Abstract
Despite the fact that humans are exposed to multiple forms of mercury (elemental, inorganic, and organic), most research on mercury toxicity has focused on methylmercury (MeHg) and on neurotoxic outcomes and mechanisms. Recent work has indicated that the immunotoxic effects of mercury compounds may be significant contributors to human disease as well as mechanistically relevant to other target organ toxicities. In this study, we compared the effects of inorganic Hg (iHg) to organic Hg species (MeHg and ethylmercury, EtHg) in human peripheral blood mononuclear cells (PBMCs) in vitro at sub -cytotoxic concentrations, using methods developed to characterize response of human PBMCs to iHg in vitro. PBMCs were isolated from six volunteer blood donors (3 males, 3 females) and cultured in the presence and absence of lipopolysaccharide (LPS) and low levels (up to 200 nM of each Hg species, separately) for 24 hours in culture. Cell culture supernatants were analyzed for cytokine concentrations with a bead-based multiplex assay.
We report that iHg and MeHg both increase pro-inflammatory cytokine release in LPS-stimulated PBMCs, while EtHg decreases IFN-γ release as well pro-inflammatory cytokine release. IL-17 release is significantly increased only in response to iHg treatment. Levels of anti-inflammatory cytokines (IL-1Ra and IL-10) were not significantly altered by any Hg treatment. These results indicate that both organic and inorganic species of Hg can affect the human immune system, but that they may exert different effects on immune function.
Keywords: mercury, immunotoxicity, humans, in vitro, cytokines
1. Introduction
In addition to its well-characterized effects on the nervous system, mercury (Hg)has also been shown to affect immune function, including the induction of a lupus-like autoimmune pathology in genetically susceptible mouse strains as well as lupus-prone strains after exposure to inorganic Hg (iHg)(Nielsen and Hultman, 2002; Pollard et al., 2001; Silbergeld et al., 2005). In some of these models, MeHg and EtHg can also induce autoimmune disease in susceptible mouse strains, though the induction of autoimmune responses by organic species of Hg is slower and somewhat attenuated as compared to iHg (Haggqvist et al., 2005; Havarinasab et al., 2005; Havarinasab and Hultman, 2005; Hultman and Hansson-Georgiadis, 1999).
This information on the immunotoxicity of these Hg compounds is relatively limited at this time. For one reason, most in vitro and in vivo studies have used iHg at relatively high concentrations compared to human exposure data in order to study the effects of Hg on the immune system (Pollard et al., 2005; Silbergeld et al., 2005), which may result in non-specific effects on immune cells through cytotoxicity. Second, most of these studies have utilized iHg (HgCl2), and not MeHg or other organic species. Most human populations are primarily exposed to MeHg through consumption of contaminated fish (Mahaffey et al., 2004; National Research Council (U.S.) Board on Environmental Studies and Toxicology., 2000). The predominant source of human exposure to other organomercurials is ethylmercury (EtHg) exposure to ethylmercury thiosalicylate (thimerosal)used in medical preparations including some vaccines. Thimerosal has been removed from most vaccines in the United States, but its use as a preservative in vaccines continues in other parts of the world (Pichichero et al., 2002).
There are also important transformations among mercury compounds in biological systems. MeHg is demethylated to iHg (Hg2+) in situ in mammalian tissues such as liver, brain, and phagocytic cell populations by an unknown mechanism (Suda and Hirayama, 1992; Suda et al., 1992). Havarinasab et al. recently reported that treatment of mice with MeHg leads to uptake of MeHg into lymphoid tissue followed by progressive demethylation to iHg within the lymph nodes; demethylation was positively correlated with the production of autoantibodies (Havarinasab et al., 2007). Studies of thimerosal toxicokinetics in nonhuman primates indicate that EtHg is more extensively dealkylated to iHg compared with MeHg (Burbacher et al., 2005). Once metabolized, the presence of iHg in brain and other body tissues is persistent (Vahter et al., 1994).
Little is known about comparative immunotoxic effects of exposure to organic versus inorganic Hg species in humans, though epidemiological data support the hypothesis that mercury compounds can affect the human immune system. In case-control studies, Hg exposure (measured as total Hg in urine) has been associated with increased risk of lupus(Cooper et al., 2004)and greater severity of scleroderma (Arnett et al., 1996). Thimerosal has been shown to be a potent allergen in human populations, with a relatively high prevalence of allergic individuals in populations worldwide(Pratt et al., 2004; Slodownik and Ingber, 2005; Wetter et al., 2005; Zoller et al., 2006; Zug et al., 2008). We have reported that environmental and occupational exposures to elemental and iHg are positively correlated with serum levels of autoantibodies (Gardner et al., 2010; Silva et al., 2004). Exposure to MeHg may have a similar, though less severe, impact on the human immune system in terms of autoantibody response, (Silva et al., 2004). These findings were confirmed in another population exposed primarily to MeHg through contaminated fish consumption (Alves et al., 2006). Further, we have shown that patterns of cytokine expression in serum from these Hg-exposed populations are similar to those found in vitro using the methods described here (Gardner et al., 2010).
The goal of this study is to compare the effects of iHg to organic Hg species (MeHg and EtHg) in human cells in vitro at environmentally -relevant, sub-cytotoxic concentrations. For these studies, we used concentrations of Hg within the range of biological exposures (blood levels) reported for each compound. Because animal models indicate that activation of the immune system may elicit the immunotoxic effects of mercury (Abedi-Valugerdi et al., 2005), we evaluated the effects of Hg species on human peripheral blood mononuclear cells (PBMCs) in the presence and absence of the immune stimulant lipopolysaccharide (LPS). Cytokine release was selected as the primary endpoint for evaluation based on the sensitivity and consistency of this endpoint as an indicator of immune response to Hg (Gardner et al., 2009). These findings are consistent with experimental results in which iHg upregulates pro-inflammatory cytokines, in addition to IL-4 and IFN-γ, (Johansson et al., 1997; Zdolsek et al., 1994). IFN-γ is required for iHg-induced disease onset in vivo (Hu et al., 1999; Kono et al., 1998), and IL-1 is required for iHg-induced T cell proliferation in vitro (Pollard and Landberg, 2001). We also examined IL-17 release by PBMCs. Although IL-17 production has not been explicitly explored in animal models of Hg immunotoxicity, it has been shown to be a major regulator of autoimmune disease progression in humans (Garrett-Sinha et al., 2008; Langrish et al., 2005; Wong et al., 2000).
2. Materials and Methods
2.1. Chemicals and reagents
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. PBS, Penicillin-Streptomycin, and L-glutamine were obtained from Mediatech (Manassas, VA). RPMI 1640 and heat-inactivated fetal bovine serum (hiFBS) were obtained from Invitrogen (Carlsbad, CA). LPS was reconstituted in sterile PBS as a 400 ng/ml stock solution and frozen at −20°C in aliquots; all experiments used freshly thawed LPS aliquots from the same batch. Each Hg species was dissolved in sterile PBS for a stock solution concentration of 3 mM, which was filter-sterilized and serially diluted to the working volumes specified below. All plastics used were endotoxin free, and the Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ) and culture media (prior to LPS treatment) had endotoxin levels of less than 0.5 EU.
2.2. Human subjects
Six volunteers (three males and three females) were recruited from the Johns Hopkins Medical Institution community and enrolled in the study following informed consent. Volunteers were required to be between the ages of 18 and 40 years old. In order to avoid obvious sources of variability within the immune system, our exclusion criteria included: personal or immediate family history of autoimmune disease, use of any steroidal medications (including birth control pills), regular use of non-steroidal anti-inflammatory drugs (NSAIDs), receipt of an organ transplant, and pregnancy. Volunteers were asked to donate 20 ml of blood and answer a brief questionnaire about their lifestyle and health status. All volunteers were required to be feeling well and free of illness (to the best of their knowledge) at the time of the blood draw.
The protocol was approved by the Johns Hopkins Bloomberg School of Public Health (JHSPH) Committee on Human Research. No volunteers were exposed by us to Hg in the course of participation in this study.
2.3. Blood collection and cell culture
We employed methods as described in our recent report utilizing human PBMCs to study immunotoxic effects of iHg (Gardner et al., 2009). Briefly, from each participating volunteer a sample of venous blood (20 ml) was collected by a trained phlebotomist (RMG) under aseptic conditions into vacutainer tubes coated with Sodium Heparin (Becton Dickinson, Franklin Lakes, NJ). The whole blood was diluted 1:1 with PBS and layered over Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ) before centrifuging at 1300×g for 30 minutes in order to separate PBMCs. Cells were washed twice with PBS and then cultured at a density of 106 cells/ml in RPMI 1640 media supplemented with 1.77 mM L-Glutamine, 76 μM streptomycin, 44 IU Penicillin, 7.44 mM HEPES, and 8.9% hiFBS and containing 0, 25, 50, 100, or 200 nM of the selected mercury species (HgCl2—iHg, methylmercuric chloride—MeHg, or thimerosal—EtHg, all with PBS as a vehicle). Cells were also separately cultured with the same concentrations of each mercury species in the presence of 50ng/ml LPS. Each treatment group was represented in triplicate. PBMCs were maintained in culture at 37°C and 5% CO2 for 24 hours. After 24 hours, cells were harvested by gentle agitation of the media, followed by centrifugation for 5 minutes at 2000×g. Cell culture supernatants were stored at −80°C until analysis. Cytokine levels were monitored in cell-culture supernatants at 12, 24, and 48 in cells from one volunteer in order to aid in the selection of the cell culture time period.
2.4. Cell viability assay
Cell viability was measured after 24 hours in culture by adding 3 -(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, 0.5 mg/ml in PBS) to each culture well. Cells were incubated with MTT for 4 hours at 37°C and 5% CO2. The MTT solution was aspirated and the cells were treated with 200μl DMSO. An aliquot from each well was then read using a MRX microplate reader (Dynex Technologies, Chantilly, VA), with absorbance read at 560nm.
2.5. Cytokine release measurement
Cell culture supernatants were analyzed for cytokine content using the Bio-Plex Suspension Array system(Bio -Rad, Hercules, CA), multiplex bead-based cytokine assay, according to the manufacturer’s instructions. The following seven cytokines were measured in all samples: Interleukin-1β (IL-1β, detection range 0.6–2527 pg/ml), IL-1 Receptor antagonist (IL-1Ra, 5.5–22,701 pg/ml), IL-4 (0.1–720 pg/ml), IL-10 (0.9–1808 pg/ml), IL-17 (3.3–6985 pg/ml), Interferon- γ (IFN-γ, 6.4–20,882 pg/ml), and Tumor Necrosis Factor-α (TNF-α, 1.6–55,716 pg/ml).
2.6. Statistical analysis
To test if there were significant changes in cell viability at any concentration of any mercury species used, MTT data for each mercury species were analyzed using ANOVA with Bonferroni-corrected pairwise post-tests.
The range of cytokine concentrations observed for each treatment group is described on the arithmetic scale (Table 1), along with the geometric mean response. Because data for cytokine release were right-skewed, the data for each treatment group were log -transformed for display and analysis. In order to examine whether each mercury species affects cell populations and cytokine production in cultures of human PBMCs, we used univariate linear regression on the concentration-response curves to test for a trend as a result of mercury treatment. P[Hg] is defined as the p-value for the slope of the concentration-response curve for each endpoint, which tests the null hypothesis that the slope of the concentration-response curve is equal to zero. Data from LPS-treated cells was analyzed separately from the data gathered for cells treated with Hg only. For analysis, any cytokine measurement that fell below the lower limit of detection (LOD) of the standard curve was assigned a value of the LOD/√2. All statistical analyses were conducted using STATA10 software (STATAcorp LP).
Table 1. Cytokine concentrations by treatment group.
The geometric mean (minimum – maximum) is shown for each treatment group. P[Hg] represents the p-value that tests the trend of the effect of mercury treatment in the concentration-response curve
| + LPS | − LPS | ||||||
|---|---|---|---|---|---|---|---|
| Cytokine | Hg | 0 nM | 200 nM | P[Hg] | 0 nM | 200 nM | P[Hg] |
| IFN-γ | MeHg | 312.6 (184.4 – 496.6) | 337.9 (204.8 – 582.2) | 0.692 | 12.1 (2.0 – 23.4) | 13.1 (4.7 – 18.4) | 0.407 |
| EtHg | 224.9 (81.8 – 358.1) | 0.003 | 11.8 (3.1 – 17.8) | 0.590 | |||
| iHg | 352.5 (199.8 – 503.3) | 0.230 | 13.4 (4.3 – 22.9) | 0.540 | |||
| IL-1β | MeHg | 523.1 (318.5 – 1049.3) | 689.5 (362.6 – 2146.8) | 0.033 | ND | ND | -- |
| EtHg | 419.4 (258.5 – 1410.3) | 0.017 | ND | -- | |||
| iHg | 792.5 (374.3 – 2573.9) | 0.002 | ND | -- | |||
| IL-1Ra | MeHg | 1019.0 (468.8 – 2734.3) | 963.9 (376.2 – 2296.0) | 0.787 | 15.0 (5.4 – 44.0) | 13.6 (7.1 – 45.6) | 0.648 |
| EtHg | 822.2 (387.4 – 2313.3) | 0.232 | 16.6 (8.2 – 51.2) | 0.490 | |||
| iHg | 783.7 (385.1 – 2049.7) | 0.107 | 15.0 (6.5 – 43.3) | 0.899 | |||
| IL-4 | MeHg | 3.3 (1.9 – 5.3) | 3.6 (2.2 – 6.0) | 0.422 | 0.3 (0.1* – 0.7) | 0.3 (0.2 – 0.7) | 0.643 |
| EtHg | 3.0 (1.6 – 4.6) | 0.426 | 0.2 (0.1* – 0.4) | 0.212 | |||
| iHg | 3.6 (1.8 – 5.2) | 0.448 | 0.4 (0.2 – 0.9) | 0.179 | |||
| IL-10 | MeHg | 999.2 (392.9 – 1921.9) | 953.0 (393.4 – 1997.9) | 0.896 | 2.5 (1.3 – 3.9) | 2.6 (1.7 – 3.5) | 0.876 |
| EtHg | 742.5 (234.0 – 1783.2) | 0.190 | 3.2 (1.6 – 3.9) | 0.460 | |||
| iHg | 762.1 (239.1 – 1731.8) | 0.219 | 2.3 (0.8 – 3.7) | 0.552 | |||
| IL-17 | MeHg | 47.4 (21.5 – 61.7) | 56.1 (39.5 – 74.8) | 0.066 | 18.4 (0.7* – 31.2) | 17.8 (0.7* – 36.6) | 0.994 |
| EtHg | 50.7 (30.5 – 78.8) | 0.368 | 20.4 (2.4 – 33.4) | 0.381 | |||
| iHg | 63.6 (48.9 – 75.1) | 0.010 | 18.4 (1.7 – 31.7) | 0.801 | |||
| TNF-α | MeHg | 362.5 (182.3 – 652.7) | 419.5 (211.2 – 830.7) | 0.203 | 2.3 (0.6 – 4.1) | 2.4 (0.7 – 4.0) | 0.570 |
| EtHg | 264.5 (145.3 – 400.0) | 0.002 | 2.3 (0.8 – 3.7) | 0.866 | |||
| iHg | 478.2 (234.4 – 801.6) | 0.015 | 2.4 (0.8 – 4.1) | 0.930 | |||
Indicates imputed value for measurement below limit of detection (LOD/√2)
3. Results
3.1. Low concentrations of inorganic and organic species of mercury do not affect cell viability
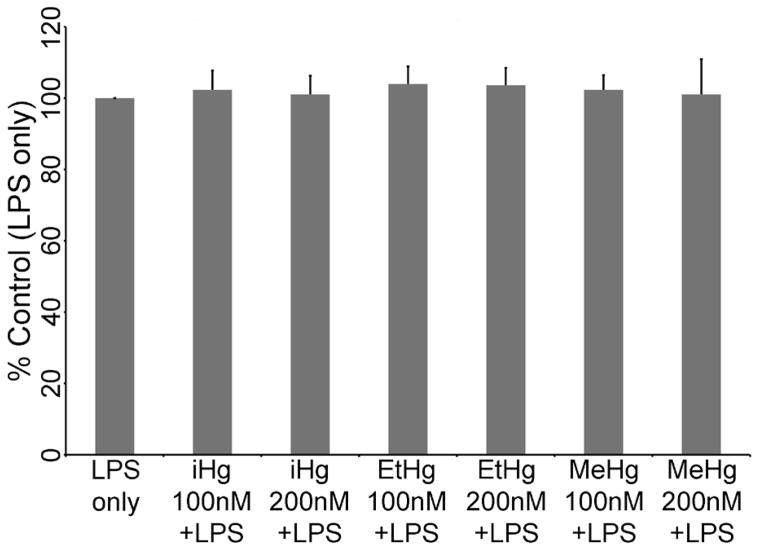
To ensure that any effects of any of the mercury species used in this study were not due to non-specific cytotoxicity, we measured cell viability in PBMCs from each of the six volunteers for cells treated with LPS only, and with LPS in addition to 100 nM or 200 nM of each species of mercury. As seen in Figure 1, these concentrations of iHg, EtHg, and MeHg do not cause significant changes to cell viability as compared to cells treated with LPS (ANOVA p-values: iHg = 0.697, EtHg = 0.421, MeHg = 0.598) after 24 or 48 (data not shown) hours in culture.
Figure 1. Low concentrations of inorganic, ethyl, or methylmercury do not affect cell viability.
PBMCs isolated from six volunteers were treated with the specified concentrations of each mercury species (up to 200 nM) in the presence of 50 ng/ml LPS. Cell viability was measured by MTT assay. The optical density for cells treated with LPS only was set to be 100% viability, and each treatment condition is compared to this control. No statistically significant changes in viability were observed.
3.2. iHg, MeHg, and EtHg treatment differentially regulate the release of pro-inflammatory cytokines in the presence of LPS
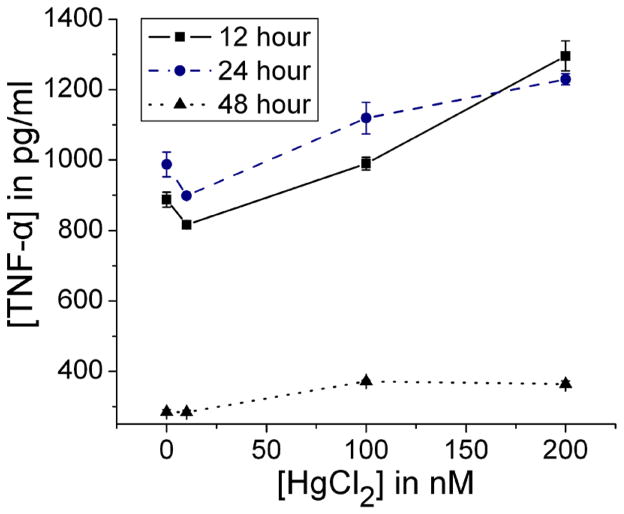
A cell culture time period of 24 hours was selected for experiments based on production of the pro-inflammatory cytokine TNF-α at 12, 24, and 48 hours using cells from a male volunteer (Figure 2).
Figure 2. TNF-α release by PBMCs over time.
PBMCs isolated from a male volunteer were treated with iHg (up to 200 nM) in the presence of 50 ng/ml LPS. TNF-α was measured in cell culture supernatants after 12, 24, and 48 hours in culture. Error bars represent the standard error of the mean. TNF-α signficantly increased in a concentration response at 12 (P[Hg]<0.001), 24 (P[Hg]<0.001), and 48 (P[Hg]<0.001) hours, though TNF-α levels were higher at the earlier time points.
As expected and previously reported for iHg (Gardner et al., 2009), levels of cytokine production were low in the absence of LPS. No changes were observed in the release of any cytokine as a result of treatment with any species of mercury tested in the absence of LPS treatment (Table 1). 93% of samples had an IL-1β below the LOD in the absence of LPS, so statistical analysis was not attempted. Non-detect values were distributed throughout all treatment groups.
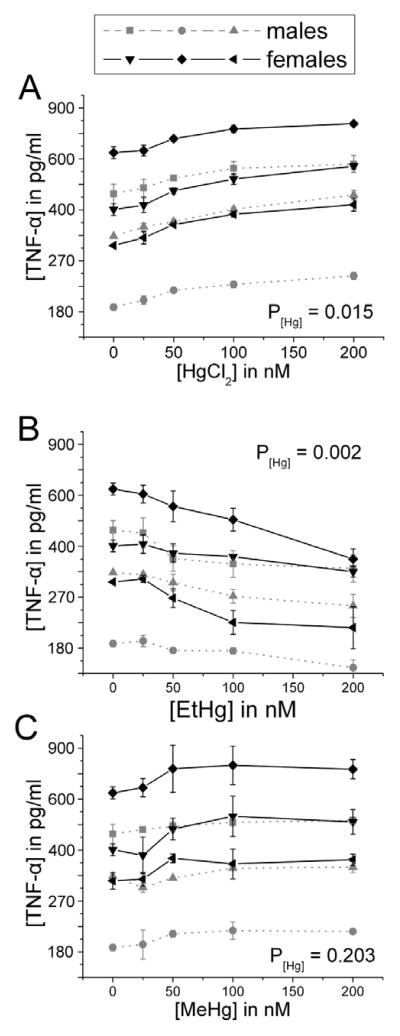
In the presence of LPS, iHg caused a concentration-dependent increase in the release of TNF-α and IL-1β by PBMCs (Figures 3A and 4A). MeHg caused a significant increase in release of IL-1β only (Figure 4C); while MeHg-associated increases in TNF-α were not statistically significant (Figure 3C). In contrast, EtHg caused a concentration -dependent decrease in the release of both TNF-α and IL -1β (Figures 3B and 4B).
Figure 3. TNF-α release increases in a concentration-dependent manner in response to iHg, but decreases in response to EtHg treatment.
PBMCs isolated from six volunteers were treated with the specified concentrations of each mercury species (up to 200 nM) in the presence of 50 ng/ml LPS. TNF-α was measured in cell culture supernatants after 24 hours in culture. Results are shown for cells treated with iHg (A), EtHg (B), and MeHg (C). P[Hg] represents the p-value for the trend in Hg effect in the concentration-response curve. Error bars represent the standard error of the mean. MeHg does not significantly affect TNF-α release, where as EtHg significantly down-regulates its release and iHg significantly up-regulates its release.
Figure 4. IL-1β release increases in a concentration-dependent manner in response to iHg and MeHg, but decreases in response to EtHg treatment.
PBMCs isolated from six volunteers were treated with the specified concentrations of each mercury species (up to 200 nM) in the presence of 50 ng/ml LPS. IL-1β was measured in cell culture supernatants after 24 hours in culture. Results are shown for cells treated with iHg (A), EtHg (B), and MeHg (C). P[Hg] represents the p-value for the trend in Hg effect in the concentration-response curve. Error bars represent the standard error of the mean. iHg and MeHg significantly up-regulate IL-1β release, whereas EtHg significantly down-regulates its release.
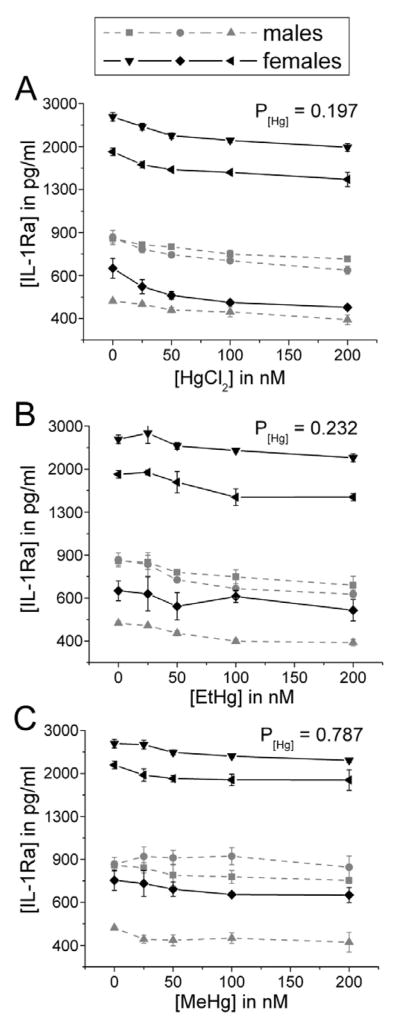
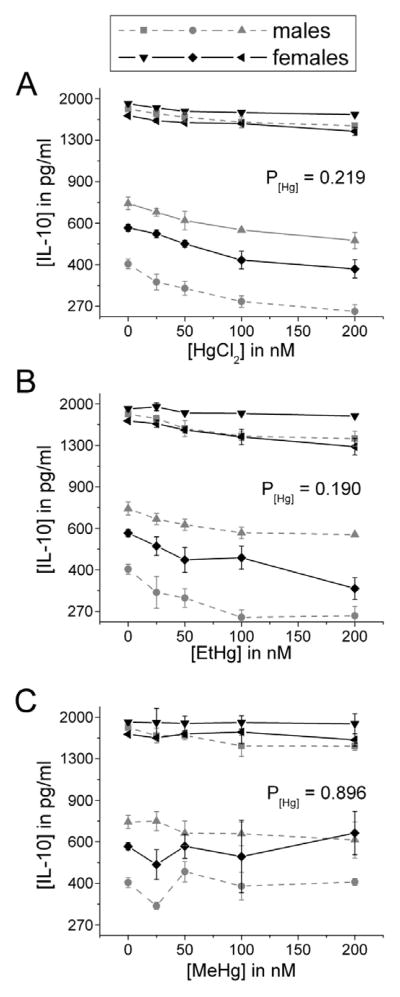
In contrast to pro-inflammatory cytokine release, treatment with these Hg species did not significantly affect LPS-stimulated release of the anti -inflammatory cytokines IL-1Ra and IL-10 (Figures 5 and 6). This unopposed pro-inflammatory cytokine release is consistent with our previous findings on the effects of iHg (Gardner etal., 2009).
Figure 5. IL-1Ra release is not significantly affected by treatment with iHg, EtHg, or MeHg.
PBMCs isolated from six volunteers were treated with the specified concentrations of each mercury species (up to 200 nM) in the presence of 50 ng/ml LPS. IL-1Ra was measured in cell culture supernatants after 24 hours in culture. Results are shown for cells treated with iHg (A), EtHg (B), and MeHg (C). P[Hg] represents the p-value for the trend in Hg effect in the concentration-response curve. Error bars represent the standard error of the mean.
Figure 6. IL-10 release is not significantly affected by treatment with iHg, EtHg, or MeHg.
PBMCs isolated from six volunteers were treated with the specified concentrations of each mercury species (up to 200 nM) in the presence of 50 ng/ml LPS. IL-10 was measured in cell culture supernatants after 24 hours in culture. Results are shown for cells treated with iHg (A), EtHg (B), and MeHg (C). P[Hg] represents the p-value for the trend in Hg effect in the concentration-response curve. Error bars represent the standard error of the mean.
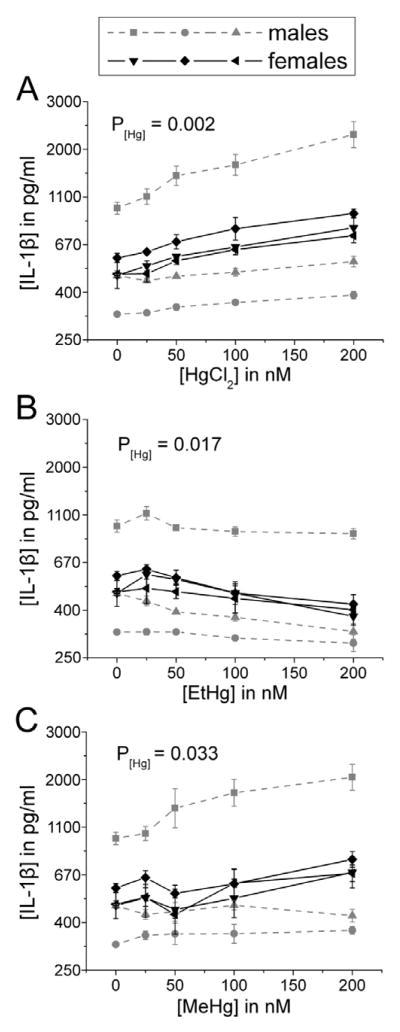
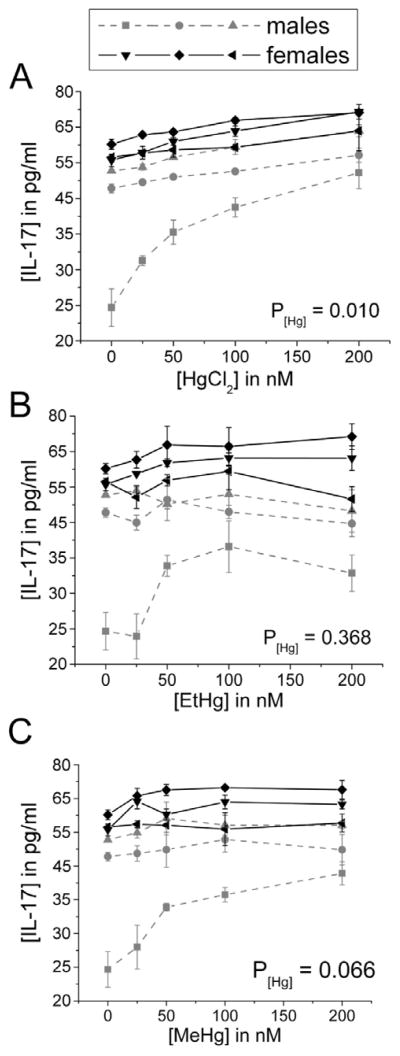
3.3. IFN-γ, IL-4, and IL-17 are differentially regulated by inorganic compared to organic Hg
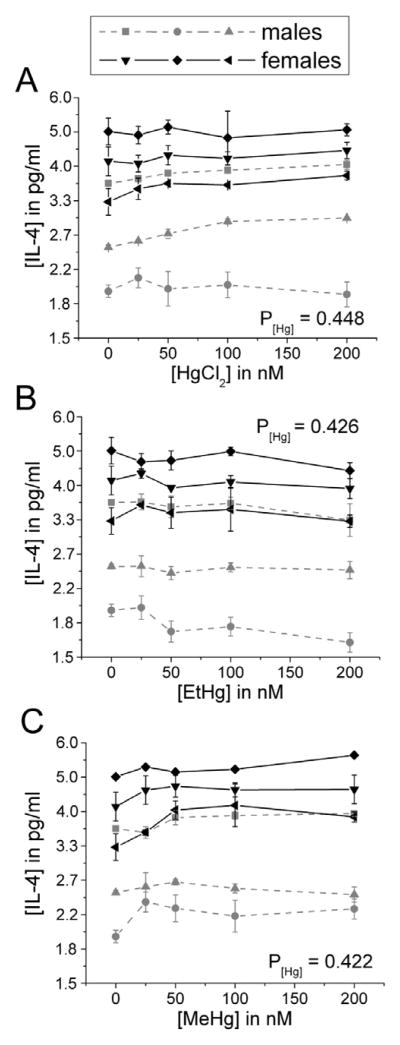
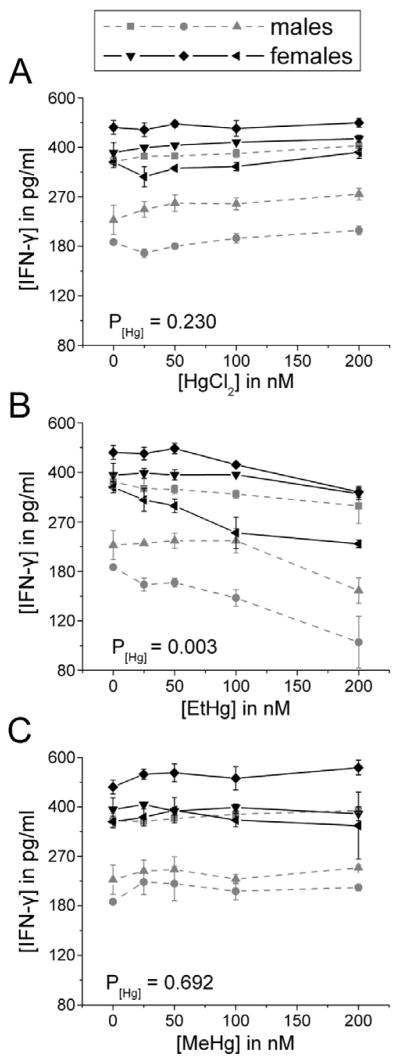
There was no effect of any Hg species on the production of IL-4 by immune cells in the presence of LPS (Figure 7). Only EtHg affected IFN-γ release (decrease) (Figure 8) and only iHg treatment increased the release of IL-17 by PBMCs (Figure 9).
Figure 7. IL-4 release is not significantly affected by treatment with iHg, EtHg, or MeHg.
PBMCs isolated from six volunteers were treated with the specified concentrations of each mercury species (up to 200 nM) in the presence of 50 ng/ml LPS. IL-4 was measured in cell culture supernatants after 24 hours in culture. Results are shown for cells treated with iHg (A), EtHg (B), and MeHg (C). P[Hg] represents the p-value for the trend in Hg effect in the concentration-response curve. Error bars represent the standard error of the mean.
Figure 8. IFN-γ release decreases in response to EtHg treatment.
PBMCs isolated from six volunteers were treated with the specified concentrations of each mercury species (up to 200 nM) in the presence of 50 ng/ml LPS. IFN-γ was measured in cell culture supernatants after 24 hours in culture. Results are shown for cells treated with iHg (A), EtHg (B), and MeHg (C). P[Hg] represents the p-value for the trend in Hg effect in the concentration-response curve. EtHg significantly down-regulates release of IFN-γ. Error bars represent the standard error of the mean.
Figure 9. IL-17 release increases in a concentration-dependent manner in response to iHg treatment.
PBMCs isolated from six volunteers were treated with the specified concentrations of each mercury species (up to 200 nM) in the presence of 50 ng/ml LPS. IL-17 was measured in cell culture supernatants after 24 hours in culture. Results are shown for cells treated with iHg (A), EtHg (B), and MeHg (C). P[Hg] represents the p-value for the trend in Hg effect in the concentration-response curve. Error bars represent the standard error of the mean.
In the absence of LPS stimulation, these cytokine levels were low, and no changes were seen as a result of treatment with any species of Hg. Of the non -LPS treated PBMCs, 44% of samples had an IL-4 concentration below the LOD, and 48% of samples had an IL-17 concentration below the limit of detection. Statistical analyses were conducted with these samples assigned a value of the LOD/√2 (See Table 1).
4. Discussion
In this study, we report that in vitro exposures of human PBMCs to iHg, EtHg, and MeHg treatment differentially affect cytokine production in the presence of LPS. We confirmed that all concentrations used in the treatment of cells for all Hg species tested neither increased nor decreased cell viability, thus ruling out a non-specific effect of any Hg species on cytokine production. Our previous studies with iHg demonstrated that this effect was unrelated to changes in cell populations (Gardner et al., 2009); these Hg species primarily acted on the production of pro-inflammatory cytokines. The release of the pro-inflammatory cytokines TNF-α and IL-1β is significantly increased in response to iHg treatment in the presence of LPS. MeHg has similar, but attenuated, effects on pro-inflammatory cytokine production compared to iHg treatment.
In contrast to iHg and MeHg, EtHg causes a significant decrease in TNF -α and IL-1β release in the presence of LPS. EtHg also causes a significant decrease in IFN-γ production, whereas iHg and MeHg had no detectable effect. This suggests that low concentrations of EtHg can significantly shift the balance of cytokine response toward an immunologically inappropriate pattern without typical co-expression of both pro- and anti-inflammatory responses, resulting in a relatively unopposed inflammatory response.
These findings are consistent with studies in experimental animals. Co-exposure to the immune stimulus LPS, can shift susceptibility to the immunotoxic effects of iHg so that non-susceptible mouse strains become susceptible and susceptible strains experience an exacerbation of the iHg-induced autoimmune disease (Abedi-Valugerdi et al., 2005). In genetically-susceptible mice MeHg induced signs of autoimmunity similar to iHg, but with less potency (Haggqvist et al., 2005; Hultman and Hansson-Georgiadis, 1999). This may be due to the kinetics of MeHg metabolism to iHg in the lymph nodes of exposed mice (Havarinasab et al., 2007). Because phagocytic immune cells (including human monocytes) can demethylate MeHg to iHg in vitro (Suda et al., 1992), we speculate that a similar metabolism of MeHg may have occurred in our in vitro culture of human PBMCs, though further studies are required to confirm this hypothesis.
None of the Hg species significantly affected anti-inflammatory cytokine production. The expression of these cytokines is typically co-regulated with pro-inflammatory cytokines as their expression is normally up-regulated concurrently with or shortly after up-regulation of pro-inflammatory responses, preventing tissue damage resulting from unchecked inflammation (Dinarello, 1996; Moore et al., 2001). In evaluating the effects of LPS alone on PMBCs, we observed physiologically-appropriate increases in the release of both pro- and anti-inflammatory cytokines as measured by appropriate production of both pro- and anti-inflammatory cytokine responses. The fact that we did not observe this balance in the presence of iHg and MeHg suggest that there may be an effect on intra-or intercellular signaling to prevent a concomitant up-regulation in anti-inflammatory or regulatory cytokines in the presence of increased pro-inflammatory cytokine production.
The effects of Hg on cytokine release were dependent upon the activation state of the cell, as no significant changes were observed in these parameters in the absence of LPS stimulation. These data are supported by studies in animal models (Silbergeld et al., 2000)in which exposure to mercury impaired host resistance to malaria. Further, epidemiological studies of persons with autoimmune diseases have indicated that while there is a heritable component to disease susceptibility, there is also substantial evidence that environmental exposure to both pathogens and xenobiotics contribute to risk of autoimmune disease (Cooper et al., 1999; Rioux and Abbas, 2005). Inappropriate early innate responses to common pathogens have been hypothesized to contribute to the breaking of self-tolerance in the etiology of autoimmune disease (Bach, 2005). The evidence presented here clearly indicates that Hg acts as an immunotoxicant by dysregulating physiologically-appropriate responses to stimuli, suggesting that Hg exposure may lead to similar dysregulation of the immune response to pathogens in exposed persons. This study provides evidence that Hg exposure can affect human immune function within the range of exposures found within populations worldwide, and that a major effect of Hg exposure is the dysregulation of signal transduction pathways, leading to excessive inflammatory cytokine release in the case of iHg and MeHg exposure and suppression of immune responses in the case of EtHg. Globally, human populations are exposed primarily to MeHg as compared to other species of Hg. While the effects that we reported for MeHg are generally more subtle than those of iHg and are different from those of EtHg, they may have the greatest implications for public health given the ubiquitous nature of MeHg exposure and the eventual metabolism of MeHg to iHg in vivo. The immunotoxic effects of MeHg exposure, in terms of both exacerbation of inflammatory diseases and risks of autoimmunity, must be evaluated in population-based studies so that the risks of MeHg exposure to human health can be appropriately assessed.
Previously we reported on the effects of Hg amalgam and fish consumption in this model system in a larger number of subjects and found no significant effect of fish consumption and a slight negative effect of Hg amalgam on response to iHg treatment in terms of pro-inflammatory cytokine response (Gardner et al., 2009). Given the small number of subjects in this study, modeling the effect Hg amalgam on in vitro cytokine response was not attempted, although we do not believe that the effect would have been significant.
The effects of iHg and MeHg to increase pro-inflammatory cytokine release may be relevant to some specific effects of mercury compounds in humans. A significant association has been observed between elevated MeHg exposures resulting from fish consumption and increased risk for myocardial infarction despite the cardio-protective effects of fish consumption (Guallar et al., 2002). There is increased information supporting an inflammatory basis of cardiovascular disease, etiology, and progression (Apostolakis et al., 2008; Boyle, 2005; Dinarello, 1996). Our results, which indicate that Hg exposure may dysregulate inflammatory signals within the human immune system, further supports the need to investigate interactions among fish consumption, Hg exposure, biomarkers of inflammation, and cardiovascular disease risk.
Acknowledgments
Funding
This research was supported by grants from Cure Autism Now and the United States National Institute of Environmental Health Sciences (Grant numbers 1R21ES014857-01, ES07141, K99/R00ES015426).
Footnotes
Conflict of interest
The authors declare that there are not conflicts of interest.
Contributor Information
Renee M. Gardner, Email: rgardner@jhsph.edu.
Jennifer F. Nyland, Email: jnyland@uscmed.sc.edu.
Ellen K. Silbergeld, Email: esilberg@jhsph.edu.
References
- Abedi-Valugerdi M, Nilsson C, Zargari A, Gharibdoost F, DePierre JW, Hassan M. Bacterial lipopolysaccharide both renders resistant mice susceptible to mercury-induced autoimmunity and exacerbates such autoimmunity in susceptible mice. Clin Exp Immunol. 2005;141:238–247. doi: 10.1111/j.1365-2249.2005.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves MF, Fraiji NA, Barbosa AC, De Lima DS, Souza JR, Dorea JG, Cordeiro GW. Fish consumption, mercury exposure and serum antinuclear antibody in Amazonians. Int J Environ Health Res. 2006;16:255–262. doi: 10.1080/09603120600734147. [DOI] [PubMed] [Google Scholar]
- Apostolakis S, Vogiatzi K, Krambovitis E, Spandidos DA. IL-1 cytokines in cardiovascular disease: diagnostic, prognostic and therapeutic implications. Cardiovasc Hematol Agents Med Chem. 2008;6:150–158. doi: 10.2174/187152508783955006. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Reveille JD, Goldstein R, Pollard KM, Leaird K, Smith EA, Leroy EC, Fritzler MJ. Autoantibodies to fibrillarin in systemic sclerosis (scleroderma). An immunogenetic, serologic, and clinical analysis. Arthritis Rheum. 1996;39:1151–1160. doi: 10.1002/art.1780390712. [DOI] [PubMed] [Google Scholar]
- Bach JF. Infections and autoimmune diseases. J Autoimmun. 2005;25(Suppl):74–80. doi: 10.1016/j.jaut.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005;3:63–68. doi: 10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ Health Perspect. 2005;113:1015–1021. doi: 10.1289/ehp.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Miller FW, Pandey JP. The role of genetic factors in autoimmune disease: implications for environmental research. Environ Health Perspect. 1999;107(Suppl 5):693–700. doi: 10.1289/ehp.99107s5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol. 2004;31:1928–1933. [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Evans SL, Wang SB, Doyle KM, Crainiceanu CM, Silbergeld EK. Mercury induces an unopposed inflammatory response in human peripheral blood mononuclear cells in vitro. Environ Health Perspect. 2009;117:1932–1938. doi: 10.1289/ehp.0900855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brasil: a cross-sectional study. Environ Res. 2010;110:345–354. doi: 10.1016/j.envres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:519–525. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gomez-Aracena J, Kark JD, Riemersma RA, Martin-Moreno JM, Kok FJ. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Haggqvist B, Havarinasab S, Bjorn E, Hultman P. The immunosuppressive effect of methylmercury does not preclude development of autoimmunity in genetically susceptible mice. Toxicology. 2005;208:149–164. doi: 10.1016/j.tox.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, Bjorn E, Nielsen JB, Hultman P. Mercury species in lymphoid and non-lymphoid tissues after exposure to methyl mercury: correlation with autoimmune parameters during and after treatment in susceptible mice. Toxicol Appl Pharmacol. 2007;221:21–28. doi: 10.1016/j.taap.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, Haggqvist B, Bjorn E, Pollard KM, Hultman P. Immunosuppressive and autoimmune effects of thimerosal in mice. Toxicol Appl Pharmacol. 2005;204:109–121. doi: 10.1016/j.taap.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, Hultman P. Organic mercury compounds and autoimmunity. Autoimmun Rev. 2005;4:270–275. doi: 10.1016/j.autrev.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hu H, Moller G, Abedi-Valugerdi M. Mechanism of mercury-induced autoimmunity: both T helper 1-and T helper 2 -type responses are involved. Immunology. 1999;96:348–357. doi: 10.1046/j.1365-2567.1999.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman P, Hansson-Georgiadis H. Methyl mercury-induced autoimmunity in mice. Toxicol Appl Pharmacol. 1999;154:203–211. doi: 10.1006/taap.1998.8576. [DOI] [PubMed] [Google Scholar]
- Johansson U, Sander B, Hultman P. Effects of the murine genotype on T cell activation and cytokine production in murine mercury-induced autoimmunity. J Autoimmun. 1997;10:347–355. doi: 10.1006/jaut.1997.0149. [DOI] [PubMed] [Google Scholar]
- Kono DH, Balomenos D, Pearson DL, Park MS, Hildebrandt B, Hultman P, Pollard KM. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN-gamma and not Th1/Th2 imbalance. J Immunol. 1998;161:234–240. [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.) Board on Environmental Studies and Toxicology. Toxicological effects of methylmercury. National Academy Press; Washington, DC: 2000. p. xvii.p. 344. [Google Scholar]
- Nielsen JB, Hultman P. Mercury-induced autoimmunity in mice. Environ Health Perspect. 2002;110(Suppl 5):877–881. doi: 10.1289/ehp.02110s5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichichero ME, Cernichiari E, Lopreiato J, Treanor J. Mercury concentrations and metabolism in infants receiving vaccines containing thiomersal: a descriptive study. Lancet. 2002;360:1737–1741. doi: 10.1016/S0140-6736(02)11682-5. [DOI] [PubMed] [Google Scholar]
- Pollard KM, Hultman P, Kono DH. Immunology and genetics of induced systemic autoimmunity. Autoimmun Rev. 2005;4:282–288. doi: 10.1016/j.autrev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Pollard KM, Landberg GP. The in vitro proliferation of murine lymphocytes to mercuric chloride is restricted to mature T cells and is interleukin 1 dependent. Int Immunopharmacol. 2001;1:581–593. doi: 10.1016/s1567-5769(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Pollard KM, Pearson DL, Hultman P, Deane TN, Lindh U, Kono DH. Xenobiotic acceleration of idiopathic systemic autoimmunity in lupus-prone bxsb mice. Environ Health Perspect. 2001;109:27–33. doi: 10.1289/ehp.0110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt MD, Belsito DV, DeLeo VA, Fowler JF, Jr, Fransway AF, Maibach HI, Marks JG, Mathias CG, Rietschel RL, Sasseville D, Sherertz EF, Storrs FJ, Taylor JS, Zug K. North American Contact Dermatitis Group patch-test results, 2001–2002 study period. Dermatitis. 2004;15:176–183. [PubMed] [Google Scholar]
- Rioux JD, Abbas AK. Paths to understanding the genetic basis of autoimmune disease. Nature. 2005;435:584–589. doi: 10.1038/nature03723. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Sacci JB, Jr, Azad AF. Mercury exposure and murine response to Plasmodium yoelii infection and immunization. Immunopharmacol Immunotoxicol. 2000;22:685–695. doi: 10.3109/08923970009016432. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Silva IA, Nyland JF. Mercury and autoimmunity: implications for occupational and environmental health. Toxicol Appl Pharmacol. 2005;207:282–292. doi: 10.1016/j.taap.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Silva IA, Nyland JF, Gorman A, Perisse A, Ventura AM, Santos EC, de Souza JM, Burek CL, Rose NR, Silbergeld EK. Mercury exposure, malaria, and serum antinuclear/antinucleolar antibodies in amazon populations in Brazil: a cross-sectional study. Environ Health. 2004;3:11–22. doi: 10.1186/1476-069X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slodownik D, Ingber A. Thimerosal--is it really irrelevant? Contact Dermatitis. 2005;53:324–326. doi: 10.1111/j.0105-1873.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- Suda I, Hirayama K. Degradation of methyl and ethyl mercury into inorganic mercury by hydroxyl radical produced from rat liver microsomes. Arch Toxicol. 1992;66:398–402. doi: 10.1007/BF02035129. [DOI] [PubMed] [Google Scholar]
- Suda I, Totoki S, Uchida T, Takahashi H. Degradation of methyl and ethyl mercury into inorganic mercury by various phagocytic cells. Arch Toxicol. 1992;66:40–44. doi: 10.1007/BF02307268. [DOI] [PubMed] [Google Scholar]
- Vahter M, Mottet NK, Friberg L, Lind B, Shen DD, Burbacher T. Speciation of mercury in the primate blood and brain following long-term exposure to methyl mercury. Toxicol Appl Pharmacol. 1994;124:221–229. doi: 10.1006/taap.1994.1026. [DOI] [PubMed] [Google Scholar]
- Wetter DA, Davis MD, Yiannias JA, Cheng JF, Connolly SM, el-Azhary RA, Farmer SA, Fett DD, Johnson JS, Linehan DL, Richardson DM, Schroeter AL. Patch test results from the Mayo Clinic Contact Dermatitis Group, 1998–2000. J Am Acad Dermatol. 2005;53:416–421. doi: 10.1016/j.jaad.2005.04.077. [DOI] [PubMed] [Google Scholar]
- Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–593. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- Zdolsek JM, Soder O, Hultman P. Mercury induces in vivo and in vitro secretion of interleukin-1 in mice. Immunopharmacology. 1994;28:201–208. doi: 10.1016/0162-3109(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Zoller L, Bergman R, Weltfriend S. Preservatives sensitivity in Israel: a 10-year overview (1995–2004) Contact Dermatitis. 2006;55:227–229. doi: 10.1111/j.1600-0536.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- Zug KA, McGinley-Smith D, Warshaw EM, Taylor JS, Rietschel RL, Maibach HI, Belsito DV, Fowler JF, Jr, Storrs FJ, DeLeo VA, Marks JG, Jr, Mathias CG, Pratt MD, Sasseville D. Contact allergy in children referred for patch testing: North American Contact Dermatitis Group data, 2001–2004. Arch Dermatol. 2008;144:1329–1336. doi: 10.1001/archderm.144.10.1329. [DOI] [PubMed] [Google Scholar]