, Abdel-Mohsen M, Liegler T, Harvill K, Hoh R, Palmer S, Bacchetti P, Hunt PW, Martin JN, McCune JM, Tracy RP, Busch MP, O’Doherty U, Shacklett BL, Wong JK, Deeks SG. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. Plos Pathog. 2013 Oct;9(10):e1003691. doi: 10.1371/journal.ppat.1003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]