Abstract
We examined gene expression in tree shrew choroid in response to three different myopiagenic conditions: minus lens (ML) wear, form deprivation (FD), and continuous darkness (DK). Four groups of tree shrews (n = 7 per group) were used. Starting 24 days after normal eye opening (days of visual experience [DVE]), the ML group wore a monocular −5 D lens for 2 days. The FD group wore a monocular translucent diffuser for 2 days. The DK group experienced continuous darkness binocularly for 11 days, starting at 17 DVE. An age-matched normal group was examined at 26 DVE. Quantitative PCR was used to measure the relative (treated eye vs. control eye) differences in mRNA levels in the choroid for 77 candidate genes. Small myopic changes were observed in the treated eyes (relative to the control eyes) of the ML group (−1.0 ± 0.2 D; mean ± SEM) and FD group (−1.9 ± 0.2 D). A larger myopia developed in the DK group (−4.4 ± 1.0 D) relative to Normal eyes (both groups, mean of right and left eyes). In the ML group, 28 genes showed significant differential mRNA expression; eighteen were down-regulated. A very similar pattern occurred in the FD group; twenty-seven of the same genes were similarly regulated, along with five additional genes. Fewer expression differences in the DK group were significant compared to normal or the control eyes of the ML and FD groups, but the pattern was similar to that of the ML and FD differential expression patterns. These data suggest that, at the level of the choroid, the gene expression signatures produced by “GO” emmetropization signals are highly similar despite the different visual conditions.
Keywords: Myopia, Choroid, Emmetropization pathway, Gene expression, Animal models, Refractive error
1. Introduction
It is well established that a visually-guided emmetropization mechanism operates during post-natal visual development in a wide range of vertebrate species, including humans (Mutti et al., 2005; Norton, 1999; Schaeffel & Howland, 1988; Smith, Hung, & Harwerth, 1999; Wallman & Winawer, 2004). This mechanism uses refractive error, detected by the retina, to adjust the axial elongation rate of the growing eye to achieve a match between the location of the retina and that of the focal plane, reducing the refractive error. Studies that either cut the optic nerve (Raviola & Wiesel, 1985; Troilo, Gottlieb, & Wallman, 1987) or suppressed retinal output (Norton, Essinger, & McBrien, 1994) have found that there is a direct pathway within the eye: emmetropization signals originate in the retina, pass into the retinal pigment epithelium (RPE) and then into the choroid, finally reaching the sclera. In tree shrews (mammals closely related to primates), stimulation of this pathway produces remodeling of the scleral extracellular matrix that alters its biomechanical properties, increasing the viscoelasticity and the axial elongation rate. We will refer to this as the “direct emmetropization pathway” because it can operate, albeit less well, in the absence of an “indirect” pathway comprised of connections from the retina, through central visual structures, that controls accommodation and other potential outputs to the eye and can affect refractive development (Dillingham, Guggenheim, & Erichsen, 2013; McFadden & Wildsoet, 2009; Schaeffel et al., 1990; Wildsoet, 2003).
Minus lens (ML) wear and form deprivation (FD) are two treatments often used to stimulate the emmetropization mechanism (Wallman & Winawer, 2004). Wearing a minus lens, held in place in front of the eye in a goggle frame, shifts the focal plane away from the cornea, creating an artificially hyperopic refractive state. This produces what has been described as a “GO” condition (Rohrer et al., 1993; Schaeffel & Howland, 1988). In response, the lens-wearing eye increases its axial elongation rate, moving the retina to the shifted focal plane at which point the hyperopia is eliminated and the GO condition has dissipated. When the minus lens is removed, the treated eye is myopic. Form deprivation with a translucent diffuser provides ample retinal illuminance but removes the possibility that sharply-focused images can occur on the retina. This also is a GO condition that causes an increase in the axial elongation rate and myopia in the treated eye. However, because elongation cannot restore clear retinal images, the GO condition continues and the elongation rate remains elevated throughout the treatment period.
A third procedure, treatment with a period of continuous darkness (DK), also produces increased axial elongation in tree shrews and in chicks. Tree shrews that were first raised in standard colony lighting with light-on and light-off periods, and then transferred to a completely dark environment, develop an increased axial elongation rate and become myopic compared with age-matched normally-raised animals (Norton, Amedo, & Siegwart, 2006). Chicks placed in DK also exhibit increased axial elongation (Troilo & Wallman, 1991). However, prolonged DK treatment also produces flattening of the cornea so that the birds eventually become refractively hyperopic despite having elongated eyes (Lauber, 1991). The retinal mechanism by which darkness produces a GO condition is still unclear.
Based on behavioral and electrophysiological studies, ML and FD treatments produce different, distinct patterns of excitation and inhibition in the retina that are communicated through the geniculostriate visual pathway to produce differing visual responses. Several studies have suggested that the retinal emmetropization-related signaling produced by these two GO conditions can be distinguished (Bartmann et al., 1994; Bitzer, Feldkaemper, & Schaeffel, 2000; Fujikado et al., 1997; Kee, Marzani, & Wallman, 2001; Schaeffel et al., 1994; Wildsoet, 2003; Yew & Wildsoet, 2003). However, in the sclera, it has been found that ML and FD produce nearly identical gene expression signatures; DK treatment also produces a similar gene expression signature (Guo et al., 2013). It appears that the different retinal activity produced by these three myopiagenic conditions may be converted into a common set of emmetropization signals as it passes through the direct RPE-choroid-sclera emmetropization pathway. Has this consolidation into a common signal occurred at the level of the choroid, or does the choroidal “compartment” of the emmetropization pathway still distinguish amongst the visual conditions that produce a retinally-generated GO condition?
Although changes in levels of proteins or other molecules presumably are key to actually transmitting signals from choroid to sclera, it has been found that changes in mRNA levels can identify the responses of the cells in tissues and are useful in identifying pathways of interest (Gao et al., 2011, 2013; Schippert et al., 2006; Shelton et al., 2008; Siegwart & Norton, 2005; Stone et al., 2011; Zhang, Liu, & Wildsoet, 2012). In a previous paper in tree shrew choroid (He et al., 2014), we examined the gene expression signatures produced by ML wear (GO) and by recovery from induced myopia (a STOP condition). Short-term ML treatment produced a GO gene expression signature that was distinct from the STOP gene expression signature. These results, involving altered gene expression in many genes, have shown that emmetropization-related signaling can be detected in the choroidal compartment of the direct emmetropization pathway. The goal of the present study was to examine alterations in gene expression in the choroid after 2 days of ML wear, 2 days of FD, and after 11 days of DK treatment. The question asked was whether the three GO conditions would produce the same, or very similar, gene expression signatures?
2. Materials and methods
2.1. Experimental groups
The methods employed in this study were generally identical to those in our previous paper (He et al., 2014). The juvenile tree shrews (Tupaia glis belangeri) used in this study were produced in our breeding colony and raised by their mothers on a 14 h light/10 h dark cycle. Tree shrew pups open their eyes about three weeks after birth. The first day both eyes are open is day one of visual experience (DVE). All procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Experimental groups were balanced to include both males and females, and avoided pups from the same parents wherever possible.
There were four groups of animals (n = 7 per group) (Fig. 1). Starting at 24 ± 1 DVE, the ML group wore a monocular −5 D (spherical power) lens for 2 days; the FD group wore a monocular translucent diffuser for 2 days; the DK group was housed in continuous darkness for 11 days, starting at 17 ± 1 DVE. In the ML and FD groups, the untreated fellow eye served as a control. A normal group (26N) was also examined at 26 DVE. Data from the ML and 26N groups were reported in the previous study (He et al., 2014) and are shown here for direct comparison with the FD and DK group results.
Fig. 1.
Experimental groups and duration of treatments. The red vertical bar indicates the point when a dental acrylic pedestal was installed under anesthesia. Filled regions indicate the type and duration of visual treatment. The right end of each bar indicates the time point when mRNA levels were measured.
2.2. Visual treatments
Animals in all groups were anesthetized (17.5 mg ketamine, 1.2 mg xylazine; supplemented with 0.5–2.0% isoflurane as needed) and received a dental acrylic pedestal. For the ML and FD groups, this occurred at 21 ± 1 DVE; in the DK group, the pedestal was installed at 16 ± 1 DVE. After pedestal installation, all animals were placed in individual cages with standard colony fluorescent lighting (GE F34CW WM ECO cool white or F32T8/25W/SPX41/ECO), 100–300 lux on the floor of the cage. In the ML and FD groups, 3 days after pedestal installation, a goggle frame holding a −5 D lens (12 mm diameter PMMA contact lens; Conforma Contact Lenses, Norfolk, VA) or a translucent diffuser was clipped to the pedestal, firmly holding the lens or diffuser in front of the randomly selected treated eye. The untreated fellow control eye had unrestricted vision through the open goggle frame. Lenses were cleaned twice daily (approximately 9:30 AM and 4:30 PM) while diffusers were cleaned only in the morning. During cleaning, goggles were briefly (<3 min) removed under dim illumination and animals were kept in a darkened nest box to minimize exposure to visual stimuli. Animals in the DK group were transferred to continuous darkness 1 day after pedestal installation (at 17 ± 1 DVE) and checked daily with night-vision goggles and infrared illumination; DK treatment ended after 11 days. The 26N group received a pedestal at 21 ± 1 DVE but did not wear a goggle.
2.3. Refractive and axial measures
Non-cycloplegic refractive measures were made, in awake animals, at the start and end of the treatment period with a Nidek ARK-700A infrared autorefractor (Marco Ophthalmic, Jacksonville, FL). Normal animals were measured just before euthanasia. Cycloplegic refractive measures were omitted to prevent any interference by atropine on retino-scleral signaling (McKanna & Casagrande, 1981). However, previous studies have shown that non-cycloplegic measures provide a valid estimate of the refractive state, and of induced myopia, in tree shrews (Norton, Siegwart, & Amedo, 2006; Norton et al., 2000). All refractive values were corrected to the corneal plane and for the small eye artifact (Glickstein & Millodot, 1970), previously shown to be approximately +4 D in tree shrews (Norton, Wu, & Siegwart, 2003).
At the time the pedestal was attached, ocular component dimensions were measured in most of the animals with a Lenstar LS-900 optical biometer (Haag-Streit USA, Mason, OH) to ensure that the two eyes did not differ significantly in axial component dimensions before treatment began. Post-treatment axial component measures were also taken, except for one DK animal. The Lenstar optical biometer allowed these measures to be made quickly, in awake animals, just before euthanasia.
2.4. Choroid dissection
On completion of the final refractive measures, approximately 2–4 h into the light phase, animals were terminally anesthetized (17.5 mg ketamine and 1.2 mg xylazine, followed by 50 mg xylazine). DK animals were euthanized at similar times. Both eyes were enucleated and placed into RNAlater solution (Life Technologies, Carlsbad, CA). Extraocular muscles, conjunctiva, and orbital fat were trimmed from the exterior surface of the eye and the cornea dissected away just behind the corneoscleral junction. While viewing through a surgical microscope, the lens and vitreous humor were removed; the retina and RPE, which were tightly bound to each other, were then lifted from the eyecup. While still immersed in RNAlater, choroid was teased from the scleral inner surface using the rounded ends of forceps, collected, and then frozen in liquid nitrogen. Because the dissection was extremely gentle, it is possible that small portions of the lamina fusca, the outermost layer of the choroid, may have adhered to the sclera in some cases and, thus, not been included in our choroidal sample. Because the retina/RPE separated cleanly from the choroid without dissection, and because the inner surface of the sclera was not disrupted by forceful scraping of the surface (and most scleral fibroblasts are within the layered matrix, not on the inner surface), there is no reason to expect significant contamination of the choroidal samples from either retina/RPE or sclera. As a further precaution, RPE65 mRNA expression was measured in a group of 1-day ML animals for both the choroid and RPE, collected, as above, from the same eye. Whilst RPE65 mRNA abundance was approximately 100 fold greater in RPE than in choroid, its differential (treated vs. control eye) expression was not significantly altered in RPE but was significantly up-regulated in the treated eyes of the choroid sample (He et al., 2014). Thus, the RPE cannot be the source of the mRNA for RPE65 in the choroid. We think it is therefore reasonable to conclude that there was no significant contamination of our choroid sample with RPE.
2.5. Gene expression analysis
Each frozen choroid was homogenized with a disposable pestle (Fisher Scientific, Pittsburgh, PA) from which total RNA was isolated using a RiboPure kit (Life Technologies) according to the manufacturer’s instructions, with the addition of an on-filter DNase treatment. The purified RNA was quantified (NanoDrop Technologies, Wilmington, DE), with an average yield per choroid of 4.5 ± 1.2 μg (mean ± SD). RNA quality was confirmed by denaturing gel electrophoresis (RNA FlashGel; Lonza, Rockland, ME). cDNA was synthesized from 1 μg of total RNA in a final reaction volume of 20 μl using a Superscript III RT kit (Life Technologies) with minor modifications (2.5 μM anchored oligo (dT) 20 primers and DTT omitted). The resultant cDNA was diluted 5-fold and stored at −20 °C until use.
Tree shrew-specific quantitative PCR (qPCR) primers were designed for 77 genes of interest (Table 1) and the reference gene RNA polymerase II (POLR2A) using Beacon Designer v7.7 (Premier Biosoft International, Palo Alto, CA). None of the treatment conditions affected the expression of the reference gene. Primer sequences, amplicon size, and efficiencies are listed in Supplementary Table S1. The selected candidate genes included representatives of three major groupings: signaling, metallopeptidases & TIMPs, and extracellular matrix (ECM) proteins. They were selected from genes that were found to change in preliminary studies of tree shrew choroid during ML along with additional genes that were suggested by a whole-transcriptome analysis of three of the ML animals. All primers were designed to work under the same cycling conditions. All amplicons were located within the coding region and most spanned at least one intron; amplicon identity was verified by gel electrophoresis and sequencing.
Table 1.
Genes examined by functional category, with cellular location of the protein encoded by the gene, and its UniProt accession ID.
| Gene symbol | Protein name | Location | UniProt ID |
|---|---|---|---|
| Signaling – cell surface | |||
| ADORA2A | Adenosine receptor A2a | Cell surface | P29274 |
| AQP4 | Aquaporin 4 | Cell surface | P55087 |
| CHRNA7 | Cholinergic receptor, nicotinic α7 | Cell surface | P36544 |
| DRD2 | Dopamine receptor D2 | Cell surface | P14416 |
| EPHA1 | EPH receptor A1 | Cell surface | P21709 |
| FGFR1 | FGF receptor 1 | Cell surface | P11362 |
| GFRA1 | GDNF family receptor α1 | Cell surface | P56159 |
| GRM5 | Metabotropic glutamate receptor 5 | Cell surface | P41594 |
| IGF2R | Insulin-like growth factor 2 receptor | Cell surface | P11717 |
| INSR | Insulin receptor | Cell surface | P06213 |
| OPN1LW | Opsin 1, long-wave-sensitive | Cell surface | P04000 |
| P2RY1 | Purinergic receptor P2Y, G-protein coupled, 1 | Cell surface | P47900 |
| SCUBE3 | Signal peptide, CUB and EGF-like domain-containing protein 3 | Cell surface | Q8IX30 |
| TNMD | Tenomodulin | Cell surface | Q9H2S6 |
| VIPR1 | VIP receptor 1 | Cell surface | P32241 |
| VIPR2 | VIP receptor 2 | Cell surface | P41587 |
| Signaling – intracellular | |||
| BCO2 | Beta-carotene oxygenase 2 | Intracellular | Q9BYV7 |
| CABP5 | Calcium binding protein 5 | Intracellular | Q9NP86 |
| CAMP | Cathelicidin antimicrobial peptide | Intracellular | P49913 |
| CDC42 | Cell division cycle 42 | Intracellular | P60953 |
| CHAT | Choline O-acetyltransferase | Intracellular | P28329 |
| CYP26B1 | Cytochrome P450 26B1 | Intracellular | Q9NR63 |
| NOS1 | Nitric oxide synthase 1 | Intracellular | P29475 |
| RASGRF1 | Ras-specific guanine nucleotide-releasing factor 1 | Intracellular | Q13972 |
| RLBP1 | Retinaldehyde binding protein 1 | Intracellular | P12271 |
| RPE65 | Retinoid isomerohydrolase | Intracellular | Q16518 |
| S100A12 | Protein S100-A12 | Intracellular | P80511 |
| ZNF185 | Zinc finger protein 185 | Intracellular | O15231 |
| Signaling – transcription regulators | |||
| EGR1 | Early growth response protein 1 | Intracellular | P18146 |
| HIF1A | Hypoxia-inducible factor 1α | Intracellular | Q16665 |
| PER2 | Period circadian clock 2 | Intracellular | O15055 |
| RXRB | Retinoid X receptor β | Intracellular | P28702 |
| VDR | Vitamin D receptor | Intracellular | P11473 |
| Signaling – secreted | |||
| ANGPTL7 | Angiopoietin-related protein 7 | Extracellular | O43827 |
| APOE | Apolipoprotein E | Extracellular | P02649 |
| BMP2 | Bone morphogenetic protein 2 | Extracellular | P12643 |
| BMP4 | Bone morphogenetic protein 4 | Extracellular | P12644 |
| CILP | Cartilage intermediate layer protein 1 | Extracellular | O75339 |
| EGF | Epidermal growth factor | Extracellular | P01133 |
| FAM180A | Family with sequence similarity 180, member A | Extracellular | Q6UWF9 |
| IGF2 | Insulin-like growth factor 2 | Extracellular | P01344 |
| IL1B | Interleukin 1β | Extracellular | P01584 |
| LTBP1 | Latent TGFβ binding protein 1 | Extracellular | Q14766 |
| LTF | Lactotransferrin | Extracellular | P02788 |
| MEST | Mesoderm specific transcript | Extracellular | Q5EB52 |
| NRG1 | Neuregulin 1 | Extracellular | Q02297 |
| NTS | Neurotensin | Extracellular | P30990 |
| PENK | Proenkephalin A | Extracellular | P01210 |
| PI15 | Peptidase inhibitor 15 | Extracellular | O43692 |
| PTX3 | Pentraxin 3 | Extracellular | P26022 |
| SOSTDC1 | Sclerostin domain-containing protein 1 | Extracellular | Q6X4U4 |
| SST | Somatostatin | Extracellular | P61278 |
| TAC1 | Protachykinin 1 | Extracellular | P20366 |
| TGFB2 | Transforming growth factor β2 | Extracellular | P61812 |
| TGFB3 | Transforming growth factorβ3 | Extracellular | P10600 |
| TGFBI | TGFβ-induced protein | Extracellular | Q15582 |
| VIP | Vasoactive intestinal peptide | Extracellular | P01282 |
| Signaling – matricellular | |||
| CYR61 | Protein CYR61 | Extracellular | O00622 |
| NOV | Nephroblastoma overexpressed gene | Extracellular | P48745 |
| THBS1 | Thrombospondin 1 | Extracellular | P07996 |
| THBS2 | Thrombospondin 2 | Extracellular | P35442 |
| TNC | Tenascin C | Extracellular | P24821 |
| MP/TIMP | |||
| ADAMTS4 | ADAM metallopeptidase with thrombospondin motif, 4 | Extracellular | O75173 |
| ADAMTS5 | ADAM metallopeptidase with thrombospondin motif, 5 | Extracellular | Q9UNA0 |
| ADAMTSL3 | ADAMTS-like 3 | Extracellular | P82987 |
| MMP14 | Matrix metallopeptidase 14 | Cell surface | P50281 |
| TIMP2 | TIMP metallopeptidase inhibitor 2 | Extracellular | P16035 |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | Extracellular | P35625 |
| Extracellular matrix | |||
| COL12A1 | Collagen type XII, α1 | Extracellular | Q99715 |
| COL6A6 | Collagen type VI, α6 | Extracellular | A6NMZ7 |
| DCN | Decorin | Extracellular | P07585 |
| FMOD | Fibromodulin | Extracellular | Q06828 |
| MXRA5 | Matrix remodeling associated protein 5 | Extracellular | Q9NR99 |
| NYX | Nyctalopin | Extracellular | Q9GZU5 |
| OGN | Mimecan | Extracellular | P20774 |
| PRELP | Prolargin | Extracellular | P51888 |
| SERPINH1 | Serpin H1 | Intracellular | P50454 |
Relative gene expression was measured by qPCR on a StepOne-Plus Real-Time PCR System using Power SYBR Green PCR Master Mix (both, Life Technologies). Reactions were performed in triplicate in a 15 μl volume containing 300 nM each primer and 0.4 μl cDNA template. Cycling parameters were the same for all assays: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 62 °C for 60 s. Single gene products were obtained for all reactions as assessed by melt curve analysis. Relative gene expression was calculated using the ΔΔCt method (Livak & Schmittgen, 2001) to first normalize the expression level of the target gene to that of the reference gene, and then to compare the relative expression of the target gene for treated vs. control eyes, treated vs. normal eyes, and control vs. normal eyes. For DK animals the average of the right and left eyes was compared with normal eyes, and with the control eyes of the ML and FD groups. The geometric group mean (for the 7 biological replicates) of these expression ratios was used to calculate the fold change in gene expression for each of the target genes.
2.6. Statistical analysis
One-way analysis of variance (ANOVA; Statistica, Statsoft, Tulsa, OK) was used to compare control and normal eye refractive data across groups of animals; paired t-tests were used to determine if significant myopia (treated eye vs. control eye, DK vs. normal) had developed. For gene expression data, paired t-tests were used to assess treated eye vs. control eye differences; unpaired t-tests were used to test for gene expression differences between all independent groups. For both t-tests and ANOVA, p < 0.05 was considered significant and no adjustment for possible false discovery rate was applied. Linear regressions between expression differences were made in SigmaPlot (Systat Software, San Jose, CA).
3. Results
3.1. Refraction
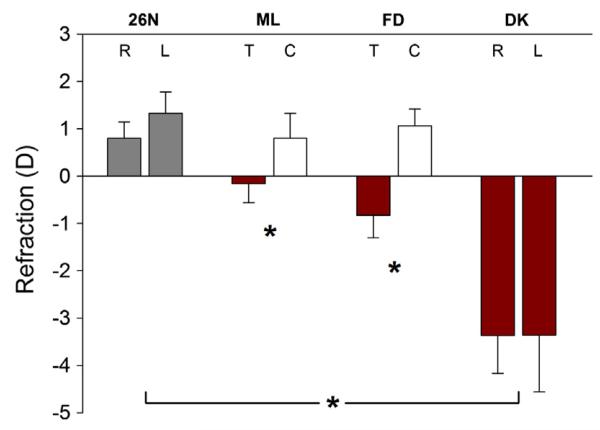
The final refractive values of the normal, treated, and control eyes in the four groups are shown in Fig. 2. As expected in tree shrews at 26 DVE, both eyes of the normal group were slightly hyperopic (right eyes, 0.8 ± 0.4 D; left eyes, 1.3 ± 0.4 D; mean ± SEM). After 2 days of treatment, the ML treated eyes showed a small, statistically-significant myopic shift; the treated eyes were −1.0 ± 0.2 D myopic in comparison to the control eyes. After 2 days of form deprivation, the treated eyes in the FD group also were significantly myopic (−1.9 ± 0.2 D) relative to control eyes. After 11 days of dark treatment, the DK group exhibited a statistically-significant myopic shift (−4.4 ± 1.0 D) compared with the 26N group (both groups, mean of right and left eyes). The control eyes in the ML and FD groups did not differ significantly from the 26 DVE normal eyes (one-way ANOVA, p = 0.79).
Fig. 2.
End-of-treatment refractive measures for the normal, ML, FD, and DK groups. Values are the mean refraction ± SEM for the right (R) and left (L) eyes of the 26N and DK groups or for the treated (T) and control (C) eyes of the ML and FD groups. ML and FD treated eyes were significantly different relative to control eyes; the DK eyes were myopic compared with the eyes of the 26N group; indicated by asterisks.
Ocular component dimensions, measured with the Lenstar, confirmed that the vitreous chamber of the treated eyes had elongated, relative to the control eyes. In the ML group, the vitreous chamber of the treated eyes (measured to the front of the retina) was 0.016 ± 0.004 mm (mean ± SEM) larger than in the control eyes. In the FD group, the treated eye vitreous chamber was 0.038 ± 0.011 mm longer than in the control eyes. The vitreous chamber in the DK group (2.94 ± 0.03 mm) was significantly larger than the vitreous in the 26N group (2.86 ± 0.02 mm). It also was significantly longer than the vitreous chamber in the control eyes of the ML (2.83 ± 0.02 mm) and FD (2.86 ± 0.02 mm) groups. Corneal thickness, anterior chamber depth, and lens thickness did not differ between treated vs. control eyes or between the 26N and DK groups. The choroid was slightly thinner in the treated eyes of the ML group (0.60 ± 0.005 μm vs. 0.65 ± 0.005 μm) and FD group (0.58 ± 0.002 μm vs. 0.062 ± 0.003 μm) but the differences were not statistically significant. Thus, the refractive myopia was primarily the result of a larger vitreous chamber depth.
3.2. Gene expression
3.2.1. Normal and DK groups
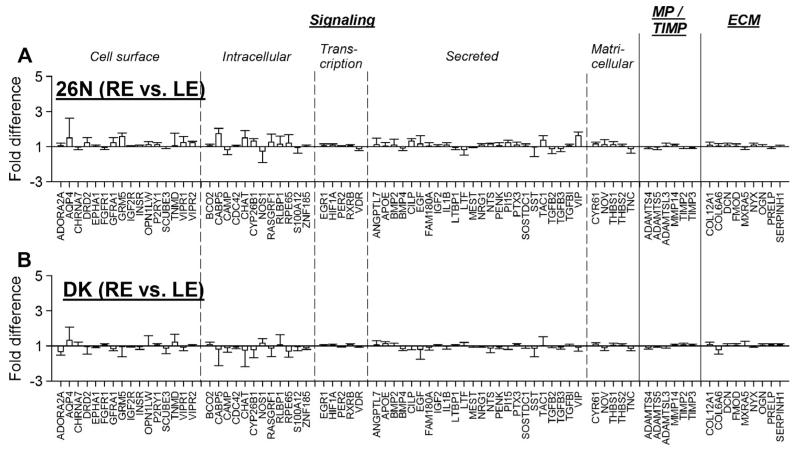
The mRNA levels in normal eyes, and the variability between normal left and right eyes, can provide a basis for comparison with the levels and variability found in treated and control eyes. Fig. 3A compares gene expression in the left and right eyes of the 26N group reported previously (He et al., 2014). Expression values for all 77 genes are provided in Table 2. Expression levels did not differ significantly between left and right eyes for any of the 77 genes. Fig. 3B compares gene expression in the left and right eyes of the DK group. There were no significant differences between left and right eye mRNA levels for any of the 77 genes. Thus, the differences found in the treatment groups can be attributed to the experimental conditions.
Fig. 3.
Gene expression fold differences between right and left eyes for the (A) 26N group and (B) DK group. Headings separated by vertical dashed lines indicate functional grouping of the protein products of the genes. Error bars = SEM. No gene showed significant regulation in either group. The data shown in (A) are reproduced from He et al. (2014).
Table 2.
Gene expression differences comparing right vs. left, treated vs. control, treated vs. normal, and control vs. normal eyes. MLC = ML control eye, FDC = FD control eye. Red text = significant down-regulation, blue = significant up-regulation, grey = expression difference not statistically significant. “X” = differential expression likely due to control eye effect.
| 26N |
ML |
FD |
DK |
DK |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RE vs. LE | T vs. C | T vs. N | C vs. N | T vs. C | T vs. N | C vs. N | T vs. MLC | T vs. FDC | T vs. 26N | RE vs. LE | |
| Signaling – Cell surface | |||||||||||
| ADORA2A | 1.06 | −1.24 | −1.88 | −1.52 | −1.01 | 1.57 | 1.58 | 1.37 | −1.74 | −1.10 | −1.32 |
| AQP4 | 1.50 | 1.46 | 1.65 | 1.12 | 1.38 | −1.24 | −1.72 | −1.74 | 1.11 | −1.55 | 1.31 |
| CHRNA7 | −1.03 | 1.15 | 1.42 | 1.23 | 1.02 | 1.19 | 1.17 | 1.57 | 1.50 | 1.94 | 1.00 |
| DRD2 | 1.21 | 1.68 | 1.70 | 1.01 | 1.07 | 1.00 | −1.06 | −1.18 | −1.10 | −1.17 | −1.04 |
| EPHA1 | 1.01 | −1.55 | −1.75 | −1.13 | −1.85 | −1.81 | 1.02 | −1.84 | −2.13 | −2.09 | −1.05 |
| FGFR1 | −1.05 | −1.68 | −1.87 | −1.11 | −1.14 | −1.43 | −1.25 | −1.47 | −1.30 | −1.63 | 1.05 |
| GFRA1 | 1.22 | −1.12 | −1.18 | −1.05 | 1.05 | −1.00 | −1.06 | −1.44 | −1.44 | −1.52 | −1.17 |
| GRM5 | 1.57 | 1.34 | 1.50 | 1.12 | −1.36 | 1.34 | 1.83 | −1.26 | −2.06 | −1.13 | −1.05 |
| IGF2R | 1.01 | −1.16 | 1.01 | 1.17 | 1.03 | 1.34 | 1.31 | −1.07 | −1.19 | 1.10 | −1.01 |
| INSR | 1.07 | −1.49 | −1.76 | −1.18 | 1.10 | 1.21 | 1.10 | −1.24 | −1.45 | −1.46 | −1.05 |
| OPN1LW | 1.12 | 1.13 | 1.07 | −1.06 | 1.80 | 1.00 | −1.79 | −1.59 | 1.06 | −1.69 | 1.00 |
| P2RY1 | 1.12 | 1.29 | −1.24 | −1.60 | 1.31 | 1.24 | −1.06 | 1.24 | −1.22 | −1.29 | 1.06 |
| SCUBE3 | −1.00 | −1.62 | −1.28 | 1.27 | −1.70 | −1.13 | 1.50 | −2.53 | −3.00 | −2.00 | −1.13 |
| TNMD | 1.06 | −1.27 | −2.23 | −1.76 | −1.69 | −2.59 | −1.53 | −1.06 | −1.22 | −1.86 | 1.22 |
| VIPR1 | 1.24 | 1.11 | 1.03 | −1.07 | 1.37 | −1.60 | −2.19 | −1.03 | 1.98 | −1.11 | −1.08 |
| VIPR2 | 1.22 | −1.55 | −1.63 | −1.05 | −1.23 | −1.26 | −1.02 | 1.06 | 1.03 | 1.01 | 1.02 |
| Signaling – Intracellular | |||||||||||
| BCO2 | 1.06 | −1.48 | −1.33 | 1.11 | −1.60 | −1.10 | 1.46 | −1.37 | −1.79 | −1.23 | 1.08 |
| CABP5 | 1.74 | 2.14 | 2.14 | 1.00 | −1.27 | −1.38 | −1.09 | −1.15 | −1.05 | −1.15 | −1.19 |
| CAMP | −1.19 | 2.25 | 4.00 | 1.78 | −1.39 | 1.63 | 2.27 | 1.05 | −1.22 | 1.86 | −1.11 |
| CDC42 | 1.02 | −1.25 | −1.46 | −1.16 | −1.05 | 1.02 | 1.07 | 1.18 | −1.05 | 1.02 | −1.07 |
| CHAT | 1.49 | 1.22 | 1.70 | 1.39 | −1.36 | 1.36 | 1.84 | −1.33 | −1.76 | 1.05 | −1.24 |
| CYP26B1 | 1.31 | −4.05 | −3.28 | 1.24 | −2.32 | −2.16 | 1.07 | −5.34 | −4.64 | −4.32 | −1.22 |
| NOS1 | −1.27 | 1.53 | −1.62 | −2.46 | 1.25 | −2.44 | −3.07 | −2.19 | −1.76 | −5.39 | 1.16 |
| RASGRF1 | 1.26 | 1.06 | 1.43 | 1.34 | −1.29 | 1.57 | 2.03 | 1.29 | −1.17 | 1.73 | −1.13 |
| RLBP1 | 1.14 | 2.76 × | −1.22 | −3.37 | 4.10 × | −2.80 | −11.47 | −1.51 | 2.26 | −5.08 | 1.06 |
| RPE65 | 1.20 | 2.28 | 1.59 | −1.43 | 4.80 × | 1.56 | −3.08 | 1.40 | 3.02 | −1.02 | −1.31 |
| S100A12 | −1.04 | 1.62 | 2.34 | 1.45 | −1.50 | 1.60 | 2.40 | −1.39 | −2.31 | 1.04 | −1.05 |
| ZNF185 | 1.01 | −1.35 | −1.62 | −1.20 | −1.36 | −1.22 | 1.12 | −1.75 | −2.35 | −2.10 | −1.11 |
| Signaling – Transcription | |||||||||||
| EGR1 | 1.05 | −1.33 | −1.42 | −1.07 | −1.31 | −1.53 | −1.17 | 1.23 | 1.33 | 1.14 | 1.04 |
| HIF1A | 1.07 | 1.20 | 1.07 | −1.12 | 1.11 | 1.09 | −1.02 | 1.22 | 1.12 | 1.09 | 1.03 |
| PER2 | 1.02 | −1.35 | −1.36 | −1.01 | −1.06 | 1.27 | 1.35 | −1.52 | −2.09 | −1.54 | −1.00 |
| RXRB | 1.05 | −1.22 | −1.28 | −1.05 | 1.01 | 1.13 | 1.11 | 1.17 | −1.13 | −1.01 | 1.07 |
| VDR | −1.12 | −1.69 | −1.86 | −1.10 | −1.03 | −1.12 | −1.09 | −1.27 | −1.28 | −1.40 | −1.03 |
| Signaling – Secreted | |||||||||||
| ANGPTL7 | 1.11 | 1.27 | 1.17 | −1.09 | 1.49 | 1.16 | −1.29 | 1.44 | 1.70 | 1.32 | 1.06 |
| APOE | 1.04 | 1.49 | 2.83 | 1.90 | 1.20 | −1.74 | −2.09 | −1.18 | 3.36 | 1.61 | 1.13 |
| BMP2 | 1.09 | 1.54 | 1.18 | −1.30 | 1.91 × | 1.13 | −1.70 | 1.40 | 1.83 | 1.08 | 1.00 |
| BMP4 | −1.08 | 1.48 | 1.48 | 1.00 | 1.58 | 2.02 | 1.28 | 3.41 | 2.68 | 3.42 | −1.16 |
| CILP | 1.31 | −3.00 | −2.52 | 1.19 | −3.03 | −2.11 | 1.43 | −2.39 | −2.88 | −2.01 | −1.01 |
| EGF | 1.16 | 2.09 | 1.38 | −1.51 | 4.13 | 1.49 | −2.77 | −8.47 | −4.62 | −12.79 | −1.21 |
| FAM180A | 1.03 | −1.60 | −1.58 | 1.01 | −1.49 | −1.34 | 1.11 | −1.78 | −1.96 | −1.76 | −1.08 |
| IGF2 | 1.01 | 1.24 | 1.44 | 1.16 | 1.32 | 1.41 | 1.07 | 1.14 | 1.23 | 1.32 | 1.02 |
| IL1B | 1.10 | 1.05 | 1.16 | 1.11 | 1.09 | 1.40 | 1.28 | 1.43 | 1.24 | 1.58 | −1.03 |
| LTBP1 | −1.05 | 1.03 | −1.15 | −1.19 | 1.01 | −1.08 | −1.09 | −1.02 | −1.12 | −1.22 | 1.02 |
| LTF | −1.19 | 1.36 | 2.20 | 1.62 | −1.32 | 1.21 | 1.59 | −1.74 | −1.71 | −1.08 | 1.01 |
| MEST | −1.02 | −1.64 | −1.55 | 1.06 | −2.30 | −1.94 | 1.19 | −2.16 | −2.41 | −2.03 | −1.01 |
| NRG1 | 1.09 | 1.47 | 1.55 | 1.06 | 1.52 | 1.76 | 1.16 | 2.38 | 2.17 | 2.52 | −1.02 |
| NTS | 1.15 | 1.41 | 1.03 | −1.36 | 1.37 | −1.01 | −1.39 | 1.63 | 1.67 | 1.20 | −1.12 |
| PENK | 1.06 | −1.73 | −1.90 | −1.09 | −1.60 | −1.13 | 1.41 | −1.96 | −3.03 | −2.14 | −1.06 |
| PI15 | 1.22 | 3.41 | 2.87 | −1.19 | 5.84 | 6.56 | 1.12 | 3.19 | 2.39 | 2.68 | −1.00 |
| PTX3 | 1.10 | −3.48 | −2.25 | 1.55 | −3.77 | −2.45 | 1.54 | −3.09 | −3.08 | −2.00 | 1.07 |
| SOSTDC1 | 1.04 | −1.70 | −2.22 | −1.31 | −2.30 | −1.74 | 1.32 | −1.01 | −1.74 | −1.32 | −1.01 |
| SST | −1.02 | −1.45 | −1.17 | 1.25 | −2.12 | −3.16 | −1.49 | −3.79 | −2.04 | −3.04 | −1.15 |
| TAC1 | 1.35 | 1.22 | 1.28 | 1.05 | −1.03 | 1.02 | 1.06 | −1.25 | −1.26 | −1.19 | 1.01 |
| TGFB2 | −1.12 | −1.03 | −1.72 | −1.67 | 1.09 | −1.56 | −1.71 | 1.44 | 1.47 | −1.16 | −1.01 |
| TGFB3 | −1.10 | −1.07 | 1.03 | 1.10 | 1.07 | −1.46 | −1.56 | −1.36 | 1.26 | −1.24 | −1.07 |
| TGFBI | 1.06 | 1.46 | 1.48 | 1.02 | 1.61 | 1.44 | −1.12 | 1.57 | 1.78 | 1.59 | 1.00 |
| VIP | 1.62 | 1.03 | −1.10 | −1.13 | −1.57 | −1.45 | 1.09 | 1.14 | −1.08 | 1.00 | −1.09 |
| Signaling – Matricellular | |||||||||||
| CYR61 | 1.15 | −1.63 | −1.20 | 1.35 | −2.15 | −1.75 | 1.23 | −1.41 | −1.28 | −1.04 | 1.10 |
| NOV | 1.11 | −2.14 | −2.75 | −1.28 | −1.99 | −2.68 | −1.34 | −1.51 | −1.44 | −1.93 | −1.11 |
| THBS1 | 1.11 | −2.97 | −3.08 | −1.04 | −1.90 | −1.96 | −1.03 | −1.30 | −1.31 | −1.35 | 1.04 |
| THBS2 | 1.04 | −1.98 | −2.25 | −1.14 | 1.02 | 1.13 | 1.10 | 1.16 | −1.08 | 1.02 | 1.04 |
| TNC | −1.12 | −1.24 | 1.21 | 1.49 | 1.20 | −1.19 | −1.43 | −1.22 | 1.43 | 1.22 | −1.15 |
|
| |||||||||||
| MP/TIMP | |||||||||||
| ADAMTS4 | −1.03 | −1.33 | 1.08 | 1.44 | −1.00 | −1.05 | −1.05 | −1.08 | 1.39 | 1.33 | −1.10 |
| ADAMTS5 | −1.03 | −1.06 | −1.20 | −1.13 | 1.03 | −1.25 | −1.28 | 1.04 | 1.17 | −1.09 | −1.02 |
| ADAMTSL3 | 1.02 | −1.99 | −1.20 | 1.65 | −1.85 | −1.19 | 1.55 | −1.69 | −1.59 | −1.02 | −1.01 |
| MMP14 | 1.07 | 1.14 | 1.05 | −1.09 | 1.27 | 1.08 | −1.18 | −1.05 | 1.04 | −1.14 | 1.03 |
| TIMP2 | −1.01 | −1.42 | −1.29 | 1.09 | 1.12 | 1.08 | −1.04 | 1.12 | 1.27 | 1.23 | 1.06 |
| TIMP3 | −1.05 | −1.28 | −1.53 | −1.20 | −1.07 | 1.05 | 1.12 | 1.03 | −1.12 | −1.01 | 1.03 |
|
| |||||||||||
| Extracellular matrix | |||||||||||
| COL12A1 | 1.08 | −1.90 | −1.97 | −1.04 | −1.49 | −1.40 | 1.07 | 1.05 | −1.05 | 1.01 | 1.08 |
| COL6A6 | 1.04 | −1.83 | −1.35 | 1.35 | −1.76 | −1.12 | 1.58 | −1.63 | −1.90 | −1.21 | −1.23 |
| DCN | 1.09 | 1.07 | 1.08 | 1.00 | 1.06 | 1.16 | 1.09 | 1.21 | 1.11 | 1.21 | 1.01 |
| FMOD | 1.01 | −1.19 | −1.82 | −1.52 | −1.15 | −1.87 | −1.63 | −1.20 | −1.12 | −1.83 | 1.03 |
| MXRA5 | −1.03 | −1.18 | −1.04 | 1.13 | −1.02 | 1.11 | 1.13 | −1.26 | −1.26 | −1.12 | 1.00 |
| NYX | 1.09 | −1.09 | −1.26 | −1.16 | −1.06 | 1.35 | 1.43 | 1.18 | −1.27 | 1.02 | −1.02 |
| OGN | 1.00 | −1.32 | −1.70 | −1.29 | −1.37 | −1.26 | 1.08 | −1.08 | −1.50 | −1.38 | 1.03 |
| PRELP | −1.02 | −1.08 | −1.29 | −1.20 | 1.10 | 1.23 | 1.12 | 1.13 | −1.19 | −1.06 | 1.06 |
| SERPINH1 | 1.00 | −1.12 | −1.09 | 1.03 | −1.08 | 1.39 | 1.50 | 1.16 | −1.26 | 1.19 | 1.05 |
3.2.2. ML and FD groups
The ML group (Fig. 4A) had a significant difference in mRNA expression levels between the treated and control eyes for 28 genes; 18 of these genes were down-regulated. Expression values are listed in Table 2.
Fig. 4.
Gene expression fold differences between the treated and control eyes for the (A) ML group and (B) FD group. Headings separated by vertical dashed lines indicate functional grouping of the protein products of the genes. Filled bars represent statistically significant differences between the treated and control eyes (p < 0.05). Bar color is arbitrary and intended to help in comparing the same gene in the two different conditions. Error bars = SEM. The X’s indicate genes for which the significant treated vs. control eye difference was a result of the control eye differing from normal (Table 2). The data shown in (A) are reproduced from He et al. (2014).
The FD group (Fig. 4B) had a pattern of differential mRNA expression that was very similar to that in the ML group in terms of which genes did, and did not, show significant differential expression, the direction of the differential expression, and, generally, in the magnitude of that fold difference. Twenty-seven genes were differentially expressed in both; 17 were down-regulated. In addition, mRNA levels for 5 genes were significantly affected in the FD group that were not altered in the ML group: VIPR2, EGR1, and COL12A1 were down-regulated; EGF and MMP14 were significantly up-regulated. One gene (TIMP3) was significantly down-regulated in ML animals but not altered in the FD group treated eyes.
In almost all cases in both the ML and FD groups, the control eye gene expression was not significantly different from that in the 26N group, suggesting that treated vs. control differences are due to a change in the treated eye. For one gene (RLBP1) in the ML group the differential effect occurred because control eye, but not treated eye, mRNA levels were significantly different from the 26N group. In the FD group, this was the case for three genes (RLBP1, RPE65, and BMP2), as indicated in Fig. 4 and Table 2.
3.2.3. Comparison between ML and FD GO signatures
The similarities in the mRNA expression patterns in the ML and FD groups are illustrated in Fig. 5, which compares differential expression in the ML group (Fig. 4A) with the differential expression in the FD group (Fig. 4B) for the 33 genes whose expression was significantly altered in either group. All of the genes were regulated in the same direction in both conditions and generally by very similar amounts. The correlation was significant (p < 0.0001) with r2 = 0.79. Although the direction of the shared differential expression was the same for these genes, the amplitude (of the fold differences) differed for several genes. PI15, RPE65, RLBP1, and EGF were more strongly up-regulated in the FD group than in the ML group. THBS1 and CYP26B1 were more strongly down-regulated in the ML group than in the FD group.
Fig. 5.
Comparison of the gene expression differences (treated eye vs. control eye) in Fig. 4A (ML) with the differences in Fig. 4B (FD), showing the similar differential expression patterns in the two GO conditions. Values near the dashed line indicate genes that responded similarly in the two conditions. Stars = significant differences for both ML and FD; triangles = significant differences only for ML; squares = significant differences only for FD.
3.2.4. DK group
Because the DK group received binocular treatment, there was not a within-animal control for comparison. In Fig. 6, the gene expression in the DK group (mean of right and left eyes) was compared with the three possible control groups: the control eyes of the ML group (Fig. 6A), the control eyes of the FD group (Fig. 6B), and with the 26N group (mean of right and left eyes) (Fig. 6C). Fold differences for these comparisons are given in Table 2. It is evident in Fig. 6 that similar patterns occurred regardless of which eyes were used as a comparison. Fewer fold differences were statistically significant than in the ML and FD groups treated vs. control eye comparisons, presumably because comparisons were made across groups of animals, rather than within animals. However, 4 genes were significantly regulated in the DK group in all three of the comparisons in Fig. 6: CYP26B1, EGF, and PTX3 were down-regulated, while BMP4 was up-regulated. Whilst EGF was strongly down-regulated in the DK group in comparison with the ML control eyes, the FD control eyes, and the 26N eyes, it was significantly up-regulated in the treated vs. control eyes of the FD group.
Fig. 6.
Gene expression fold differences. (A) DK group (mean of the R and L eyes) vs. ML control eyes. (B) DK group vs. FD control eyes. (C) DK group vs. the 26N group (mean of the R and L eyes). Headings separated by vertical dashed lines indicate functional grouping of the protein products of the genes. Filled bars represent statistically significant differences (p < 0.05). Bar color is arbitrary and intended to help in comparing the same gene in the three different conditions. Error bars = SEM. In (A and C), the off-scale fold differences for EGF are indicated next to the bar.
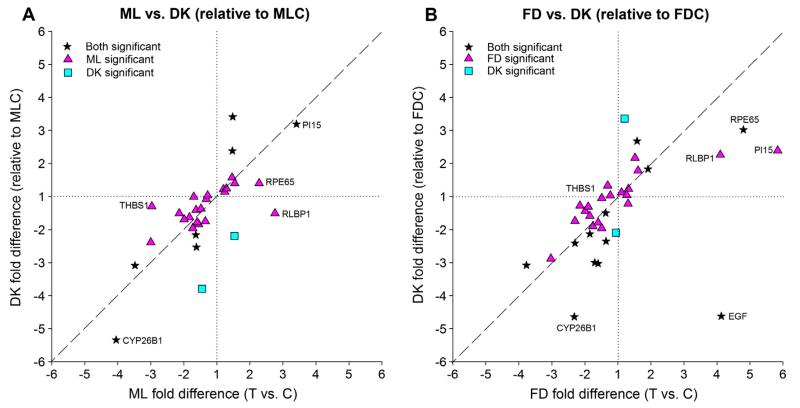
3.2.5. Comparison of DK GO signature with ML and FD GO signatures
Gene expression in the DK group eyes, compared with either the ML or FD control eyes, was similar to that found for the treated vs. control differences for the ML and FD groups. Fig. 7 is organized similarly to Fig. 5. Fig. 7A plots the DK group (relative to the ML control eyes) (Fig. 6A) against the ML treated vs. control eye fold differences (Fig. 4A). Included in the figure are 31 genes that were significantly regulated in either ML (T vs. C), DK relative to the ML control eyes, or both. Fig. 7B plots the DK group (relative to the FD control eyes) (Fig. 6B) against the FD treated vs. control eye differences (Fig. 4B), including 34 genes which were significantly regulated in either FD (T vs. C), DK relative to the FD control eyes, or both. Fig. 7A and B are similar. The comparisons of gene expression in the DK group with the ML and FD groups are more variable than was the comparison between the ML and FD groups (Fig. 5). Yet, the fold differences for nearly all genes are in the upper right or lower left quadrant indicating that they were up- or down-regulated in both DK and ML groups (Fig. 7A) or in both the DK and FD groups (Fig. 7B). The notable exception, in Fig. 7B, is EGF, which was significantly up-regulated in the FD group but down-regulated in the DK group. Also in Fig 7A, EGF is strongly down-regulated in the DK group (−8.47) and so is not shown given the figure’s scale. The correlations (excluding EGF) were significant in both Fig. 7A (p < 0.0001, r2 = 0.50) and Fig. 7B (p < 0.0001, r2 = 0.51).
Fig. 7.
Comparison of the gene expression differences for (A) Fig. 4A (ML treated eyes vs. control eyes) with the differences in Fig. 6A (DK vs. ML control eyes; MLC) and (B) Fig. 4B (FD treated eyes vs. control eyes) with the differences in Fig. 6B (DK vs. FD control eyes; FDC). Values near the dashed line indicate genes that responded with similar fold differences in the two conditions. Stars = significant fold differences for both ML and DK (in A) or both FD and DK (in B); triangles = significant fold differences only for ML or FD; squares = significant fold differences only for DK.
4. Discussion
4.1. A common choroidal GO signature?
In response to myopiagenic visual stimuli, the retinal compartment of the direct emmetropization pathway generates GO signals that are conveyed into the RPE (He, Frost, & Norton, 2014), thence into the choroid (He et al., 2014), and eventually reach the sclera (Guo et al., 2013) where they modulate the axial elongation rate of the growing postnatal eye (Siegwart & Norton, 1998). It seems evident that the three GO conditions used in this study, minus lens wear, form deprivation, and continuous darkness, must produce dramatically different responses in many of the retinal horizontal, bipolar, and amacrine cells and in the patterns of retinal ganglion cell activity that are sent through the optic nerve to central visual structures, yet all three produce a nearly identical response in the scleral fibroblasts (Guo et al., 2013). There is evidence in chicks that the emmetropization-related signaling may differ between ML and FD conditions, but this is a topic of ongoing debate (Morgan, Ashby, & Nickla, 2013). If they do differ, it seems likely that the differences are in the responses of retinal neurons, whose influence is then passed on to the RPE and choroid.
To what degree does the direct emmetropization pathway (as described in the Introduction) distinguish between these different myopiagenic visual conditions after the signals leave the retina? Although efferent influences to the choroid from the “indirect” emmetropization pathway cannot be completely ruled out, the present results suggest that the direct emmetropization mechanism may not distinguish between the different visual stimuli. Similarly, at the level of the scleral fibroblast response Guo et al. (2013) found in tree shrews that ML and FD produce virtually identical scleral gene expression signatures in a sample of 55 genes, and that DK also produces a very similar gene expression pattern. It thus seems relevant to assess whether these three GO conditions produce distinct or similar gene expression patterns in the choroid, which presumably is the primary source of the signals that produce the scleral fibroblast response.
The present study found that the three GO conditions produce very similar gene expression signatures in our sample of 77 genes in the choroid. The ML and FD gene expression signatures are extremely similar. These similarities extend not only to which genes are differentially expressed, and the magnitude of the fold differences, but also to which genes in the sample did not show significant differential expression. The DK signature may be less similar, but it is difficult to be certain without having a within-animal control eye for comparison.
We found only limited evidence of genes that respond specifically to one GO condition but not the others. mRNA expression for EGF was significantly up-regulated (4.13-fold) in FD. It was also up-regulated (2.09-fold) in ML, but the up-regulation was not statistically significant. Whether this represents a real difference between these conditions, or was due to variability in the relatively small groups of seven animals is unclear. However, EGF was dramatically down-regulated in DK choroid when compared with the ML control eyes (−8.47-fold), FD control eyes (−4.62-fold), and normal eyes (−12.79-fold). Evidence for other possible differences is less compelling; mRNA levels for three additional genes, NOS1, PER2, and SST, were significantly down-regulated in the DK eyes in two of the three comparisons but not altered in ML or FD. mRNA for APOE was significantly up-regulated in one of the three comparisons (DK vs. the FD control eyes); it was not regulated in the treated eyes vs. control eyes of the ML and FD groups. Thus, mRNA for EGF, in the DK group, was the only example in the present study of a gene whose regulation might be related to a particular myopiagenic visual condition. However, the overall similarity of the three choroidal GO gene expression signatures suggests that the details of the visual conditions that produce the initial GO signal in the retina are substantially less important in the choroid than they are in the retina.
4.2. Components of the GO signal
A reason to examine the response in choroid to these three myopiagenic stimuli is to learn if there is an essential “core” of key genes that modulate the signaling cascade into the sclera, causing it to remodel, and increasing the rate of axial elongation. The strongest evidence for a single GO response/signal would seem to be the changes that occur in all three myopiagenic conditions. Table 3 shows those genes from our sample whose mRNA levels were significantly modulated during GO. Three genes were consistently modulated in the same direction in all three GO conditions (shaded in Table 3): the treated vs. control eyes of the ML and FD groups, and in the DK treated eyes when compared with all three possible “control” eyes. mRNA for CYP26B1 and PTX3 were down-regulated and mRNA for BMP4 was up-regulated. Using a less stringent criterion, significant differences in mRNA levels in the ML, FD, and two of the three DK group comparisons, six additional genes could be included in the core of the potential GO signal/response. EPHA1, SCUBE3, ZNF185, and MEST were down-regulated; NRG1 and PI15 were up-regulated.
Table 3.
Genes that were significantly regulated under ML and FD (treated vs. control) or DK (vs. ML control, FD control, or Normal) conditions. Red gene symbols = down-regulation, blue = up-regulation, grey shading = significant regulation in all five comparisons, italics = significant regulation in four of the five comparisons.
| Functional category | ML | FD | DK | ||
|---|---|---|---|---|---|
| vs. MLC | vs. FDC | vs. 26N | |||
| Signaling - Cell surface | EPHA1 | EPHA1 | EPHA1 | EPHA1 | |
| SCUBE3 | SCUBE3 | SCUBE3 | SCUBE3 | ||
| VIPR2 | |||||
| P2RY1 | P2RY1 | ||||
| Signaling - Intracellular | BCO2 | BCO2 | |||
| CYP26B1 | CYP26B1 | CYP26B1 | CYP26B1 | CYP26B1 | |
| ZNF185 | ZNF185 | ZNF185 | ZNF185 | ||
| NOS1 | NOS1 | ||||
| RLBP1 | RLBP1 | RLBP1 | |||
| RPE65 | RPE65 | RPE65 | |||
| Signaling - Transcription | EGR1 | ||||
| PER2 | PER2 | ||||
| HIF1A | HIF1A | ||||
| Signaling - Secreted | CILP | CILP | |||
| FAM180A | FAM180A | ||||
| MEST | MEST | MEST | MEST | ||
| PENK | PENK | PENK | |||
| PTX3 | PTX3 | PTX3 | PTX3 | PTX3 | |
| SOSTDC1 | SOSTDC1 | ||||
| SST | SST | ||||
| APOE | |||||
| BMP2 | BMP2 | BMP2 | |||
| BMP4 | BMP4 | BMP4 | BMP4 | BMP4 | |
| IGF2 | IGF2 | ||||
| NRG1 | NRG1 | NRG1 | NRG1 | ||
| PI15 | PI15 | PI15 | PI15 | ||
| TGFBI | TGFBI | ||||
| EGF | EGF | EGF | EGF | ||
| Signaling - Matricellular | CYR61 | CYR61 | |||
| NOV | NOV | ||||
| THBS1 | THBS1 | ||||
| MP/TIMP | ADAMTSL3 | ADAMTSL3 | |||
| TIMP3 | |||||
| MMP14 | |||||
| Extracellular matrix | COL6A6 | COL6A6 | |||
| OGN | OGN | OGN | |||
| COL12A1 | |||||
| FMOD | |||||
The suggestion that these genes might be the essential components of a choroidal GO response must be tempered by several limitations. One is that the “essential GO” signal may change over time and may also reflect the “strength” of the GO signal. The ML and FD groups were examined after 2 days of treatment, and developed slightly different amounts of myopia (−1.0 ± 0.2 D vs. −1.9 ± 0.2 D). The similarity of the mRNA signatures (Fig. 5) may reflect that very similar alterations in gene expression are important early in the development of induced myopia. The differences in expression levels of a few more strongly-affected genes (Fig. 5) may reflect their importance in the amount or rate of myopia development. For instance, the stronger up-regulation of PI15, RPE65, RLBP1, and EGF in the FD group may suggest that they are involved in the higher rate of axial elongation and myopia development. The lower down-regulation of THBS1 and CYP26B1 during FD may also contribute to the more rapid myopia development. The DK group experienced a longer (11 day) treatment period. As is the case with form deprivation, whatever signal is produced by continuous darkness to cause increased axial elongation did not change over time – the darkness was continuous throughout the treatment period. Thus is seems that the choroids in the DK group likely were in the same “GO” situation after 11 days as they were earlier in the treatment period. However, if the choroidal response to the GO condition changes with the duration of treatment, it might help account for the less similar constellation of differentially expressed genes compared with the FD and ML groups. For instance, the dramatic down-regulation of EGF after 11 days of DK may be a late-developing signal related to alterations in circadian signaling (Morgan, Ashby, & Nickla, 2013; Nickla, Wildsoet, & Wallman, 1998).
It also is possible that some of the shared gene expression changes occur because the choroid physically responds similarly in all three myopiagenic conditions (Nickla & Wallman, 2010; Summers, 2013) in ways that may, or may not, be part of the emmetropization signaling cascade. Although the choroid is much thinner in mammals than in chicks, there is evidence in tree shrews (Siegwart & Norton, 1998) that, as in chick, it becomes thinner during myopia development (though the thinning was not statistically significant in the present study). In chicks, there is reduced choroidal blood flow and there could be remodeling of the choroidal extracellular matrix (Summers, 2013). A subset of ten genes in our sample that were significantly altered in GO conditions play a role in vascular regulation and/or angiogenesis (BMP2, BMP4, CYR61, EGF, HIF1A, IGF2, NRG1, PTX3, THBS1, and TIMP3); the altered mRNA level for these genes may have been part of a common choroidal response. It is, of course, unknown if such vascular and/or extracellular matrix changes may not only be part of a local choroidal response, but also part of the emmetropization signal as well. To directly test the possibility that the shared gene expression changes may reflect simply changes in choroidal thinning or vascular flow, future studies might examine these genes in treatments in which the eye elongates without choroidal thinning, or slows its elongation without choroidal thickening (Nickla & Wallman, 2010). It also could not be distinguished in this study the degree to which the choroidal signatures represent the response of choroidal cells to incoming signals from the RPE and the degree to which they are involved in generating signals that are transmitted to the sclera.
An additional limitation in the ability to define the key genes involved in the choroidal compartment of the direct emmetropization pathway is that although our sample is large enough to show that there are similarities and, potentially, to have found differences if they exist, this is nonetheless a relatively small sample. A preliminary whole-transcriptome analysis using mRNA from three of the ML animals suggested that over 300 distinct genes (from the just over 14,000 found to be expressed in the tree shrew choroid) may be up- or down-regulated by at least 1.2-fold (Frost, personal communication, 2013). It may be that genes not included in our sample also respond in the same way to ML, FD, and DK and thus are part of a “core” of genes in the choroidal compartment of the direct emmetropization pathway.
If, as the data of this study suggest, there is a consistent group of genes whose expression responds in the same manner to ML, FD, and DK, it would raise the question of whether the incoming signals from RPE do, or do not, distinguish between these conditions? Once information about defocus has been extracted by retinal neurons, how important, for the direct emmetropization pathway, are the details of the visual scene? The emmetropization mechanism seems to function in a wide range of vertebrate species that exist in many different visual environments. The visual system uses channels comprised of differing neurons to respond to light increments and decrements, to color, direction of motion, etc. Neurons in these channels are specialized to respond to stimuli matched to their receptive-field properties; they have limited sensitivity to other characteristics of the visual scene. Similarly, perhaps, the direct emmetropization pathway may retain only the defocus-related signaling and be insensitive to the visual details. Thus far, we only have evidence in tree shrew choroid and sclera suggesting that vision-specific information may not be encoded. It will be interesting to learn if this is also the case in other species and also in the RPE.
Supplementary Material
Acknowledgments
This study was supported by NIH Grants EY005922 and EY003039 (P30). Li He was supported in part by a supplement to EY005922 and by funds from the Department of Vision Sciences. This work was performed in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Alabama at Birmingham (Li He). Preliminary results were presented in abstract form (the 14th International Myopia Conference, 2013).
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.visres.2014.07.005.
References
- Bartmann M, Schaeffel F, Hagel G, Zrenner E. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Visual Neuroscience. 1994;11:199–208. doi: 10.1017/s0952523800001565. [DOI] [PubMed] [Google Scholar]
- Bitzer M, Feldkaemper M, Schaeffel F. Visually induced changes in components of the retinoic acid system in fundal layers of the chick. Experimental Eye Research. 2000;70:97–106. doi: 10.1006/exer.1999.0762. [DOI] [PubMed] [Google Scholar]
- Dillingham CM, Guggenheim JA, Erichsen JT. Disruption of the centrifugal visual system inhibits early eye growth in chicks. Investigative Ophthalmology & Visual Science. 2013;54:3632–3643. doi: 10.1167/iovs.12-11548. [DOI] [PubMed] [Google Scholar]
- Fujikado T, Kawasaki Y, Suzuki A, Ohmi G, Tano Y. Retinal function with lens-induced myopia compared with form-deprivation myopia in chicks. Graefes Archive for Clinical and Experimental Ophthalmology. 1997;235:320–324. doi: 10.1007/BF01739642. [DOI] [PubMed] [Google Scholar]
- Gao H, Frost MR, Siegwart JT, Jr., Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Molecular Vision. 2011;17:903–919. [PMC free article] [PubMed] [Google Scholar]
- Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- Guo L, Frost MR, He L, Siegwart JT, Jr., Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Investigative Ophthalmology & Visual Science. 2013;54:6806–6819. doi: 10.1167/iovs.13-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Frost MR, Norton TT. Differential gene expression in tree shrew retina compared with retinal pigment epithelium (RPE) in response to six hours of minus-lens wear. Investigative Ophthalmology & Visual Science. 2014;55 ARVO E-Abstract 3037. [Google Scholar]
- He L, Frost MR, Siegwart JT, Jr., Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Experimental Eye Research. 2014;123:56–71. doi: 10.1016/j.exer.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Investigative Ophthalmology & Visual Science. 2001;42:575–583. [PubMed] [Google Scholar]
- Lauber JK. Three avian eye enlargement protocols as myopia models: Effects of pharmacological intervention. Journal of Ocular Pharmacology and Therapeutics. 1991;7:65–75. doi: 10.1089/jop.1991.7.65. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McFadden SA, Wildsoet C. Mammalian eyes need an intact optic nerve to detect the sign of defocus during emmetropisation. Investigative Ophthalmology & Visual Science. 2009;50 ARVO E-Abstract 1620. [Google Scholar]
- McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development. Documenta Ophthalmologica Proceedings Series. 1981;28:187–192. [Google Scholar]
- Morgan IG, Ashby RS, Nickla DL. Form deprivation and lens-induced myopia: Are they different? Ophthalmic and Physiological Optics. 2013;33:355–361. doi: 10.1111/opo.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Investigative Ophthalmology & Visual Science. 2005;46:3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wallman J. The multifunctional choroid. Progress in Retinal and Eye Research. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. The circadian rhythm in intraocular pressure and its relation to diurnal ocular growth changes in chicks. Experimental Eye Research. 1998;66:183–193. doi: 10.1006/exer.1997.0425. [DOI] [PubMed] [Google Scholar]
- Norton TT. Animal models of myopia: Learning how vision controls the size of the eye. ILAR Journal. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT., Jr. Darkness causes myopia in visually experienced tree shrews. Investigative Ophthalmology & Visual Science. 2006;47:4700–4707. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Visual Neuroscience. 1994;11:143–153. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, Jr., Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Investigative Ophthalmology & Visual Science. 2006;47:4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, German AJ, Robertson J, Wu WW. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, induced myopia. Investigative Ophthalmology & Visual Science. 2000;41 ARVO Abstract 563. [Google Scholar]
- Norton TT, Wu WW, Siegwart JT., Jr. Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optometry and Vision Science. 2003;80:623–631. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, Wiesel TN. An animal model of myopia. New England Journal of Medicine. 1985;312:1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Negishi K, Tao J, Stell WK. A role for basic fibroblast growth factor (bFGF) in the visually guided regulation of eye growth in the chick. Investigative Ophthalmology & Visual Science. 1993;34 ARVO Abstract 2489. [Google Scholar]
- Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Research. 1994;34:143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland HC. Mathematical model of emmetropization in the chicken. Journal of the Optical Society of America. 1988;5:2080–2086. doi: 10.1364/josaa.5.002080. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Troilo D, Wallman J, Howland HC. Developing eyes that lack accommodation grow to compensate for imposed defocus. Visual Neuroscience. 1990;4:177–183. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- Schippert R, Brand C, Schaeffel F, Feldkaemper MP. Changes in scleral MMP-2, TIMP-2 and TGFβ-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Experimental Eye Research. 2006;82:710–719. doi: 10.1016/j.exer.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Shelton L, Troilo D, Lerner MR, Gusev Y, Brackett DJ, Rada JS. Microarray analysis of choroid/RPE gene expression in marmoset eyes undergoing changes in ocular growth and refraction. Molecular Vision. 2008;14:1465–1479. [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT, Jr., Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Research. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr., Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Investigative Ophthalmology & Visual Science. 2005;46:3484–3492. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Harwerth RS. Developmental visual system anomalies and the limits of emmetropization. Ophthalmic and Physiological Optics. 1999;19:90–102. [PubMed] [Google Scholar]
- Stone RA, McGlinn AM, Baldwin DA, Tobias JW, Iuvone PM, Khurana TS. Image defocus and altered retinal gene expression in chick: Clues to the pathogenesis of ametropia. Investigative Ophthalmology & Visual Science. 2011;52:5765–5777. doi: 10.1167/iovs.10-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers JA. The choroid as a sclera growth regulator. Experimental Eye Research. 2013;114:120–127. doi: 10.1016/j.exer.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Current Eye Research. 1987;6:993–999. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- Troilo D, Wallman J. The regulation of eye growth and refractive state: An experimental study of emmetropization. Vision Research. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks – Insights from selective lesions of the optic nerve and ciliary nerve. Current Eye Research. 2003;27:371–385. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- Yew K, Wildsoet CF. The usual effects of high-power negative lens and diffusers show differential susceptibility to disruption to the diurnal light cycle. Investigative Ophthalmology & Visual Science. 2003;44 ARVO E-Abstract 1979. [Google Scholar]
- Zhang Y, Liu Y, Wildsoet CF. Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium. Investigative Ophthalmology & Visual Science. 2012;53:6072–6080. doi: 10.1167/iovs.12-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.